Abstract
Controlled radical copolymerization of chlorotrifluoroethylene (CTFE) and vinyl acetate (VAc) was successfully achieved in the presence of bis(acetylacetonato)cobalt(II) (Co(acac)2) as a mediated agent and 2,2′-azo-bis-isobutyronitrile (AIBN) as initiator. Both the molar mass and the fluorinated unit content of the copolymer could be controlled, and the chain extension polymerization of the obtained fluorinated copolymer was also achieved.
1. Introduction
Fluorinated polymers usually exhibit many remarkable properties due to the high electronegativity of the fluorine atom and the strong dissociation energy of the C-F bond (485 kJ·mol−1) [1,2,3,4,5]. However, perfluoropolymers often suffer from poor solubility in common organic solvents and have difficult processing characteristics. As a result, an increasing amount of research in recent years have been focusing on the copolymerization of fluoroolefins and non-fluoro monomers to prepare semi-fluorinated polymers [6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25]. Iodine transfer terpolymerization (ITP) was developed in the late 1970s by Tatemoto et al. [26] and believed to be a suitable controlled radical polymerization (CRP) method for the polymerization of some fluoroolefins, especially for vinylidene fluoride (VDF) [27,28,29]. However, there are very few successful cases publicly reported using this method. Since the mid-1990s, methods of CRP have been extensively studied and developed as a powerful tool for the synthesis of well-defined polymers with a predetermined molar mass, low dispersity, and various architectures [30,31,32,33,34]. Some of the CRP methods have also been used in the (co)polymerization of fluoroolefins, such as VDF, chlorotrifluoroethylene (CTFE), 3,3,3-trifluoropropene (TFP), and hexafluoropropylene (HFP). Ameduri et al. reported the synthesis of poly(VDF-co-TFP)-b-oligo(vinyl acetate) via the RAFT/MADIX process with fluorinated xanthate as a mediated agent [14]. Later on, another similar copolymerization process of perfluoro(methyl vinyl ether) and VDF was presented by Girard et al. [9]. In 2011, Liu et al. achieved the living/controlled radical copolymerization of CTFE and butyl vinyl ether (BVE) at room temperature under 60Co γ-ray irradiation with an O-ethyl xanthate [11]. Later in 2014, living/controlled radical copolymerization of CTFE and N-vinylpyrrolidone under 60Co γ-ray irradiation with S-benzyl O-ethyl dithiocarbonate as the RAFT/MADIX agent at room temperature was reported [6]. As mentioned before, VDF has been involved in many CRP reactions. Recently, Ameduri et al. reported another case in [35], where an organometallically mediated radical polymerization of VDF with an organocobalt compound R0(VAc)nCoIII(acac)2 (R0 = primary radical generated by V-70, n ≈ 4) as initiator was achieved. Besides these, there were also some studies reporting the cobalt-mediated radical copolymerization of fluorinated monomers and VAc in the last two years [36,37,38]. However, the fluorinated monomers used in these works have no fluorine atom on the double bond, meaning that they have very similar chemical properties with non-fluorinated monomers.
CTFE is a gas-state fluoroolefin, and still poses as an interesting challenge for the CRP of CTFE. In this work, we investigated the cobalt-mediated radical copolymerization of CTFE and VAc in the presence of bis(acetylacetonato)cobalt(II) (Co(acac)2) as a mediated compound. A more stable and easily available initiator, 2,2′-azo-bis-isobutyronitrile (AIBN), in replacement of V-70 was also used in the polymerization. The results indicate that the process has the features of CRP. Molar masses of the obtained copolymers increased linearly with the monomer conversions, and a linear relationship between ln([M]0/[M]) and the polymerization time was found to exist. Moreover, a block copolymer was prepared by chain extension polymerization of VAc using the obtained polymer as a macro-initiator and mediated agent. The Co(acac)2-mediated radical copolymerization process is depicted in Scheme 1.
Scheme 1.
Schematic representation of Co(acac)2-mediated radical copolymerization of chlorotrifluoroethylene (CTFE) and vinyl acetate (VAc).
2. Experimental
2.1. Materials
CTFE was purchased from Zhejiang Juhua Co., Ltd., Quzhou, China. VAc and ethyl acetate were purchased from Sinopharm Chemical Reagent Co, Ltd., Shanghai, China. VAc was purified by passing through a basic alumina column, and subsequently distilled. Ethyl acetate was refluxed and distilled over CaH2. AIBN and Co(acac)2 were purchased from Aladdin Industrial Corporation (Shanghai, China). AIBN was recrystallized from methanol twice before use. All other chemicals were used as received, unless otherwise noted.
