Highly Permeable Matrimid®/PIM-EA(H2)-TB Blend Membrane for Gas Separation
Abstract
1. Introduction
2. Materials and Methods
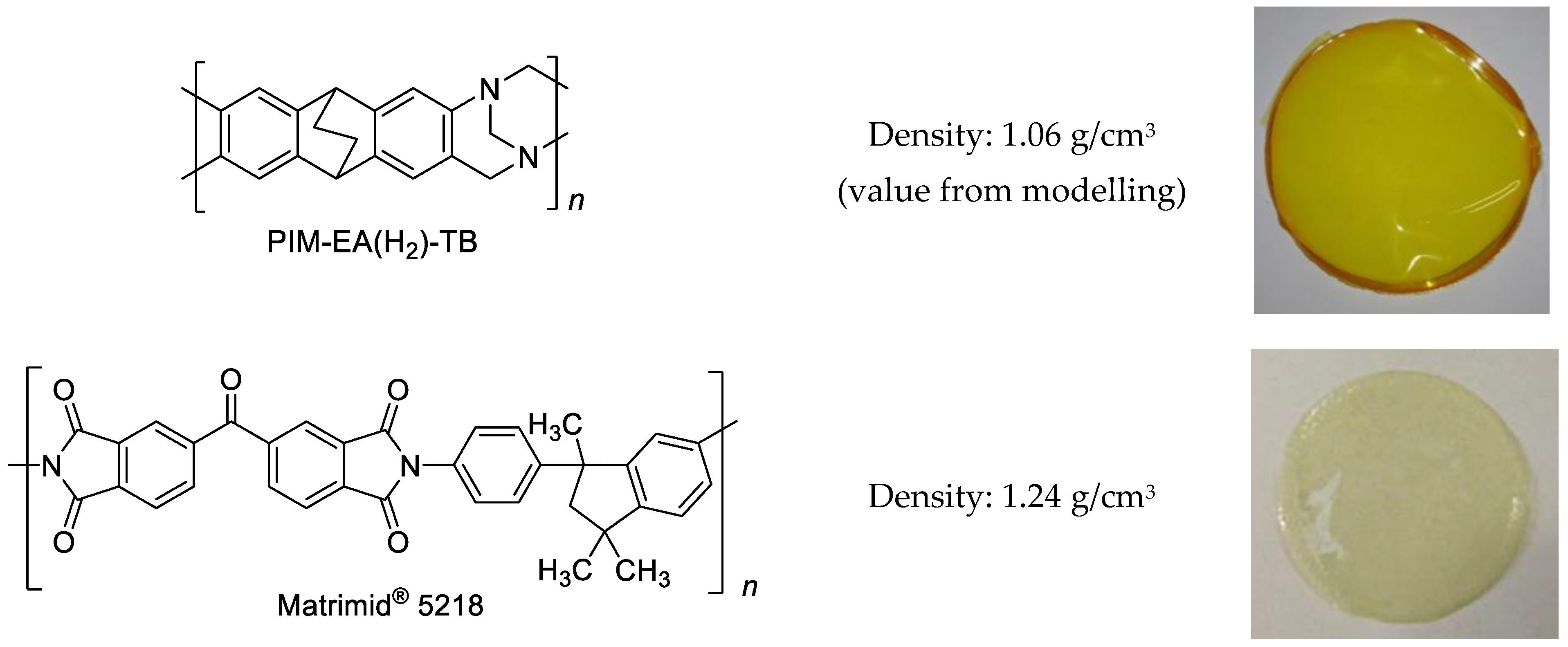
2.1. Materials
2.2. Preparation of Matrimid®5218/PIM-EA(H2)-TB Blend Membranes
2.3. Membranes Characterization
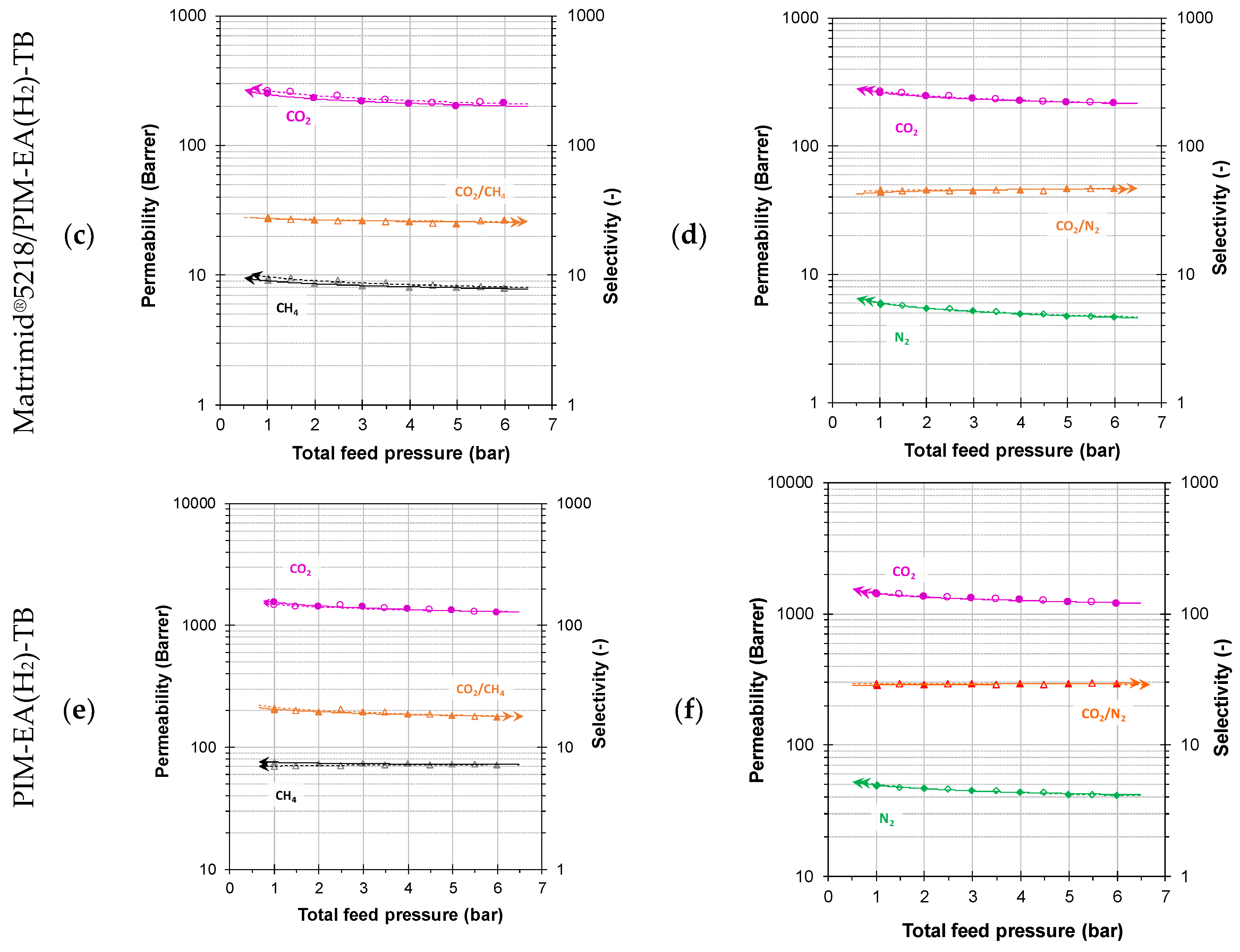
3. Results and Discussion
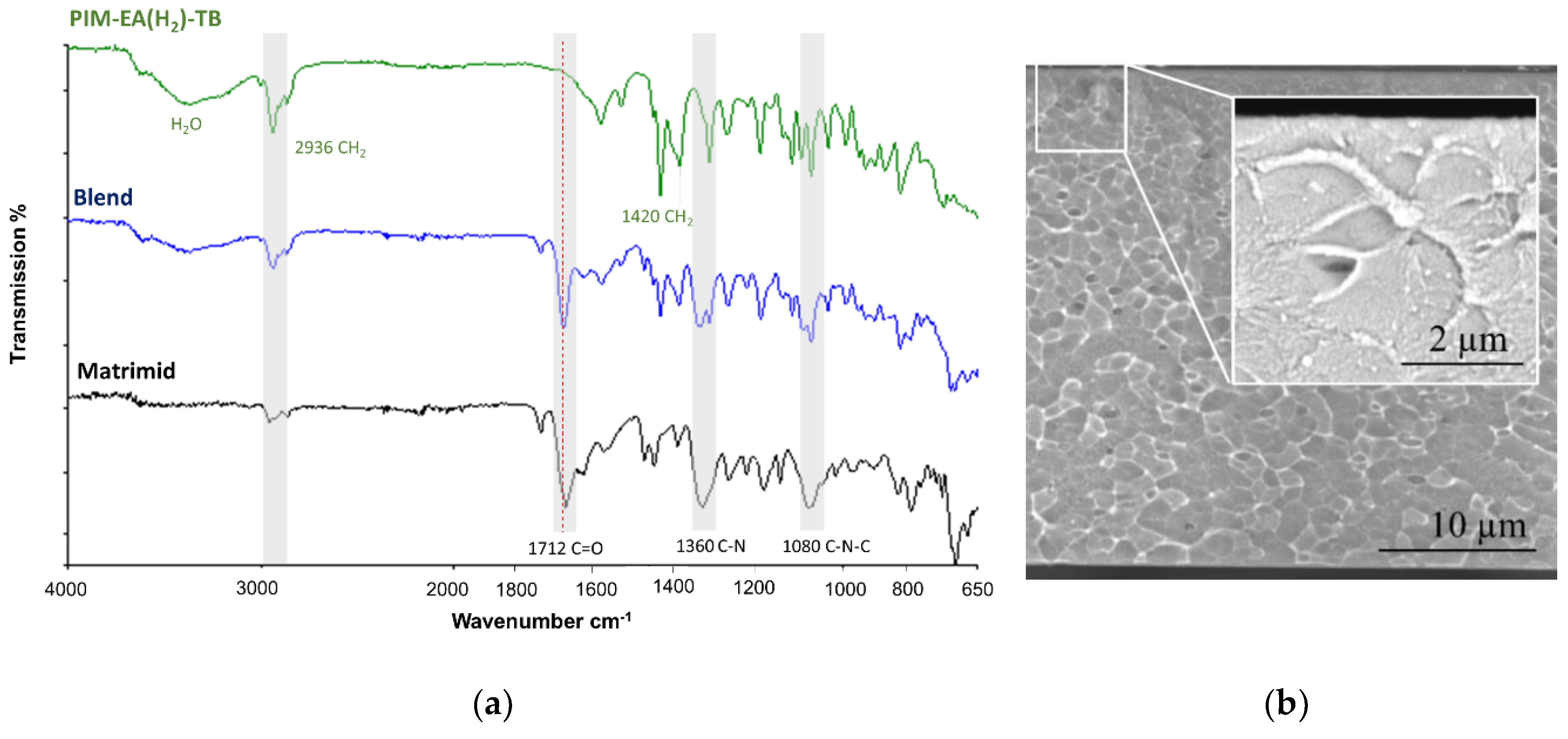
3.1. Chemical and Morphological Characterization
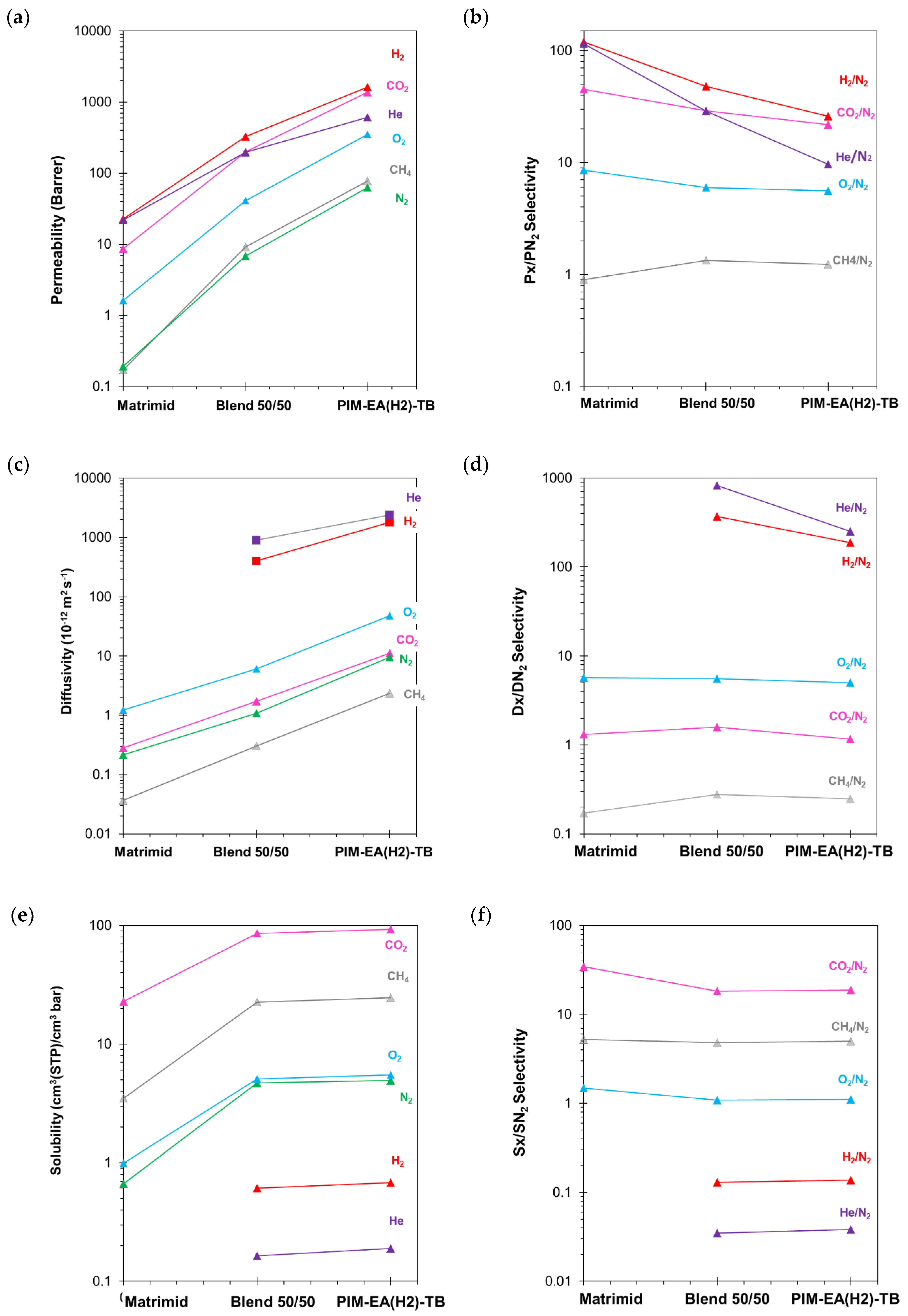
3.2. Pure Gas Transport Properties
3.3. Mixed Gas Transport Properties
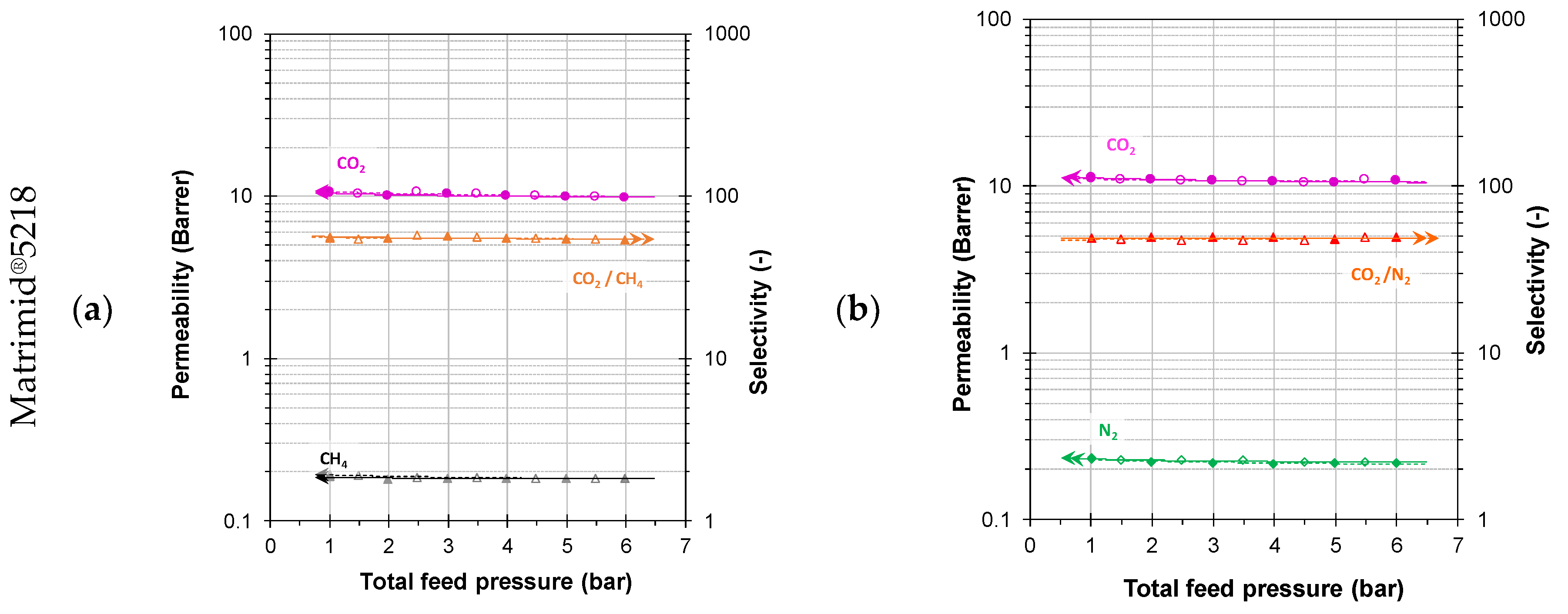
3.3.1. Mixed Gas Permeability
3.3.2. Mixed Gas Diffusion and Solubility
3.4. Robeson Plots and Comparison with Literature Blend-Data
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A. Mixed Gas Permeation Data
| Matrimid | Pressure | P, (Barrer) | Selectivity (−) | Pressure | P, (Barrer) | Selectivity (−) | ||
| (bar(a)) | CH4 | CO2 | CO2/CH4 | (bar(a)) | N2 | CO2 | CO2/N2 | |
| 1 | 0.19 | 10.5 | 55.7 | 1 | 0.23 | 11.3 | 48.5 | |
| 2 | 0.18 | 10.0 | 55.6 | 2 | 0.22 | 11.0 | 49.8 | |
| 3 | 0.18 | 10.3 | 56.7 | 3 | 0.22 | 10.8 | 49.3 | |
| 4 | 0.18 | 10.1 | 55.3 | 4 | 0.22 | 10.7 | 49.3 | |
| 5 | 0.18 | 9.9 | 54.0 | 5 | 0.22 | 10.4 | 48.0 | |
| 6 | 0.18 | 9.8 | 53.6 | 6 | 0.22 | 10.8 | 49.4 | |
| 5.5 | 0.18 | 9.9 | 54.3 | 5.5 | 0.22 | 10.9 | 49.5 | |
| 4.5 | 0.18 | 10.1 | 55.3 | 4.5 | 0.22 | 10.5 | 47.3 | |
| 3.5 | 0.18 | 10.3 | 56.3 | 3.5 | 0.23 | 10.7 | 47.1 | |
| 2.5 | 0.19 | 10.6 | 57.2 | 2.5 | 0.23 | 10.8 | 47.7 | |
| 1.5 | 0.19 | 10.4 | 54.4 | 1.5 | 0.23 | 11.0 | 48.3 | |
| 1 | 0.19 | 10.6 | 55.2 | 1 | 0.23 | 11.1 | 48.5 | |
| Matrimid®5218/PIM-EA(H2)-TB blend | Pressure | P, (Barrer) | Selectivity (−) | Pressure | P, (Barrer) | Selectivity (−) | ||
| (bar(a)) | CH4 | CO2 | CO2/CH4 | (bar(a)) | N2 | CO2 | CO2/N2 | |
| 1 | 9.09 | 250 | 27.5 | 1 | 6.0 | 260 | 43.5 | |
| 2 | 8.59 | 231 | 26.8 | 2 | 5.4 | 246 | 45.3 | |
| 3 | 8.29 | 217 | 26.2 | 3 | 5.2 | 236 | 45.0 | |
| 4 | 8.09 | 210 | 25.9 | 4 | 5.0 | 225 | 45.6 | |
| 5 | 8.08 | 200 | 24.8 | 5 | 4.7 | 219 | 46.6 | |
| 6 | 7.91 | 212 | 26.8 | 6 | 4.6 | 217 | 47.0 | |
| 5.5 | 8.16 | 215 | 26.4 | 5.5 | 4.7 | 219 | 46.7 | |
| 4.5 | 8.46 | 213 | 25.2 | 4.5 | 4.9 | 224 | 45.2 | |
| 3.5 | 8.70 | 226 | 26.0 | 3.5 | 5.2 | 233 | 45.3 | |
| 2.5 | 9.09 | 240 | 26.4 | 2.5 | 5.4 | 244 | 45.2 | |
