A Comparative Study on the Characterization of Nanofibers with Cellulose I, I/II, and II Polymorphs from Wood
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of Cellulose Nanofibers with Different Polymorphs
2.3. Preparation of CNF Films
2.4. Characterization
2.4.1. Chemical Composition Measurement
2.4.2. X-ray Diffraction (XRD)
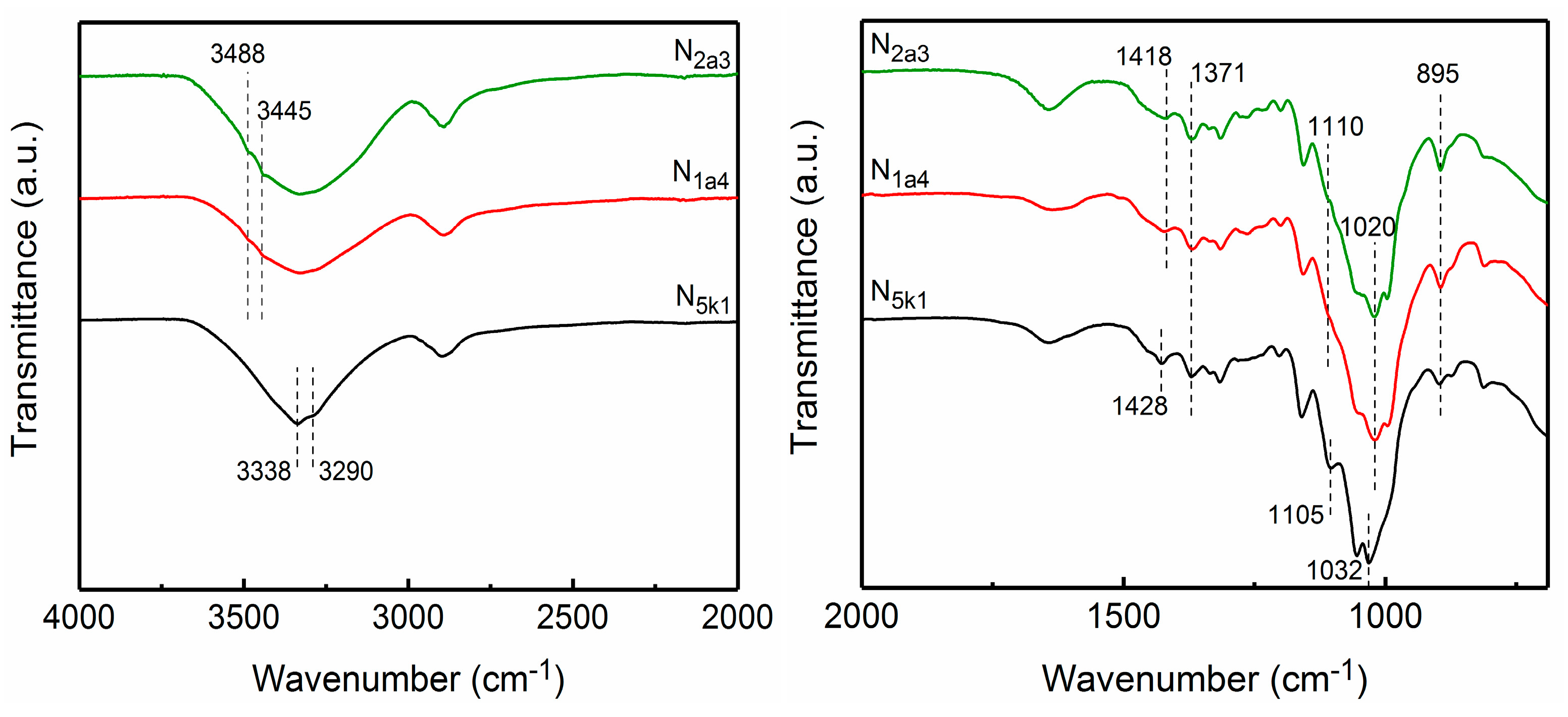
2.4.3. Fourier Transform Infrared (FTIR) Spectroscopy
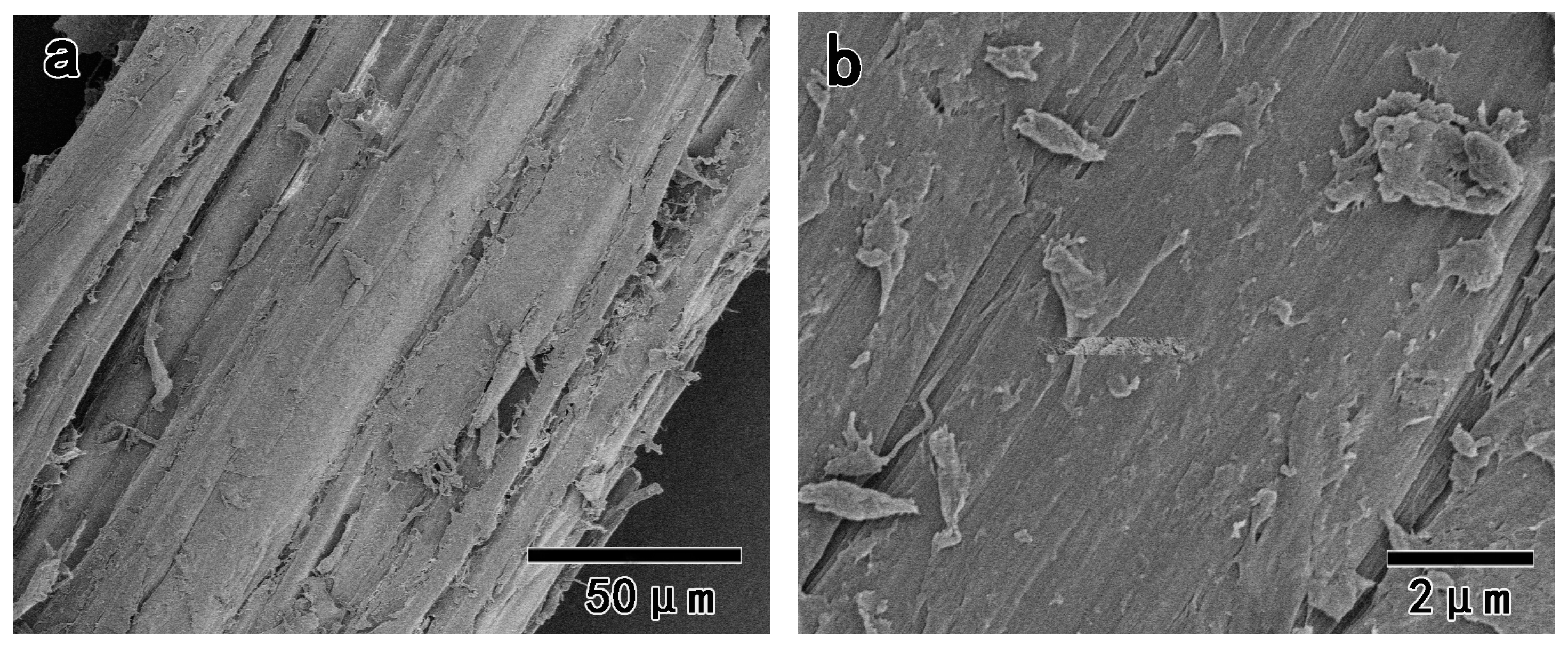
2.4.4. Field Emission Scanning Electron Microscopy (FE-SEM)
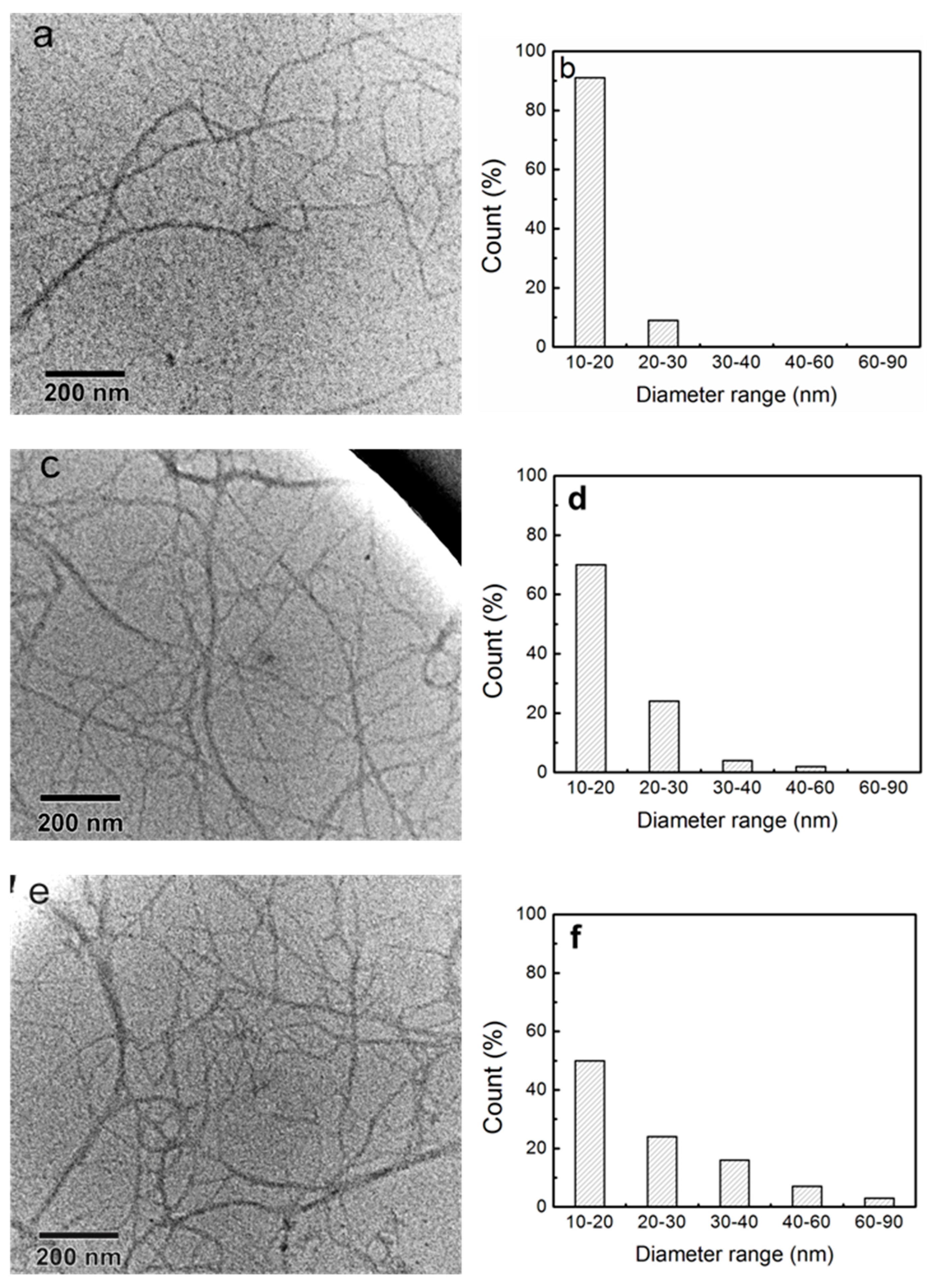
2.4.5. Transmission Electron Microscopy (TEM)
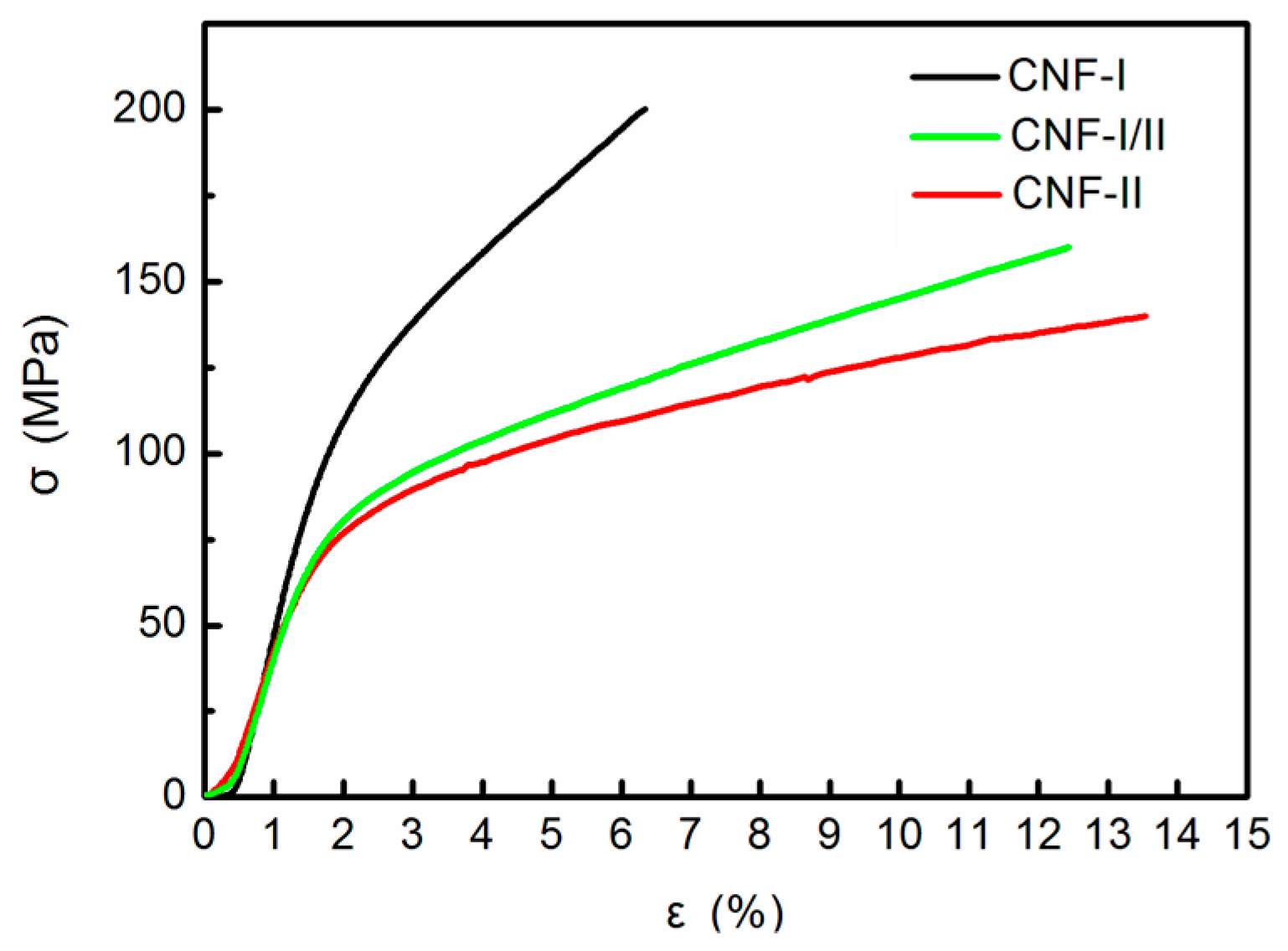
2.4.6. Tensile Performance
2.4.7. Thermomechanical Analysis (TMA)
2.4.8. Thermogravimetric Analysis (TGA)
2.4.9. Light Transmittance
3. Results and Discussion
3.1. Chemical Composition Measurement
3.2. X-ray Diffraction (XRD) Studies
3.3. Fourier Transform Infrared (FTIR) Spectroscopy
3.4. The Morphology of Pulps and Fibrillated Nanofibers
3.5. Tensile Performance
3.6. Thermomechanical Analysis (TMA)
3.7. Thermogravimetric Analysis (TGA)
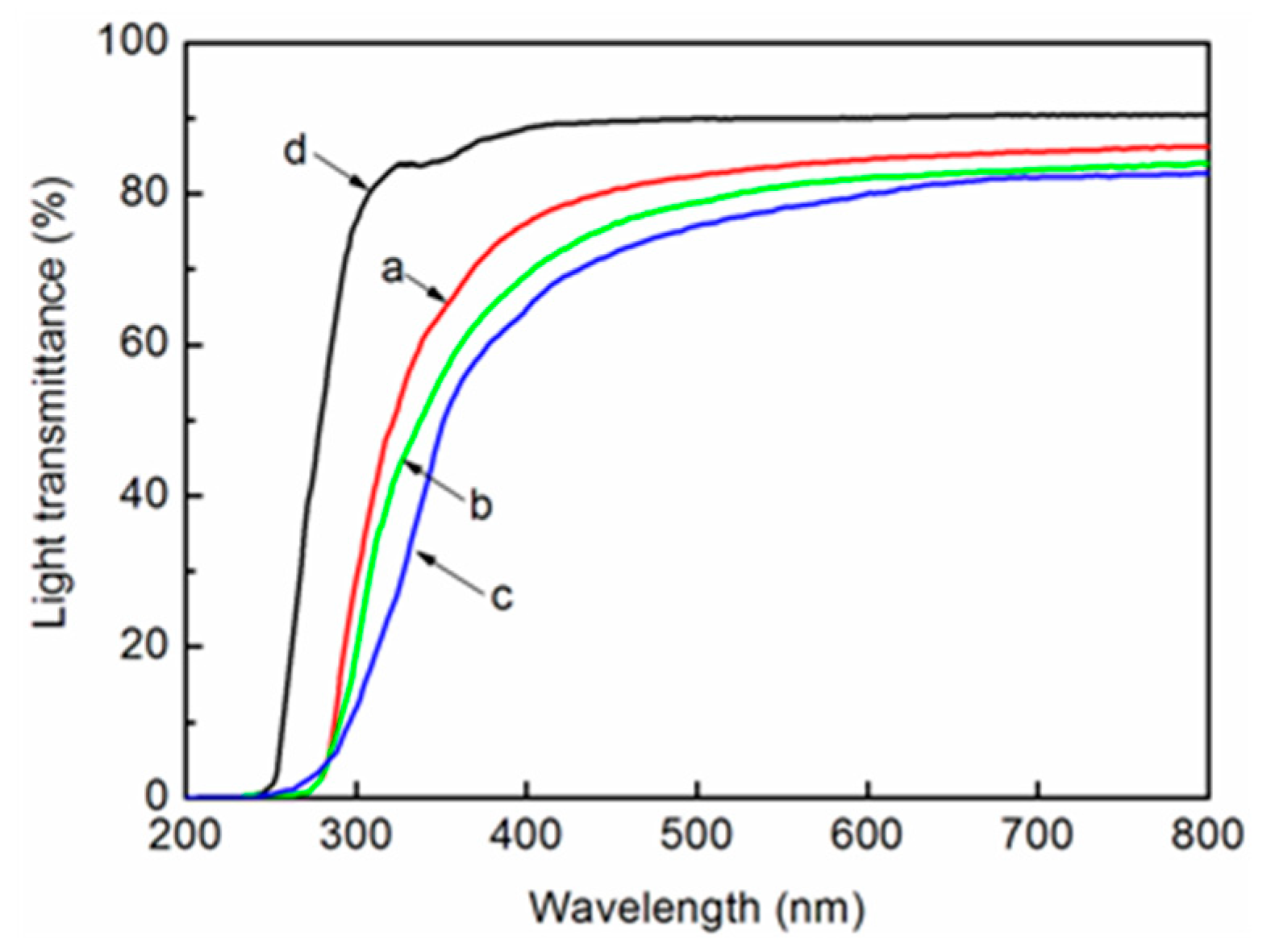
3.8. Light Transmittance
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose nanomaterials review: Structure, properties and nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994. [Google Scholar] [CrossRef] [PubMed]