2.2. Characterization
Weight percentages of carbon and hydrogen were assessed by elemental analysis on a Vario EL cube (Elementar, Langenselbold, Germany) instrument.
The values of the number-average molar mass (Mn,GPC) and dispersity (Mw/Mn) were determined by means of a Waters 150 C gel permeation equipped with 103, 104, and 105 Å Waters Ultrastyragel columns and a light scattering detector, using THF (1.0 mL/min) as the eluent, and the calibration was carried out with polystyrene standards.
1H and 19F NMR spectra were recorded on a Bruker DPX-400 spectrometer at room temperature, using CDCl3 as the solvent.
Thermogravimetric analysis (TGA) was performed with a DTG-60H (Shimadzu, Kyoto, Japan) apparatus, under nitrogen atmosphere, at a heating rate of 10 °C/min from room temperature up to a maximum of 700 °C.
2.3. Synthetic Procedures
2.3.1. General Procedure for Co(acac)2-Mediated Copolymerization of CTFE and VAc
The reaction was performed in a 30 mL stainless steel autoclave equipped with a manometer, a safety inlet valve, and a magnetic stirrer. First, the mixture of 18 mg AIBN (0.11 mmol), 16 mg Co(acac)2 (0.055 mmol), 1.00 mL VAc (10.8 mmol), and 5.0 mL ethyl acetate were introduced into the autoclave, after which the vessel was closed and immersed in liquid nitrogen for 15 min. Several nitrogen-vacuum cycles were conducted to remove any traces of oxygen. After that, the required quantity (a certain molar fold of VAc (see in Table 1); 1 fold is 2.5 g) of CTFE was condensed into the autoclave via a mass flow meter. The exact mass value of CTFE was assessed by calculating the weight difference of the autoclave before and after filling with CTFE. Then, the polymerization reaction was conducted at 70 °C for a predetermined time. The reaction was quenched by cooling with ice water, and the residual CTFE was slowly vented. The solution was concentrated on a rotary evaporator and precipitated in petroleum ether. The obtained precipitate was finally dried under a vacuum at 40 °C until a constant weight was attained.

Table 1.
Results of Co(acac)2-mediated radical copolymerization of CTFE and VAc.
2.3.2. General Procedure for Chain Extension Polymerization with VAc
The chain extension polymerization reaction was performed in the same autoclave we mentioned before. The obtained copolymer (0.215 g, Sample P7 in Table 1) and 1.00 mL (10.8 mmol) VAc was dissolved in 5 mL ethyl acetate and charged in the autoclave. Then, the vessel was closed and immersed in the liquid nitrogen for 15 min. Five cycles of nitrogen-vacuum were conducted to remove any traces of oxygen. After that, the autoclave was immersed in a thermostatic oil bath at 30 °C for 6 h. Then, the autoclave was cooled down by ice water and the solution was precipitated in petroleum ether. The resultant product was collected and dried under a vacuum at 40 °C until it reached a constant weight.
3. Results and Discussion
The results of Co(acac)2-mediated copolymerization of CTFE and VAc are shown in Table 1. In this work, AIBN was used to initiate the copolymerization instead of labile V70 at a higher temperature of 70 °C, which probably caused relatively higher values of dispersity of the resulted copolymers.
Equations (1) and (2) were used to determine the molar fractions of CTFE and VAc in the copolymer (CTFE% and VAc%, respectively) according to the molecular formulas of CTFE and VAc (CTFE = C2ClF3, VAc = C4H6O2):
where C% is the weight percentage of carbon in the copolymer accessed by elemental analysis. MC is the atomic weight of carbon. MCTFE and MVAc stand for the molar mass of CTFE and VAc, respectively.
C% = (2MC × CTFE% + 4MC × VAc%)/(MCTFE × CTFE% + MVAc × VAc%)
CTFE% = 1 − VAc%
The conversion of VAc (Conv.) could be calculated according to Equation (3):
where mp is the mass of resultant copolymer, and mVAc is the initial mass of VAc in the solution.
The theoretical molar mass (Mn,th) of poly(CTFE-co-VAc) was calculated according to Equation (4):
where the coefficient 200 corresponds to the initial molar ratio of VAc to Co(acac)2.
