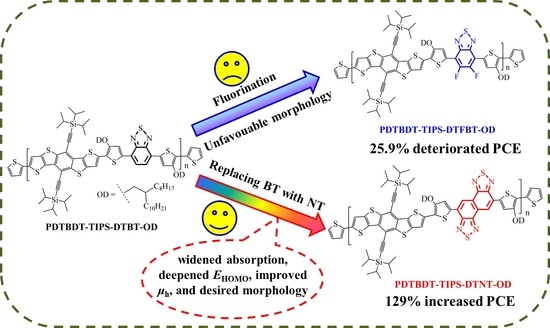
Enhanced Photovoltaic Performance in D-π-A Copolymers Containing Triisopropylsilylethynyl-Substituted Dithienobenzodithiophene by Modulating the Electron-Deficient Units
Abstract
1. Introduction
2. Materials and Methods
2.1. Characterization
2.2. Materials
2.3. Polymer Synthesis
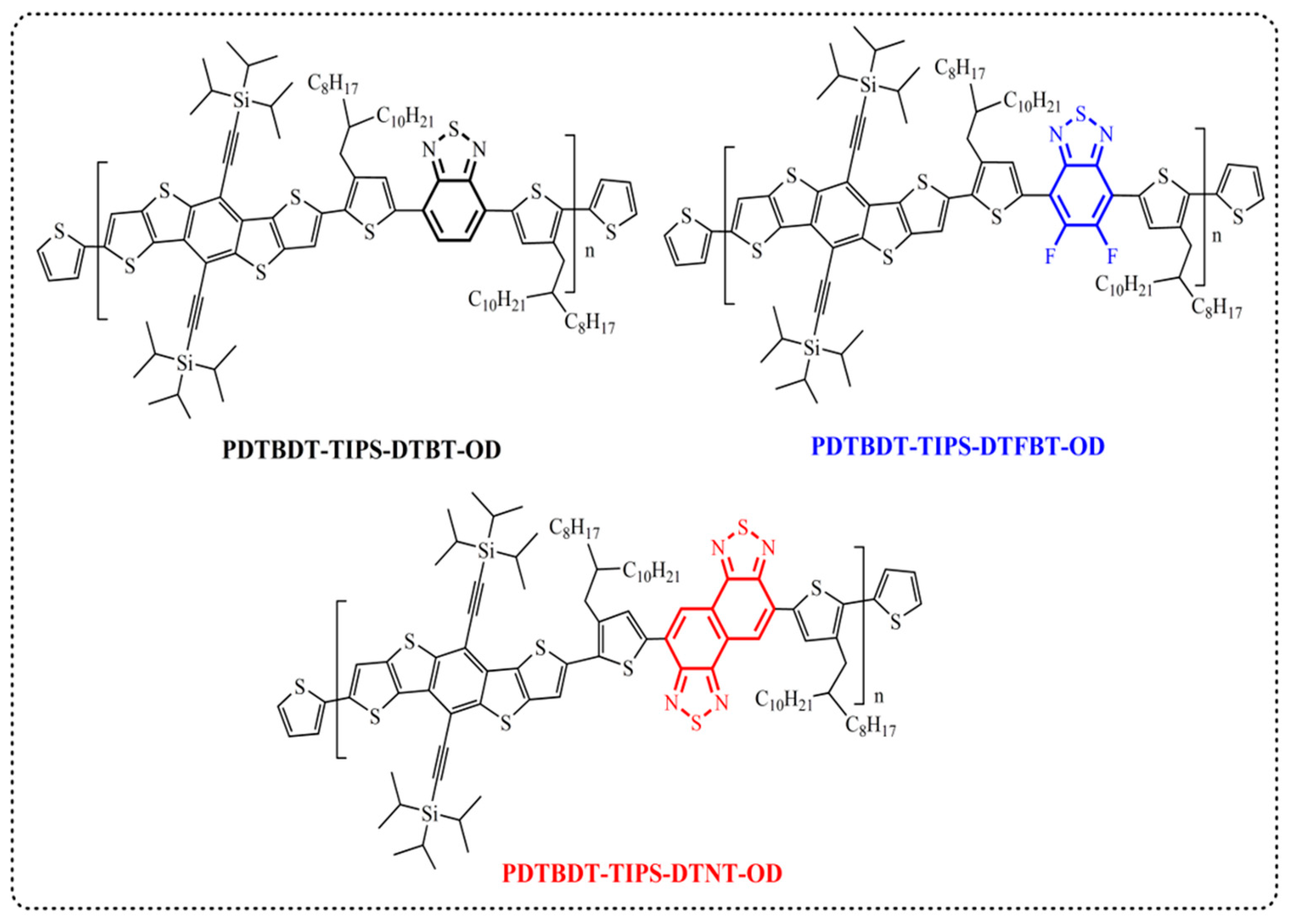
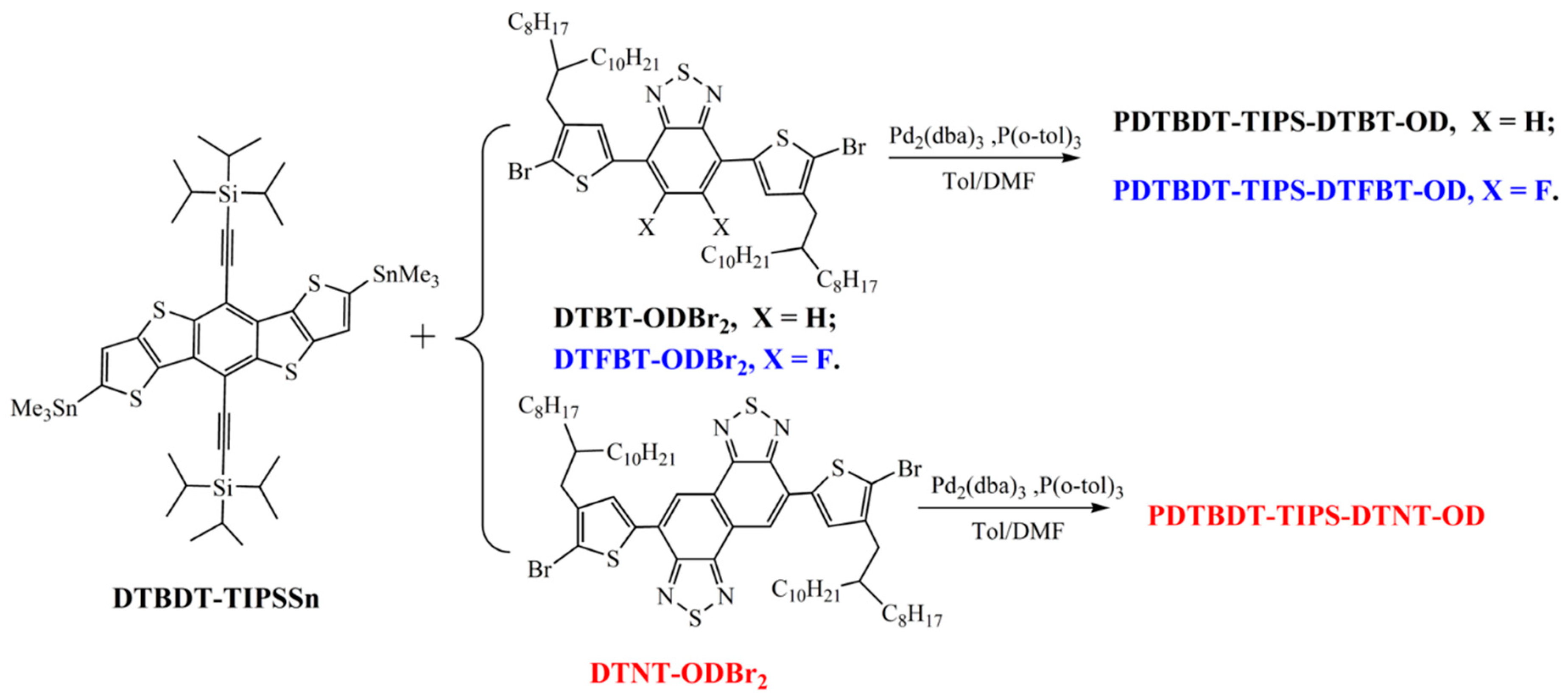
2.3.1. Poly[5,10-bis(triisopropylsilylethynyl)dithieno[2,3-d:2′,3′-d′]benzo[1,2-b:4,5-b′]dithiophene-2,7-diyl-alt-4,7-bis(4-(2-octyldodecyl)thien-2-yl)benzo[c][1,2,5]thiadiazole-5,5′-diyl] (PDTBDT-TIPS-DTBT-OD)
2.3.2. Poly[5,10-bis(triisopropylsilylethynyl)dithieno[2,3-d:2′,3′-d′]benzo[1,2-b:4,5-b′]dithiophene-2,7-diyl-alt-4,7-bis(4-(2-octyldodecyl)thien-2-yl)-5,6-difluorobenzo[c][1,2,5]thiadiazole-5,5′-diyl] (PDTBDT-TIPS-DTFBT-OD)
2.3.3. Poly[5,10-bis(triisopropylsilylethynyl)dithieno[2,3-d:2′,3′-d′]benzo[1,2-b:4,5-b′]dithiophene-2,7-diyl-alt-4,9-bis(4-(2-octyldodecyl)thien-2-yl)naphto[1,2-c:5,6-c′]bis[1,2,5]thiadiazole-5,5′-diyl] (PDTBDT-TIPS-DTNT-OD)
2.4. Fabrication and Characterization of the PSCs
2.5. Hole-Only Device Fabrication and Measurement
3. Results
3.1. Molecular Design, Synthesis, and Characterization
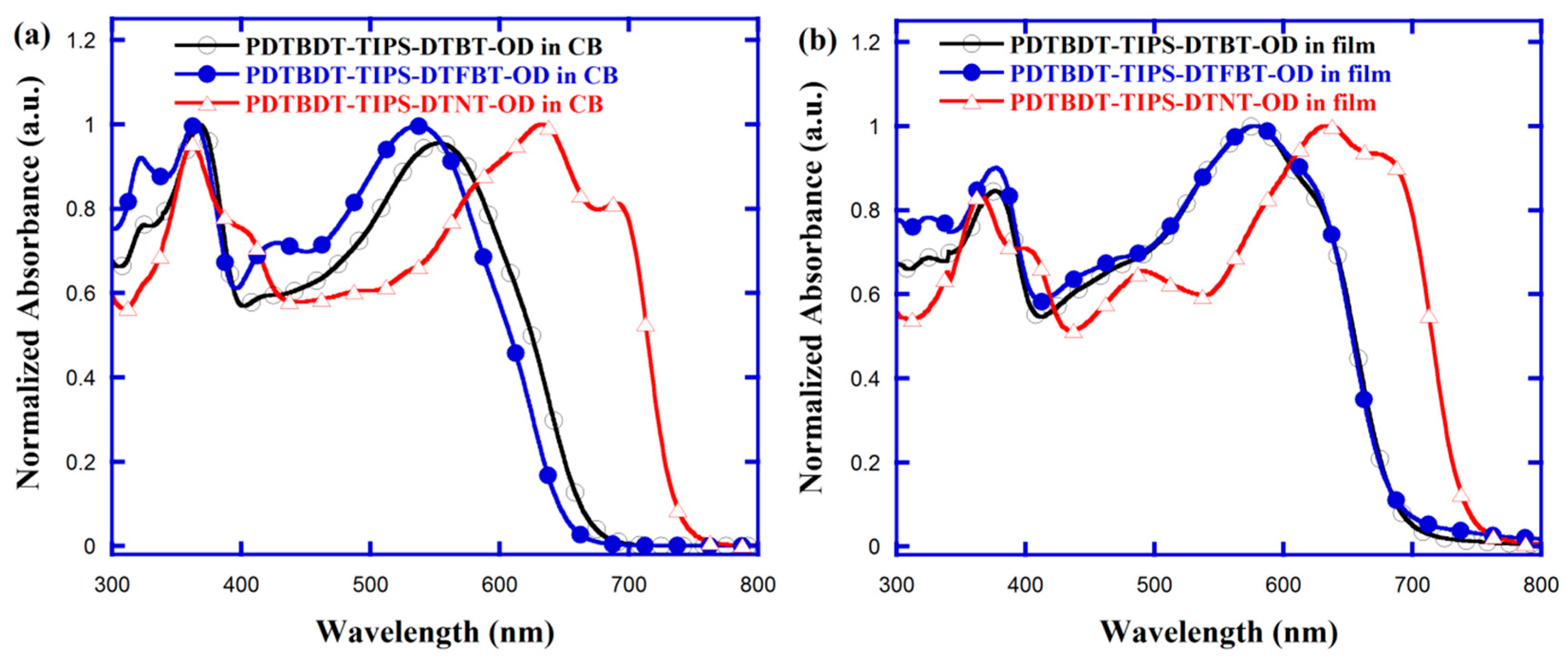
3.2. Optical Property
3.3. X-Ray Diffraction (XRD) Analysis
3.4. Electrochemical Property
3.5. Theoretical Calculation
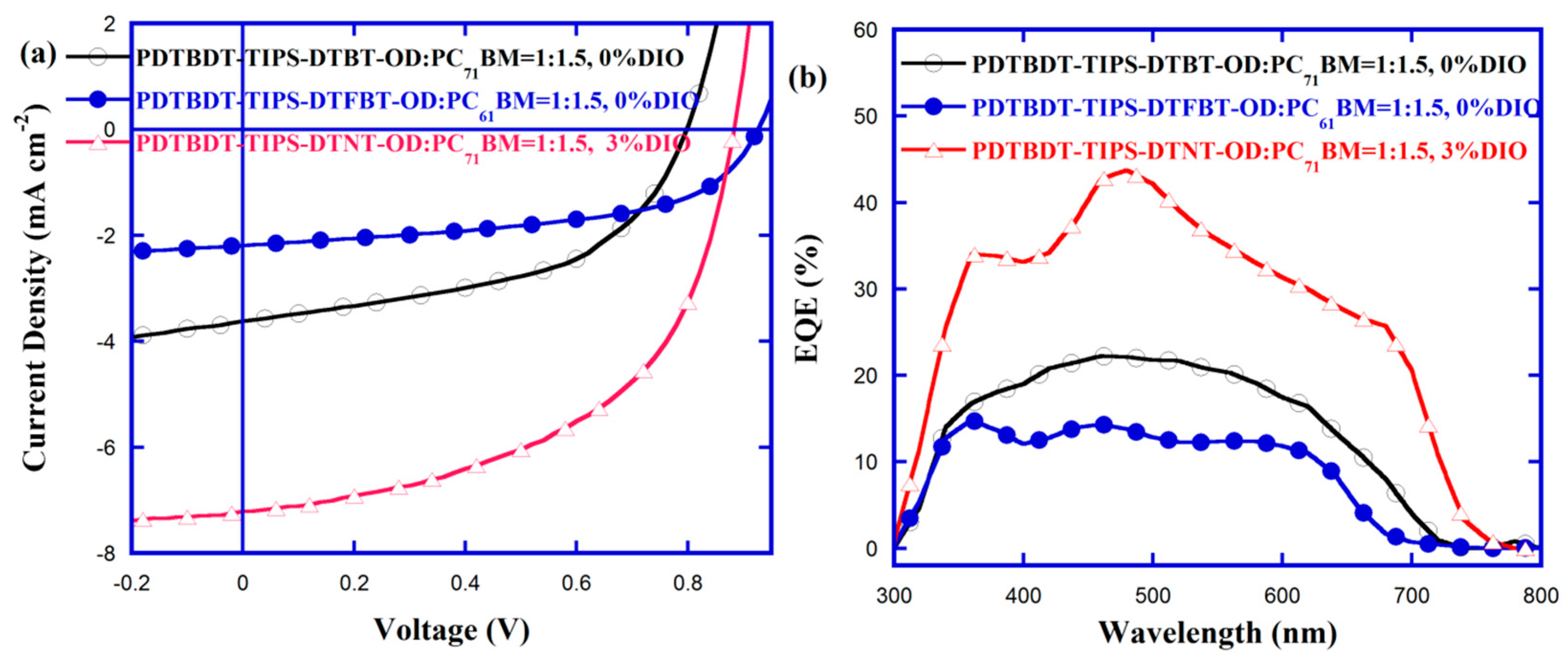
3.6. Photovoltaic Properties
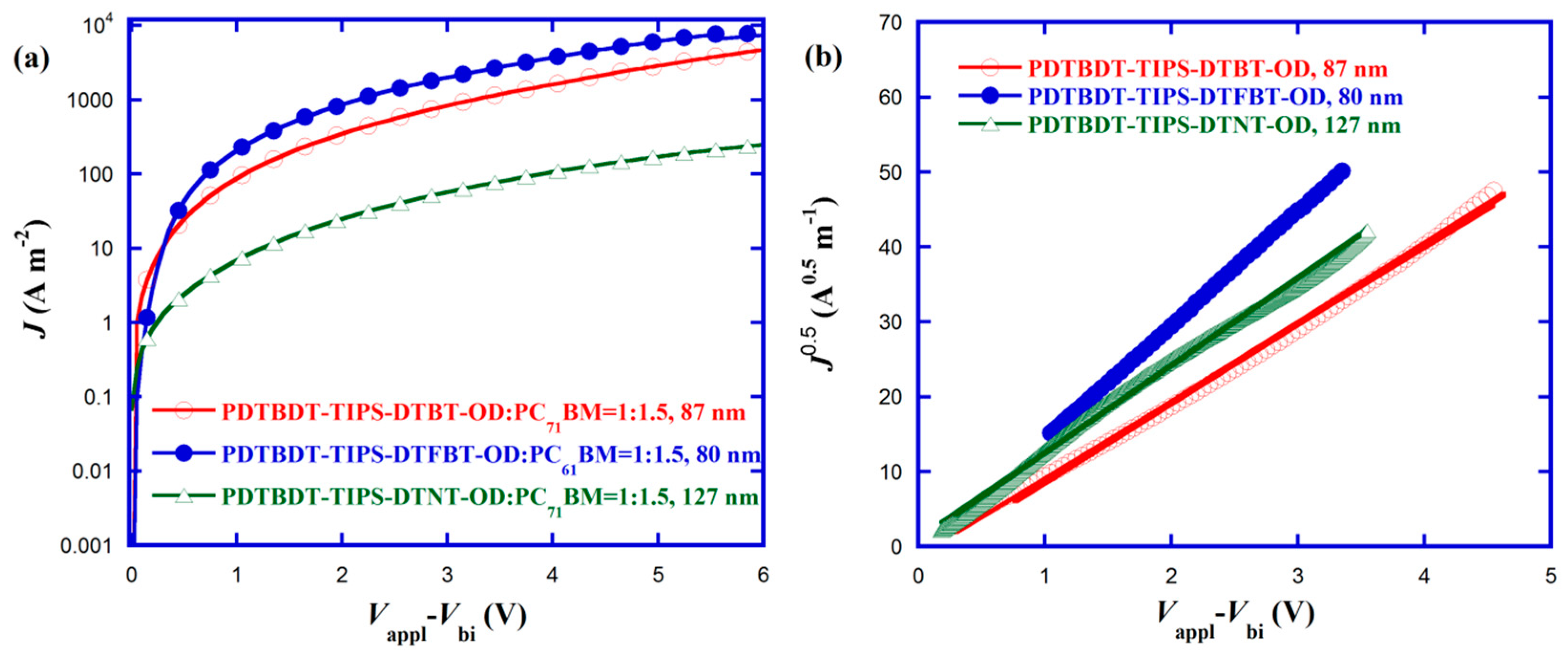
3.7. Charge Mobilities
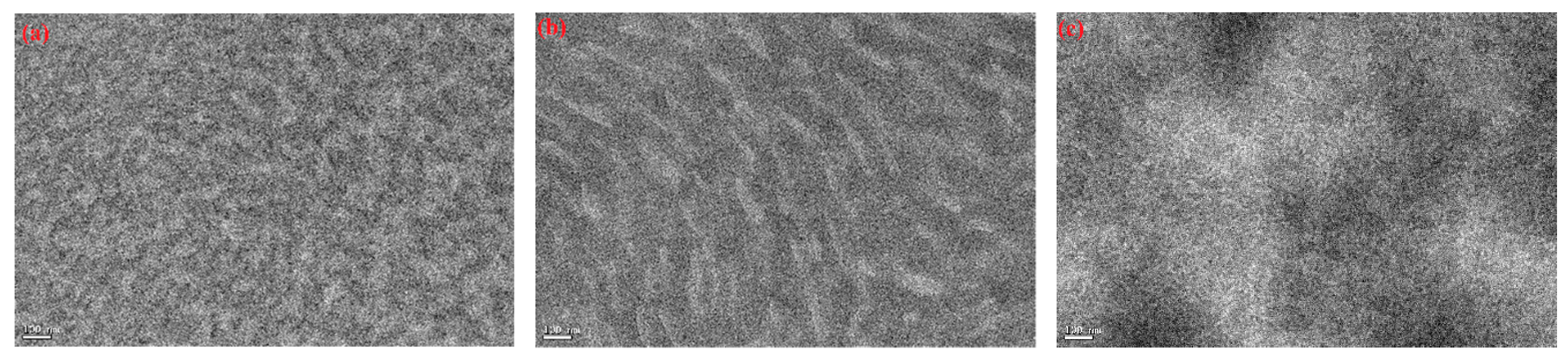
3.8. Film Morphology
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cheng, P.; Zhan, X. Stability of organic solar cells: Challenges and strategies. Chem. Soc. Rev. 2016, 45, 2544–2582. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Liu, S.; Gu, H.; Dai, W.; Luo, X. Highly flattened donor-acceptor polymers based on fluoride-substituent acceptors for efficient heterojunction solar cells. Sol. Energy 2018, 166, 450–457. [Google Scholar] [CrossRef]
- Li, G.; Zhu, R.; Yang, Y. Polymer solar cells. Nat. Photonics 2012, 6, 153–161. [Google Scholar] [CrossRef]
- Li, Y. Molecular design of photovoltaic materials for polymer solar cells: Toward suitable electronic energy levels and broad absorption. Acc. Chem. Res. 2012, 45, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhao, J.; Li, Z.; Mu, C.; Ma, W.; Hu, H.; Jiang, K.; Lin, H.; Ade, H.; Yan, H. Aggregation and morphology control enables multiple cases of high-efficiency polymer solar cells. Nat. Commun. 2014, 5, 5293. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, Y.; Yang, G.; Jiang, K.; Lin, H.; Ade, H.; Ma, W.; Yan, H. Efficient organic solar cells processed from hydrocarbon solvents. Nat. Energy 2016, 1, 15027. [Google Scholar] [CrossRef]
- Jin, Y.; Chen, Z.; Xiao, M.; Peng, J.; Fan, B.; Ying, L.; Zhang, G.; Jiang, X.-F.; Yin, Q.; Liang, Z.; et al. Thick film polymer solar cells based on naphtho[1,2-c:5,6-c′]bis[1,2,5]thiadiazole conjugated polymers with efficiency over 11%. Adv. Energy Mater. 2017, 7, 1700944. [Google Scholar] [CrossRef]
