Adsorption Separation of Analgesic Pharmaceuticals from Ultrapure and Waste Water: Batch Studies Using a Polymeric Resin and an Activated Carbon
Abstract
:1. Introduction
2. Materials and Methods
2.1. Adsorbent Materials
2.2. Chemicals and Analytic Methods
2.3. Aqueous Matrices for Adsorption Experiments
2.4. Adsorption Experiments
2.5. Modeling of Adsorption Results
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Farré, M.; Pérez, S.; Kantiani, L.; Barceló, D. Fate and toxicity of emerging pollutants, their metabolites and transformation products in the aquatic environment. TrAC Trends Anal. Chem. 2008, 27, 991–1007. [Google Scholar] [CrossRef]
- Gogoi, A.; Mazumder, P.; Tyagi, V.K.; Tushara, C.; An, A.K.; Kumar, M. Occurrence and fate of emerging contaminants in water environment: A review. Groundw. Sustain. Dev. 2018, 6, 169–180. [Google Scholar] [CrossRef]
- Tarpani, R.R.Z.; Azapagic, A. A methodology for estimating concentrations of pharmaceuticals and personal care products (PPCPs) in wastewater treatment plants and in freshwaters. Sci. Total Environ. 2018, 622–623, 1417–1430. [Google Scholar] [CrossRef] [PubMed]
- Alfonsín, C.; Hospido, A.; Omil, F.; Moreira, M.T.; Feijoo, G. PPCPs in wastewater—Update and calculation of characterization factors for their inclusion in LCA studies. J. Clean. Prod. 2014, 83, 245–255. [Google Scholar] [CrossRef]
- De Andrade, J.R.; Oliveira, M.F.; Da Silva, M.G.C.; Vieira, M.G.A. Adsorption of Pharmaceuticals from Water and Wastewater Using Nonconventional Low-Cost Materials: A Review. Ind. Eng. Chem. Res. 2018, 57, 3103–3127. [Google Scholar] [CrossRef]
- Domínguez, J.R.; González, T.; Palo, P.; Cuerda-Correa, E.M. Removal of common pharmaceuticals present in surface waters by Amberlite XAD-7 acrylic-ester-resin: Influence of pH and presence of other drugs. Desalination 2011, 269, 231–238. [Google Scholar] [CrossRef]