Mn,th = 200 × Conv. × [MVAc + (CTFE%/VAc%) × MCTFE]
Given that CTFE is a gas-state monomer, it is not a homogeneous reaction system for the copolymerization between CTFE and VAc. Only part of CTFE which we condensed in the autoclave dissolved in the reaction solution, and the undissolved CTFE would gradually permeate into the solution as the dissolved CTFE was consumed during the copolymerization reaction. There is no suitable method to determine the reactivity ratios for this kind of copolymerization system. Thus, we had to introduce some indirect parameters to describe the kinetics of the copolymerization.
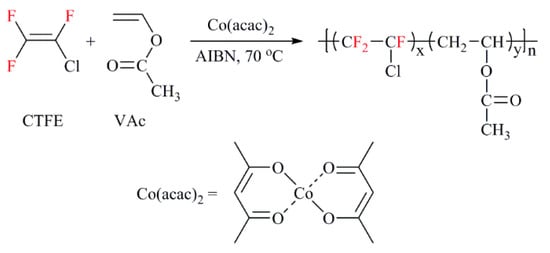
Figure 1 shows the molar content of CTFE in the obtained copolymers prepared under the same condition. As can be seen, the molar fractions of CTFE increase with the initial molar ratio of CTFE to VAc. Meanwhile, the rising trend comes to an equilibrium when [CTFE]0/[VAc]0 reaches up to 4. In the kinetic study to be described later, a four-fold initial molar ratio of CTFE to VAc was used in the polymerization process.
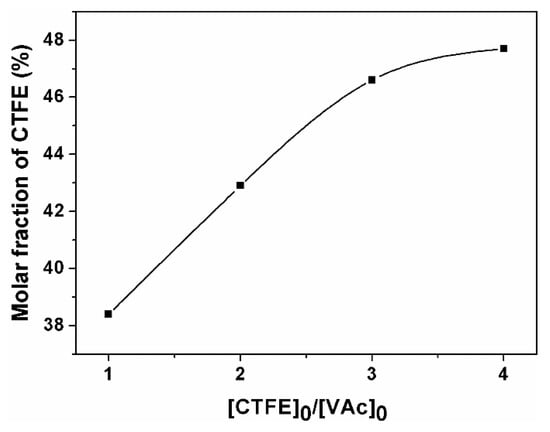
Figure 1.
Molar content of CTFE in the copolymer as a function of the initial molar ratio of CTFE to VAc (Sample P1, P2, P3, and P4).
When the initial molar ratio of CTFE to VAc is fixed at four-fold, molar fractions of CTFE in the resulted copolymers increase with the monomer conversions (shown in Figure 2), due to the increase in the real-time molar ratio of CTFE to VAc in the polymerization process.
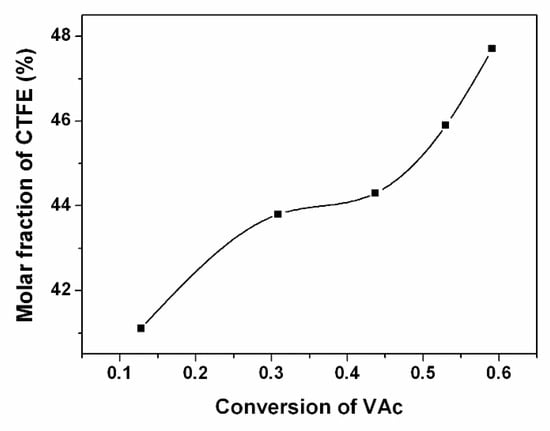
Figure 2.
Molar content of CTFE in the copolymer as a function of VAc conversion (P4, P6, P7, P8, and P9).
It can be observed that molar masses determined by GPC (Mn,GPC) are higher than the theoretical values (Mn,th), which is probably due to the standards of polystyrenes used in the calibration. The molar masses of poly(CTFE-co-VAc) increase linearly with the monomer conversions, and the dispersities (Mw/Mn) remain at about 1.5 up to high conversions (shown in Figure 3). The linear kinetic curve could be ascribed to the quasi-alternating copolymerization of CTFE and VAc. Meanwhile, a control experiment with the same condition of sample P8 but without adding Co(acac)2 was conducted. The Mn,GPC of the obtained copolymer is 15,500, and the value of dispersity is as high as 2.59. Figure 4 shows the GPC traces of these copolymers.
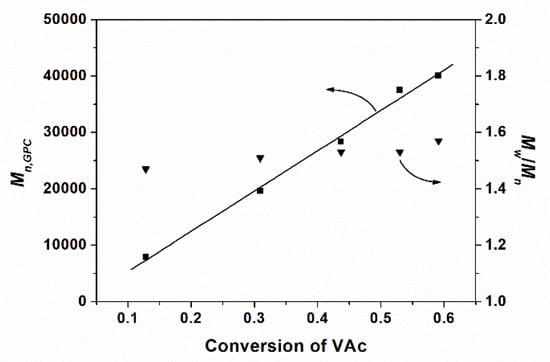
Figure 3.
