Solubility Difference between Pectic Fractions from Creeping Fig Seeds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction of WEP
2.3. Fractionation of WEP by DEAE Fast Flow Chromatography
2.4. General Physicochemical Properties Analysis and Solubility
2.5. X-ray Diffraction (XRD) Analysis
2.6. Environmental Scanning Electron Microscopy (ESEM) Analysis
2.7. FT-IR Spectroscopy
2.8. Mineral Analysis
2.9. Statistical Analysis
3. Results
3.1. Fractionation of WEP
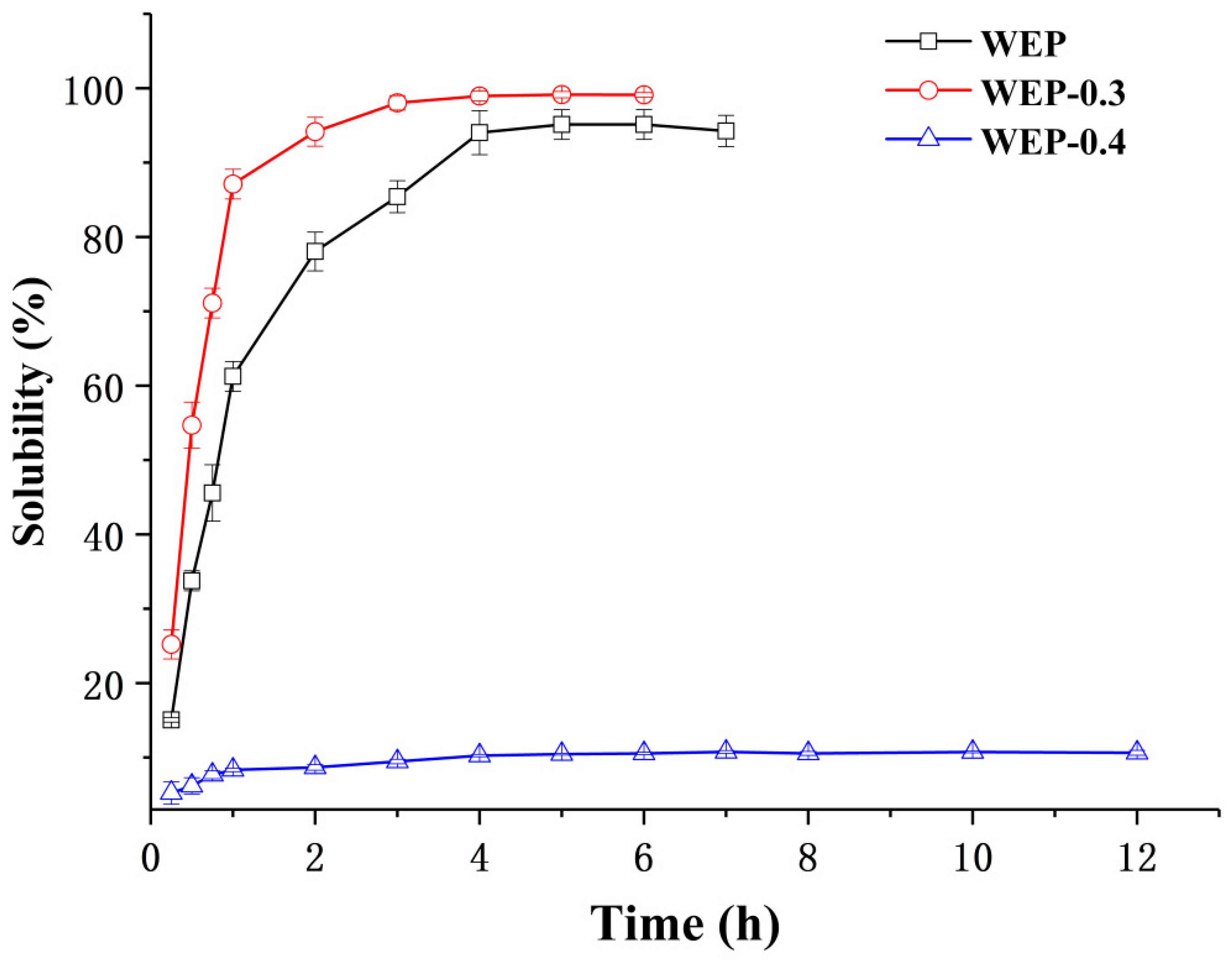
3.2. Solubility
3.3. General Physicochemical Properties Analysis
3.4. X-ray Diffraction Analysis
3.5. Environmental Scanning Electron Microscope (ESEM)
3.6. FT-IR Spectrum Characterization
3.7. Mineral Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Taboada, E.; Fisher, P.; Jara, R.; Zúñiga, E.; Gidekel, M.; Cabrera, J.C.; Pereira, E.; Gutiérrez-Moraga, A.; Villalonga, R.; Cabrera, G. Isolation and characterisation of pectic substances from murta (Ugni molinae Turcz) fruits. Food Chem. 2010, 123, 669–678. [Google Scholar] [CrossRef]
- Grassino, A.N.; Barba, F.J.; Brnčić, M.; Lorenzo, J.M.; Lucini, L.; Brnčić, S.R. Analytical tools used for the identification and quantification of pectin extracted from plant food matrices, wastes and by-products: A review. Food Chem. 2018, 266, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Ridley, B.L.; O’Neill, M.A.; Mohnen, D. Pectins: Structure, biosynthesis, and oligogalacturonide-related signaling. Phytochemistry 2001, 57, 929–967. [Google Scholar] [CrossRef]
- Christiaens, S.; Uwibambe, D.; Uyttebroek, M.; Droogenbroeck, B.V.; Loey, A.M.V.; Hendrickx, M.E. Pectin characterisation in vegetable waste streams: A starting point for waste valorisation in the food industry. LWT—Food Sci. Technol. 2015, 61, 275–282. [Google Scholar] [CrossRef]
- Kumar, A.; Garg, S. Immobilization of enzymes and biotechnological perspective. In Biotechnology Applications; Mishra, S.K., Champaign, P., Eds.; IK International, New. Delhi. Publisher: Delhi, India, 2009; pp. 39–52. [Google Scholar]
- Marić, M.; Grassino, A.N.; Zhu, Z.; Brnčić, M.; Brnčić, S.R. An overview of the traditional and innovative approaches for pectin extraction from plant food wastes and by-products: Ultrasound-, microwaves-, and enzyme-assisted extraction. Trends Food Sci. Technol. 2018, 76, 28–37. [Google Scholar] [CrossRef]
- Shi, X.; Chang, K.; Schwarz, J.; Wiesenborn, D.; Shih, M. Optimizing pectin extraction from sunflower heads by alkaline washing. Bioresour. Technol. 1996, 58, 291–297. [Google Scholar] [CrossRef]
- Guo, X.; Han, D.; Xi, H.; Rao, L.; Liao, X.; Hu, X.; Wu, J. Extraction of pectin from navel orange peel assisted by ultra-high pressure, microwave or traditional heating: A comparison. Carbohydr. Polym. 2012, 88, 441–448. [Google Scholar] [CrossRef]