- Akhtar, J.; Amin, N.A.S.; Shahzad, K. A review on removal of pharmaceuticals from water by adsorption. Desalin. Water Treat. 2016, 57, 12842–12860. [Google Scholar] [CrossRef]
- Mansour, F.; Al-Hindi, M.; Yahfoufi, R.; Ayoub, G.M.; Ahmad, M.N. The use of activated carbon for the removal of pharmaceuticals from aqueous solutions: a review. Rev. Environ. Sci. Bio/Technol. 2018, 17, 109–145. [Google Scholar] [CrossRef]
- Coimbra, R.N.; Escapa, C.; Paniagua, S.; Otero, M. Adsorptive removal of diclofenac from ultrapure and wastewater: A comparative assessment on the performance of a polymeric resin and activated carbons. Desalin. Water Treat. 2016, 57, 27914–27923. [Google Scholar] [CrossRef]
- Otero, M.; Grande, C.A.; Rodrigues, A.E. Adsorption of salicylic acid onto polymeric adsorbents and activated charcoal. React. Funct. Polym. 2004, 60, 203–213. [Google Scholar] [CrossRef]
- Otero, M.; Zabkova, M.; Rodrigues, A.E. Comparative study of the adsorption of phenol and salicylic acid from aqueous solution onto nonionic polymeric resins. Sep. Purif. Technol. 2005, 45, 86–95. [Google Scholar] [CrossRef]
- Otero, M.; Zabkova, M.; Rodrigues, A.E. Salicylic acid adsorption onto sephabeads SP206 in view of its purification by thermal parametric pumping. Adsorption 2005, 11, 887–892. [Google Scholar] [CrossRef]
- Ramos, A.M.; Otero, M.; Rodrigues, A.E. Recovery of Vitamin B12 and cephalosporin-C from aqueous solutions by adsorption on non-ionic polymeric adsorbents. Sep. Purif. Technol. 2004, 38, 85–98. [Google Scholar] [CrossRef]
- Robberson, K.A.; Waghe, A.B.; Sabatini, D.A.; Butler, E.C. Adsorption of the quinolone antibiotic nalidixic acid onto anion-exchange and neutral polymers. Chemosphere 2006, 63, 934–941. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, L.; Persson, P.-O.; Rydén, L.; Darozhka, S.; Zaliauskiene, A. Water pollution reduction. In Cleaner Production—Technologies and Tools for Resource Efficient Production; Nilsson, L., Persson, P.-O., Rydén, L., Darozhka, S., Zaliauskiene, A., Eds.; Baltic University Press: Uppsala, Sweden, 2007; p. 129. [Google Scholar]