| 1.5 | 9.48 | 257 | 27.1 | 1.5 | 5.7 | 258 | 45.0 | |
| 1 | 9.46 | 263 | 27.8 | 1 | 5.8 | 265 | 45.5 | |
| PIM-EA(H2)-TB | Pressure | P, (Barrer) | Selectivity (−) | Pressure | P, (Barrer) | Selectivity (−) | ||
| (bar(a)) | CH4 | CO2 | CO2/CH4 | (bar(a)) | N2 | CO2 | CO2/N2 | |
| 1 | 75.7 | 1540 | 20.3 | 1 | 49.6 | 1410 | 28.4 | |
| 2 | 73.4 | 1430 | 19.5 | 2 | 47.1 | 1350 | 28.7 | |
| 3 | 73.7 | 1430 | 19.4 | 3 | 44.8 | 1320 | 29.4 | |
| 4 | 73.4 | 1370 | 18.7 | 4 | 43.4 | 1280 | 29.5 | |
| 5 | 72.6 | 1320 | 18.2 | 5 | 42.0 | 1230 | 29.4 | |
| 6 | 72.1 | 1270 | 17.6 | 6 | 41.1 | 1200 | 29.2 | |
| 5.5 | 72.2 | 1290 | 17.8 | 5.5 | 41.6 | 1230 | 29.5 | |
| 4.5 | 71.6 | 1340 | 18.8 | 4.5 | 43.7 | 1260 | 28.8 | |
| 3.5 | 71.6 | 1390 | 19.4 | 3.5 | 44.9 | 1290 | 28.8 | |
| 2.5 | 71.1 | 1460 | 20.6 | 2.5 | 46.1 | 1340 | 29.1 | |
| 1.5 | 71.1 | 1430 | 20.2 | 1.5 | 47.7 | 1410 | 29.5 | |
| 1 | 69.5 | 1460 | 21.0 | 1 | 48.6 | 1430 | 29.4 | |
References
- Robeson, L.M. Correlation of separation factor versus permeability for polymeric membranes. J. Memb. Sci. 1991, 62, 165–185. [Google Scholar] [CrossRef]
- Robeson, L.M. The upper bound revisited. J. Memb. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Swaidan, R.; Ghanem, B.; Pinnau, I. Fine-Tuned Intrinsically Ultramicroporous Polymers Redefine the Permeability/Selectivity Upper Bounds of Membrane-Based Air and Hydrogen Separations. ACS Macro Lett. 2015, 4, 947–951. [Google Scholar] [CrossRef]
- Freeman, B.D. Basis of permeability/selectivity tradeoff relations in polymeric gas separation membranes. Macromolecules 1999, 32, 375–380. [Google Scholar] [CrossRef]
- Budd, P.M.; Elabas, E.S.; Ghanem, B.S.; Makhseed, S.; McKeown, N.B.; Msayib, K.J.; Tattershall, C.E.; Wang, D. Solution-Processed, Organophilic Membrane Derived from a Polymer of Intrinsic Microporosity. Adv. Mater. 2004, 16, 456–459. [Google Scholar] [CrossRef]
- Williams, R.; Burt, L.A.; Esposito, E.; Jansen, J.C.; Tocci, E.; Rizzuto, C.; Lanč, M.; Carta, M.; McKeown, N.B. A highly rigid and gas selective methanopentacene-based polymer of intrinsic microporosity derived from Tröger’s base polymerization. J. Mater. Chem. A 2018, 6, 5661–5667. [Google Scholar] [CrossRef]
- Rose, I.; Bezzu, C.G.; Carta, M.; Comesanã-Gándara, B.; Lasseuguette, E.; Ferrari, M.C.; Bernardo, P.; Clarizia, G.; Fuoco, A.; Jansen, J.C.; et al. Polymer ultrapermeability from the inefficient packing of 2D chains. Nat. Mater. 2017, 16, 932–937. [Google Scholar] [CrossRef] [PubMed]