- El-Nawawi, S.; Heikal, Y. Production of a low ester pectin by de-esterification of high ester citrus pectin. Carbohydr. Polym. 1995, 27, 191–195. [Google Scholar] [CrossRef]
- Renard, C.M.; Thibault, J.-F. Degradation of pectins in alkaline conditions: Kinetics of demethylation. Carbohydr. Res. 1996, 286, 139–150. [Google Scholar] [CrossRef]
- Ralet, M.-C.; Dronnet, V.; Buchholt, H.C.; Thibault, J.-F. Enzymatically and chemically de-esterified lime pectins: Characterisation, polyelectrolyte behaviour and calcium binding properties. Carbohydr. Res. 2001, 336, 117–125. [Google Scholar] [CrossRef]
- Kim, W.; Smit, C.; Rao, V. Demethylation of pectin using acid and ammonia. J. Food Sci. 1978, 43, 74–78. [Google Scholar] [CrossRef]
- Liang, R.-H.; Chen, J.; Liu, W.; Liu, C.-M.; Yu, W.; Yuan, M.; Zhou, X.-Q. Extraction, characterization and spontaneous gel-forming property of pectin from creeping fig (Ficus pumila Linn.) seeds. Carbohydr. Polym. 2012, 87, 76–83. [Google Scholar] [CrossRef]
- Sun, Y.; Tang, J.; Gu, X.; Li, D. Water-soluble polysaccharides from Angelica sinensis (Oliv.) Diels: Preparation, characterization and bioactivity. Int. J. Biol. Macromol. 2005, 36, 283–289. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Blumenkrantz, N.; Asboe-Hansen, G. New method for quantitative determination of uronic acids. Anal. Biochem. 1973, 54, 484–489. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Monsoor, M.A. Effect of drying methods on the functional properties of soy hull pectin. Carbohydr. Polym. 2005, 61, 362–367. [Google Scholar] [CrossRef]
- Chen, J.; Liang, R.-H.; Liu, W.; Liu, C.-M.; Li, T.; Tu, Z.-C.; Wan, J. Degradation of high-methoxyl pectin by dynamic high pressure microfluidization and its mechanism. Food Hydrocoll. 2012, 28, 121–129. [Google Scholar] [CrossRef]
- Gnanasambandam, R.; Proctor, A. Determination of pectin degree of esterification by diffuse reflectance Fourier transform infrared spectroscopy. Food Chem. 2000, 68, 327–332. [Google Scholar] [CrossRef]
- Jie, C.; Sarma, B.; Evans, J.M.B.; Myerson, A.S. Pharmaceutical Crystallization. Cryst. Growth Des. 2011, 11, 887–895. [Google Scholar] [CrossRef]
- Whistler, R.L. Solubility of Polysaccharides and Their Behavior in Solution; ACS Publications: Washington, DC, USA, 1973. [Google Scholar]
- Agata, Z.; Cédric, G.; Alain, B.; Bruno, P.; Catherine, G.; Jean-Francois, T.; Marie-Christine, R. Assessment of in vitro binding of isolated pectic domains to cellulose by adsorption isotherms, electron microscopy, and X-ray diffraction methods. Biomacromolecules 2007, 8, 223–232. [Google Scholar] [CrossRef]