- Ling, S.J.; Chen, W.; Fan, Y.M.; Zheng, K.; Jin, K.; Yu, H.P.; Buehler, M.J.; Kaplan, D.L. Biopolymer nanofibrils: Structure, modeling, preparation, and applications. Prog. Polym. Sci. 2018, 5, 1–56. [Google Scholar] [CrossRef]
- Jin, E.; Guo, J.G.; Yang, F.; Zhu, Y.Y.; Song, J.L.; Jin, Y.C.; Rojas, O.J. On the polymorphic and morphological changes of cellulose nanocrystals (CNC-I) upon mercerization and conversion to CNC-II. Carbohydr. Polym. 2016, 143, 327–335. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, A.C. Cellulose: The structure slowly unravels. Cellulose 1997, 4, 173–207. [Google Scholar] [CrossRef]
- Okano, T.; Sarko, A. Mercerization of cellulose. II. Alkali-cellulose intermediates and a possible mercerization mechanism. J. Appl. Polym. Sci. 1985, 30, 325–332. [Google Scholar] [CrossRef]
- Yue, Y.Y.; Han, J.Q.; Han, G.P.; Zhang, Q.G.; French, A.D.; Wu, Q.L. Characterization of cellulose I/II hybrid fibers isolated from energycane bagasse during the delignification process: Morphology, crystallinity and percentage estimation. Carbohydr. Polym. 2015, 133, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Muhd Julkapli, N.; Bagheri, S. Nanocellulose as a green and sustainable emerging material in energy applications: A review. Polym. Adv. Technol. 2017, 28, 1583–1594. [Google Scholar] [CrossRef]