- Zhao, W.; Li, S.; Yao, H.; Zhang, S.; Zhang, Y.; Yang, B.; Hou, J. Molecular optimization enables over 13% efficiency in organic solar cells. J. Am. Chem. Soc. 2017, 139, 7148–7151. [Google Scholar] [CrossRef]
- Bundgaard, E.; Krebs, F.C. Low band gap polymers for organic photovoltaics. Sol. Energy Mater. Sol. Cells 2007, 91, 954–985. [Google Scholar] [CrossRef]
- Zhou, H.; Yang, L.; You, W. Rational design of high performance conjugated polymers for organic solar cells. Macromolecules 2012, 45, 607–632. [Google Scholar] [CrossRef]
- Xu, T.; Yu, L. How to design low bandgap polymers for highly efficient organic solar cells. Mater. Today 2014, 17, 11–15. [Google Scholar] [CrossRef]
- Qi, B.; Wang, J. Open-circuit voltage in organic solar cells. J. Mater. Chem. 2012, 22, 24315–24325. [Google Scholar] [CrossRef]
- Elumalai, N.K.; Uddin, A. Open circuit voltage of organic solar cells: An in-depth review. Energy Environ. Sci. 2016, 9, 391–410. [Google Scholar] [CrossRef]
- Chen, Z.; Cai, P.; Chen, J.; Liu, X.; Zhang, L.; Lan, L.; Peng, J.; Ma, Y.; Cao, Y. Low band-gap conjugated polymers with strong interchain aggregation and very high hole mobility towards highly efficient thick-film polymer solar cells. Adv. Mater. 2014, 26, 2586–2591. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Chen, H.; Tang, Y.; Wang, J.-Y.; Ma, W.; Zheng, Q. Modulation of bulk heterojunction morphology through small π-bridge changes for polymer solar cells with enhanced performance. J. Mater. Chem. C 2018, 6, 5999–6007. [Google Scholar] [CrossRef]
- Zhang, Z.-G.; Wang, J. Structures and properties of conjugated Donor-Acceptor copolymers for solar cell applications. J. Mater. Chem. 2012, 22, 4178–4187. [Google Scholar] [CrossRef]
- Kim, H.G.; Jo, S.B.; Shim, C.; Lee, J.; Shin, J.; Cho, E.C.; Ihn, S.-G.; Choi, Y.S.; Kim, Y.; Cho, K. Synthesis and photovoltaic properties of benzo[1,2-b:4,5-b′]dithiophene derivative-based polymers with deep HOMO levels. J. Mater. Chem. 2012, 22, 17709–17717. [Google Scholar] [CrossRef]
- Hou, J.; Park, M.-H.; Zhang, S.; Yao, Y.; Chen, L.-M.; Li, J.-H.; Yang, Y. Bandgap and molecular energy level control of conjugated polymer photovoltaic materials based on benzo[1,2-b:4,5-b′]dithiophene. Maromolecules 2008, 41, 6012–6601. [Google Scholar] [CrossRef]
- Du, J.; Biewer, M.C.; Stefan, M.C. Benzothiadiazole building units in solution-processable small molecules for organic photovoltaics. J. Mater. Chem. A 2016, 4, 15771–15787. [Google Scholar] [CrossRef]
- Wang, Y.; Michinobu, T. Benzothiadiazole and its π-extended, heteroannulated derivatives: Useful acceptor building blocks for high-performance donor-acceptor polymers in organic electronics. J. Mater. Chem. C 2016, 4, 6200–6214. [Google Scholar] [CrossRef]
- Mori, H.; Nishinaga, S.; Takahashi, R.; Nishihara, Y. Alkoxy-substituted anthra[1,2-c:5,6-c′]bis([1,2,5]-thiadiazole) (ATz): A new electron-acceptor unit in the semiconducting polymers for organic electronics. Macromolecules 2018, 51, 5473–5484. [Google Scholar] [CrossRef]