- Carta, M.; Malpass-Evans, R.; Croad, M.; Rogan, Y.; Jansen, J.C.; Bernardo, P.; Bazzarelli, F.; McKeown, N.B. An Efficient Polymer Molecular Sieve for Membrane Gas Separations. Science 2013, 339, 303–307. [Google Scholar] [CrossRef]
- Paul, D.R.; Barlow, J.W. A binary interaction model for miscibility of copolymers in blends. Polymer 1984, 25, 487–494. [Google Scholar] [CrossRef]
- Mannan, H.A.; Mukhtar, H.; Murugesan, T.; Nasir, R.; Mohshim, D.F.; Mushtaq, A. Recent applications of polymer blends in gas separation membranes. Chem. Eng. Technol. 2013, 36, 1838–1846. [Google Scholar] [CrossRef]
- Grobelny, J.; Rice, D.M.; Karasz, F.E.; MacKnight, W.J. High-resolution solid-state carbon-13 nuclear magnetic resonance study of polybenzimidazole/polyimide blends. Macromolecules 1990, 23, 2139–2144. [Google Scholar] [CrossRef]
- Chung, T.S.; Guo, W.F.; Liu, Y. Enhanced Matrimid membranes for pervaporation by homogenous blends with polybenzimidazole (PBI). J. Memb. Sci. 2006, 271, 221–231. [Google Scholar] [CrossRef]
- Jansen, J.C.; Darvishmanesh, S.; Tasselli, F.; Bazzarelli, F.; Bernardo, P.; Tocci, E.; Friess, K.; Randova, A.; Drioli, E.; Van der Bruggen, B. Influence of the blend composition on the properties and separation performance of novel solvent resistant polyphenylsulfone/polyimide nanofiltration membranes. J. Memb. Sci. 2013, 447, 107–118. [Google Scholar] [CrossRef]
- Han, J.; Lee, W.; Choi, J.M.; Patel, R.; Min, B.-R. Characterization of polyethersulfone/polyimide blend membranes prepared by a dry/wet phase inversion: Precipitation kinetics, morphology and gas separation. J. Memb. Sci. 2010, 351, 141–148. [Google Scholar] [CrossRef]
- Kapantaidakis, G.C.; Kaldis, S.P.; Dabou, X.S.; Sakellaropoulos, G.P. Gas permeation through PSF-PI miscible blend membranes. J. Memb. Sci. 1996, 110, 239–247. [Google Scholar] [CrossRef]
- Kapantaidakis, G.C.; Koops, G.H. High flux polyethersulfone–polyimide blend hollow fiber membranes for gas separation. J. Memb. Sci. 2002, 204, 153–171. [Google Scholar] [CrossRef]
- Basu, S.; Cano-Odena, A.; Vankelecom, I.F.J. Asymmetric membrane based on Matrimid® and polysulphone blends for enhanced permeance and stability in binary gas (CO2/CH4) mixture separations. Sep. Purif. Technol. 2010, 75, 15–21. [Google Scholar] [CrossRef]