- Lin, G.; Wang, S.; Zhang, L.; Hu, T.; Peng, J.; Cheng, S.; Fu, L. Selective Adsorption of Ag+ on a New Cyanuric-Thiosemicarbazide Chelating Resin with High Capacity from Acid Solutions. Polymers 2017, 9, 568. [Google Scholar] [CrossRef]
- Bolong, N.; Ismail, A.F.; Salim, M.R.; Matsuurad, T. A review of the effects of emerging contaminants in wastewater and options for their removal. Desalination 2009, 239, 229–246. [Google Scholar] [CrossRef]
- Priac, A.; Morin-Crini, N.; Druart, C.; Gavoille, S; Bradu, C.; Lagarrigue, C.; Torri, G.; Winterton, P.; Crini, G. Alkylphenol and alkylphenol polyethoxylates in water and wastewater: A review of options for their elimination. Arabian J. Chem. 2017, 10, S3749–S3773. [Google Scholar] [CrossRef]
- Sophia, A.C.; Lima, E.C. Removal of emerging contaminants from the environment by adsorption. Ecotoxicol. Environ. Saf. 2018, 150, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.M.P.T.; Silva, L.J.G.; Laranjeiro, C.S.M.; Meisel, L.M.; Lino, C.M.; Pena, A. Human pharmaceuticals in Portuguese rivers: The impact of water scarcity in the environmental risk. Sci. Total Environ. 2017, 609, 1182–1191. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Li, H.; Chen, L.; Li, L.; Yin, L.; Lee, H.; Yang, Z. Mass loading and emission of thirty-seven pharmaceuticals in a typical municipal wastewater treatment plant in Hunan Province, Southern China. Ecotoxicol. Environ. Saf. 2018, 147, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Bellver-Domingo, A.; Fuentes, R.; Hernández-Sancho, F. Shadow prices of emerging pollutants in wastewater treatment plants: Quantification of environmental externalities. J. Environ. Manag. 2017, 203, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Escapa, C.; Coimbra, R.N.; Nuevo, C.; Vega, S.; Paniagua, S.; García, A.I.; Calvo, L.F.; Otero, M. Valorization of microalgae biomass by its use for the removal of paracetamol from contaminated water. Water 2017, 9, 312. [Google Scholar] [CrossRef]