- Blouin, N.; Michaud, A.; Leclerc, M. A low-bandgap poly(2,7-carbazole) derivative for use in high-performance solar cells. Adv. Mater. 2007, 19, 2295–2300. [Google Scholar] [CrossRef]
- Peet, J.; Kim, J.Y.; Coates, N.E.; Ma, W.L.; Moses, D.; Heeger, A.J.; Bazan, G.C. Efficiency enhancement in low-bandgap polymer solar cells by processing with alkane dithiols. Nat. Mater. 2007, 6, 497–500. [Google Scholar] [CrossRef]
- Osaka, I.; Shimawaki, M.; Mori, H.; Doi, I.; Miyazaki, E.; Koganezawa, T.; Takimiya, K. Synthesis, characterization, and transistor and solar cell applications of a naphthobisthiadiazole-based semi-conducting polymer. J. Am. Chem. Soc. 2012, 134, 3498–3507. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Hu, X.; Liu, P.; Li, W.; Gong, X.; Huang, F.; Cao, Y. Donor-acceptor conjugated polymer based on naphtho[1,2-c:5,6-c′]bis[1,2,5]thiadiazole for high-performance polymer solar cells. J. Am. Chem. Soc. 2011, 133, 9638–9641. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Xia, Y.; Huang, F.; Luo, G.; Li, J.; Zhang, P.; Zhu, Y.; Yang, C.; Wu, H.; Cao, Y. An alkylthieno-2-yl flanked dithieno[2,3-d:2′,3′-d′]benzo[1,2-b:4,5-b′]dithiophene-based low band gap conjugated polymer for high performance photovoltaic solar cells. RSC Adv. 2015, 5, 12879–12885. [Google Scholar] [CrossRef]
- Zhou, H.; Yang, L.; Stuart, A.C.; Price, S.C.; Liu, S.; You, W. Development of fluorinated benzothiadi-azole as a structural unit for a polymer solar cell of 7% efficiency. Angew. Chem. 2011, 123, 3051–3054. [Google Scholar] [CrossRef]
- Jo, J.W.; Jung, J.W.; Wang, H.-W.; Kim, P.; Russell, T.P.; Jo, W.H. Fluorination of polythiophene derivatives for high performance organic photovoltaics. Chem. Mater. 2014, 26, 4214–4220. [Google Scholar] [CrossRef]
- Li, Z.; Lin, H.; Jiang, K.; Carpenter, J.; Li, Y.; Liu, Y.; Hu, H.; Zhao, J.; Ma, W.; Ade, H.; et al. Dramatic performance enhancement for large bandgap thick-film polymer solar cells introduced by a difluorinated donor unit. Nano Energy 2015, 15, 607–615. [Google Scholar] [CrossRef]
- Wolf, J.; Cruciani, F.; El Labban, A.; Beaujuge, P.M. Wide band-gap 3,4-difluorothiophene-based polymer with 7% solar cell efficiency: An alternative to P3HT. Chem. Mater. 2015, 27, 4184–4187. [Google Scholar] [CrossRef]
- Hu, H.; Jiang, K.; Kim, J.-H.; Yang, G.; Li, Z.; Ma, T.; Lu, G.; Qu, Y.; Ade, H.; Yan, H. Influence of fluorination on the properties and performance of isoindigo-quaterthiophene-based polymers. J. Mater. Chem. A 2016, 4, 5039–5043. [Google Scholar] [CrossRef]
- Leclerc, N.; Chávez, P.; Ibraikulov, O.A.; Heiser, T.; Lévêque, P. Impact of backbone fluorination on π-conjugated polymers in organic photovoltaic devices: A review. Polymers 2016, 8, 11. [Google Scholar] [CrossRef]
- Lee, H.S.; Song, H.G.; Jung, H.; Kim, M.H.; Cho, C.; Lee, J.-Y.; Park, S.; Son, H.J.; Yun, H.-J.; Kwon, S.-K.; et al. Effects of backbone planarity and tightly packed alkyl chains in the donor-acceptor polymers for high photostability. Macromolecules 2016, 49, 7844–7856. [Google Scholar] [CrossRef]
