Nanomechanics and Nanorheology of Microgels at Interfaces
Abstract
1. Introduction
2. Atomic Force Microscopy
2.1. Static Force Measurements
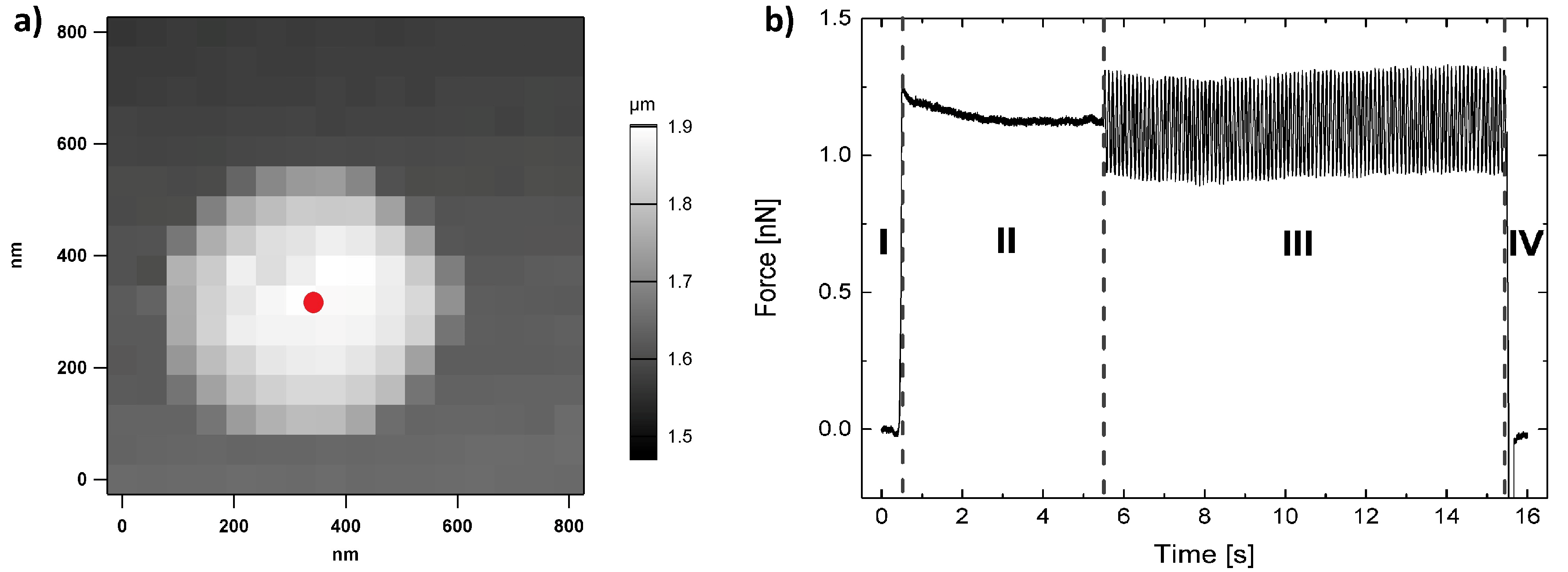
2.2. Dynamic Force Measurements
3. Nanomechanics and Nanorheology: Effect of Different Parameters
3.1. Outer Parameter
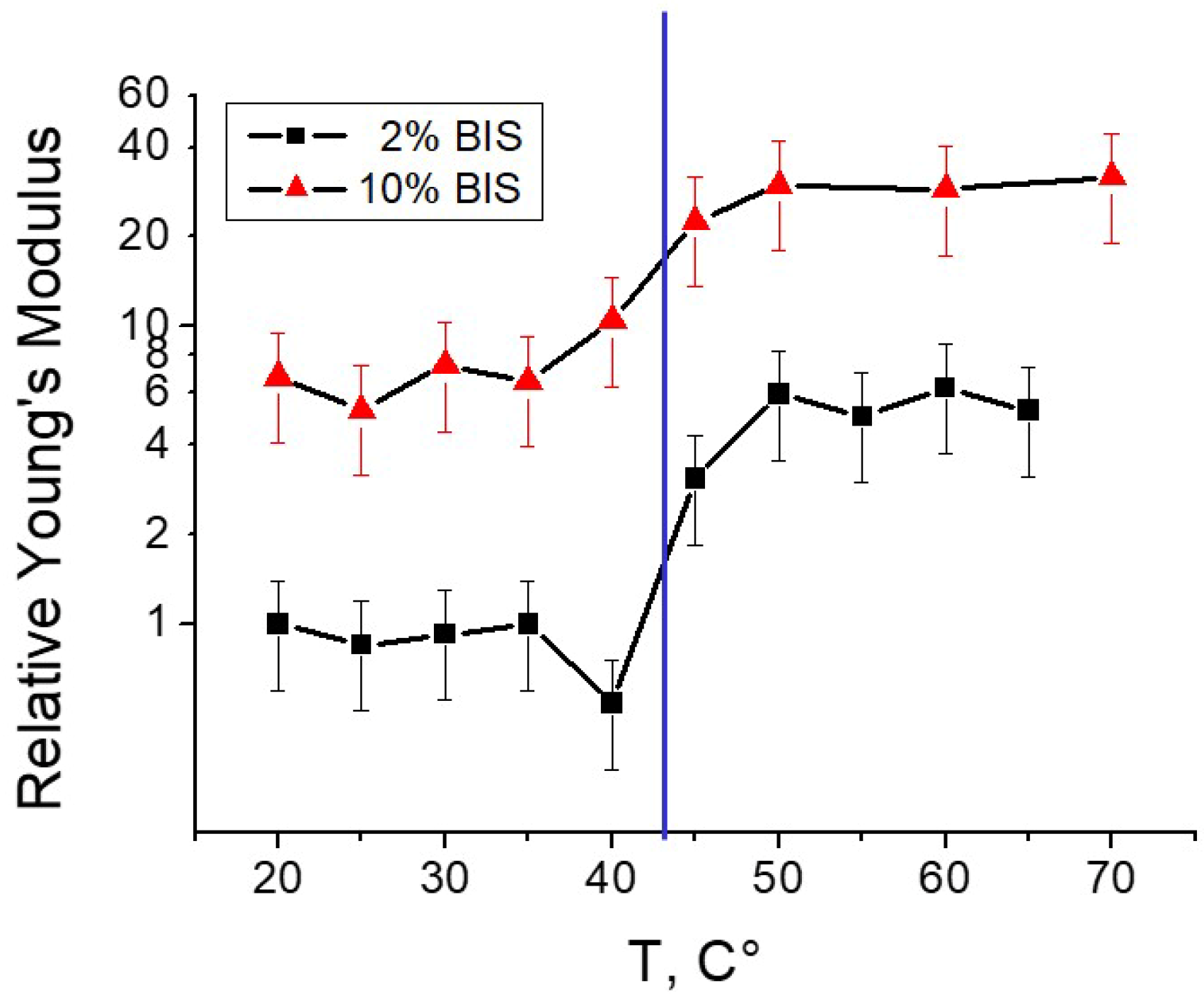
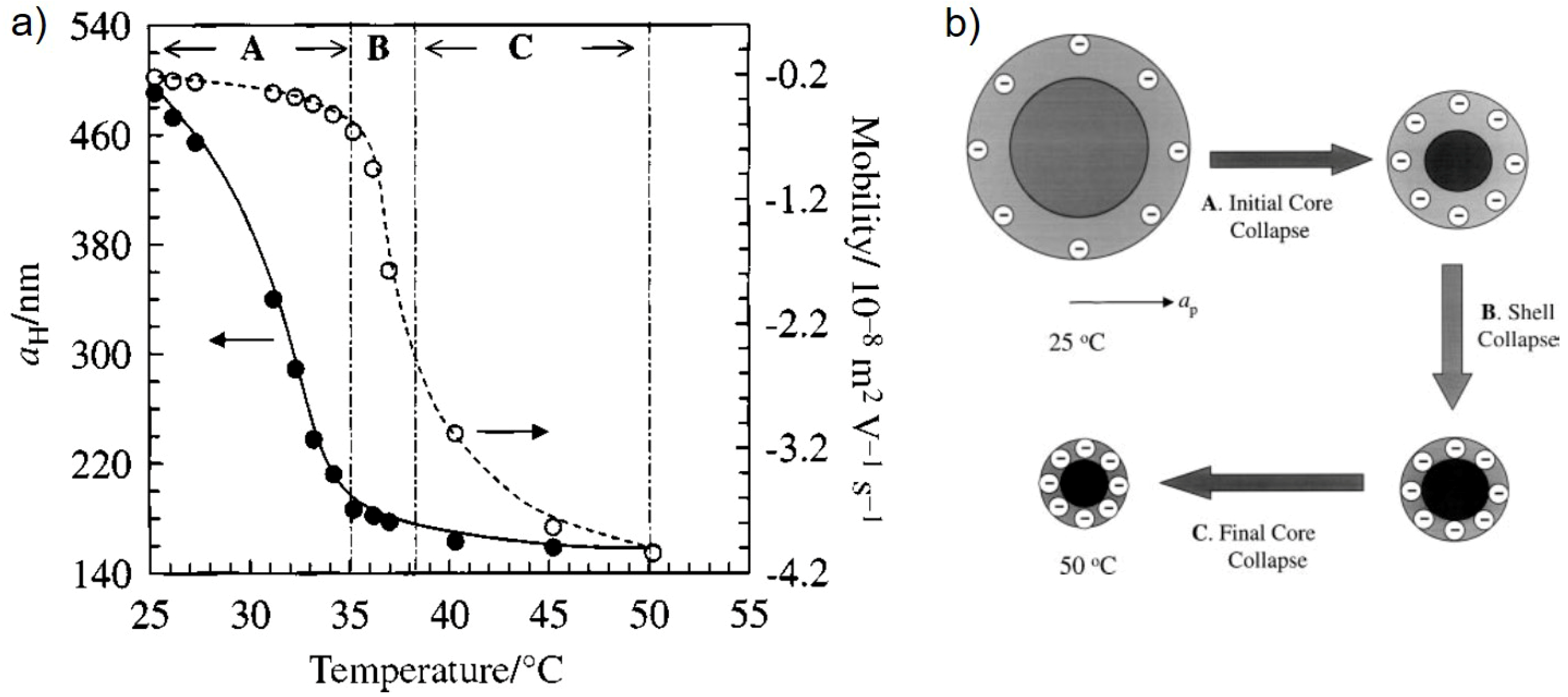
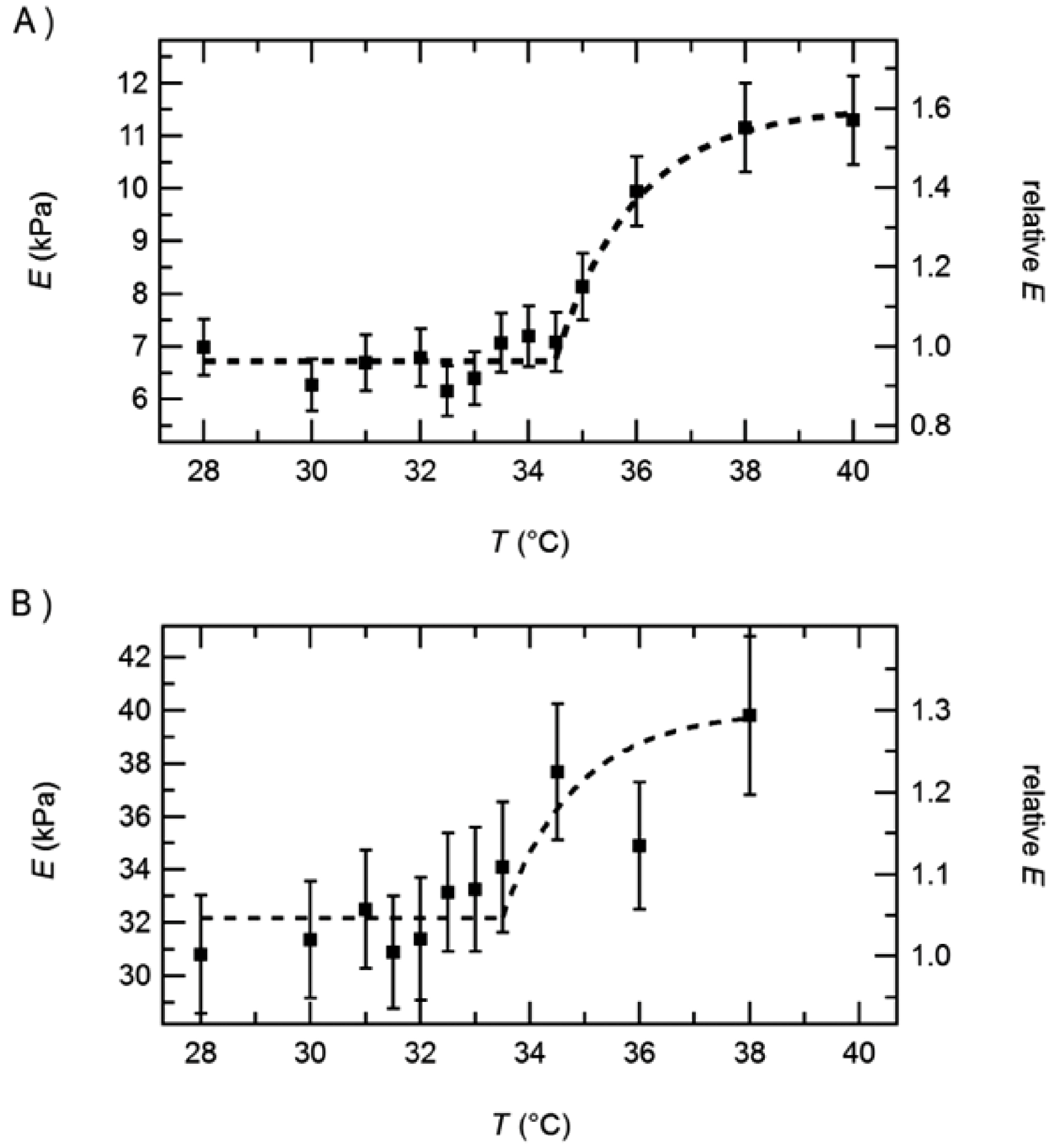
3.1.1. Effect of Temperature
3.1.2. Effect of Solvent Composition: The Cononsolvency Effect
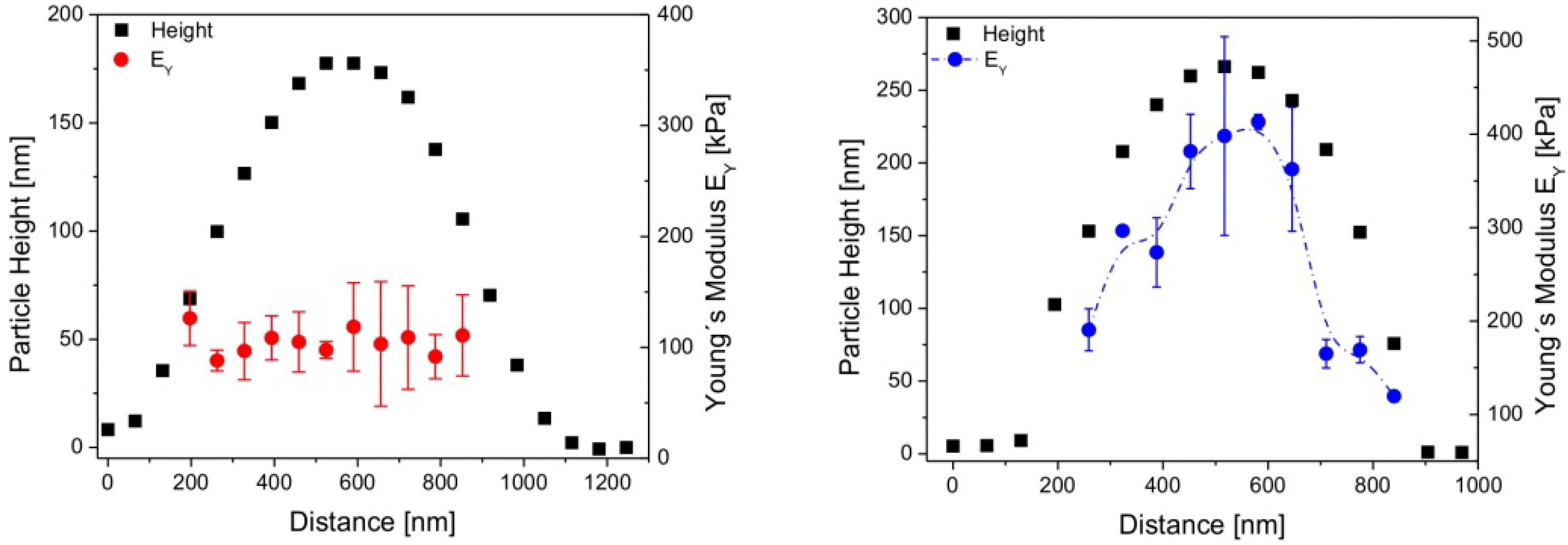
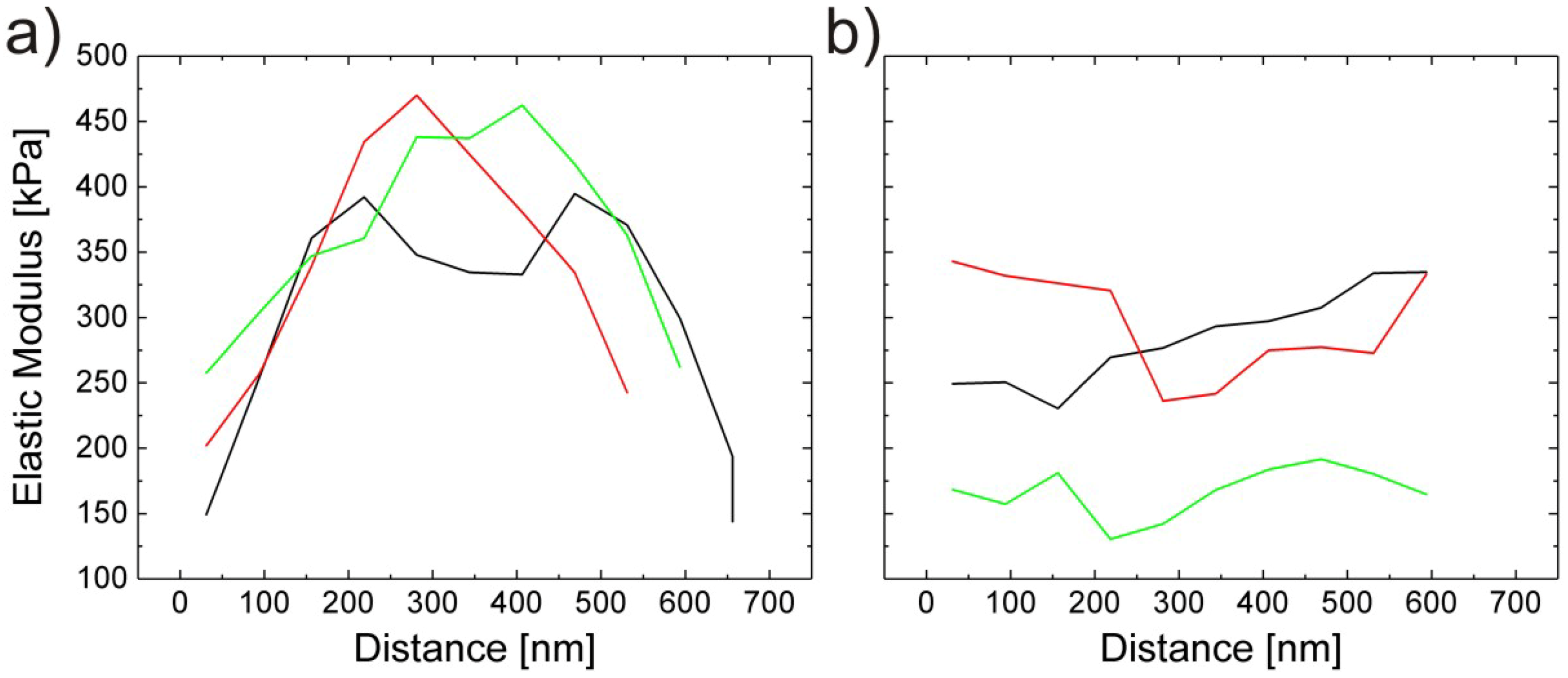
3.2. Composition of the Microgels (Inner Parameters)
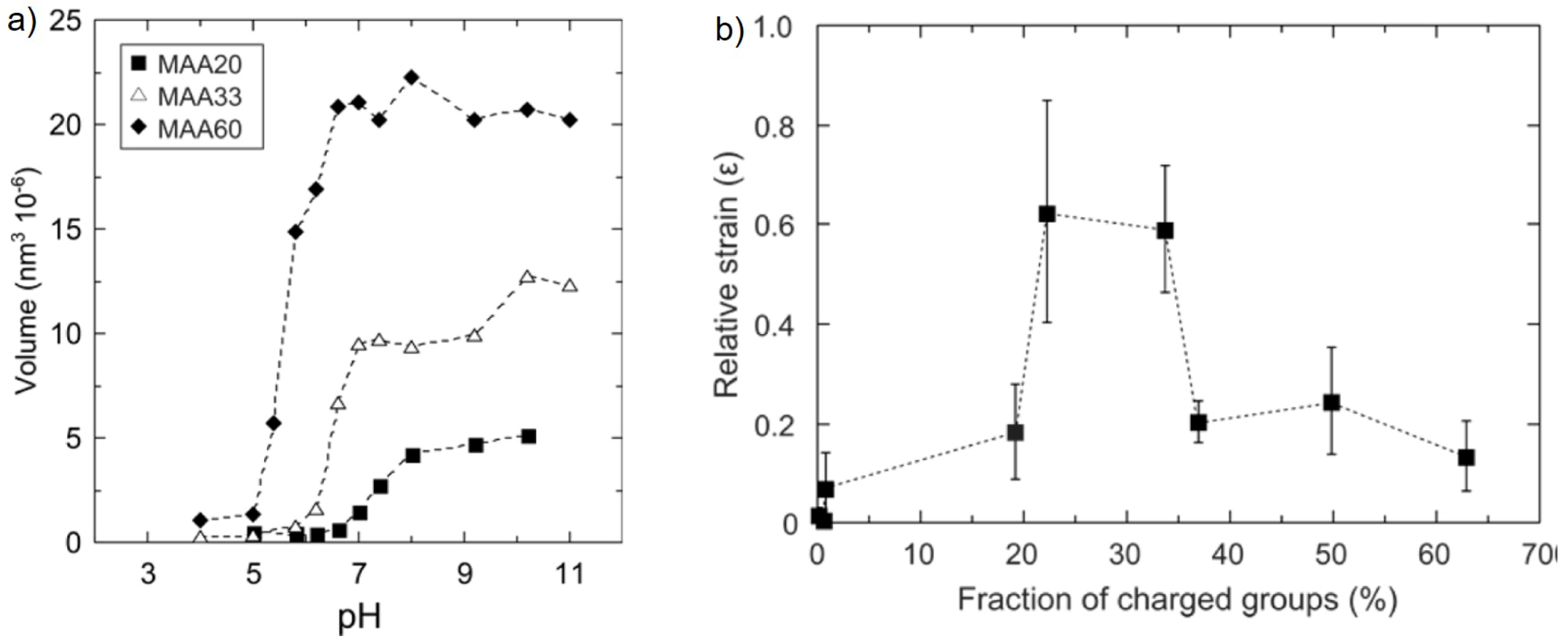
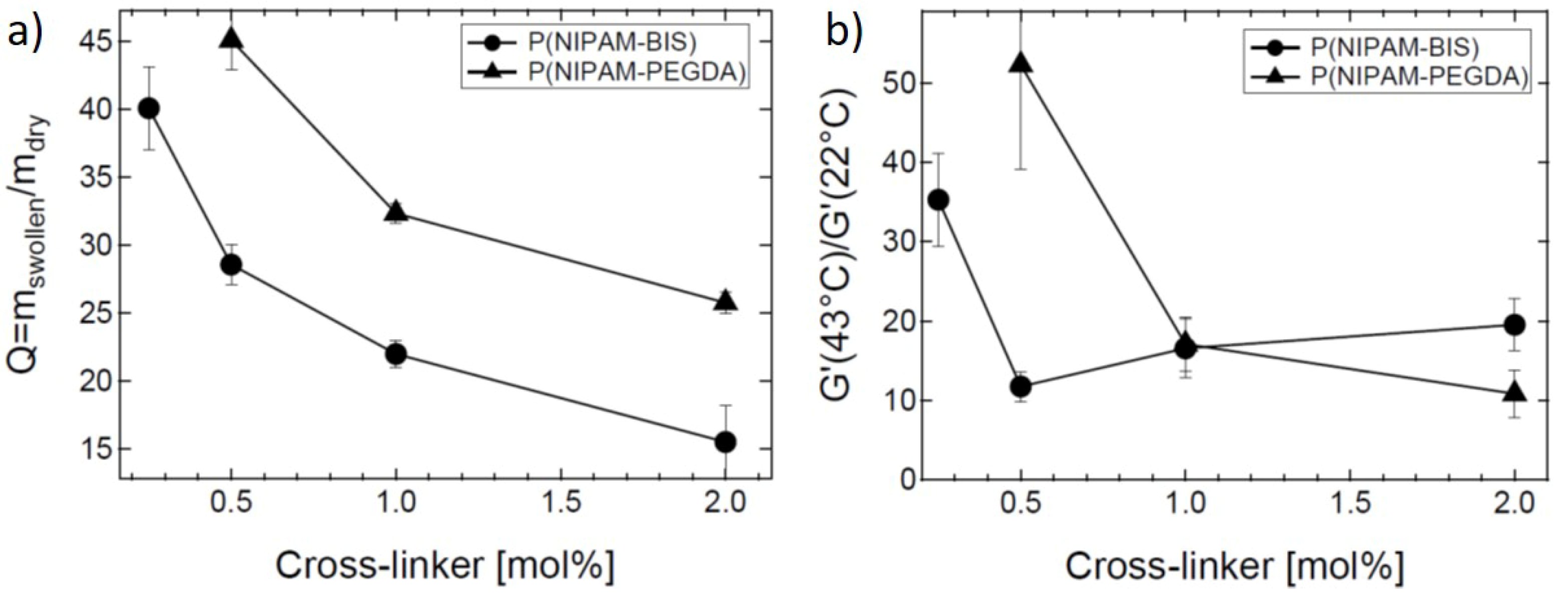
Effect of Cross-Linker Content
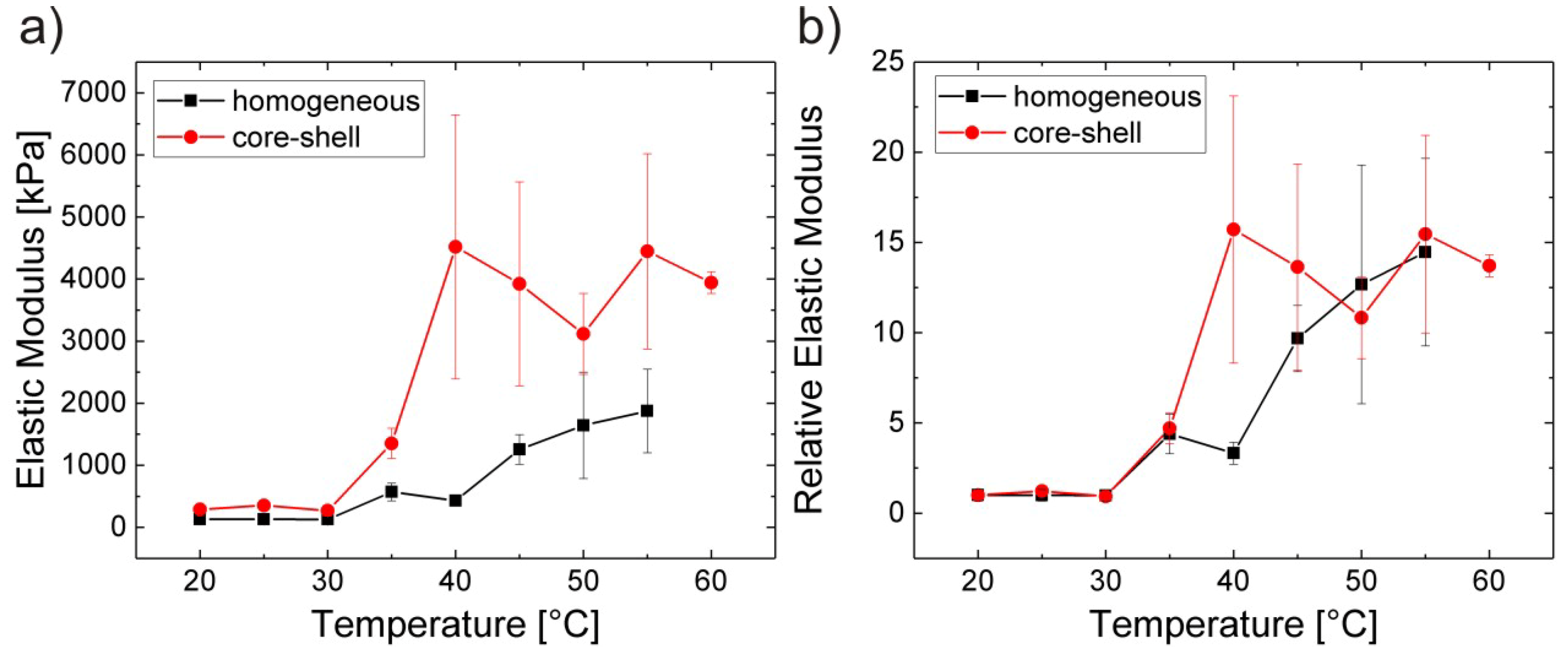
3.3. Homogeneous It vs. Core-Shell Microgels
Effect of Charge Density
3.4. Composite Microgels
3.5. Microgels with a Stimuli-Insensitive Shell
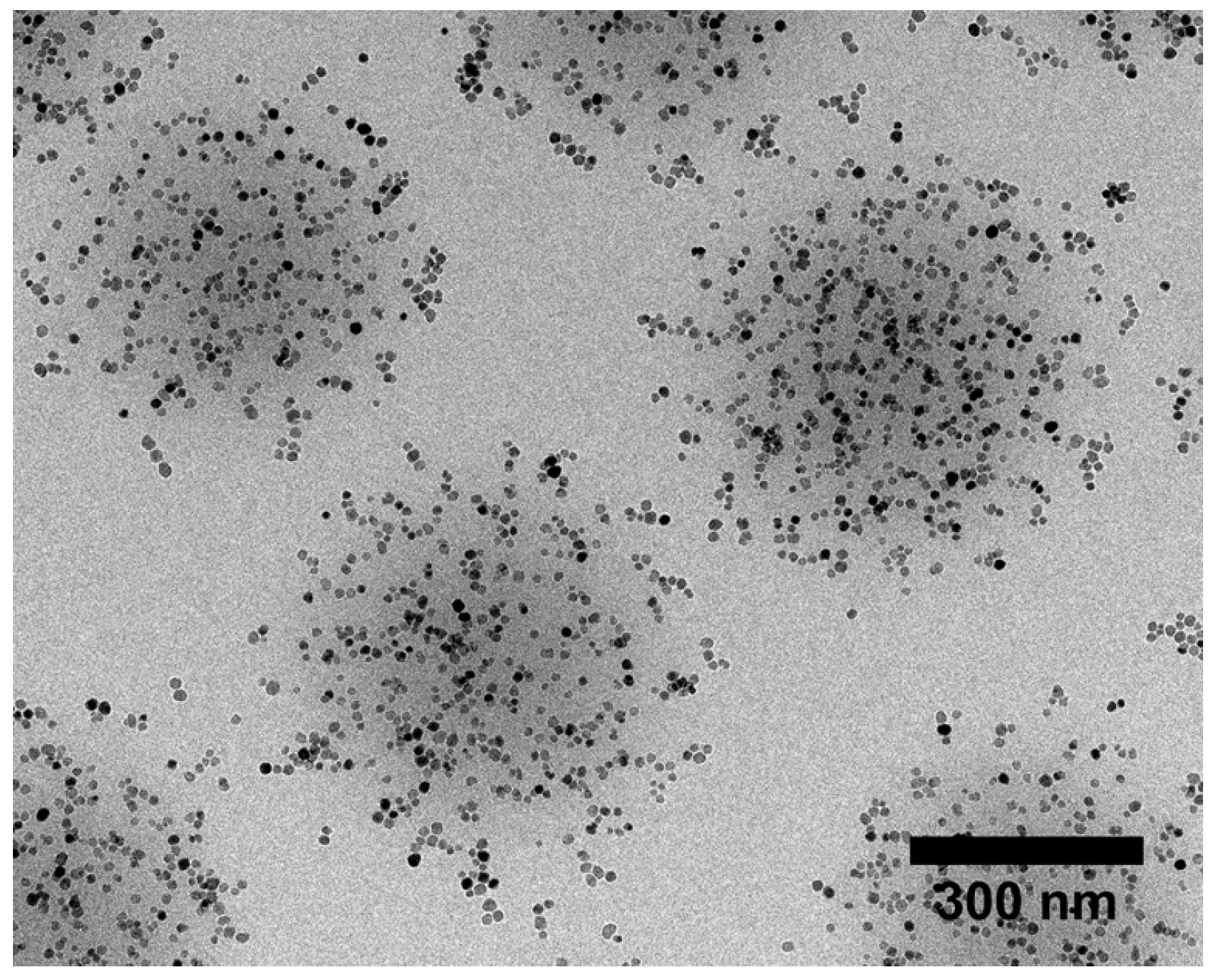
Microgels Doped with Metal Nanoparticles
4. Applications
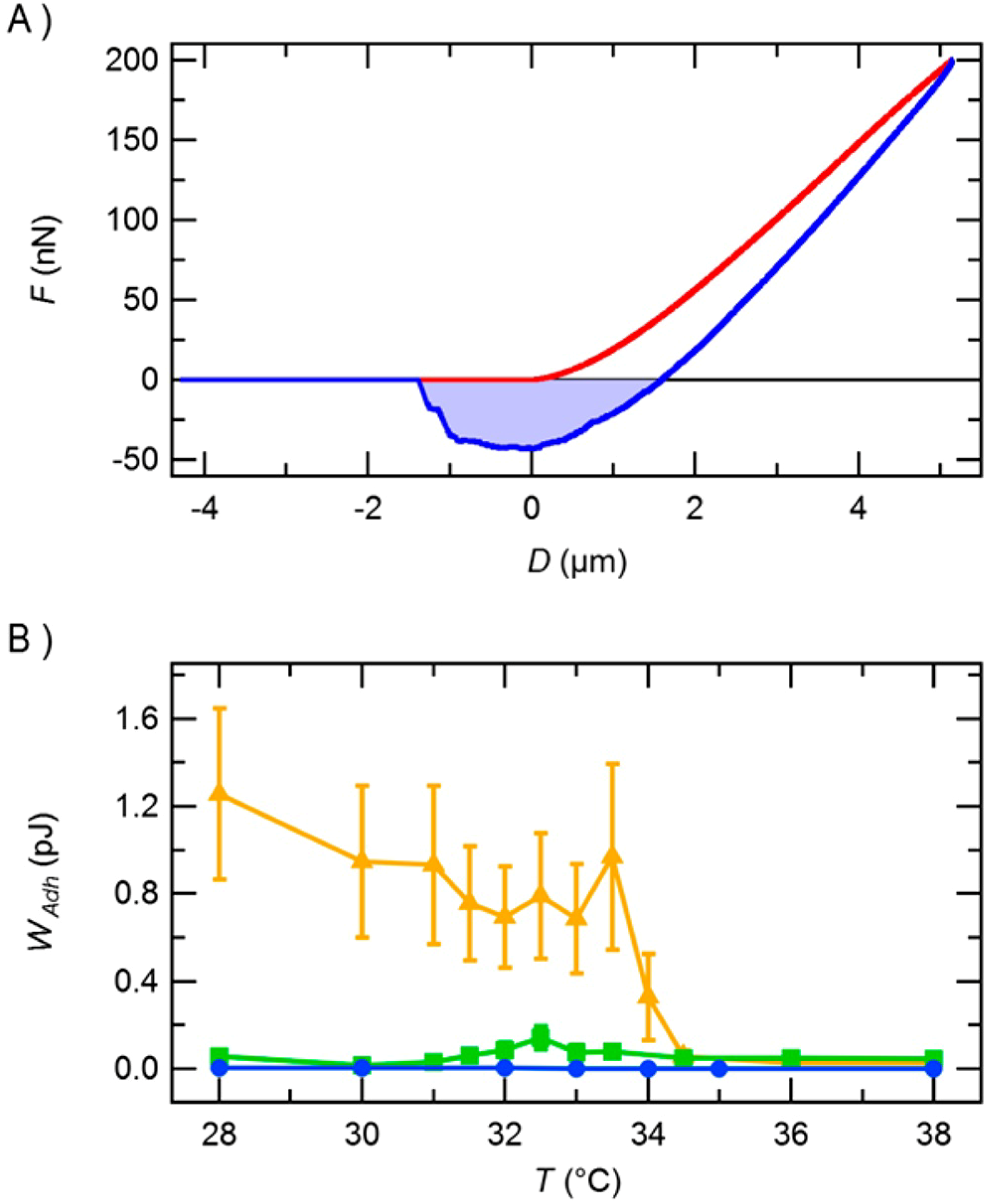
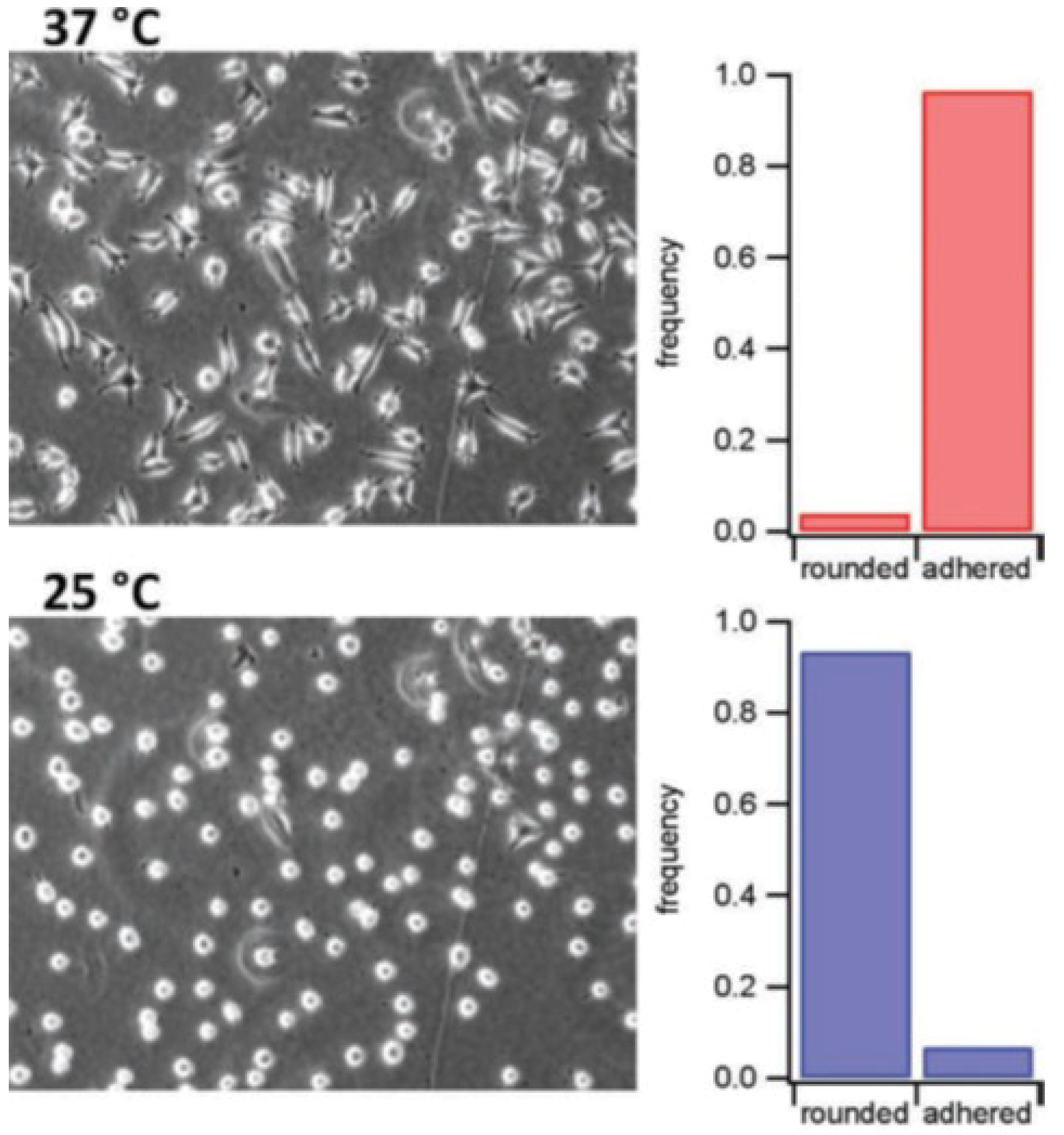
4.1. Microgels Acting as Cellular Micro-Environments
4.2. Tactile Feed-Back Devices
4.3. From Pickering Emulsions to Mickering Emulsions
5. Summary, Conclusions and Outlook
Funding
Conflicts of Interest
References
- Li, Y.; Tanaka, T. Kinetics of swelling and shrinking of gels. J. Chem. Phys. 1990, 92, 1365–1371. [Google Scholar] [CrossRef]
- Snowden, M.J.; Chowdhry, B.Z.; Vincent, B.; Morris, G.E. Colloidal copolymer microgels of N-isopropylacrylamide and acrylic acid: pH, ionic strength and temperature effects. J. Chem. Soc. Faraday Trans. 1996, 92, 5013–5016. [Google Scholar] [CrossRef]
- Jones, C.D.; Lyon, L.A. Synthesis and characterization of multiresponsive core-shell microgels. Macromolecules 2000, 33, 8301–8306. [Google Scholar] [CrossRef]
- Suzuki, A.; Tanaka, T. Phase transition in polymer gels induced by visible light. Nature 1990, 346, 345. [Google Scholar] [CrossRef]
- Fuhrer, R.; Athanassiou, E.K.; Luechinger, N.A.; Stark, W.J. Crosslinking metal nanoparticles into the polymer backbone of hydrogels enables preparation of soft, magnetic field-driven actuators with muscle-like flexibility. Small 2009, 5, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Messing, R.; Frickel, N.; Belkoura, L.; Strey, R.; Rahn, H.; Odenbach, S.; Schmidt, A.M. Cobalt ferrite nanoparticles as multifunctional cross-linkers in PAAm ferrohydrogels. Macromolecules 2011, 44, 2990–2999. [Google Scholar] [CrossRef]
- Tanaka, T.; Fimore, D.; Sun, S.-T.; Nishio, I.; Swislow, G.; Shah, A. Phase transitions in ionic gels. Phys. Rev. Lett. 1980, 45, 1636. [Google Scholar] [CrossRef]
- Deng, Y.; Pelton, R. Synthesis and solution properties of poly (N-isopropylacrylamide-co-diallyldimethylammonium chloride). Macromolecules 1995, 28, 4617–4621. [Google Scholar] [CrossRef]
- Kratz, K.; Eimer, W. Swelling properties of colloidal poly (N-isopropylacrylamide) microgels in solution. Ber. Bunsenges. Phys. Chem. 1998, 102, 848–854. [Google Scholar] [CrossRef]
- Kratz, K.; Hellweg, T.; Eimer, W. Effect of connectivity and charge density on the swelling and local structural and dynamic properties of colloidal PNIPAM microgels. Ber. Bunsenges. Phys. Chem. 1998, 102, 1603–1608. [Google Scholar] [CrossRef]