- Rafiq, S.; Man, Z.; Maitra, S.; Maulud, A.; Ahmad, F.; Muhammad, N. Preparation of asymmetric polysulfone/polyimide blended membranes for CO2 separation. Korean J. Chem. Eng. 2011, 28, 2050–2056. [Google Scholar] [CrossRef]
- Budd, P.M.; McKeown, N.B.; Ghanem, B.S.; Msayib, K.J.; Fritsch, D.; Starannikova, L.; Belov, N.; Sanfirova, O.; Yampolskii, Y.; Shantarovich, V. Gas permeation parameters and other physicochemical properties of a polymer of intrinsic microporosity: Polybenzodioxane PIM-1. J. Memb. Sci. 2008, 325, 851–860. [Google Scholar] [CrossRef]
- Mason, C.R.; Maynard-Atem, L.; Al-Harbi, N.M.; Budd, P.M.; Bernardo, P.; Bazzarelli, F.; Clarizia, G.; Jansen, J.C. Polymer of intrinsic microporosity incorporating thioamide functionality: Preparation and gas transport properties. Macromolecules 2011, 44, 6471–6479. [Google Scholar] [CrossRef]
- Bezzu, C.G.; Carta, M.; Tonkins, A.; Jansen, J.C.; Bernardo, P.; Bazzarelli, F.; Mckeown, N.B. A spirobifluorene-based polymer of intrinsic microporosity with improved performance for gas separation. Adv. Mater. 2012, 24, 5930–5933. [Google Scholar] [CrossRef] [PubMed]
- Mason, C.R.; Maynard-Atem, L.; Heard, K.W.J.; Satilmis, B.; Budd, P.M.; Friess, K.; Lanč, M.; Bernardo, P.; Clarizia, G.; Jansen, J.C. Enhancement of CO2 Affinity in a Polymer of Intrinsic Microporosity by Amine Modification. Macromolecules 2014, 47, 1021–1029. [Google Scholar] [CrossRef] [PubMed]
- Carta, M.; Croad, M.; Malpass-Evans, R.; Jansen, J.C.; Bernardo, P.; Clarizia, G.; Friess, K.; Lanč, M.; McKeown, N.B. Triptycene Induced Enhancement of Membrane Gas Selectivity for Microporous Tröger’s Base Polymers. Adv. Mater. 2014, 26, 3526–3531. [Google Scholar] [CrossRef] [PubMed]
- Bezzu, C.G.; Carta, M.; Ferrari, M.-C.; Jansen, J.C.; Monteleone, M.; Esposito, E.; Fuoco, A.; Hart, K.; Liyana-Arachchi, T.P.; Colina, C.M.; et al. The synthesis, chain-packing simulation and long-term gas permeability of highly selective spirobifluorene-based polymers of intrinsic microporosity. J. Mater. Chem. A 2018, 6. [Google Scholar] [CrossRef]
- Du, N.; Robertson, G.P.; Song, J.; Pinnau, I.; Guiver, M.D. High-performance carboxylated polymers of intrinsic microporosity (PIMs) with tunable gas transport properties. Macromolecules 2009, 42, 6038–6043. [Google Scholar] [CrossRef]