- Xu, X.-P.; Li, Y.; Luo, M.-M.; Peng, Q. Recent progress towards fluorinated copolymers for efficient photovoltaic applications. Chin. Chem. Lett. 2016, 27, 1241–1249. [Google Scholar] [CrossRef]
- Zhang, S.; Qin, Y.; Uddin, M.A.; Jang, B.; Zhao, W.; Liu, D.; Woo, H.Y. Hou, J. A fluorinated polythiophene derivative with stabilized backbone conformation for highly efficient fullerene and non-fullerene polymer solar cells. Macromolecules 2016, 49, 2993–3000. [Google Scholar] [CrossRef]
- Wang, T.; Lau, T.-K.; Lu, X.; Yuan, J.; Feng, L.; Jiang, L.; Deng, W.; Peng, H.; Li, Y.; Zou, Y. A medium bandgap D-A copolymer based on 4-alkyl-3,5-difluorophenyl substituted quinoxaline unit for high performance solar cells. Macromolecules 2018, 51, 2838–2846. [Google Scholar] [CrossRef]
- Zhang, Y.; Chien, S.-C.; Chen, K.-S.; Yip, H.-L.; Sun, Y.; Davies, J.A.; Chen, F.-C.; Jen, A.K.-Y. Increased open circuit voltage in fluorinated benzothiadiazole-based alternating conjugated polymers. Chem. Commun. 2011, 47, 11026–11028. [Google Scholar] [CrossRef]
- Min, J.; Zhang, Z.-G.; Zhang, S.; Li, Y. Conjugated side-chain-isolated D-A copolymers based on benzo[1,2-b:4,5-b′]dithiophene-alt-dithienylbenzotriazole: Synthesis and photovoltaic properties. Chem. Mater. 2012, 24, 3247–3254. [Google Scholar] [CrossRef]
- Stuart, A.C.; Tumbleston, J.R.; Zhou, H.; Li, W.; Liu, S.; Ade, H.; You, W. Fluorine substituents reduce charge recombination and drive structure and morphology development in polymer solar cells. J. Am. Chem. Soc. 2013, 135, 1806–1815. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, K.; Fukuhara, T.; Suda, Y.; Suzuki, Y.; Koganezawa, T.; Yoshida, H.; Ohkita, H.; Osaka, I.; Takimiya, K. Implication of fluorine atom on electronic properties, ordering structures, and photovoltaic performance in naphthobisthiadiazole-based semiconducting polymers. J. Am. Chem. Soc. 2016, 138, 10265–10275. [Google Scholar] [CrossRef]
- Price, S.C.; Stuart, A.C.; Yang, L.; Zhou, H.; You, W. Fluorine substituted conjugated polymer of medium band gap yields 7% efficiency in polymer-fullerene solar cells. J. Am. Chem. Soc. 2011, 133, 4625–4631. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Lu, J.; Tse, S.-C.; Zhou, J.; Du, X.; Tao, Y.; Ding, J. Synthesis and applications of difluorobenzo-thiadiazole based conjugated polymers for organic photovoltaics. J. Mater. Chem. 2012, 21, 3226–3233. [Google Scholar] [CrossRef]
- Jo, J.W.; Bae, S.; Liu, F.; Russell, T.P.; Jo, W.H. Comparison of two D-A type polymers with each being fluorinated on D and A unit for high performance solar cells. Adv. Funct. Mater. 2015, 2, 120–125. [Google Scholar] [CrossRef]
- Zhang, Y.; Zou, J.; Cheuh, C.-C.; Yip, H.-L.; Jen, A.K.-Y. Significant improved performance of photovoltaic cells made from a partially fluorinated cyclopentadithiophene/benzothiadiazole conjugated polymer. Macromolecules 2012, 45, 5427–5435. [Google Scholar] [CrossRef]
- Iyer, A.; Bjorgaard, J.; Anderson, T.; Köse, M.E. Quinoxaline-based semiconducting polymers: Effect of fluorination on the photophysical, thermal, and charge transport properties. Macromolecules 2012, 45, 6380–6389. [Google Scholar] [CrossRef]