- Lessa, E.F.; Nunes, M.L.; Fajardo, A.R. Chitosan/waste coffee-grounds composite: An efficient and eco-friendly adsorbent for removal of pharmaceutical contaminants from water. Carbohydr. Polym. 2018, 189, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Sumalinog, D.A.G.; Capareda, S.C.; de Luna, M.D.G. Evaluation of the effectiveness and mechanisms of acetaminophen and methylene blue dye adsorption on activated biochar derived from municipal solid wastes. J. Environ. Manag. 2018, 210, 255–262. [Google Scholar] [CrossRef] [PubMed]
- An, H.J.; Bhadra, B.N.; Khan, N.A.; Jhung, S.H. Adsorptive removal of wide range of pharmaceutical and personal care products from water by using metal azolate framework-6-derived porous carbon. Chem. Eng. J. 2018, 343, 447–454. [Google Scholar] [CrossRef]
- Ondarts, M.; Reinert, L.; Guittonneau, S.; Baupc, S.; Delpeux, S.; Lévêque, J.-M.; Duclaux, L. Improving the adsorption kinetics of ibuprofen on an activated carbon fabric through ultrasound irradiation: Simulation and experimental studies. Chem. Eng. J. 2018, 343, 163–172. [Google Scholar] [CrossRef]
- Verma, V.K.; Subbiah, S. Prospects of Silk Sericin as an Adsorbent for Removal of Ibuprofen from Aqueous Solution. Ind. Eng. Chem. Res. 2017, 56, 10142–10154. [Google Scholar] [CrossRef]
- Suntisukaseam, U.; Weschayanwiwat, P.; Sabatini, D.A. Sorption of amphiphile pharmaceutical compounds onto polar and nonpolar adsorbents. Environ. Eng. Sci. 2007, 24, 1457–1465. [Google Scholar] [CrossRef]
- Silva, C.P.; Jaria, G.; Otero, M.; Esteves, V.I.; Calisto, V. Waste-based alternative adsorbents for the remediation of pharmaceutical contaminated waters: Has a step forward already been taken? Bioresour. Technol. 2018, 250, 888–901. [Google Scholar] [CrossRef] [PubMed]