- Du, N.; Robertson, G.P.; Pinnau, I.; Guiver, M.D. Polymers of intrinsic microporosity derived from novel disulfone-based monomers. Macromolecules 2009. [Google Scholar] [CrossRef]
- Du, N.; Robertson, G.P.; Pinnau, I.; Guiver, M.D. Polymers of intrinsic microporosity with dinaphthyl and thianthrene segments. Macromolecules 2010. [Google Scholar] [CrossRef]
- Swaidan, R.; Ghanem, B.S.; Litwiller, E.; Pinnau, I. Pure- and mixed-gas CO2/CH4 separation properties of PIM-1 and an amidoxime-functionalized PIM-1. J. Memb. Sci. 2014, 457, 95–102. [Google Scholar] [CrossRef]
- Ma, C.; Urban, J.J. Polymers of Intrinsic Microporosity (PIMs) Gas Separation Membranes: A mini Review. Proc. Nat. Res. Soc. 2018, 2, 2002. [Google Scholar] [CrossRef]
- Yong, W.F.; Lee, Z.K.; Chung, T.S. Blends of a polymer of intrinsic microporosity and partially sulfonated polyphenylenesulfone for Gas separation. ChemSusChem 2016, 9, 1953–1962. [Google Scholar] [CrossRef]
- Salehian, P.; Yong, W.F.; Chung, T.-S. Development of high performance carboxylated PIM-1/P84 blend membranes for pervaporation dehydration of isopropanol and CO2/CH4 separation. J. Memb. Sci. 2016, 518, 110–119. [Google Scholar] [CrossRef]
- Yong, W.F.; Li, F.Y.; Xiao, Y.C.; Li, P.; Pramoda, K.P.; Tong, Y.W.; Chung, T.S. Molecular engineering of PIM-1/Matrimid blend membranes for gas separation. J. Memb. Sci. 2012, 407–408, 47–57. [Google Scholar] [CrossRef]
- Yong, W.F.; Li, F.Y.; Chung, T.S.; Tong, Y.W. Molecular interaction, gas transport properties and plasticization behavior of cPIM-1/Torlon blend membranes. J. Memb. Sci. 2014, 462, 119–130. [Google Scholar] [CrossRef]
- Yong, W.F.; Chung, T.S. Miscible blends of carboxylated polymers of intrinsic microporosity (cPIM-1) and Matrimid. Polymer 2015, 59, 290–297. [Google Scholar] [CrossRef]
- Yong, W.F.; Li, F.Y.; Xiao, Y.C.; Chung, T.S.; Tong, Y.W. High performance PIM-1/Matrimid hollow fiber membranes for CO2/CH4, O2/N2 and CO2/N2 separation. J. Memb. Sci. 2013, 443, 156–169. [Google Scholar] [CrossRef]
- Zhao, S.; Liao, J.; Li, D.; Wang, X.; Li, N. Blending of compatible polymer of intrinsic microporosity (PIM-1) with Tröger’s Base polymer for gas separation membranes. J. Memb. Sci. 2018, 566, 77–86. [Google Scholar] [CrossRef]