Molar mass and dispersity of the copolymer as a function of VAc conversion.
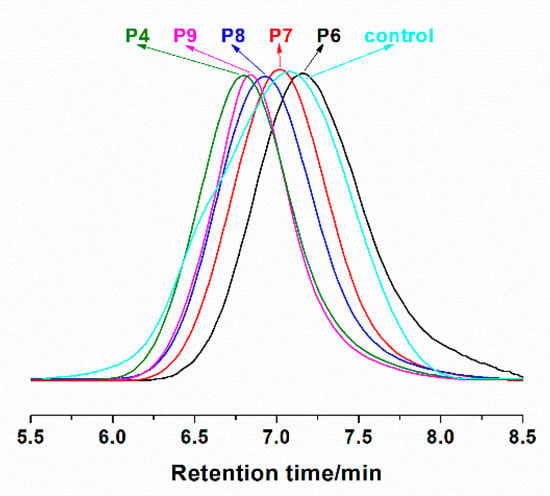
Figure 4.
GPC traces of some representative copolymers.
Figure 5 shows the linear relationship between ln([M]0/[M]) and the polymerization time, which indicates that the Co(acac)2-mediated radical copolymerization of CTFE and VAc is a first-order reaction with respect to the monomer concentration. Meanwhile, an induction period of about one hour prior to polymerization was observed, which corresponds to the time required to inject a certain amount of radicals in the medium sufficient to convert the CoII species into organocobalt(III) derivatives. In fact, the induction period of the copolymerization reaction in this work is much shorter than that previously reported for the polymerization of VAc by other researchers, which is as long as several hours [39,40]. This is probably due to the higher temperature and different chemical composition of the cobalt compound we used in the polymerization reaction. In fact, the exact denotation of the cobalt compound we used is Co(acac)2·2H2O, which means that there was at least a two-fold molar ratio of water to Co(acac)2 in the reaction medium. Water is a typical Lewis base, and would add to the coordination sphere of cobalt and facilitate the homolytic cleavage of the Co-C bond, which would open access to the reversible-termination mechanism from the very beginning of the process [41].
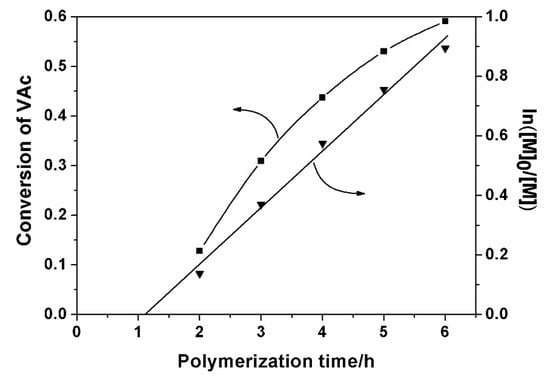
Figure 5.
Conversion and ln([M]0/[M]) of VAc as a function of polymerization time for the copolymerization of CTFE and VAc.
The chemical structure of the fluorinated copolymer was characterized by 1H and 19F NMR spectroscopy, and Sample P7 was chosen as a representative. For Sample P7, the molar ratio of the CTFE unit to VAc unit in the copolymer is 43.8/56.2, which is very close to the alternating structure. Figure 6 shows the 19F NMR spectrum of sample P7. There are a series of multiplets located between −108 and −132 ppm, which are attributed to the groups of CF2 and CFCl in the chain backbone. Meanwhile, no signal appears at −100 ppm in 19F NMR spectrum, which indicates that there are no CTFE-CTFE diads in the polymer chains [42]. Figure 7 shows the 1H NMR spectrum of poly(CTFE-co-VAc), where A represents the CTFE unit and B represents the VAc unit. The resonances located between 6.60 and 4.70 ppm can be attributed to the proton of methine in the VAc unit. As can be seen, there are four kinds of chain sequences in the copolymer, most of which have an alternating structure (ABA) that is followed by ABB or BBA, and very few BBB chain sequences exist. Signals appearing between 3.10 and 0.80 ppm belong to the methene of the VAc unit in the backbone and the methyl of the VAc unit in the pendent group.