- Senff, H.; Richtering, W. Temperature sensitive microgel suspensions: Colloidal phase behavior and rheology of soft spheres. J. Chem. Phys. 1999, 111, 1705–1711. [Google Scholar] [CrossRef]
- Pelton, R. Temperature-sensitive aqueous microgels. Adv. Coll. Int. Sci. 2000, 85, 1–33. [Google Scholar] [CrossRef]
- Tanaka, T. Kinetics of Phase Transition in Polymer Gels. Physica A 1986, 140, 261. [Google Scholar] [CrossRef]
- Wu, C.; Zhou, S. Volume phase transition of swollen gels: Discontinuous or continuous? Macromolecules 1997, 30, 574–576. [Google Scholar] [CrossRef]
- Nystroöm, L.; Alvarez-Asencio, R.; Frenning, G.; Saunders, B.R.; Rutland, M.W.; Malmsten, M. Electrostatic swelling transitions in surface-bound microgels. ACS Appl. Mater. Interfaces 2016, 8, 27129–27139. [Google Scholar] [CrossRef] [PubMed]
- Burmistrova, A.; Richter, M.; Eisele, M.; Üzüm, C.; von Klitzing, R. The effect of co-monomer content on the swelling/shrinking and mechanical behaviour of individually adsorbed pnipam microgel particles. Polymers 2011, 3, 1575–1590. [Google Scholar] [CrossRef]
- Singh, N.; Bridges, A.W.; Garcia, A.J.; Lyon, L.A. Covalent tethering of functional microgel films onto poly (ethylene terephthalate) surfaces. Biomacromolecules 2007, 8, 3271–3275. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, D.; McGrath, J.G.; Kawaguchi, H.; Lyon, L.A. Colloidal crystals of thermosensitive, core/shell hybrid microgels. Phys. Chem. C 2007, 111, 5667–5672. [Google Scholar] [CrossRef]
- Serpe, M.J.; Jones, C.D.; Lyon, L.A. Layer-by-layer deposition of thermoresponsive microgel thin films. Langmuir 2003, 19, 8759–8764. [Google Scholar] [CrossRef]
- Burmistrova, A.; von Klitzing, R. Control of number density and swelling/shrinking behavior of p(nipam–aac) particles at solid surfaces. J. Mater. Chem. 2010, 20, 3502–3507. [Google Scholar] [CrossRef]
- Burmistrova, A.; Richter, M.; Üzüm, C.; von Klitzing, R. Effect of cross-linker density of p(NIPAM-co-AAC) microgels at solid surfaces on the swelling/shrinking behaviour and the young’s modulus. Coll. Polym. Sci. 2011, 289, 613–624. [Google Scholar] [CrossRef]
- Wellert, S.; Hertle, Y.; Richter, M.; Medebach, M.; Magerl, D.; Wang, W.; Deme, B.; Müller-Buschbaum, P.; Hellweg, T.; von Klitzing, R. Inner structure of adsorbed ionic microgel particles. Langmuir 2014, 30, 7168–7176. [Google Scholar] [CrossRef] [PubMed]
- Gawlitza, K.; Ivanova, O.; Holderer, O.; Radulescu, A.; von Klitzing, R.; Wellert, S. Bulk phase and surface dynamics of peg microgel particles. Macromolecules 2015, 48, 5807–5815. [Google Scholar] [CrossRef]
- Hertz, H. On the contact of elastic solids. Reine Angew. Math. 1882, 92, 156–171. [Google Scholar]
- Dimitriadis, E.K.; Horkay, F.; Maresca, J.; Kachar, B.; Chadwick, R.S. Determination of elastic moduli of thin layers of soft material using the atomic force microscope. Biophys. J. 2002, 82, 2798–2810. [Google Scholar] [CrossRef]
- Backes, S.; Witt, M.U.; Roeben, E.; Kuhrts, L.; Aleed, S.; Schmidt, A.M.; von Klitzing, R. Loading of PNIPAM based microgels with CoFe2O4 nanoparticles and their magnetic response in bulk and at surfaces. J. Phys. Chem. B 2015, 119, 12129–12137. [Google Scholar] [CrossRef] [PubMed]