- Bernardo, P.; Scorzafave, V.; Clarizia, G.; Tocci, E.; Jansen, J.C.; Borgogno, A.; Malpass-Evans, R.; McKeown, N.B.; Carta, M.; Tasselli, F. Thin film composite membranes based on a polymer of intrinsic microporosity derived from Tröger’s base: A combined experimental and computational investigation of the role of residual casting solvent. J. Memb. Sci. 2019, 569, 17–31. [Google Scholar] [CrossRef]
- Benito, J.; Sánchez-Laínez, J.; Zornoza, B.; Martín, S.; Carta, M.; Malpass-Evans, R.; Téllez, C.; McKeown, N.B.; Coronas, J.; Gascón, I. Ultrathin Composite Polymeric Membranes for CO2/N2 Separation with Minimum Thickness and High CO2 Permeance. ChemSusChem 2017, 10, 4014–4017. [Google Scholar] [CrossRef]
- Benito, J.; Vidal, J.; Sánchez-Laínez, J.; Zornoza, B.; Téllez, C.; Martín, S.; Msayib, K.J.; Comesaña-Gándara, B.; McKeown, N.B.; Coronas, J.; et al. The fabrication of ultrathin films and their gas separation performance from polymers of intrinsic microporosity with two-dimensional (2D) and three-dimensional (3D) chain conformations. J. Colloid Interface Sci. 2019, 536, 474–482. [Google Scholar] [CrossRef]
- Sanchez-Lainez, J.; Zornoza, B.; Carta, M.; Malpass-Evans, R.; McKeown, N.B.; Téllez, C.; Coronas, J. Hydrogen Separation at High Temperature with Dense and Asymmetric Membranes based on PIM-EA(H2)-TB/PBI Blends. Ind. Eng. Chem. Res. 2018, 57, 16909–16916. [Google Scholar] [CrossRef]
- Carta, M.; Croad, M.; Jansen, J.C.; Bernardo, P.; Clarizia, G.; McKeown, N.B. Synthesis of cardo-polymers using Tröger’s base formation. Polym. Chem. 2014, 5, 5255. [Google Scholar] [CrossRef]
- Weigelt, F.; Georgopanos, P.; Shishatskiy, S.; Filiz, V.; Brinkmann, T.; Abetz, V. Development and characterization of defect-free Matrimid® mixed-matrix membranes containing activated carbon particles for gas separation. Polymers 2018, 10, 51. [Google Scholar] [CrossRef]
- Jansen, J.C.; Friess, K.; Drioli, E. Organic vapour transport in glassy perfluoropolymer membranes: A simple semi-quantitative approach to analyze clustering phenomena by time lag measurements. J. Memb. Sci. 2011, 367, 141–151. [Google Scholar] [CrossRef]
- Crank, J. The Mathematics of Diffusion, 2nd ed.; Clarendon Press: Oxford, UK, 1975; ISBN 0198533446. [Google Scholar]
- Fraga, S.C.; Monteleone, M.; Lanč, M.; Esposito, E.; Fuoco, A.; Giorno, L.; Pilnáček, K.; Friess, K.; Carta, M.; McKeown, N.B.; et al. A novel time lag method for the analysis of mixed gas diffusion in polymeric membranes by on-line mass spectrometry: Method development and validation. J. Memb. Sci. 2018, 561, 39–58. [Google Scholar] [CrossRef]