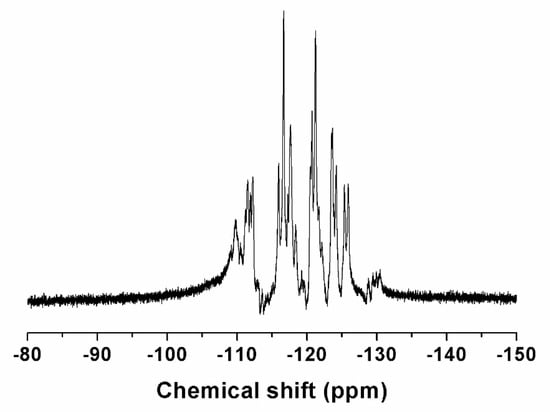
Figure 6.
19F NMR spectrum of poly(CTFE-co-VAc) (Sample P7) recorded in CDCl3 at room temperature.
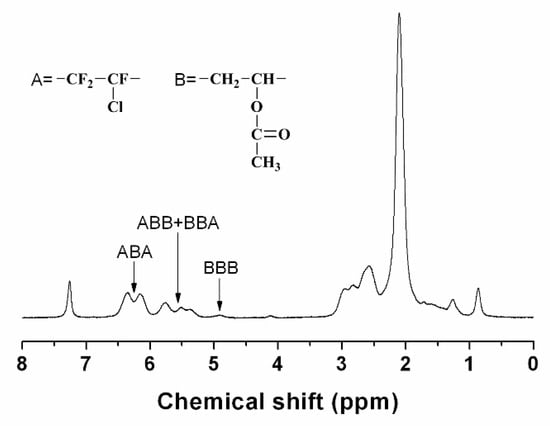
Figure 7.
1H NMR spectrum of poly(CTFE-co-VAc) (Sample P7) recorded in CDCl3 at room temperature.
Chain extension polymerization was conducted with Sample P7 as a macro-initiator and mediated agent, and VAc as the monomer. A really low reaction temperature of 30 °C avoided the initiating reaction of possible residual AIBN. Figure 8 shows the GPC traces of Sample P7 and the block copolymer (poly(CTFE-co-VAc)-b-PVAc) prepared by chain extension polymerization. As can be seen, the GPC curve of the block copolymer shifts toward a significantly higher molar mass compared with that of Sample P7, which indicates that the chain extension polymerization has been successfully achieved.
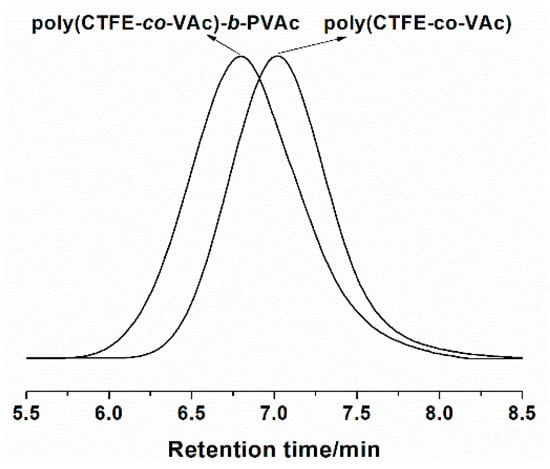
Figure 8.
GPC traces of poly(CTFE-co-VAc) (Sample P7, Mn,GPC = 19,600, Mw/Mn = 1.51) and poly(CTFE-co-VAc)-b-PVAc (Mn,GPC = 39,300, Mw/Mn = 1.57).
Thermal stability of the obtained fluorinated copolymers was examined by thermogravimetric analysis (TGA) under a nitrogen condition. Figure 9 shows the thermograms of the copolymers. Severe degradation starts from about 300 °C, which demonstrates that the obtained fluorinated copolymers have good thermal stability.
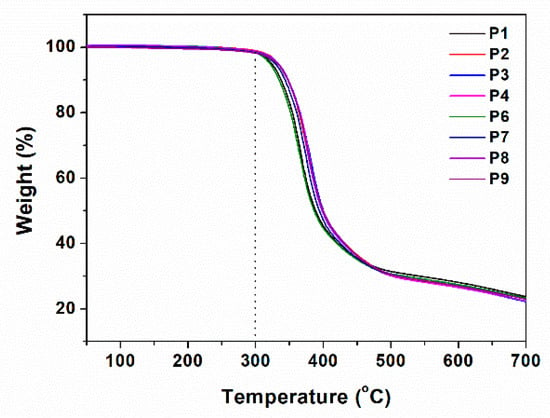
Figure 9.
Thermogravimetric analysis (TGA) thermograms (under nitrogen, at 10 °C·min−1) of the copolymers.