- Schroeder, B.C.; Huang, Z.; Ashraf, R.S.; Smith, J.; D’Angelo, P.; Watkins, S.E.; Anthopoulos, T.D.; Durrant, J.R.; McCulloch, I. Silaindacenodithiophene-based low band gap polymers-the effect of fluorine substitution on device performances and film morphologies. Adv. Funct. Mater. 2012, 22, 1663–1670. [Google Scholar] [CrossRef]
- Jin, Y.; Chen, Z.; Dong, S.; Zheng, N.; Ying, L.; Jiang, X.-F.; Liu, F.; Huang, F.; Cao, Y. A novel naphtho[1,2-c:5,6-c′]bis([1,2,5]thiadiazole)-based narrow-bandgap π-conjugated polymer with power conversion efficiency over 10%. Adv. Mater. 2016, 28, 9811–9818. [Google Scholar] [CrossRef]
- Osaka, I.; Takimiya, K. Naphthobischalcogenadiazole conjugated polymers: Emerging materials for organic electronics. Adv. Mater. 2017, 29, 1605218. [Google Scholar] [CrossRef]
- Tong, J.; Li, J.; Zhang, P.; Ma, X.; Wang, M.; An, L.; Sun, J.; Guo, P.; Yang, C.; Xia, Y. Naphtho[1,2-c:5,6-c′]-bis[1,2,5]thiadiazole-based conjugated polymers consisting of oligothiophenes for efficient polymer solar cells. Polymer 2017, 121, 183–195. [Google Scholar] [CrossRef]
- Xu, X.; Feng, K.; Li, K.; Peng, Q. Synthesis and photovoltaic properties of two dimensional benzodi- thiophene-thiophene copolymers with pendent rational naphtho[1,2-c:5,6-c]bis[1,2,5]thiadiazole side chains. J. Mater. Chem. A 2017, 3, 23149–23161. [Google Scholar] [CrossRef]
- Shin, N.; Yun, H.-J.; Yoon, Y.; Son, H.J.; Ju, S.-Y.; Kwon, S.-K.; Kim, B.S.; Kim, Y.-H. Highly stable polymer solar cells based on poly(dithienobenzodithiophene-co-thienothiophene). Macromolecules 2015, 48, 3890–3899. [Google Scholar] [CrossRef]
- Son, H.; Lu, L.; Chen, W.; Xu, T.; Zheng, T.; Carsten, B.; Strzalka, J.; Darling, S.; Chen, L.; Yu, L. Synthesis and photovoltaic effect in dithieno[2,3-d:2′,3′-d′]benzo[1,2-b:4,5-b′]dithiophene-based conjugated polymers. Adv. Mater. 2013, 25, 838–843. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, Z.; Ma, W.; Huang, Y.; Huo, L.; Guo, X.; Zhang, M.; Ade, H.; Hou, J. PDT-S-T: A new polymer with optimized molecular conformation for controlled aggregation and π–π stacking and its application in efficient photovoltaic devices. Adv. Mater. 2013, 25, 3449–3455. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Zhang, P.; Li, J.; Li, Y.; Wang, J.; Zhang, S.; Xia, Y.; Meng, X.; Fan, D.; Chu, J. Synthetically controlling the optoelectronic properties of dithieno[2,3-d:2′,3′-d′]benzo[1,2-b:4,5-b′]dithiophene-alt-di-ketopyrrolopyrrole-conjugated polymers for efficient solar cells. J. Mater. Chem. A 2014, 2, 15316–15325. [Google Scholar] [CrossRef]
- Huo, L.; Liu, T.; Sun, X.; Cai, Y.; Heeger, A.J.; Sun, Y. Single-junction organic solar cells based on a novel wide-bandgap polymer with efficiency of 9.7%. Adv. Mater. 2015, 27, 2938–2944. [Google Scholar] [CrossRef]
- Zhong, W.; Xiao, J.; Sun, S.; Jiang, X.-F.; Lan, L.; Ying, L.; Yang, W.; Yip, H.-L.; Huang, F.; Cao, Y. Wide bandgap dithienobenzodithiophene-based π-conjugated polymers consisting of fluorinated benzotriazole and benzothiadiazole for polymer solar cells. J. Mater. Chem. C 2016, 4, 4719–4727. [Google Scholar] [CrossRef]