- Seuss, M.; Schmolke, W.; Drechsler, A.; Fery, A.; Seiffert, S. Core-shell microgels with switchable elasticity at constant interfacial interaction. ACS Appl. Mater. Interfaces 2016, 8, 16317–16327. [Google Scholar] [CrossRef] [PubMed]
- Welsch, N.; Lyon, L.A. Oligo(ethylene glycol)-sidechain microgels prepared in absence of cross-linking agent: Polymerization, characterization and variation of particle deformability. PLoS ONE 2017, 12, e0181369. [Google Scholar] [CrossRef] [PubMed]
- Backes, S.; Krause, P.; Tabaka, W.; Witt, M.U.; Mukherji, D.; Kremer, K.; von Klitzing, R. Poly(N-isopropylacrylamide) microgels under alcoholic intoxication: When a lcst polymer shows swelling with increasing temperature. ACS Macro Lett. 2017, 6, 1042–1046. [Google Scholar] [CrossRef]
- Backes, S.; Krause, P.; Tabaka, W.; Witt, M.U.; von Klitzing, R. Combined cononsolvency and temperature effects on adsorbed pnipam microgels. Langmuir 2017, 33, 14269–14277. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.C.; Horkay, F. Nanomechanics of polymer gels and biological tissues: A critical review of analytical approaches in the hertzian regime and beyond. Soft Matter 2008, 4, 669–682. [Google Scholar] [CrossRef]
- Kim, M.T. Influence of substrate on the elastic reaction of films for the microindentation tests. Thin Solid Films 1996, 283, 12–16. [Google Scholar] [CrossRef]
- Domke, J.; Radmacher, M. Measuring the elastic properties of thin polymer films with the atomic force microscope. Langmuir 1998, 14, 3320–3325. [Google Scholar] [CrossRef]
- Akhremitchev, B.B.; Walker, G.C. Finite sample thickness effects on elasticity determination using atomic force microscopy. Langmuir 1999, 15, 5630–5634. [Google Scholar] [CrossRef]
- Hashmi, S.; Dufresne, E.R. Mechanical properties of individual microgel particles through the deswelling transition. Soft Matter 2009, 5, 3682–3688. [Google Scholar] [CrossRef]
- Mahaffy, R.; Sikh, C.; MacKintosh, F.; Kas, J. Scanning probe-based frequency-dependent microrheology of polymer gels and biological cells. Phys. Rev. Lett. 2000, 85, 880–883. [Google Scholar] [CrossRef] [PubMed]
- Alcaraz, J.; Buscemi, L.; Grabulosa, M.; Trepat, X.; Fabry, B.; Farre, R.; Navajas, D. Microrheology of human lung epithelial cells measured by atomic force microscopy. Biophys. J. 2003, 84, 2071–2079. [Google Scholar] [CrossRef]
- Rother, J.; Nöding, H.; Mey, I.; Janshoff, A. Atomic force microscopy-based microrheology reveals significant differences in the viscoelastic response between malign and benign cell lines. Open Biol. 2014, 4, 140046. [Google Scholar] [CrossRef] [PubMed]
- Hellwig, J.; Micciulla, S.; Strebe, J.; von Klitzing, R. Separation of storage and loss modulus of polyelectrolyte multilayers on a nanoscale: A dynamic AFM study. Langmuir 2016, 32, 10505–10512. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.L. Contact Mechanics; Cambridge University Press: Cambridge, UK, 1987. [Google Scholar]
- Hirotsu, S. Critical points of the volume phase transition in N-isopropylacrylamide. J. Chem. Phys. 1988, 88, 427–431. [Google Scholar] [CrossRef]
- Winnik, F.M.; Ringsdorf, H.; Venzmer, J. Methanol-water as a co-nonsolvent system for poly(N-isopropylacrylamide). Macromolecules 1990, 23, 2415–2416. [Google Scholar] [CrossRef]