4. Conclusions
We successfully conducted the radical copolymerization of CTFE and VAc in the presence of Co(acac)2 as a controlling agent. Molar fractions of the CTFE unit in the obtained copolymer gradually increased with the initial molar ratios of CTFE to VAc and the monomer conversions. The molar masses determined by GPC of the copolymers increased linearly with the VAc conversions. Moreover, a linear relationship between ln([M]0/[M]) and the polymerization time was found to exist. Besides, a block copolymer was prepared by chain extension polymerization of VAc based on the obtained fluorinated copolymer without adding any other extra agents. All these results demonstrated that Co(acac)2-mediated controlled radical copolymerization of CTFE and VAc initiated by AIBN could be achieved. Furthermore, we believe that this method would also be suitable for other electron-deficient fluoroolefins, and more detailed work needs to be done. VAc units in the polymer backbone could be transferred into hydroxyl groups, so that we could prepare various functional fluorinated polymers based on the method introduced in this work.
Author Contributions
Data curation, H.W.; Formal analysis, Q.D.; Investigation, P.W.; Writing—original draft, P.W.; Writing—review & editing, R.B.
Funding
The authors are grateful for financial support from the National Natural Science Foundation (NNSF) of China (NO. 21504086) and Science Challenge Project (TZ2018004).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hougham, G.G.; Cassidy, P.E.; Johns, K.; Davidson, T. Fluoropolymers 1: Synthesis; Springer Science & Business Media: New York, NY, USA, 1999. [Google Scholar]
- Smart, B. Properties of fluorinated compounds, physical and physicochemical properties. Chem. Organ. Fluorine Compd. II 1995, 187, 979. [Google Scholar]
- Yamabe, M. A Challenge to Novel Fluoropolymers; Macromolecular Symposia; Wiley Online Library: Hoboken, NJ, USA, 1992; pp. 11–18. [Google Scholar]
- Ameduri, B.; Boutevin, B. Well-Architectured Fluoropolymers: Synthesis, Properties and Applications: Synthesis, Properties and Applications; Elsevier: Amsterdam, The Netherlands, 2004. [Google Scholar]
- Wang, W.; Yan, D.; Bratton, D.; Howdle, S.M.; Wang, Q.; Lecomte, P. Charge transfer complex inimer: A facile route to dendritic materials. Adv. Mater. 2003, 15, 1348–1352. [Google Scholar] [CrossRef]
- Wang, P.; Dai, J.; Liu, L.; Dong, Q.; Wang, H.; Bai, R. Synthesis and properties of a well-defined copolymer of chlorotrifluoroethylene and n-vinylpyrrolidone by xanthate-mediated radical copolymerization under 60 co γ-ray irradiation. Polym. Chem. 2014, 5, 6358–6364. [Google Scholar] [CrossRef]
- Wang, P.; Dai, J.; Liu, L.; Jin, B.; Bai, R. Xanthate-mediated living/controlled radical copolymerization of hexafluoropropylene and butyl vinyl ether under 60co γ-ray irradiation and preparation of fluorinated polymers end-capped with a fluoroalkyl sulfonic acid group. Polym. Chem. 2013, 4, 1760–1764. [Google Scholar] [CrossRef]
- Boschet, F.; Ameduri, B. (co)polymers of chlorotrifluoroethylene: Synthesis, properties, and applications. Chem. Rev. 2013, 114, 927–980. [Google Scholar] [CrossRef] [PubMed]
- Girard, E.; Marty, J.-D.; Ameduri, B.; Destarac, M. Direct synthesis of vinylidene fluoride-based amphiphilic diblock copolymers by raft/madix polymerization. ACS Macro Lett. 2012, 1, 270–274. [Google Scholar] [CrossRef]
- Alaaeddine, A.; Boschet, F.; Ameduri, B.; Boutevin, B. Synthesis and characterization of original alternated fluorinated copolymers bearing glycidyl carbonate side groups. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 3303–3312. [Google Scholar] [CrossRef]