- Monteleone, M.; Esposito, E.; Fuoco, A.; Lanč, M.; Pilnáček, K.; Friess, K.; Bezzu, C.G.; Carta, M.; McKeown, N.B.; Jansen, J.C. A novel time lag method for the analysis of mixed gas diffusion in polymeric membranes by on-line mass spectrometry: Pressure dependence of transport parameters. Membranes 2018, 8, 73. [Google Scholar] [CrossRef] [PubMed]
- Pilnáček, K.; Vopička, O.; Lanč, M.; Dendisová, M.; Zgažar, M.; Budd, P.M.; Carta, M.; Malpass-Evans, R.; McKeown, N.B.; Friess, K. Aging of polymers of intrinsic microporosity tracked by methanol vapour permeation. J. Memb. Sci. 2016, 520, 895–906. [Google Scholar] [CrossRef]
- Robeson, L.M. Polymer blends in membrane transport processes. Ind. Eng. Chem. Res. 2010, 49, 11859–11865. [Google Scholar] [CrossRef]
- Lanč, M.; Pilnáček, K.; Mason, C.R.; Budd, P.M.; Rogan, Y.; Malpass-Evans, R.; Carta, M.; Gándara, B.C.; McKeown, N.B.; Jansen, J.C.; et al. Gas sorption in polymers of intrinsic microporosity: The difference between solubility coefficients determined via time-lag and direct sorption experiments. J. Memb. Sci. 2019, 570–571, 522–536. [Google Scholar] [CrossRef]
 Matrimid®5218, X PIM-EA(H2)-TB and Matrimid®5218/PIM blend membranes for CO2/N2 (a), CO2/CH4 (b), O2/N2 (c) and H2/CH4 (d) gas pairs (pure gas filled symbols and Mixed gas open symbols). Reference data for other matrimid-based blends (■) and other PIM-based blends (■) are plotted for comparison.
Matrimid®5218, X PIM-EA(H2)-TB and Matrimid®5218/PIM blend membranes for CO2/N2 (a), CO2/CH4 (b), O2/N2 (c) and H2/CH4 (d) gas pairs (pure gas filled symbols and Mixed gas open symbols). Reference data for other matrimid-based blends (■) and other PIM-based blends (■) are plotted for comparison.
 Matrimid®5218, X PIM-EA(H2)-TB and Matrimid®5218/PIM blend membranes for CO2/N2 (a), CO2/CH4 (b), O2/N2 (c) and H2/CH4 (d) gas pairs (pure gas filled symbols and Mixed gas open symbols). Reference data for other matrimid-based blends (■) and other PIM-based blends (■) are plotted for comparison.
Matrimid®5218, X PIM-EA(H2)-TB and Matrimid®5218/PIM blend membranes for CO2/N2 (a), CO2/CH4 (b), O2/N2 (c) and H2/CH4 (d) gas pairs (pure gas filled symbols and Mixed gas open symbols). Reference data for other matrimid-based blends (■) and other PIM-based blends (■) are plotted for comparison.