- Nagarajan, S.; Skillen, N.C.; Irvine, J.T.S.; Lawton, L.A.; Robertson, P.K.J. Cellulose II as bioethanol feedstock and its advantages over native cellulose. Renew. Sustain. Energy Rev. 2017, 77, 182–192. [Google Scholar] [CrossRef]
- Serizawa, T.; Kato, M.; Okura, H.; Sawada, T.; Wada, M. Hydrolytic activities of artificial nanocellulose synthesized via phosphorylase-catalyzed enzymatic reactions. Polym. J. 2016, 48, 539–544. [Google Scholar] [CrossRef]
- Wu, Q.L.; Mei, C.T.; Han, J.Q.; Yue, Y.Y.; Xu, X.W. Preparation technology and industrialization status of nanocellulose. J. For. Eng. 2018, 3, 1–9. [Google Scholar]
- Siro, I.; Plackett, D. Microfibrillated cellulose and new nanocomposite materials: A review. Cellulose 2010, 17, 459–494. [Google Scholar] [CrossRef]
- Kargarzadeh, H.; Mariano, M.; Huang, J.; Lin, N.; Ahmad, I.; Dufresne, A.; Thomas, S. Recent developments on nanocellulose reinforced polymer nanocomposites: A review. Polymer 2017, 132, 368–393. [Google Scholar] [CrossRef]
- Abe, K.; Yano, H. Formation of hydrogels from cellulose nanofibers. Carbohydr. Polym. 2011, 85, 733–737. [Google Scholar] [CrossRef]
- Yue, Y.Y.; Zhou, C.J.; French, A.D.; Xia, G.; Han, G.P.; Wang, Q.W.; Wu, Q.L. Comparative properties of cellulose nano-crystals from native and mercerized cotton fibers. Cellulose 2012, 19, 1173–1187. [Google Scholar] [CrossRef]
- Gong, J.; Mo, L.H.; Li, J. A comparative study on the preparation and characterization of cellulose nanocrystals with various polymorphs. Carbohydr. Polym. 2018, 195, 18–28. [Google Scholar] [CrossRef]