- Liu, L.; Lu, D.; Wang, H.; Dong, Q.; Wang, P.; Bai, R. Living/controlled free radical copolymerization of chlorotrifluoroethene and butyl vinyl ether under 60co γ-ray irradiation in the presence of s-benzyl o-ethyl dithiocarbonate. Chem. Commun. 2011, 47, 7839–7841. [Google Scholar] [CrossRef]
- Kostov, G.; Boschet, F.; Buller, J.; Badache, L.; Brandsadter, S.; Ameduri, B. First amphiphilic poly (vinylidene fluoride-co-3,3,3-trifluoropropene)-b-oligo (vinyl alcohol) block copolymers as potential nonpersistent fluorosurfactants from radical polymerization controlled by xanthate. Macromolecules 2011, 44, 1841–1855. [Google Scholar] [CrossRef]
- Santhosh, K.K.S.; Ameduri, B. Synthesis and characterization of epoxy functionalized cooligomers based on chlorotrifluoroethylene and allyl glycidyl ether. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 3587–3595. [Google Scholar] [CrossRef]
- Kyulavska, M.; Kostov, G.; Ameduri, B.; Mateva, R. Unexpected alternating radical copolymerization of chlorotrifluoroethylene with 3-isopropenyl-α,α′-dimethylbenzyl isocyanate. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 2681–2697. [Google Scholar] [CrossRef]
- Boschet, F.D.R.; Cracowski, J.-M.; Montembault, V.R.; Ameduri, B. Radical copolymerization of α,β-difluoroacrylic acid with vinylidene fluoride. Macromolecules 2010, 43, 4879–4888. [Google Scholar] [CrossRef]
- Valade, D.; Boschet, F.; Améduri, B. Synthesis and modification of alternating copolymers based on vinyl ethers, chlorotrifluoroethylene, and hexafluoropropylene. Macromolecules 2009, 42, 7689–7700. [Google Scholar] [CrossRef]
- Lu, Y.; Claude, J.; Neese, B.; Zhang, Q.; Wang, Q. A modular approach to ferroelectric polymers with chemically tunable curie temperatures and dielectric constants. J. Am. Chem. Soc. 2006, 128, 8120–8121. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Holdcroft, S. Synthesis and proton conductivity of partially sulfonated poly ([vinylidene difluoride-co-hexafluoropropylene]-b-styrene) block copolymers. Macromolecules 2005, 38, 4193–4201. [Google Scholar] [CrossRef]
- Gaboyard, M.; Hervaud, Y.; Boutevin, B. Photoinitiated alternating copolymerization of vinyl ethers with chlorotrifluoroethylene. Polym. Int. 2002, 51, 577–584. [Google Scholar] [CrossRef]
- Boutevin, B.; Cersosimo, F.; Youssef, B. Studies of the alternating copolymerization of vinyl ethers with chlorotrifluoroethylene. Macromolecules 1992, 25, 2842–2846. [Google Scholar] [CrossRef]
- Jin, C.; Otsuhata, K.; Tabata, Y. Radiation-induced copolymerization of n-vinylpyrrolidone with monochlorotrifluoroethylene. J. Macromol. Sci. -Chem. 1985, 22, 379–386. [Google Scholar] [CrossRef]
- Tabata, Y.; Du Plessis, T. Radiation-induced copolymerization of chlorotrifluoroethylene with ethyl vinyl ether. J. Polym. Sci. Part A Polym. Chem. 1971, 9, 3425–3435. [Google Scholar] [CrossRef]
- Ragazzini, M.; Garbuglio, C.; Carcano, D.; Minasso, B.; Cevidalli, G. Copolymerization of ethylene and chlorotrifluoroethylene by trialkylboron catalysts—I. Polymerization and reactivity ratios. Eur. Polym. J. 1967, 3, 129–136. [Google Scholar] [CrossRef]
- Garbuglio, C.; Ragazzini, M.; Pilati, O.; Carcano, D.; Cevidalli, G. Copolymerization of ethylene and chlorotrifluoroethylene by trialkylboron catalysts—II. Physico-chemical characterization of the copolymers. Eur. Polym. J. 1967, 3, 137–144. [Google Scholar] [CrossRef]
- Guerre, M.; Uchiyama, M.; Lopez, G.; Améduri, B.; Satoh, K.; Kamigaito, M.; Ladmiral, V. Synthesis of peve-b-p(ctfe-alt-eve) block copolymers by sequential cationic and radical raft polymerization. Polym. Chem. 2018, 9, 352–361. [Google Scholar] [CrossRef]
- Tatemoto, M.; Nakagawa, T. German patent 2,729,671. Chem. Abstr. 1978, 88, 137374. [Google Scholar]