- Kårelid, V.; Larsson, G.; Björlenius, B. Pilot-scale removal of pharmaceuticals in municipal wastewater: Comparison of granular and powdered activated carbon treatment at three wastewater treatment plants. J. Environ. Manag. 2017, 193, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hansen, C.; Allen, K. Extraction of anthocyanins from black bean canning wastewater with macroporous resins. J. Food Sci. 2014, 79, E184–E188. [Google Scholar] [CrossRef] [PubMed]
- APHA-AWWA-WPCF. Standard Methods for the Examination of Water and Wastewater, 20th ed.; American Public Health Association: Washington, DC, USA, 2001. [Google Scholar]
- Lagergren, S. Zur theorie der sogenannten adsorption gelöster stoffe. K. Sven. Vetenskapsakad. Hand. 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Freundlich, H. Über die Adsorption in Lösungen. Z. Phys. Chem. 1906, 57, 385–470. [Google Scholar] [CrossRef]
- Langmuir, I. The Adsorption of Gases on Plane Surfaces of Glass, Mica and Platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Sips, R. Combined form of Langmuir and Freundlich equations. J. Chem. Phys. 1948, 16, 490–495. [Google Scholar] [CrossRef]
- Sotelo, J.L.; Rodríguez, A.R.; Mateos, M.M.; Hernández, S.D.; Torrellas, S.A.; Rodríguez, J.G. Adsorption of pharmaceutical compounds and an endocrine disruptor from aqueous solutions by carbon materials. J. Environ. Sci. Health Part B 2012, 47, 640–652. [Google Scholar] [CrossRef] [PubMed]
- Kovalova, L.; Knappe, D.R.U.; Lehnberg, K.; Kazner, C.; Hollender, J. Removal of highly polar micropollutants from wastewater by powder activated carbon. Environ. Sci. Pollut. Res. 2013, 20, 3607–3615. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Díaz, J.D.; Abdel daiem, M.M.; Rivera-Utrilla, J.; Sánchez-Polo, M.; Bautista-Toledo, I. Adsorption/bioadsorption of phthalic acid, an organic micropollutant present in landfill leachates, on activated carbons. J. Colloid Interface Sci. 2012, 369, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Combarros, R.G.; Rosas, I.; Lavín, A.G.; Rendueles, M.; Díaz, M. Influence of biofilm on activated carbon on the adsorption and biodegradation of salicylic acid in wastewater. Water Air Soil Pollut. 2014, 225, 1858. [Google Scholar] [CrossRef]
| Adsorbent | GPP20 | Sepabeads SP207 |
|---|---|---|
| Matrix | Coal based steam activated carbon | Styrene-divinylbenzene copolymer |
| Colour | Black carbon | Yellowish opaque beads |
| Specific surface area (m2 g−1) | 1000 | 650 |
| Mean particle diameter (mm) | 0.04 | 0.4 |
| Pharmaceutical (Formula) | Structure | Mw (g mol−1) | Sw a (mg L−1) | pKa | Log Kow | PSA (A2) | HBAC |
|---|---|---|---|---|---|---|---|
| Ibuprofen Sodium (C3H17NaO2) | 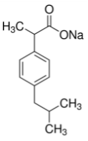 | 228.26 | 100,000 | 4.91 | 3.8 | 40.1 | 2 |
| Acetaminophen (C8H9NO2) | 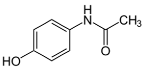 | 151.17 | 14,000 | 9.48 | 0.46 | 49.3 | 2 |
| pH | Conductivity | TSS | BOD5 | COD | NTK | N–NH4 | N–NO3 | N–NO2 | Total P–PO4 |
|---|---|---|---|---|---|---|---|---|---|
| (µS cm−1) | (mg L−1) | (mg L−1) | (mg L−1) | (mg L−1) | (mg L−1) | (mg L−1) | (mg L−1) | (mg L−1) | |
| 7.8 ± 0.2 | 612 ± 3 | 22 ± 1 | 21 ± 2 | 47 ± 3 | 17 ± 2 | 13.10 ± 0.42 | 1.73 ± 0.18 | 0.48 ± 0.09 | 1.75 ± 0.13 |
| 35 * | 25 * | 125 * |
| Parameters | Acetaminophen | Ibuprofen | |||||||
|---|---|---|---|---|---|---|---|---|---|
| GPP20 | SP207 | GPP20 | SP207 | ||||||
| UPW | WW | UPW | WW | UPW | WW | UPW | WW | ||
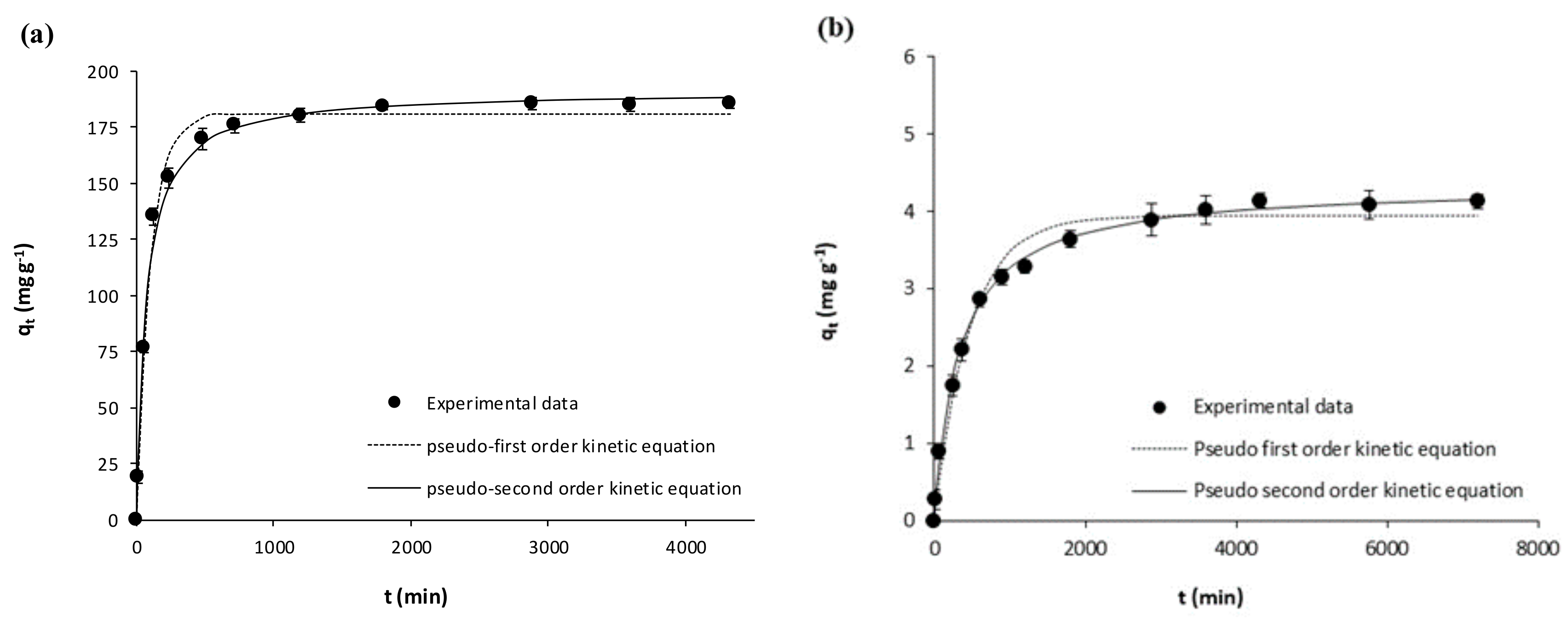
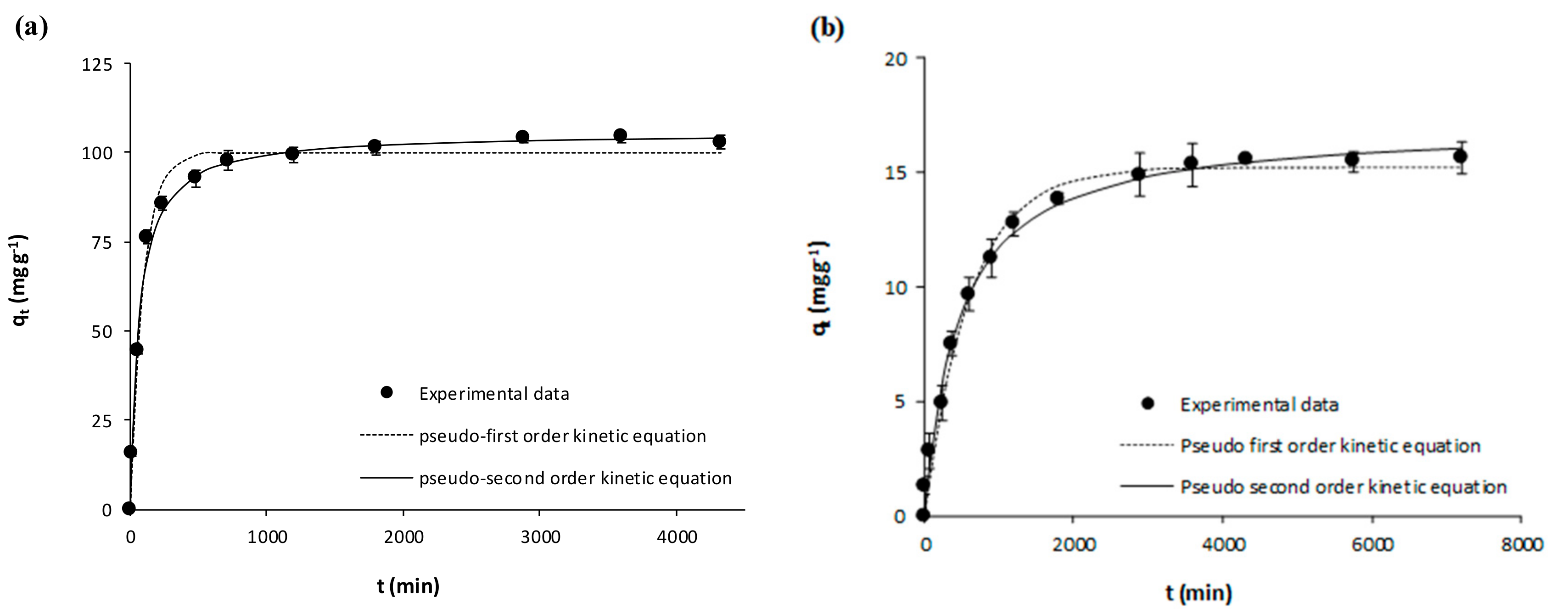
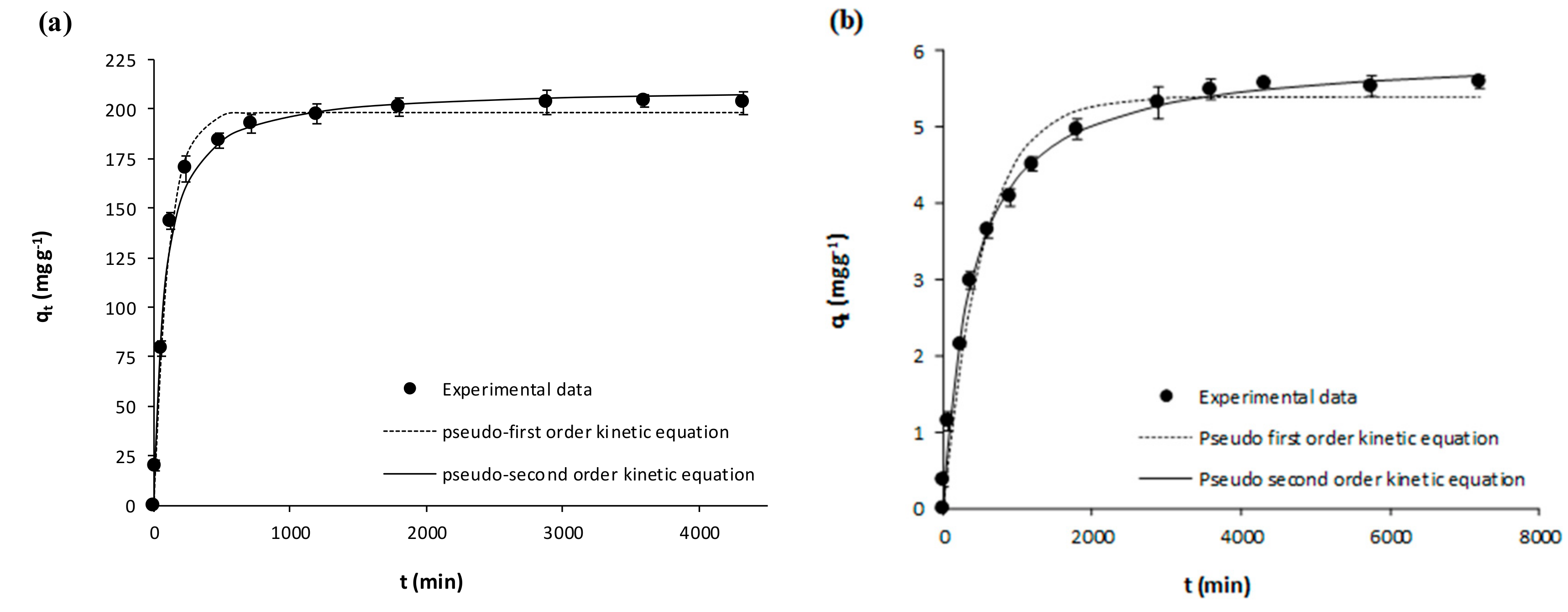
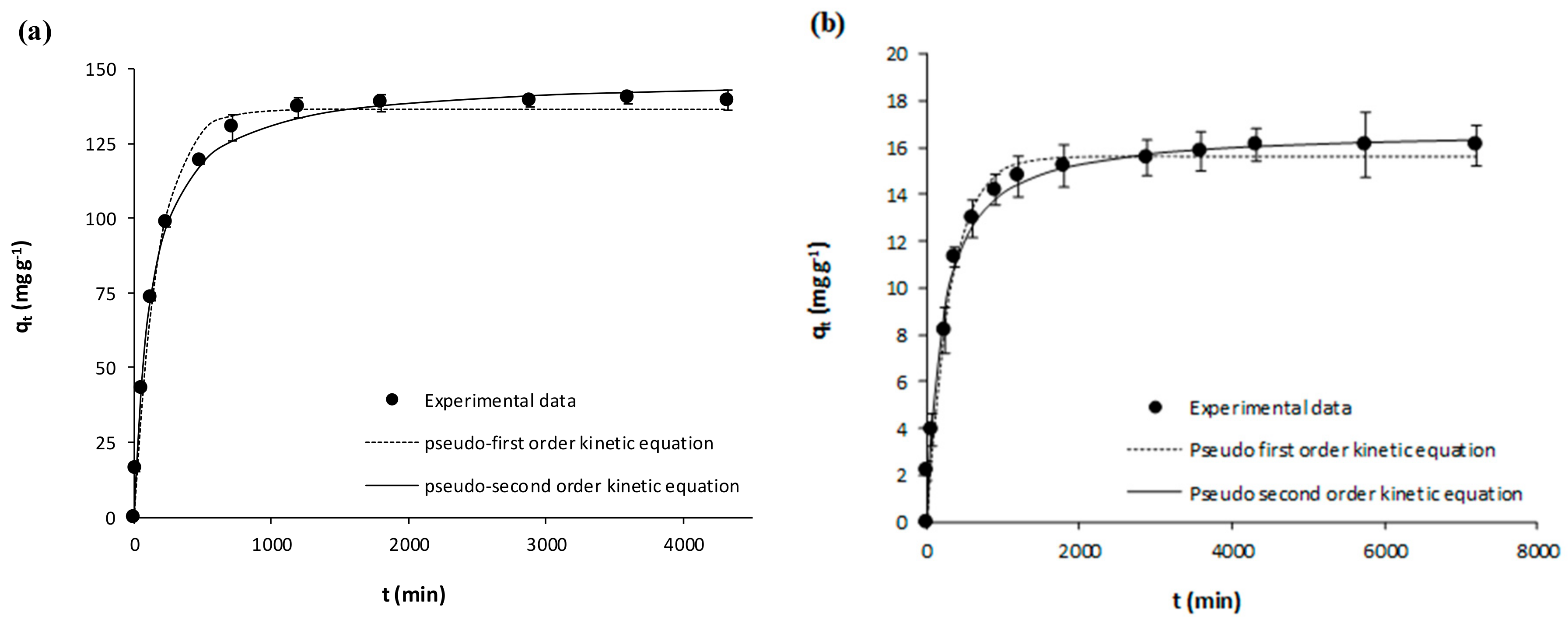