- Abe, K.; Iwamoto, S.; Yano, H. Obtaining cellulose nanofibers with a uniform width of 15 nm from wood. Biomacromolecules 2007, 8, 3276–3278. [Google Scholar] [CrossRef]
- Sharma, S.; Nair, S.S.; Zhang, Z.; Ragauskas, A.J.; Deng, Y.L. Characterization of micro fibrillation process of cellulose and mercerized cellulose pulp. RSC Adv. 2015, 5, 63111–63122. [Google Scholar] [CrossRef]
- Wang, H.Y.; Li, D.G.; Yano, H.; Abe, K. Preparation of tough cellulose II nanofibers with high thermal stability from wood. Cellulose 2014, 21, 1505–1515. [Google Scholar] [CrossRef]
- Wang, H.Y.; Chen, C.C.; Fang, L.; Li, S.Y.; Chen, N.; Pang, J.W.; Li, D.G. Effect of delignification technique on the ease of fibrillation of cellulose II nanofibers from wood. Cellulose 2018, 25, 7003–7015. [Google Scholar] [CrossRef]
- Han, J.Q.; Zhou, C.J.; Wu, Y.Q.; Liu, F.Y.; Wu, Q.L. Self-assembling behavior of cellulose nanoparticles during freeze-drying: Effect of suspension concentration, particle size, crystal structure, and surface charge. Biomacromolecules 2013, 14, 1529–1540. [Google Scholar] [CrossRef]
- Stevanic, J.S.; Salme, L. Orientation of the wood polymers in the cell wall of spruce wood fibres. Holzforschung 2009, 63, 497–503. [Google Scholar] [CrossRef]