- David, G.; Boyer, C.; Tonnar, J.; Ameduri, B.; Lacroix-Desmazes, P.; Boutevin, B. Use of iodocompounds in radical polymerization. Chem. Rev. 2006, 106, 3936–3962. [Google Scholar] [CrossRef] [PubMed]
- Boyer, C.; Ameduri, B.; Hung, M.H. Telechelic diiodopoly(vdf-co-pmve) copolymers by iodine transfer copolymerization of vinylidene fluoride (vdf) with perfluoromethyl vinyl ether (pmve). Macromolecules 2010, 43, 3652–3663. [Google Scholar] [CrossRef]
- Sawada, H.; Tashima, T.; Nishiyama, Y.; Kikuchi, M.; Goto, Y.; Kostov, G.; Ameduri, B. Iodine transfer terpolymerization of vinylidene fluoride, α-trifluoromethacrylic acid and hexafluoropropylene for exceptional thermostable fluoropolymers/silica nanocomposites. Macromolecules 2011, 44, 1114–1124. [Google Scholar] [CrossRef]
- Kamigaito, M.; Ando, T.; Sawamoto, M. Metal-catalyzed living radical polymerization. Chem. Rev. 2001, 101, 3689–3746. [Google Scholar] [CrossRef] [PubMed]
- Moad, G.; Rizzardo, E.; Thang, S.H. Living radical polymerization by the raft process-a second update. Aust. J. Chem. 2009, 62, 1402–1472. [Google Scholar] [CrossRef]
- Hawker, C.J.; Bosman, A.W.; Harth, E. New polymer synthesis by nitroxide mediated living radical polymerizations. Chem. Rev. 2001, 101, 3661–3688. [Google Scholar] [CrossRef] [PubMed]
- Wayland, B.B.; Poszmik, G.; Mukerjee, S.L.; Fryd, M. Living radical polymerization of acrylates by organocobalt porphyrin complexes. J. Am. Chem. Soc. 1994, 116, 7943–7944. [Google Scholar] [CrossRef]
- Arvanitopoulos, L.D.; Greuel, M.P.; Harwood, H.J. Living free-radical polymerization using alkyl cobaloximes as photoinitiators. Abstr. Pap. Am. Chem. Soc. 1994, 208, 402. [Google Scholar]
- Banerjee, S.; Ladmiral, V.; Debuigne, A.; Detrembleur, C.; Poli, R.; Ameduri, B. Organometallic-mediated radical polymerization of vinylidene fluoride. Angew. Chem. 2018, 57, 2934–2937. [Google Scholar] [CrossRef] [PubMed]
- Demarteau, J.; Améduri, B.; Ladmiral, V.; Mees, M.A.; Hoogenboom, R.; Debuigne, A.; Detrembleur, C. Controlled synthesis of fluorinated copolymers via cobalt-mediated radical copolymerization of perfluorohexylethylene and vinyl acetate. Macromolecules 2017, 50, 3750–3760. [Google Scholar] [CrossRef]
- Banerjee, S.; Ladmiral, V.; Debuigne, A.; Detrembleur, C.; Rahaman, S.M.W.; Poli, R.; Ameduri, B. Organometallic-mediated alternating radical copolymerization of tert-butyl-2-trifluoromethacrylate with vinyl acetate and synthesis of block copolymers thereof. Macromol. Rapid Commun. 2017, 38. [Google Scholar] [CrossRef]
- Banerjee, S.; Bellan, E.; Gayet, F.; Debuigne, A.; Detrembleur, C.; Poli, R.; Améduri, B.; Ladmiral, V. Bis(formylphenolato)cobalt(ii)-mediated alternating radical copolymerization of tert-butyl 2-trifluoromethylacrylate with vinyl acetate. Polymers 2017, 9, 702. [Google Scholar] [CrossRef]
- Peng, C.H.; Yang, T.Y.; Zhao, Y.; Fu, X. Reversible deactivation radical polymerization mediated by cobalt complexes: Recent progress and perspectives. Organ. Biomol. Chem. 2014, 12, 8580–8587. [Google Scholar] [CrossRef]
- Debuigne, A.; Poli, R.; Jérôme, C.; Jérôme, R.; Detrembleur, C. Overview of cobalt-mediated radical polymerization: Roots, state of the art and future prospects. Prog. Polym. Sci. 2009, 34, 211–239. [Google Scholar] [CrossRef]
- Debuigne, A.; Champouret, Y.; Jerome, R.; Poli, R.; Detrembleur, C. Mechanistic insights into the cobalt-mediated radical polymerization (cmrp) of vinyl acetate with cobalt(iii) adducts as initiators. Chem. -Eur. J. 2008, 14, 4046–4059. [Google Scholar] [CrossRef]
- Carnevale, D.; Wormald, P.; Ameduri, B.; Tayouo, R.; Ashbrook, S.E. Multinuclear magnetic resonance and dft studies of the poly (chlorotrifluoroethylene-alt-ethyl vinyl ether) copolymers. Macromolecules 2009, 42, 5652–5659. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).