- Schild, H.G.; Muthukumar, M.; Tirrell, D.A. Cononsolvency in mixed aqueous solutions of poly(N-isopropylacrylamide). Macromolecules 1991, 24, 948–952. [Google Scholar] [CrossRef]
- Crowther, H.M.; Vincent, B. Swelling behavior of poly- N-isopropylacrylamide microgel particles in alcoholic solutions. Colloid Polym. Sci. 1998, 276, 45–51. [Google Scholar] [CrossRef]
- Richter, M.; Hunnenmörder, M.; von Klitzing, R. The impact of the cononsolvency effect on poly(N-isopropylacrylamide) based microgels at interfaces. Colloid Polym. Sci. 2014, 292, 2439–2452. [Google Scholar] [CrossRef]
- Kojima, H.; Tanaka, F.; Scherzinger, C.; Richtering, W. Temperature dependent phase behavior of PNIPAM microgels in mixed water/methanol solvents. J. Polym. Sci. B Polym. Phys. 2013, 51, 1100–1111. [Google Scholar] [CrossRef]
- Bischofberger, I.; Calzolari, D.C.E.; Trappe, V. Co-nonsolvency of PNIPAM at the transition between solvation mechanisms. Soft Matter 2014, 10, 8288–8295. [Google Scholar] [CrossRef] [PubMed]
- Pritz, T. Frequency dependencies of complex moduli and complex poisson’s ratio of real solid materials. J. Sound Vib. 1998, 214, 83–104. [Google Scholar] [CrossRef]
- Walter, J.; Sehrt, J.; Vrabec, J.; Hasse, H. Molecular dynamics and experimental study of conformation change of poly(N-isopropylacrylamide) hydrogels in mixtures of water and methanol. J. Phys. Chem. B 2012, 116, 5251–5259. [Google Scholar] [CrossRef] [PubMed]
- Mukherji, D.; Kremer, K. Coil-globule-coil transition of PNIPAM in aqueous methanol: Coupling all-atom simulations to semi-grand canonical coarse-grained reservoir. Macromolecules 2013, 46, 9158–9163. [Google Scholar] [CrossRef]
- Heyda, J.; Muzdalo, A.; Dzubiella, J. Rationalizing polymer swelling and collapse under attractive cosolvent conditions. Macromolecules 2013, 46, 1231–1238. [Google Scholar] [CrossRef]
- Mukherji, D.; Marques, C.M.; Kremer, K. Polymer collapse in miscible good solvents is a generic phenomenon driven by preferential adsorption. Nat. Commun. 2014, 5, 4882. [Google Scholar] [CrossRef] [PubMed]
- Mukherji, D.; Wagner, M.; Watson, M.D.; Winzen, S.; de Oliveira, T.E.; Marques, C.M.; Kremer, K. Relating side chain organization of PNIPAM with its conformation in aqueous methanol. Soft Matter 2016, 12, 7995–8003. [Google Scholar] [CrossRef] [PubMed]
- Landau, L.D.; Lifshitz, E.M. Theory of Elasticity; Pergamon Press: Oxford, UK, 1970. [Google Scholar]
- Stieger, M.; Pedersen, J.S.; Lindner, P.; Richtering, W. Are thermoresponsive microgels model systems for concentrated colloidal suspensions? A rheology and small-angle neutron scattering study. Langmuir 2004, 20, 7283–7292. [Google Scholar] [CrossRef] [PubMed]
- Guillermo, A.; Addad, J.P.C.; Bazile, J.P.; Duracher, D.; Elaissari, A.; Pichot, C. NMR investigations into heterogeneous structures of thermosensitive microgel particles. J. Polym. Sci. B Polym. Phys. 2000, 38, 889–898. [Google Scholar] [CrossRef]
- Daly, E.; Saunders, B.R. Temperature–dependent electrophoretic mobility and hydrodynamic radius measurements of poly(N-isopropylacrylamide) microgel particles: Structural insights. Phys. Chem. Chem. Phys. 2000, 2, 3187–3193. [Google Scholar] [CrossRef]
- Varga, I.; Gilanyi, T.; Meszaros, R.; Filipcsei, G.; Zrinyi, M. Effect of cross-link density on the internal structure of poly(N-isopropylacrylamide) microgels. J. Phys. Chem. B 2001, 105, 9071–9076. [Google Scholar] [CrossRef]