- Guo, P.; Luo, G.; Su, Q.; Li, J.; Zhang, P.; Tong, J.; Yang, C.; Xia, Y.; Wu, H. Boosting up performance of inverted photovoltaic cells from bis(alkylthien-2-yl)dithieno[2,3-d:2′,3′-d′]benzo[1,2-b:4′,5′-b′]dithiophene-based copolymers by advantageous vertical phase separation. ACS Appl. Mater. Interfaces 2017, 9, 10937–10945. [Google Scholar] [CrossRef]
- Gao, P.; Tong, J.; Guo, P.; Li, J.; Wang, N.; Li, C.; Ma, X.; Zhang, P.; Wang, C.; Xia, Y. Medium band gap conjugated polymers from thienoacene derivatives and pentacyclic aromatic lactam as promising alternatives of poly(3-hexylthiophene) in photovoltaic application. J. Polym. Sci. Part A Polym. Chem. 2018, 56, 85–95. [Google Scholar] [CrossRef]
- Shi, Q.; Fan, H.; Liu, Y.; Hu, W.; Li, Y.; Zhan, X. A copolymer of benzodithiophene with TIPS side chains for enhanced photovoltaic performance. Macromolecules 2011, 44, 9173–9179. [Google Scholar] [CrossRef]
- Bathula, C.; Song, C.E.; Badgujar, S.; Hong, S.-J.; Kang, I.-N.; Moon, S.-J.; Lee, J.; Cho, S.; Shim, H.-K.; Lee, S.K. New TIPS-substituted benzo[1,2-b:4,5-b′]dithiophene-based copolymers for application in polymer solar cells. J. Mater. Chem. 2012, 22, 22224–22232. [Google Scholar] [CrossRef]
- Kim, J.-H.; Song, C.E.; Shin, N.; Kang, H.; Wood, S.; Kang, I.-N.; Kim, B.J.; Kim, B.S.; Kim, J.-S.; Shin, W.S.; et al. High-crystalline medium-band-gap polymers consisting of benzodithiophene and benzotriazole derivatives for organic photovoltaic cells. ACS Appl. Mater. Interfaces 2013, 5, 12820–12831. [Google Scholar] [CrossRef]
- Kim, J.-H.; Lee, M.; Yang, H.; Hwang, D.-H. A high molecular weight triisopropylsilylethynyl (TIPS)-benzodithiophene and diketopyrrolopyrrole-based copolymer for high performance organic photovoltaic cells. J. Mater. Chem. A 2014, 2, 6348–6352. [Google Scholar] [CrossRef]
- Wang, L.; Liu, H.; Huai, Z.; Yang, S. Wide band gap and highly conjugated copolymers incorporating 2-(triisopropylsilylethynyl)thiophene-substituted benzodithiophene for efficient non-fullerene organic solar cells. ACS Appl. Mater. Interfaces 2017, 9, 28828–28837. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Wu, H.; Wang, D.; Yang, W.; Cao, Y. Novel electroluminescent conjugated polyelectrolytes based on polyfluorene. Chem. Mater. 2014, 16, 708–716. [Google Scholar] [CrossRef]
- Tong, J.; An, L.; Li, J.; Lv, J.; Guo, P.; Li, L.; Zhang, P.; Yang, C.; Xia, Y.; Wang, C. Effects of alkyl side chain length of low bandgap naphtho[1,2-c:5,6-c′]bis[1,2,5]thiadiazole-based copolymers on the optoelectronic properties of polymer solar cells. J. Polym. Sci. Part A Polym. Chem. 2018, 56, 2059–2071. [Google Scholar] [CrossRef]
- Guo, X.; Zhou, N.; Lou, S.J.; Smith, J.; Tice, D.B.; Hennek, J.W.; Ortiz, R.P.; Navarrete, J.T.L.; Li, S.; Strzalka, J.; et al. Polymer solar cells with enhanced fill factors. Nat. Photonics 2015, 7, 825–833. [Google Scholar] [CrossRef]
- Carsten, B.; He, F.; Son, H.J.; Xu, T.; Yu, L. Stille polycondensation for synthesis of functional materials. Chem. Rev. 2011, 111, 1493–1528. [Google Scholar] [CrossRef]