- Sharma, R.; Kamboj, S.; Khurana, R.; Singh, G.; Rana, V. Physicochemical and functional performance of pectin extracted by QbD approach from Tamarindus indica L. pulp. Carbohyd. Polym. 2015, 134, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Du, Y.; Zhu, X.; Xiong, H.; Meng, W.W.; Hu, J. Physicochemical and comparative properties of pectins extracted from Akebia trifoliata var. australis peel. Carbohyd. Polym. 2012, 87, 1663–1669. [Google Scholar] [CrossRef]
- Kravtchenko, T.; Renoir, J.; Parker, A.; Brigand, G. A novel method for determining the dissolution kinetics of hydrocolloid powders. Food Hydrocoll. 1999, 13, 219–225. [Google Scholar] [CrossRef]
- Parker, A.; Vigouroux, F.; Reed, W.F. Dissolution kinetics of polymer powders. AIChE J. 2000, 46, 1290–1299. [Google Scholar] [CrossRef]
- Monsoor, M.; Proctor, A. Preparation and functional properties of soy hull pectin. J. Am. Oil Chem. Soc. 2001, 78, 709–713. [Google Scholar] [CrossRef]




| Method | WEP | WEP-0.3 | WEP-0.4 |
|---|---|---|---|
| Color observation | Light yellow | Snow white | Snow white |
| Iodination reaction | (−) a | (−) | (−) |
| Fehling’s test | (−) | (−) | (−) |
| FeCl3 reaction | (−) | (−) | (−) |
| Carbazole reaction | (+) b | (+) | (+) |
| Peak at UV 280 nm | (+) | (−) | (−) |
| Coomassie brilliant blue reaction | (+) | (−) | (−) |
| Samples | Metals(mg/g) | ||||
|---|---|---|---|---|---|
| K | Na | Ca | Mg | Fe | |
| Tap Water | 2.59 ± 0.01 * | 5.40 ± 0.02 | 4.68 ± 0.02 | 0.25 ± 0.00 | 0.01 ± 0.00 |
| Deionzed Water | ND | ND | ND | ND | ND |
| WEP | 0.23 ± 0.01 a** | 0.38 ± 0.01 a | 2.67 ± 0.01 a | 1.08 ± 0.00 a | 0.07 ± 0.01 a |
| WEP-0.3 | 0.18 ± 0.02 a | 0.41 ± 0.00 a | 3.26 ± 0.03 b | 0.15 ± 0.01 b | 0.04 ± 0.01 a |
| WEP-0.4 | 0.24 ± 0.01 a | 0.06 ± 0.01 b | 6.84 ± 0.03 c | 0.34 ± 0.01 c | 0.02 ± 0.02 a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, R.-s.; He, X.-h.; Lin, H.; Liang, R.-h.; Liang, L.; Chen, J.; Liu, C.-m. Solubility Difference between Pectic Fractions from Creeping Fig Seeds. Polymers 2019, 11, 159. https://doi.org/10.3390/polym11010159
Wang R-s, He X-h, Lin H, Liang R-h, Liang L, Chen J, Liu C-m. Solubility Difference between Pectic Fractions from Creeping Fig Seeds. Polymers. 2019; 11(1):159. https://doi.org/10.3390/polym11010159
Chicago/Turabian StyleWang, Ri-si, Xiao-hong He, Hong Lin, Rui-hong Liang, Lu Liang, Jun Chen, and Cheng-mei Liu. 2019. "Solubility Difference between Pectic Fractions from Creeping Fig Seeds" Polymers 11, no. 1: 159. https://doi.org/10.3390/polym11010159
APA StyleWang, R.-s., He, X.-h., Lin, H., Liang, R.-h., Liang, L., Chen, J., & Liu, C.-m. (2019). Solubility Difference between Pectic Fractions from Creeping Fig Seeds. Polymers, 11(1), 159. https://doi.org/10.3390/polym11010159