- Sierra-Martin, B.; Choi, Y.; Romero-Cano, M.S.; Cosgrove, T.; Vincent, B.; Fernandez-Barbero, A. Microscopic signature of a microgel volume phase transition. Macromolecules 2005, 38, 10782–10787. [Google Scholar] [CrossRef]
- Tong, Z.; Zeng, F.; Zheng, X. Inverse molecular weight dependence of cloud points for aqueous poly(N-isopropylacrylamide) solutions. Macromolecules 1999, 32, 4488–4490. [Google Scholar] [CrossRef]
- McPhee, W.; Tam, K.C.; Pelton, R. Poly(N-isopropylacrylamide) latices prepared with sodium dodecyl sulfate. J. Colloid Interface Sci. 1993, 156, 24–30. [Google Scholar] [CrossRef]
- Gao, J.; Frisken, B.J. Cross-linker-free N-isopropylacrylamide gel nanospheres. Langmuir 2003, 19, 5212–5216. [Google Scholar] [CrossRef]
- Gao, J.; Frisken, B.J. Influence of reaction conditions on the synthesis of self-cross-linked N-isopropylacrylamide microgels. Langmuir 2003, 19, 5217–5222. [Google Scholar] [CrossRef]
- Acciaro, R.; Gilanyi, T.; Varga, I. Preparation of monodisperse poly(N-isopropylacrylamide) microgel particles with homogenous cross-link density distribution. Langmuir 2011, 27, 7917–7925. [Google Scholar] [CrossRef] [PubMed]
- Backes, S. PNIPAM Microgels in External Fields: Temperature Response in Cononsolvent Systems and Under the Influence of Magnetic Fields. Ph.D. Thesis, Technical University of Berlin, Berlin, Germany, April 2018. [Google Scholar]
- Krause, P. Studies of Elastic Properties of PNIPAM Hydrogels for Programmable Tactile Surface Applications. Mater’s Thesis, Technical University of Berlin, Berlin, Germany, December 2017. [Google Scholar]
- Richter, M.; Eisele, M.; von Klitzing, R. Unpublished results.
- Pelton, R.H.; Pelton, H.M.; Morphesis, A.; Rowell, R.L. Particle sizes and electrophoretic mobilities of poly (N-isopropylacrylamide) latex. Langmuir 1989, 5, 816–818. [Google Scholar] [CrossRef]
- Berndt, I.; Pedersen, J.S.; Lindner, P.; Richtering, W. Influence of shell thickness and cross-llink density on the structure of temperature-sensitive-poly-N-isopropylacrylamide-poly-N-isopropylmethacrylamide core-shell microgels investigated by small-angle neutron scattering. Langmuir 2006, 22, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; Sanson, N.; Fava, D.; Kumacheva, E. Microgels loaded with gold nanorods: Photothermally triggered volume transitions under physiological conditions. Langmuir 2007, 23, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Karg, M.; Lu, Y.; Carbocos-Argibay, E.; Pastoriza-Santos, I.; Prez-Juste, J.; Liz-Marzan, L.M.; Hellweg, T. Multiresponsive Hybrid Colloids Based on Gold Nanorods and Poly(NIPAMco- allylacetic acid) Microgels: Temperature- and pH-Tunable Plasmon Resonance. Langmuir 2009, 25, 3163. [Google Scholar] [CrossRef] [PubMed]
- Lange, H.; Bastus, N.G.; Carl, A.; Richter, M.; Weller, H.; Juarez, B.H.; Thomsen, C.; von Klitzing, R.; Knorr, A. Tunable plasmon coupling in distance-controlled gold nanoparticles. Langmuir 2012, 28, 8862–8866. [Google Scholar] [CrossRef] [PubMed]