- Zhan, T.Y.; Jiang, J.L.; Lu, J.X.; Zhang, Y.L.; Chang, J.M. Influence of hygrothermal condition on dynamic viscoelasticity of chinese fir (cunninghamia lanceolata). Part 1: Moisture adsorption. Holzforschung 2018, 72, 567–578. [Google Scholar] [CrossRef]
- Rabetafika, H.N.; Bchir, B.; Blecker, C.; Paquot, M.; Wathelet, B. Comparative study of alkaline extraction process of hemicelluloses from pear pomace. Biomass Bioenergy 2014, 61, 254–264. [Google Scholar] [CrossRef]
- French, A.D. Idealized powder diffraction patterns for cellulose polymorphs. Cellulose 2014, 21, 885–896. [Google Scholar] [CrossRef]
- Chen, W.S.; Yu, H.P.; Liu, Y.X.; Hai, Y.F.; Zhang, M.X.; Chen, P. Isolation and characterization of cellulose nanofibers from four plant cellulose fibers using a chemical-ultrasonic process. Cellulose 2011, 18, 433–442. [Google Scholar] [CrossRef]
- Chen, W.S.; Yu, H.P.; Liu, Y.X.; Chen, P.; Zhang, M.X.; Hai, Y.F. Individualization of cellulose nanofibers from wood using high-intensity ultrasonication combined with chemical pretreatments. Carbohydr. Polym. 2011, 83, 1804–1811. [Google Scholar] [CrossRef]
- Sain, M.; Panthapulakkal, S. Bioprocess preparation of wheat straw fibers and their characterization. Ind. Crops Prod. 2006, 23, 1–8. [Google Scholar] [CrossRef]
- Sun, R.C.; Tomkinson, J.; Wang, Y.X.; Xiao, B. Physico-chemical and structural characterization of hemicelluloses from wheat straw by alkaline peroxideextraction. Polymer 2000, 41, 2647–2656. [Google Scholar] [CrossRef]