- Zhu, E.; Luo, G.; Liu, Y.; Yu, J.; Zhang, F.; Che, G.; Wu, H.; Tang, W. Triisopropylsilylethynyl substituted benzodithiophene copolymers: Synthesis, properties and photovoltaic characterization. J. Mater. Chem. C 2015, 3, 1595–1603. [Google Scholar] [CrossRef]
- Murugesan, V.; de Bettignies, R.; Mercier, R.; Guillerez, S.; Perrin, L. Synthesis and characterizations of benzotriazole based donor-acceptor copolymers for organic photovoltaic applications. Synth. Met. 2012, 162, 1037–1045. [Google Scholar] [CrossRef]
- Han, L.; Hu, T.; Bao, X.; Qiu, M.; Shen, W.; Sun, M.; Chen, W.; Yang, R. Steric minimization towards high planarity and molecular weight for aggregation and photovoltaic studies. J. Mater. Chem. A 2015, 3, 23587–23596. [Google Scholar] [CrossRef]
- Li, G.; Zhao, B.; Kang, C.; Lu, Z.; Li, C.; Dong, H.; Hu, W.; Wu, H.; Bo, Z. Side chain influence on the morphology and photovoltaic performance of 5-fluoro-6-alkyloxybenzothiadiazole and benzodithiophene based conjugated polymers. ACS Appl. Mater. Interfaces 2015, 7, 10710–10717. [Google Scholar] [CrossRef]
- Pommerehne, J.; Vestweber, H.; Guss, W.; Mahrt, R.F.; Bässler, H.; Porsch, M.; Daub, J. Efficient two layer leds on a polymer blend basis. Adv. Mater. 1995, 7, 551–554. [Google Scholar] [CrossRef]
- Wu, P.-T.; Kim, F.S.; Champion, R.D.; Jenekhe, S.A. Conjugated Donor-Acceptor copolymer semiconductors. Synthesis, optical properties, electrochemistry, and field-effect carrier mobility of pyridopyrazine-based copolymers. Macromolecules 2008, 41, 7021–7028. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Revision A. 01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- He, Z.; Zhong, C.; Su, S.; Xu, M.; Wu, H.; Cao, Y. Enhanced power-conversion efficiency in polymer solar cells using an inverted device structure. Nat. Photonics 2012, 6, 591–595. [Google Scholar] [CrossRef]
- Bai, Y.; Yang, B.; Zhao, C.; Shi, Z.; Hayat, T.; Alsaedi, A.; Tan, Z. Synergy of a titanium chelate electron collection layer and a vertical phase separated photoactive layer for efficient inverted polymer solar cells. J. Mater. Chem. A 2018, 6, 7257–7264. [Google Scholar] [CrossRef]
- Blom, P.W.M.; Mihailetchi, V.D.; Koster, L.J.A.; Markov, D.E. Device physics of polymer: Fullerene bulk heterojunction solar cells. Adv. Mater. 2007, 19, 1551–1566. [Google Scholar] [CrossRef]
- He, Z.; Xiao, B.; Liu, F.; Wu, H.; Yang, Y.; Xiao, S.; Wang, C.; Russell, T.P.; Cao, Y. Single-junction polymer solar cells with high efficiency and photovoltage. Nat. Photonics 2015, 9, 174–179. [Google Scholar] [CrossRef]
- Güldal, N.S.; Berlinghof, M.; Kassar, T.; Du, X.; Jiao, X.; Meyer, M.; Ameri, T.; Osvet, A.; Li, N.; Destri, G.L.; et al. Controlling additive behavior to reveal an alternative morphology formation mechanism in polymer:fullerene bulk-heterojunctions. J. Mater. Chem. A 2016, 4, 16136–16147. [Google Scholar] [CrossRef]
- Lee, J.K.; Ma, W.L.; Brabec, C.J.; Yuen, J.; Moon, J.S.; Kim, J.Y.; Lee, K.; Bazan, G.C.; Heeger, A.J. Processing additives for improved efficiency from bulk heterojunction solar cells. J. Am. Chem. Soc. 2018, 130, 3619–3623. [Google Scholar] [CrossRef]