| Pseudo-first Order | k1 (min−1) | 0.0098 ± 0.0007 | 0.0093 ± 0.0006 | 0.0021 ± 0.0002 | 0.0019 ± 0.0002 | 0.0105 ± 0.0009 | 0.0058 ± 0.0004 | 0.0016 ± 0.00001 | 0.0033 ± 0.0003 |
| qe (mg g−1) | 180.60 ± 2.48 | 198.20 ± 2.56 | 3.95 ± 0.09 | 5.39 ± 0.11 | 99.95 ± 1.63 | 136.60 ± 2.04 | 15.24 ±0.27 | 15.63 ± 0.23 | |
| R2 | 0.9914 | 0.9925 | 0.9800 | 0.9832 | 0.9872 | 0.9914 | 0.9889 | 0.9849 | |
| Sxy | 6.59 | 6.76 | 0.22 | 0.27 | 4.35 | 5.04 | 0.63 | 0.73 | |
| Pseudo-second Order | k2 (g mg−1 min−1) | 0.00008 ± 0.00001 | 0.00006 ± 0.00001 | 0.00069 ± 0.00005 | 0.00045 ± 0.00003 | 0.00015 ± 0.00001 | 0.00006 ± 0.000004 | 0.00013 ± 0.00001 | 0.00031 ± 0.00003 |
| qe (mg g−1) | 191.20 ± 2.99 | 210.30 ± 3.40 | 4.34 ± 0.05 | 5.96 ± 0.08 | 105.60 ± 1.49 | 146.90 ± 1.75 | 17.04 ± 0.32 | 16.77 ± 0.27 | |
| R2 | 0.9916 | 0.9913 | 0.9961 | 0.9958 | 0.9926 | 0.9961 | 0.9927 | 0.9911 | |
| Sxy | 6.48 | 7.30 | 0.10 | 0.13 | 3.29 | 3.39 | 0.51 | 0.55 | |
| Parameters | Acetaminophen | Ibuprofen | |||||||
|---|---|---|---|---|---|---|---|---|---|
| GPP20 | SP207 | GPP20 | SP207 | ||||||
| UPW | WW | UPW | WW | UPW | WW | UPW | WW | ||
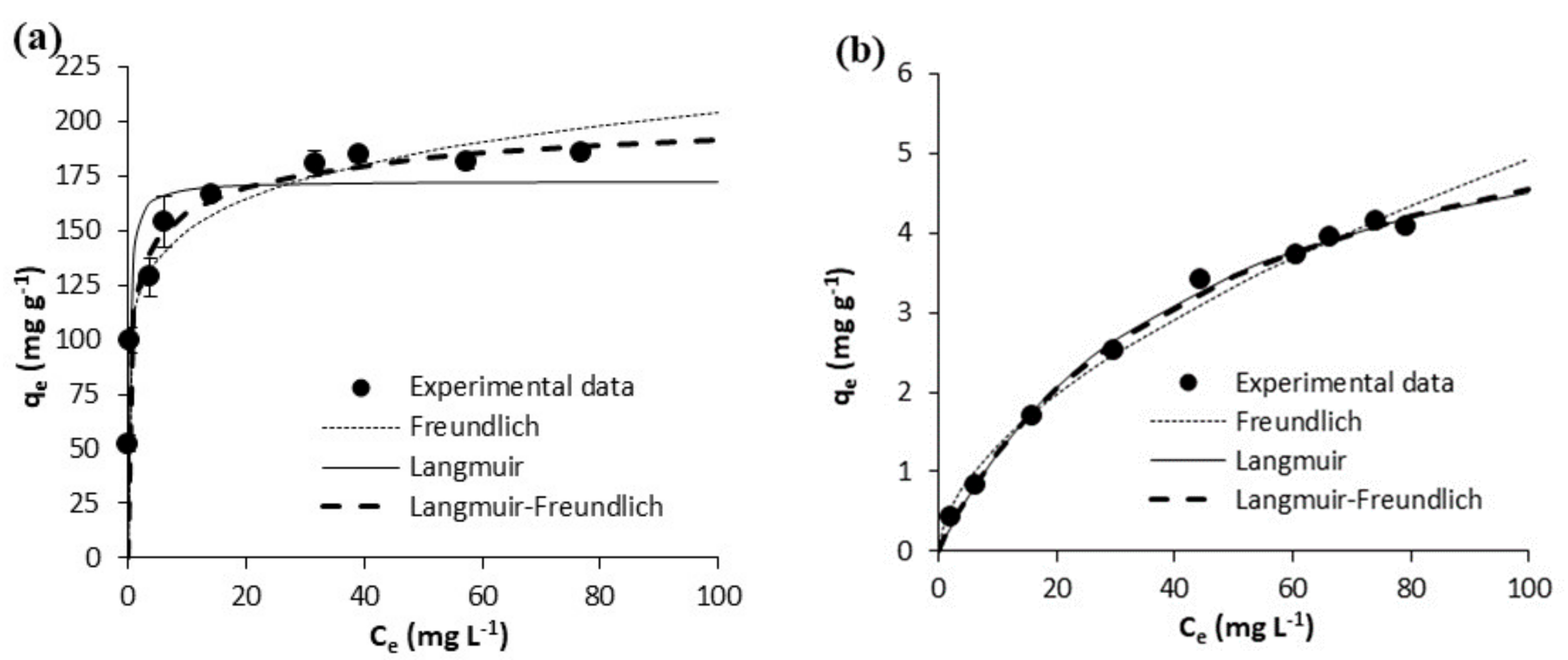
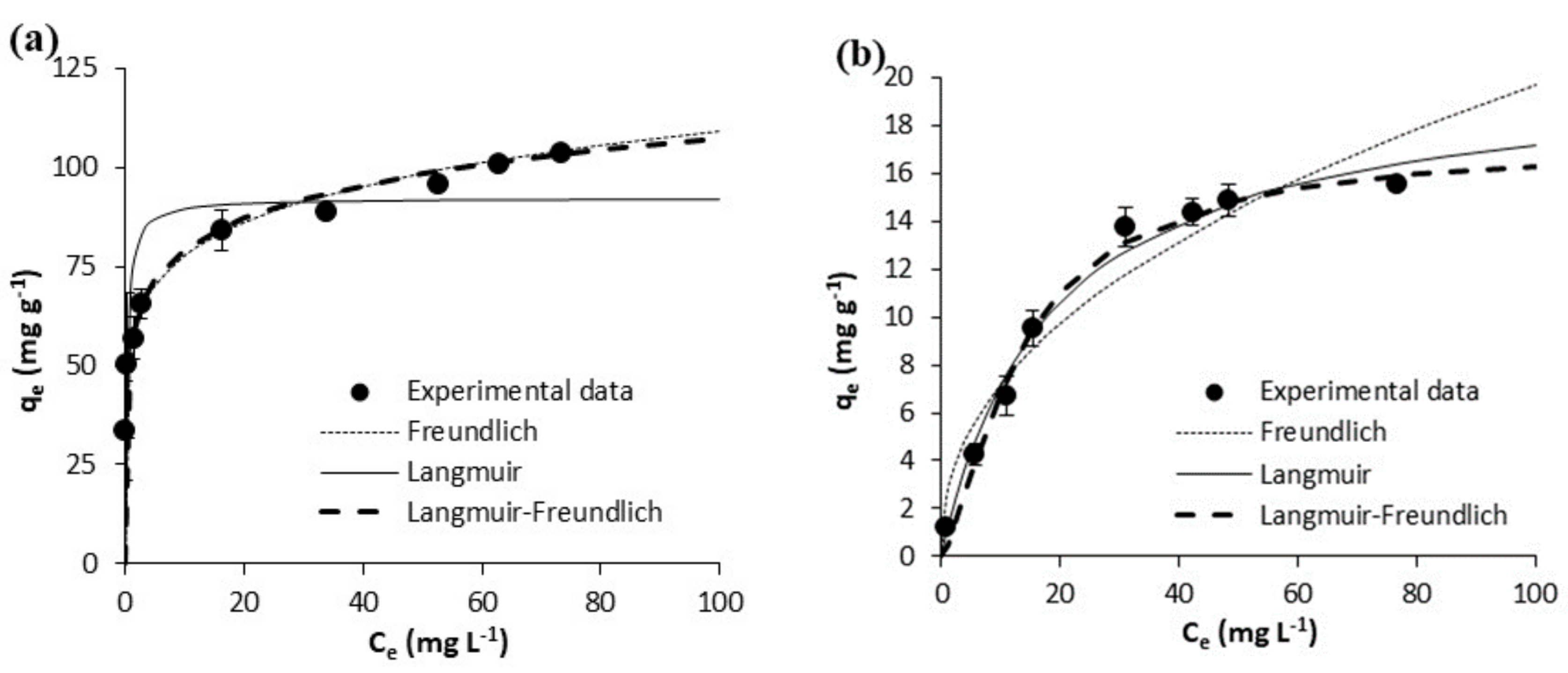
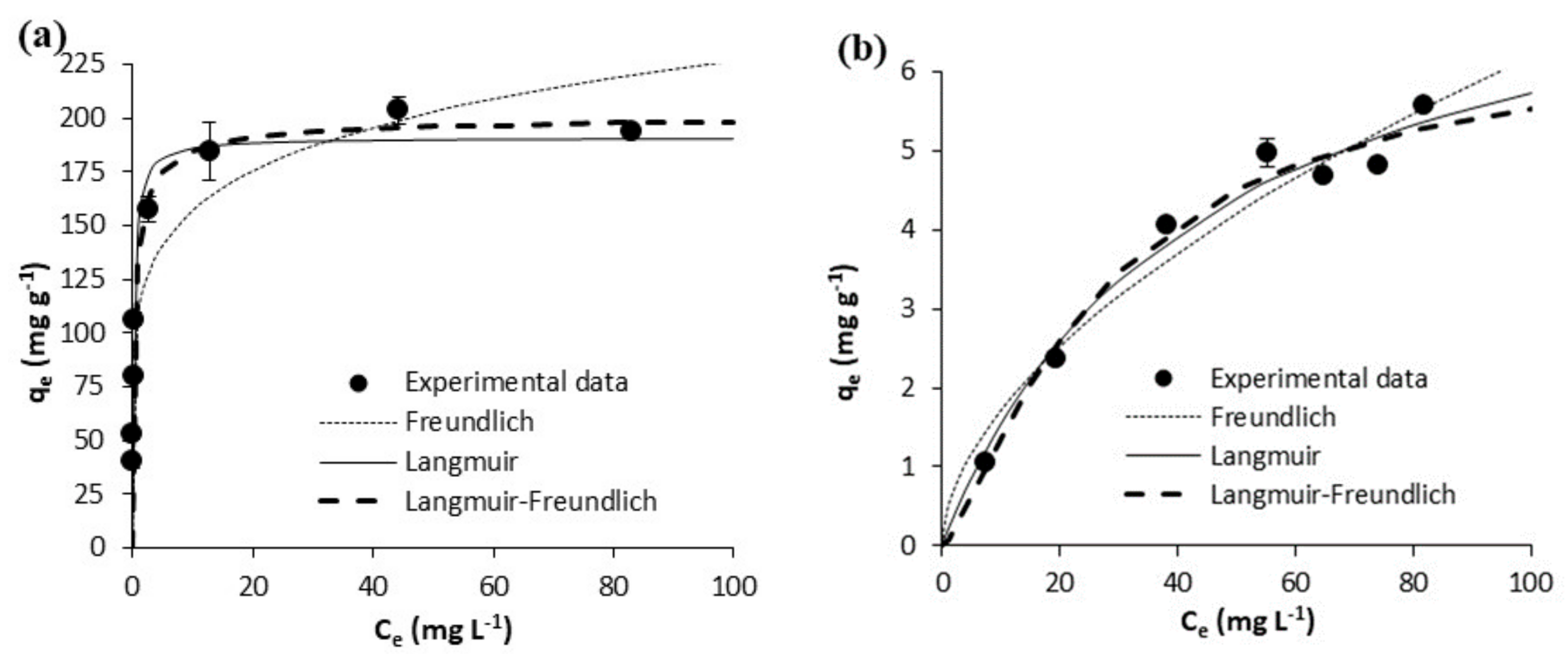
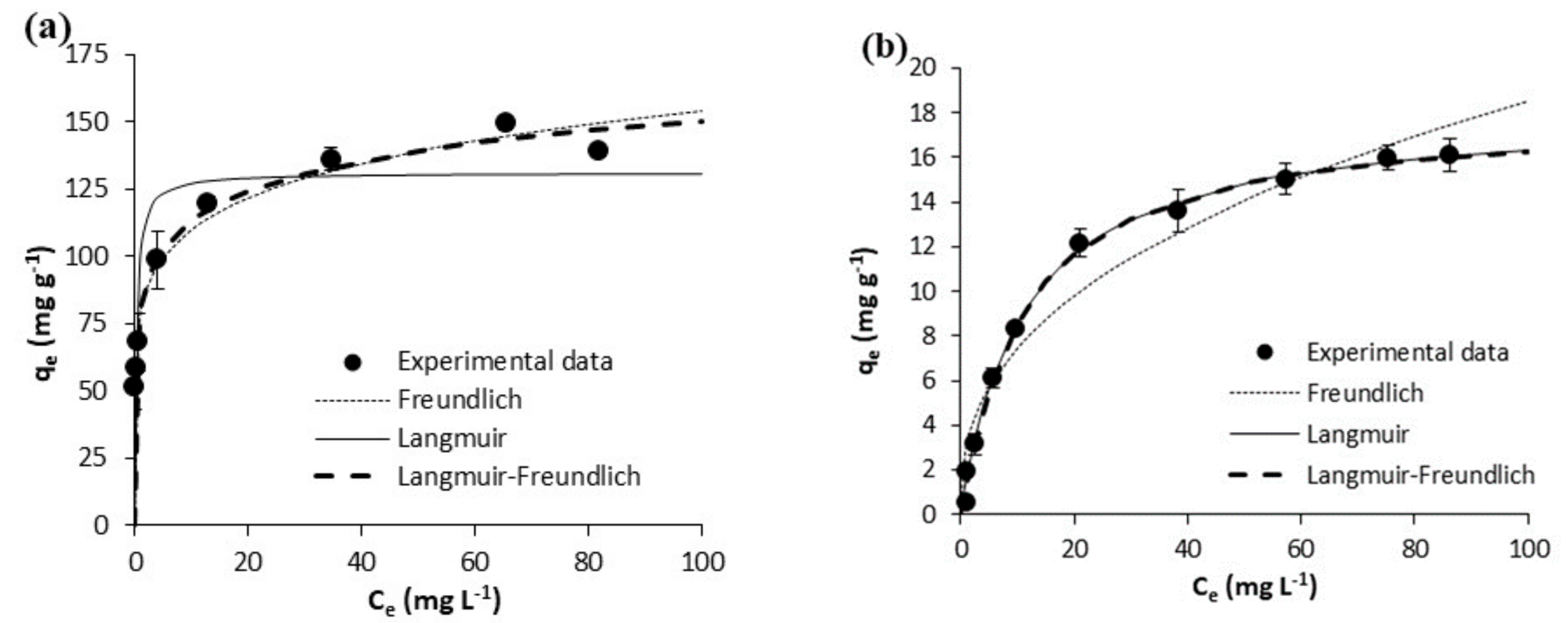
| Freundlich | KF [mg g−1 (mg L−1)−1/n] | 110.10 ± 5.42 | 108.60 ± 10.44 | 0.36 ± 0.06 | 0.47 ± 0.16 | 55.30 ± 1.51 | 78.39 ± 2.88 | 2.60 ± 0.65 | 2.97 ± 0.54 |
| N | 7.45 ± 0.81 | 6.27 ± 1.12 | 1.75 ± 0.12 | 1.79 ± 0.27 | 6.78 ± 0.36 | 6.80 ± 0.49 | 2.27 ± 0.35 | 2.51 ± 0.30 | |
| R2 | 0.9519 | 0.8761 | 0.9878 | 0.947 | 0.9865 | 0.9788 | 0.9342 | 0.9491 | |
| Syx | 10.85 | 24.97 | 0.1704 | 0.41 | 3.19 | 6.16 | 1.52 | 1.45 | |
| Langmuir | Qm(mg g−1) | 172.40 ± 8.34 | 191.20 ± 5.81 | 6.43 ± 0.28 | 8.25 ± 1.01 | 92.26 ± 5.60 | 131.40 ± 9.66 | 20.33 ± 1.16 | 18.17 ± 0.75 |
| KL(L mg-1) | 4.181 ± 2.019 | 3.688 ± 0.545 | 0.023 ± 0.002 | 0.023 ± 0.006 | 3.273 ± 1.447 | 3.144 ± 1.448 | 0.054 ± 0.009 | 0.088 ± 0.006 | |