- Široký, J.; Blackburn, R.S.; Bechtold, T.; Taylor, J.; White, P. Attenuated total reflectance fourier-transform infrared spectroscopy analysis of crystallinity changes in lyocell following continuous treatment with sodium hydroxide. Cellulose 2009, 17, 103–115. [Google Scholar] [CrossRef]
- Horikawa, Y.; Konakahara, N.; Imai, T.; Kentaro, A.; Kobayashi, Y.; Sugiyama, J. The structural changes in crystalline cellulose and effects on enzymatic digestibility. Polym. Degrad. Stab. 2013, 98, 2351–2356. [Google Scholar] [CrossRef]
- Revol, J.F.; Goring, D.A.I. On the mechanism of the mercerization of cellulose in wood. J. Appl. Polym. Sci. 1981, 26, 1275–1282. [Google Scholar] [CrossRef]
- Iwamoto, S.; Abe, K.; Yano, H. The effect of hemicelluloses on wood pulp nanofibrillation and nanofiber network characteristics. Biomacromolecules 2008, 9, 1022–1026. [Google Scholar] [CrossRef] [PubMed]
- Goda, K.; Sreekala, M.S.; Gomes, A.; Kaji, T.; Ohgi, J. Improvement of plant based natural fibers for toughening green composites—Effect of load application during mercerization of ramie fibers. Compos. Part A Appl. Sci. Manuf. 2006, 37, 2213–2220. [Google Scholar] [CrossRef]
- Ishikawa, A.; Okano, T.; Sugiyama, J. Fine structure and tensile properties of ramie fibres in the crystalline form of cellulose I, II, III and IV. Polymer 1997, 38, 463–468. [Google Scholar] [CrossRef]