- Gawlitza, K.; Turner, S.T.; Polzer, F.; Wellert, S.; Karg, M.; Mulvaney, P.; von Klitzing, R. Interaction of gold nanoparticles with thermoresponsive microgels: Influence of the cross-linker density on optical properties. Phys. Chem. Chem. Phys. 2013, 15, 15623–15631. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, M.; Tabaka, W.; Möller, T.; Oppermann, A.; Wöll, D.; Volodkin, D.; Wellert, S.; von Klitzing, R. Dls setup for in situ measurements of photoinduced size changes of microgel-based hybrid particles. Langmuir 2018, 34, 3597–3603. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Guo, J.; Ji, M.L.; Yang, W.L.; Wang, C.C.; Fu, S.K. Preparation and characterization of multiresponsive polymer composite microspheres with core-shell structure. Coll. Polym. Sci. 2007, 285, 1607–1615. [Google Scholar] [CrossRef]
- Li, P.; Zhu, A.M.; Liu, Q.L.; Zhang, Q.G. Fe3O4/poly(N-isopropylacrylamide)/chitosan composite microspheres with multiresponsive properties. Ind. Eng. Chem. Res. 2008, 47, 7700–7706. [Google Scholar] [CrossRef]
- Liu, C.Y.; Guo, J.; Yang, W.L.; Hu, J.H.; Wang, C.C.; Fu, S.K. Magnetic mesoporous silica microspheres with thermo-sensitive polymer shell for controlled drug release. J. Mater. Chem. 2009, 19, 4764–4770. [Google Scholar] [CrossRef]
- Sahiner, N.; Ilgin, P. Soft core-shell polymeric nanoparticles with magnetic property for potential guided drug delivery. Curr. Nanosci. 2010, 6, 483–491. [Google Scholar] [CrossRef]
- Dagallier, C.; Dietsch, H.; Schurtenberger, P.; Scheffold, F. Thermoresponsive hybrid microgel particles with intrinsic optical and magnetic anisotropy. Soft Matter 2010, 6, 2174–2177. [Google Scholar] [CrossRef]
- Gui, R.J.; Jin, H. Temperature-regulated polymerization and swelling/collapsing/flocculation properties of hybrid nanospheres with magnetic cores and thermo/ph-sensitive nanogel shells. RCS Adv. 2014, 4, 2797–2806. [Google Scholar] [CrossRef]
- Wong, J.E.; Gaharwar, A.K.; Mueller-Schulte, D.; Bahadur, D.; Richtering, W. Layer-by-layer assembly of a magnetic nanoparticle shell on a thermoresponsive microgel core. J. Magn. Magn. Mater. 2007, 311, 219–223. [Google Scholar] [CrossRef]
- Turcu, R.; Socoliuc, V.; Craciunescu, I.; Petran, A.; Paulus, A.; Franzreb, M.; Vasile, E.; Vekas, L. Magnetic microgels, a promising candidate for enhanced magnetic adsorbent particles in bioseparation: Synthesis, physicochemical characterization, and separation performance. Soft Matter 2015, 11, 1008–1018. [Google Scholar] [CrossRef] [PubMed]
- Pich, A.; Bhattacharya, S.; Lu, Y.; Boyko, V.; Adler, H.-J.P. Temperature-sensitive hybrid microgels with magnetic properties. Langmuir 2004, 20, 10706–10711. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Eckert, F.; Boyko, V.; Pich, A. Temperature-, ph-, and magnetic-field-sensitive hybrid microgels. SMALL 2007, 3, 650–657. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.M.; Fang, Y.H.; Zhang, G.X. Tio2- or tio2/fe3o4-containing PVA-based microgels for controlled photocatalytic degradation of methyl orange. Polym. Compos. 2017, 38, 132–137. [Google Scholar] [CrossRef]
- Wiemer, K.; Dörmbach, K.; Slabu, I.; Agrawal, G.; Schrader, F.; Caumanns, T.; Bourone, S.D.M.; Mayer, J.; Steitz, J.; Simona, U.; et al. Hydrophobic superparamagnetic FePt nanoparticles in hydrophilic poly(N-vinylcaprolactam) microgels: A new multifunctional hybrid system. J. Mater. Chem. B 2017, 5, 1284–1292. [Google Scholar] [CrossRef]