| R2 | 0.8285 | 0.9772 | 0.9964 | 0.9714 | 0.7751 | 0.7729 | 0.857 | 0.9967 | |
| Syx | 20.47 | 10.72 | 0.09326 | 0.30 | 13.00 | 20.16 | 0.70 | 0.37 | |
| Langmuir-Freundlich | Qm (mg g−1) | 235.00 ± 26.83 | 201.70 ± 7.49 | 6.90 ± 1.12 | 6.53 ± 1.26 | 260.10 ± 108.70 | 272.90 ± 133.30 | 17.25 ± 1.13 | 17.82 ± 0.68 |
| KLF [mg g−1 (mg L−1)−1/n] | 0.954 ± 0.254 | 2.055 ± 0.471 | 0.025 ± 0.004 | 0.073 ± 0.010 | 0.273 ± 0.283 | 0.414 ± 0.298 | 0.026 ± 0.011 | 0.084 ± 0.040 | |
| N | 2.99 ± 0.52 | 1.39 ± 0.19 | 1.06 ± 0.12 | 0.76 ± 0.22 | 4.88 ± 1.55 | 4.23 ± 1.527 | 0.71 ± 0.11 | 0.96 ± 0.070 | |
| R2 | 0.9852 | 0.9890 | 0.9965 | 0.9760 | 0.9880 | 0.9844 | 0.9920 | 0.9968 | |
| Syx | 6.49 | 8.15 | 0.10 | 0.31 | 3.24 | 5.80 | 0.58 | 0.39 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coimbra, R.N.; Escapa, C.; Otero, M. Adsorption Separation of Analgesic Pharmaceuticals from Ultrapure and Waste Water: Batch Studies Using a Polymeric Resin and an Activated Carbon. Polymers 2018, 10, 958. https://doi.org/10.3390/polym10090958
Coimbra RN, Escapa C, Otero M. Adsorption Separation of Analgesic Pharmaceuticals from Ultrapure and Waste Water: Batch Studies Using a Polymeric Resin and an Activated Carbon. Polymers. 2018; 10(9):958. https://doi.org/10.3390/polym10090958
Chicago/Turabian StyleCoimbra, Ricardo N., Carla Escapa, and Marta Otero. 2018. "Adsorption Separation of Analgesic Pharmaceuticals from Ultrapure and Waste Water: Batch Studies Using a Polymeric Resin and an Activated Carbon" Polymers 10, no. 9: 958. https://doi.org/10.3390/polym10090958
APA StyleCoimbra, R. N., Escapa, C., & Otero, M. (2018). Adsorption Separation of Analgesic Pharmaceuticals from Ultrapure and Waste Water: Batch Studies Using a Polymeric Resin and an Activated Carbon. Polymers, 10(9), 958. https://doi.org/10.3390/polym10090958