- Sun, X.X.; Wu, Q.L.; Zhang, X.Q.; Ren, S.X.; Lei, T.Z.; Li, W.C.; Xu, G.Y.; Zhang, Q.G. Nanocellulose films with combined cellulose nanofibers and nanocrystals: Tailored thermal, optical and mechanical properties. Cellulose 2017, 25, 1103–1115. [Google Scholar] [CrossRef]
- Diaz, J.A.; Wu, X.; Martini, A.; Youngblood, J.P.; Moon, R.J. Thermal expansion of self-organized and shear-oriented cellulose nanocrystal films. Biomacromolecules 2013, 14, 2900–2908. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Eom, Y.G. Thermogravimetric analysis of rice husk flour for a new raw material of lignocellulosic fiber-thermoplastic polymer composites. Mokchae Konghak 2001, 29, 59–67. [Google Scholar]
- Yang, H.P.; Yan, R.; Chen, H.P.; Lee, D.H.; Zheng, C.G. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Kabir, M.M.; Wang, H.; Lau, K.T.; Cardona, F. Effects of chemical treatments on hemp fibre structure. Appl. Surf. Sci. 2013, 276, 13–23. [Google Scholar] [CrossRef]






| Sample | α-Cellulose (%) | Lignin (%) | Hemicelluloses (%) |
|---|---|---|---|
| Extracted wood | 45.9 (1.8 a) | 25.5 (1.7 a) | 28.1 (2.1 a) |
| S1 | 50.2 (1.7 a) | 17.8 (1.4 a) | 29.2 (1.5 a) |
| S2 | 52.6 (1.8 a) | 13.0 (1.2 a) | 30.4 (1.8 a) |
| S5 | 64.5 (2.1 a) | 4.7 (0.8 a) | 28.3 (1.9 a) |
| S5k1 | 84.5 (1.2 a) | 1.2 (0.2 a) | 11.8 (2.2 a) |
| S1a4 | 76.3 (2.5 a) | 3.1 (0.7 a) | 16.7 (2.2 a) |