- Rodkate, N.; Rutnakornpituk, M. Multi-responsive magnetic microsphere of poly(N-isopropylacrylamide)/carboxymethylchitosan hydrogel for drug controlled release. Carbohyd. Polym. 2016, 151, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.L.; Liu, W.J.; Wu, F.H. Novel multi-responsive polymer magnetic microgels with folate or methyltetrahydrofolate ligand as anticancer drug carriers. RSC Adv. 2017, 7, 10333–10344. [Google Scholar] [CrossRef]
- Zhao, W.; Nugroho, R.W.N.; Odelius, K.; Edlund, U.; Zhao, C.; Albertsson, A. In situ cross-linking of stimuli-responsive hemicellulose microgels during spray drying. ACS Appl. Mater. Interfaces 2015, 7, 4202–4215. [Google Scholar] [CrossRef] [PubMed]
- Nikitin, L.V.; Mironova, L.S.; Stepanov, G.V.; Samus, A.N. The influence of a magnetic field on the elastic and viscous properties of magnetoelastics. Polym. Sci. Ser. A 2001, 43, 443–450. [Google Scholar]
- Bonini, M.; Lenz, S.; Falletta, E.; Ridi, F.; Carretti, E.; Fratini, E.; Wiedenmann, A.; Baglioni, P. Acrylamide-based magnetic nanosponges: A new smart nanocomposite material. Langmuir 2008, 24, 12644–12650. [Google Scholar] [CrossRef] [PubMed]
- Ivaneyko, D.; Toshchevikov, V.P.; Saphiannikova, M.; Heinrich, G. Magneto-sensitive Elastomers in a Homogeneous Magnetic Field: A Regular Rectangular Lattice Model. Macromol. Theory Simul. 2011, 20, 411–424. [Google Scholar] [CrossRef]
- Bender, P.; Günther, A.; Tschöpe, A.; Birringer, R. Synthesis and characterization of uniaxial ferrogels with ni nanorods as magnetic phase. J. Magn. Magn. Mater. 2011, 323, 2055–2063. [Google Scholar] [CrossRef]
- Bender, P.; Tschöpe, A.; Birringer, R. Magnetization measurements reveal the local shear stiffness of hydrogels probed by ferromagnetic nanorods. J. Magn. Magn. Mater. 2014, 372, 187–194. [Google Scholar] [CrossRef]
- MacKintosh, F.; Schmidt, C. Microrheology. Curr. Opin. Colloid Interface Sci. 1999, 4, 300–307. [Google Scholar] [CrossRef]
- Kumachev, A.; Tumarkin, E.; Walker, G.C.; Kumacheva, E. Characterization of the mechanical properties of microgels acting as cellular microenvironments. Soft Matter 2013, 9, 2959–2965. [Google Scholar] [CrossRef]
- Gu, W.Y.; Yao, H.; Huang, C.Y.; Cheung, H.S. New insight into deformation-dependent hydraulic permeability of gels and cartilage, and dynamic behavior of agarose gels in confined compression. J. Biomech. Eng. 2003, 36, 593–598. [Google Scholar] [CrossRef]
- Fratzl, P. Collagen: Structure and Mechanics; Springer: New York, NY, USA, 2008. [Google Scholar]
- Schmidt, S.; Zeiser, M.; Hellweg, T.; Duschl, C.; Fery, A.; Möhwald, H. Adhesion and mechanical properties of pnipam microgel films and their potential use as switchable cell culture substrates. Adv. Funct. Mater. 2010, 20, 3235–3243. [Google Scholar] [CrossRef]
- Haq, M.A.; Su, Y.; Wang, D. Mechanical properties of PNIPAM based hydrogels: A review. Mater. Sci. Eng. C 2017, 70, 842–855. [Google Scholar] [CrossRef] [PubMed]
- Miruchna, V.; Walter, R.; Lindlbauer, D.; Lehmann, M.; von Klitzing, R. Geltouch: Localized tactile feedback through thin, programmable gel. In Proceedings of the 28th Annual ACM Symposium on User Interface Software & Technology (UIST ’15), Charlotte, NC, USA, 11–15 November 2015. [Google Scholar]
- Kim, C.C.; Lee, H.H.; Oh, K.H.; Sun, J.Y. Highly stretchable, transparent ionic touch panel. Science 2016, 353, 682–687. [Google Scholar] [CrossRef] [PubMed]