| Permeability (Barrer) | Selectivity (Px/Py) | ||||||||||||
| Membrane | N2 | CH4 | O2 | He | H2 | CO2 | H2/N2 | He/N2 | O2/N2 | CH4/N2 | O2/CH4 | CO2/CH4 | CO2/N2 |
| Matrimid | 0.19 | 0.17 | 1.63 | 21.9 | 22.8 | 8.62 | 120 | 115 | 8.54 | 0.90 | 9.54 | 50.6 | 45.3 |
| Blend | 6.83 | 9.14 | 41.0 | 197 | 328 | 198 | 48.0 | 28.8 | 6.00 | 1.34 | 4.48 | 21.6 | 29.0 |
| PIM-EA(H2)-TB | 62.8 | 77.6 | 350 | 606 | 1630 | 1380 | 25.9 | 9.64 | 5.57 | 1.23 | 4.51 | 17.7 | 21.9 |
| Diffusivity (10−12 m2/s) | Diffusion selectivity (Dx/Dy) | ||||||||||||
| Membrane | N2 | CH4 | O2 | He | H2 | CO2 | H2/N2 | He/N2 | O2/N2 | CH4/N2 | O2/CH4 | CO2/CH4 | CO2/N2 |
| Matrimid | 0.21 | 0.04 | 1.23 | 0.28 | 5.74 | 0.17 | 33.6 | 7.69 | 1.31 | ||||
| Blend | 1.09 | 0.30 | 6.04 | 900 | 403 | 1.73 | 370 | 826 | 5.54 | 0.28 | 19.9 | 5.71 | 1.59 |
| PIM-EA(H2)-TB | 9.6 | 2.36 | 47.9 | 2400 | 1790 | 11.2 | 187 | 251 | 5.01 | 0.25 | 20.3 | 4.72 | 1.17 |
| Solubility (cm3(STP)/cm3 bar) | Solubility selectivity (Sx/Sy) | ||||||||||||
| Membrane | N2 | CH4 | O2 | He | H2 | CO2 | H2/N2 | He/N2 | O2/N2 | CH4/N2 | O2/CH4 | CO2/CH4 | CO2/N2 |
| Matrimid | 0.67 | 3.49 | 0.99 | 22.9 | 1.49 | 5.24 | 0.28 | 6.58 | 34.4 | ||||
| Blend | 4.70 | 22.6 | 5.08 | 0.16 | 0.61 | 85.7 | 0.13 | 0.03 | 1.08 | 4.81 | 0.22 | 3.79 | 18.2 |
| PIM-EA(H2)-TB | 4.93 | 24.6 | 5.47 | 0.19 | 0.68 | 92.5 | 0.14 | 0.04 | 1.11 | 5.00 | 0.22 | 3.76 | 18.8 |
| Permeability (Barrer) | Px/Py Selectivity (−) | |||||
|---|---|---|---|---|---|---|
| CO2 | CH4 | N2 | CO2/CH4 | CO2/N2 | ||
| Matrimid®5218 | Pure gas | 10.4 | 0.20 | 0.24 | 52 | 43.3 |
| Mix (CO2/CH4) | 10.5 | 0.19 | - | 55 | - | |
| Mix (CO2/N2) | 11.3 | - | 0.23 | - | 49.1 | |
| Blend | Pure gas | 198 | 9.1 | 6.83 | 21.66 | 28.99 |
| Mix (CO2/CH4) | 250 | 9.09 | - | 27.49 | - | |
| Mix (CO2/N2) | 260 | - | 6.00 | - | 43.37 | |
| PIM-EA(H2)-TB | Pure gas | 1391 | 62.6 | 53.1 | 22.22 | 26.20 |
| Mix (CO2/CH4) | 1527 | 76.1 | - | 20.07 | - | |
| Mix (CO2/N2) | 1445 | - | 49.4 | - | 29.25 | |
| Diffusivity (10−12 m2 s−1) | Dx/Dy Selectivity (−) | |||||
| CO2 | CH4 | N2 | CO2/CH4 | CO2/N2 | ||
| Matrimid®5218 | Pure gas | 0.35 | 0.05 | 0.28 | 7.04 | 1.24 |
| Mix (CO2/CH4) | 0.29 | 0.06 | - | 5.12 | - | |
| Mix (CO2/N2) | 0.24 | - | 0.30 | - | 0.80 | |
| Blend | Pure gas | 2.36 | 0.46 | 1.58 | 5.71 | 1.59 |
| Mix (CO2/CH4) | 2.10 | 0.66 | - | 3.18 | - | |
| Mix (CO2/N2) | 1.40 | - | 2.11 | - | 0.61 | |
| PIM-EA(H2)-TB | Pure gas | 10.7 | 2.31 | 9.22 | 4.63 | 1.16 |
| Mix (CO2/CH4) | 10.6 | 3.61 | - | 2.94 | - | |
| Mix (CO2/N2) | 7.14 | - | 10.5 | - | 0.68 | |
| Solubility (cm3(STP) cm−3 bar−1) | Sx/Sy Selectivity (−) | |||||
| CO2 | CH4 | N2 | CO2/CH4 | CO2/N2 | ||
| Matrimid®5218 | Pure gas | 22.2 | 3.00 | 0.64 | 7.39 | 34.8 |
| Mix (CO2/CH4) | 27.0 | 2.50 | - | 10.8 | - | |
| Mix (CO2/N2) | 34.9 | - | 0.57 | - | 61.5 | |
| Blend | Pure gas | 85.8 | 22.6 | 4.70 | 3.79 | 18.3 |
| Mix (CO2/CH4) | 89.2 | 10.3 | - | 8.66 | - | |
| Mix (CO2/N2) | 138 | - | 1.94 | - | 71.0 | |
| PIM-EA(H2)-TB | Pure gas | 97.5 | 20.3 | 4.32 | 4.80 | 22.3 |
| Mix (CO2/CH4) | 109 | 15.7 | - | 6.91 | - | |
| Mix (CO2/N2) | 148 | - | 3.53 | - | 42.0 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esposito, E.; Mazzei, I.; Monteleone, M.; Fuoco, A.; Carta, M.; McKeown, N.B.; Malpass-Evans, R.; Jansen, J.C. Highly Permeable Matrimid®/PIM-EA(H2)-TB Blend Membrane for Gas Separation. Polymers 2019, 11, 46. https://doi.org/10.3390/polym11010046
Esposito E, Mazzei I, Monteleone M, Fuoco A, Carta M, McKeown NB, Malpass-Evans R, Jansen JC. Highly Permeable Matrimid®/PIM-EA(H2)-TB Blend Membrane for Gas Separation. Polymers. 2019; 11(1):46. https://doi.org/10.3390/polym11010046
Chicago/Turabian StyleEsposito, Elisa, Irene Mazzei, Marcello Monteleone, Alessio Fuoco, Mariolino Carta, Neil B. McKeown, Richard Malpass-Evans, and Johannes C. Jansen. 2019. "Highly Permeable Matrimid®/PIM-EA(H2)-TB Blend Membrane for Gas Separation" Polymers 11, no. 1: 46. https://doi.org/10.3390/polym11010046
APA StyleEsposito, E., Mazzei, I., Monteleone, M., Fuoco, A., Carta, M., McKeown, N. B., Malpass-Evans, R., & Jansen, J. C. (2019). Highly Permeable Matrimid®/PIM-EA(H2)-TB Blend Membrane for Gas Separation. Polymers, 11(1), 46. https://doi.org/10.3390/polym11010046