| S2a3 | 83.6 (2.3 a) | 2.7 (0.6 a) | 10.9 (1.6 a) |
| Sample | Polymorph | CTE (ppm/K) | Tensile Strength (MPa) | Young’s Modulus (GPa) | Fracture Strain (%) |
|---|---|---|---|---|---|
| N5k1 | CNF-I | 9.4 | 202 (11 a) | 9.5 (0.7 a) | 6.0 (0.7 a) |
| N1a4 | CNF-I/II | 17.1 | 157 (7 a) | 6.8 (0.6 a) | 11.8 (1.4 a) |
| N2a3 | CNF-II | 17.3 | 137 (6 a) | 6.0 (0.5 a) | 13.0 (1.1 a) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Li, S.; Wu, T.; Wang, X.; Cheng, X.; Li, D. A Comparative Study on the Characterization of Nanofibers with Cellulose I, I/II, and II Polymorphs from Wood. Polymers 2019, 11, 153. https://doi.org/10.3390/polym11010153
Wang H, Li S, Wu T, Wang X, Cheng X, Li D. A Comparative Study on the Characterization of Nanofibers with Cellulose I, I/II, and II Polymorphs from Wood. Polymers. 2019; 11(1):153. https://doi.org/10.3390/polym11010153
Chicago/Turabian StyleWang, Haiying, Suiyi Li, Tiantian Wu, Xiaoxuan Wang, Xudong Cheng, and Dagang Li. 2019. "A Comparative Study on the Characterization of Nanofibers with Cellulose I, I/II, and II Polymorphs from Wood" Polymers 11, no. 1: 153. https://doi.org/10.3390/polym11010153
APA StyleWang, H., Li, S., Wu, T., Wang, X., Cheng, X., & Li, D. (2019). A Comparative Study on the Characterization of Nanofibers with Cellulose I, I/II, and II Polymorphs from Wood. Polymers, 11(1), 153. https://doi.org/10.3390/polym11010153