- Wienk, M.M.; Kroon, J.M.; Verhees, W.J.H.; Knol, J.; Hummelen, J.C.; van Hal, P.A.; Janssen, R.A.J. Efficient methano[70]fullerene/MDMO-PPV bulk heterojunction photovoltaic cells. Angew. Chem. 2003, 115, 3493–3497. [Google Scholar] [CrossRef]
- Li, J.; Liang, Z.; Wang, Y.; Li, H.; Tong, J.; Bao, X.; Xia, Y. Enhanced efficiency of polymer solar cells through synergistic optimization of mobility and tuning donor alloys by adding high-mobility conjugated polymers. J. Mater. Chem. C 2018, 6, 11015–11022. [Google Scholar] [CrossRef]
- Kim, T.; Lee, J.Y.; Heo, J.; Lim, B.; Kim, J.Y. Highly efficient polymer solar cells with a thieno-pyrroledione and benzodithiophene containing planar random copolymer. Polym. Chem. 2018, 9, 1216–1222. [Google Scholar] [CrossRef]
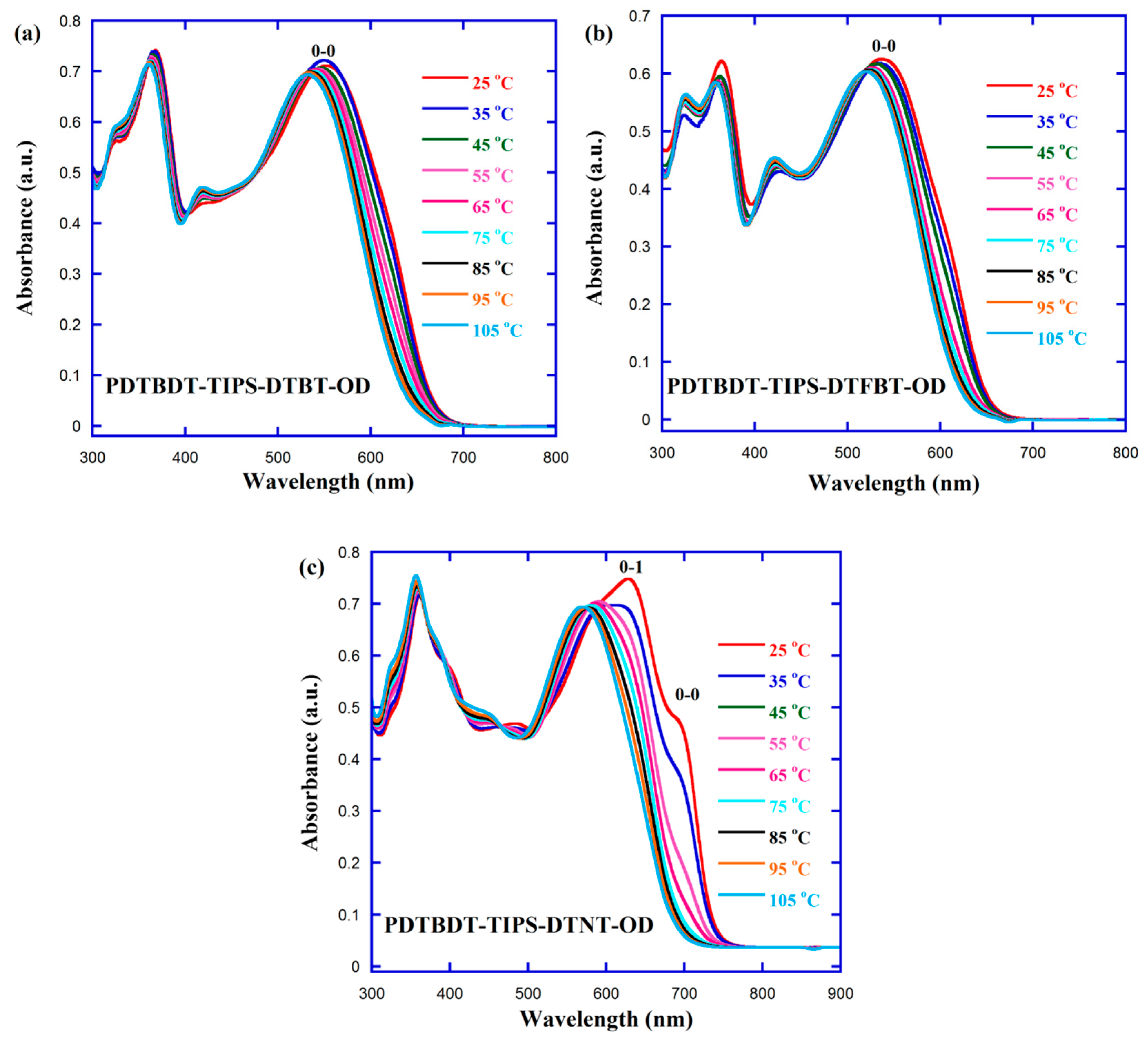
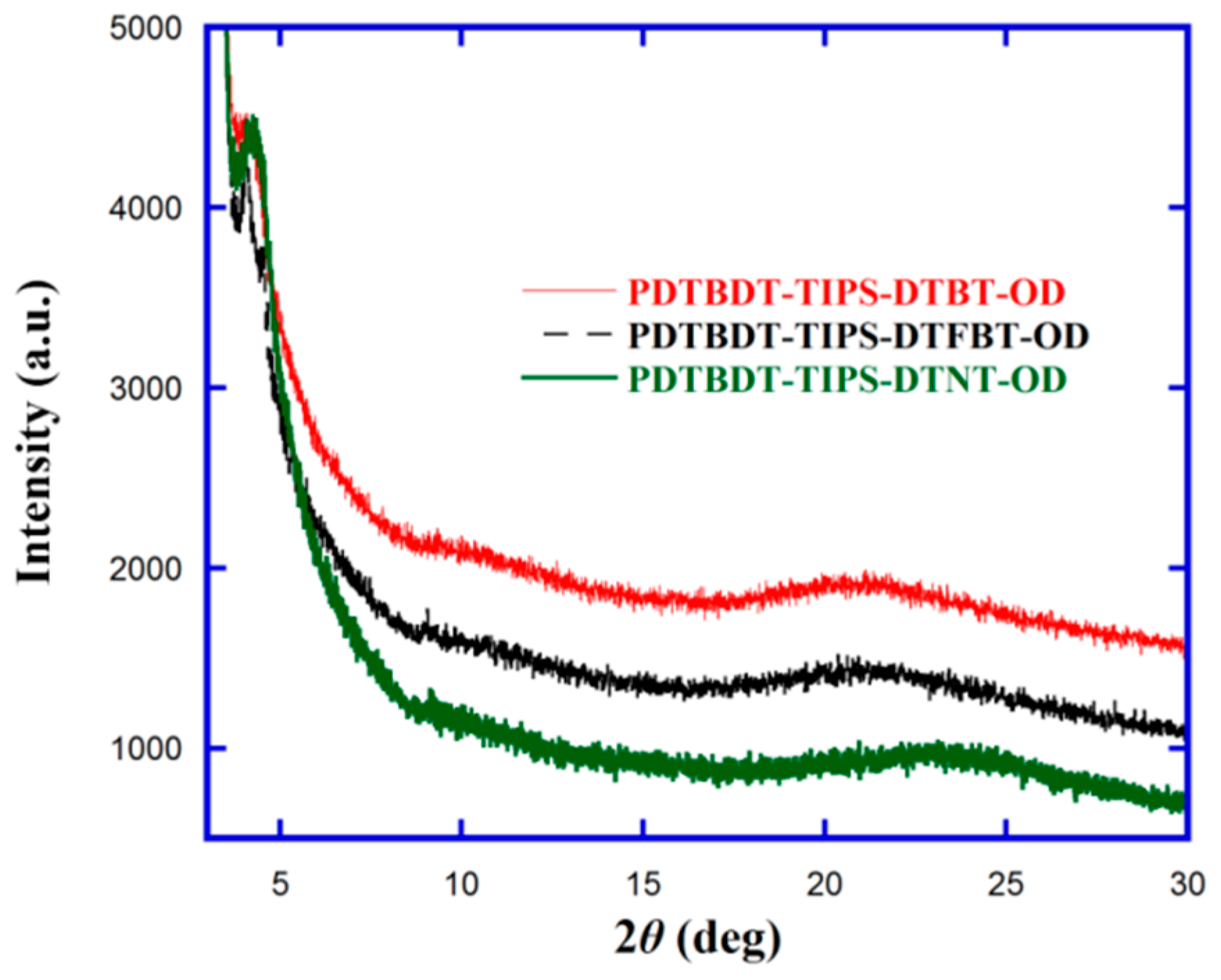



| Polymer | Solution | Film | 1 (eV) | (V) | (V) | EHOMO2 (eV) | ELUMO3 (eV) | 4 (eV) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| λmax (nm) | λsh (nm) | λmax (nm) | λsh (nm) | λonset (nm) | |||||||
| PDTBDT-TIPS-DTBT-OD | 366,425,552 | – | 375,582 | 620 | 680 | 1.82 | 0.67 | −1.22 | −5.35 | −3.46 | 1.89 |
| PDTBDT-TIPS-DTFBT-OD | 365,427,535 | – | 375,582 | 622 | 678 | 1.83 | 0.78 | −1.14 | −5.46 | −3.54 | 1.92 |
| PDTBDT-TIPS-DTNT-OD | 361,402,632 | 692 | 363, 405,492, 635 | 678 | 740 | 1.67 | 0.75 | −1.02 | −5.43 | −3.66 | 1.77 |
| Active Layer | Ratio/Additive | VOC (V) | JSC (mA cm−2) a | FF (%) | PCE (%) |
|---|---|---|---|---|---|
| PDTBDT-TIPS-DTBT-OD/PC71BM | 1:1.5/0%DIO | 0.80 | 3.62 (3.50) | 50.96 | 1.47 |
| PDTBDT-TIPS-DTFBT-OD/PC61BM | 1:1.5/0%DIO | 0.93 | 2.19 (2.08) | 53.59 | 1.09 |
| PDTBDT-TIPS-DTNT-OD/PC71BM | 1:1.5/3%DIO | 0.88 | 7.21 (7.09) | 52.99 | 3.37 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tong, J.; An, L.; Lv, J.; Guo, P.; Wang, X.; Yang, C.; Xia, Y. Enhanced Photovoltaic Performance in D-π-A Copolymers Containing Triisopropylsilylethynyl-Substituted Dithienobenzodithiophene by Modulating the Electron-Deficient Units. Polymers 2019, 11, 12. https://doi.org/10.3390/polym11010012
Tong J, An L, Lv J, Guo P, Wang X, Yang C, Xia Y. Enhanced Photovoltaic Performance in D-π-A Copolymers Containing Triisopropylsilylethynyl-Substituted Dithienobenzodithiophene by Modulating the Electron-Deficient Units. Polymers. 2019; 11(1):12. https://doi.org/10.3390/polym11010012
Chicago/Turabian StyleTong, Junfeng, Lili An, Jie Lv, Pengzhi Guo, Xunchang Wang, Chunyan Yang, and Yangjun Xia. 2019. "Enhanced Photovoltaic Performance in D-π-A Copolymers Containing Triisopropylsilylethynyl-Substituted Dithienobenzodithiophene by Modulating the Electron-Deficient Units" Polymers 11, no. 1: 12. https://doi.org/10.3390/polym11010012
APA StyleTong, J., An, L., Lv, J., Guo, P., Wang, X., Yang, C., & Xia, Y. (2019). Enhanced Photovoltaic Performance in D-π-A Copolymers Containing Triisopropylsilylethynyl-Substituted Dithienobenzodithiophene by Modulating the Electron-Deficient Units. Polymers, 11(1), 12. https://doi.org/10.3390/polym11010012