- Son, K.H.; Lee, J.W. Synthesis and characterization of poly (Ethylene Glycol) based thermo-responsive hydrogels for cell sheet engineering. Materials 2016, 9, 854. [Google Scholar] [CrossRef] [PubMed]
- Style, R.W.; Isa, L.; Dufresne, E.R. Adsorption of soft particles at fluid interfaces. Soft Matter 2015, 11, 7412. [Google Scholar] [CrossRef] [PubMed]
- Rey, M.; Fernandez-Rodryguez, M.A.; Steinacher, M.; Scheidegger, L.; Geisel, K.; Richtering, W.; Squires, T.M.; Isa, L. Isostructural solid–solid phase transition in monolayers of soft core–shell particles at fluid interfaces: Structure and mechanics. Soft Matter 2016, 12, 3545. [Google Scholar] [CrossRef] [PubMed]
- Pinaud, F.; Geisel, K.; Masse, P.; Catargi, B.; Isa, L.; Richtering, W.; Ravaine, V.; Schmitt, V. Adsorption of microgels at and oil-water interface: Correlation between packing and 2d elasticity. Soft Matter 2014, 10, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Picard, C.; Garrigue, P.; Tatry, M.-C.; Lapeyre, V.; Ravaine, S.; Schmitt, V.; Ravaine, V. Organization of microgels at the air—Water interface under compression: Role of electrostatics and cross-linking density. Langmuir 2017, 33, 7968–7981. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Quinlan, P.J.; Tam, K.C. Stimuli responsive pickering emulsions: Recent advances and potential applications. Soft Matter 2015, 11, 3512–3529. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.; Liu, T.; Rutten, S.; Phan, K.H.; Moller, M.; Richtering, W. Influence of microgel architecture and oil polarity on stabilization of emulsions by stimuli-sensitive core-shell poly (N-isopropylacrylamide-co-methacrylic acid) microgels: Mickering versus Pickering behavior? Langmuir 2011, 27, 9801–9806. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, F.; Koga, T.; Winnik, F.M. Temperature-responsive polymers in mixed solvents: Competetive hydrogen bonds cause cononsolvency. Phys. Rev. Let. 2008, 101, 028302. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Ru, G.; Wang, L.; Feng, J. 1h mas nmr studies of the phase separation of poly(N-isopropylacrylamide) gel in binary solvents. Langmuir 2009, 25, 5898–5902. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, F.; Hellwig, J.; von Klitzing, R.; Seiffert, S. Macroscopic and microscopic elasticity of heterogeneous polymer gels. ACS Macro Lett. 2015, 4, 698–703. [Google Scholar] [CrossRef]
- Seliktar, D. Designing cell-compatible hydrogels for biomedical applications. Science 2012, 336, 1124–1128. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Backes, S.; Von Klitzing, R. Nanomechanics and Nanorheology of Microgels at Interfaces. Polymers 2018, 10, 978. https://doi.org/10.3390/polym10090978
Backes S, Von Klitzing R. Nanomechanics and Nanorheology of Microgels at Interfaces. Polymers. 2018; 10(9):978. https://doi.org/10.3390/polym10090978
Chicago/Turabian StyleBackes, Sebastian, and Regine Von Klitzing. 2018. "Nanomechanics and Nanorheology of Microgels at Interfaces" Polymers 10, no. 9: 978. https://doi.org/10.3390/polym10090978
APA StyleBackes, S., & Von Klitzing, R. (2018). Nanomechanics and Nanorheology of Microgels at Interfaces. Polymers, 10(9), 978. https://doi.org/10.3390/polym10090978




