Synthesis and Characterization of a Novel Green Cationic Polyfluorene and Its Potential Use as a Fluorescent Membrane Probe
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis and Characterization of Polymers
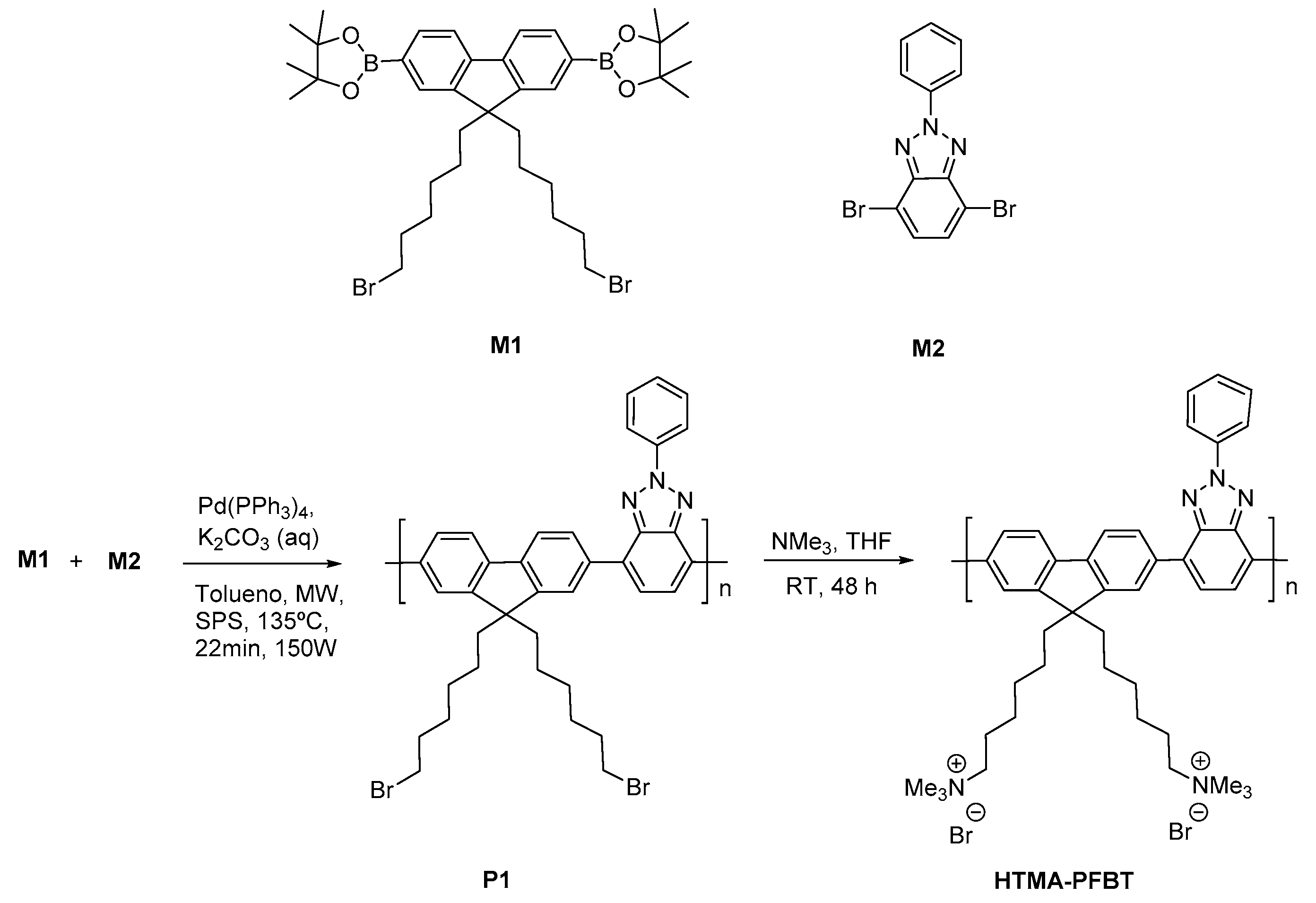
2.2.1. Synthesis of Copoly-{9,9-bis(6′-bromohexyl)-2,7-fluorene-alt-4,7-(2-(phenyl)benzo [d] [1,2,3] Triazole)} (P1)
2.2.2. Synthesis of Copoly-{[9,9-bis(6′-N,N,N-trimethylammonium)hexyl]-2,7-(fluorene)-alt-4,7-(2-(phenyl)benzo[d][1,2,3]triazole)} bromide (HTMA-PFBT)
2.2.3. Microwaves
2.2.4. Nuclear Magnetic Resonance (NMR)
2.2.5. Fourier Transform Infrared Spectroscopy (FTIR)
2.2.6. Gel Permeation Chromatography (GPC)
2.2.7. Absorption and Fluorescence Measurements
2.2.8. Fluorescence Microscopy
2.3. Studies of Interaction with Liposomes
2.3.1. Large Unilamellar Vesicles (LUVs) Preparation
2.3.2. Giant Unilamellar Vesicles (GUVs) Preparation
2.3.3. Preparation of HTMA-PFBT/Lipid Samples
2.3.4. Dynamic Light Scattering (DLS) Measurements
2.3.5. Measurements of Partition Coefficient
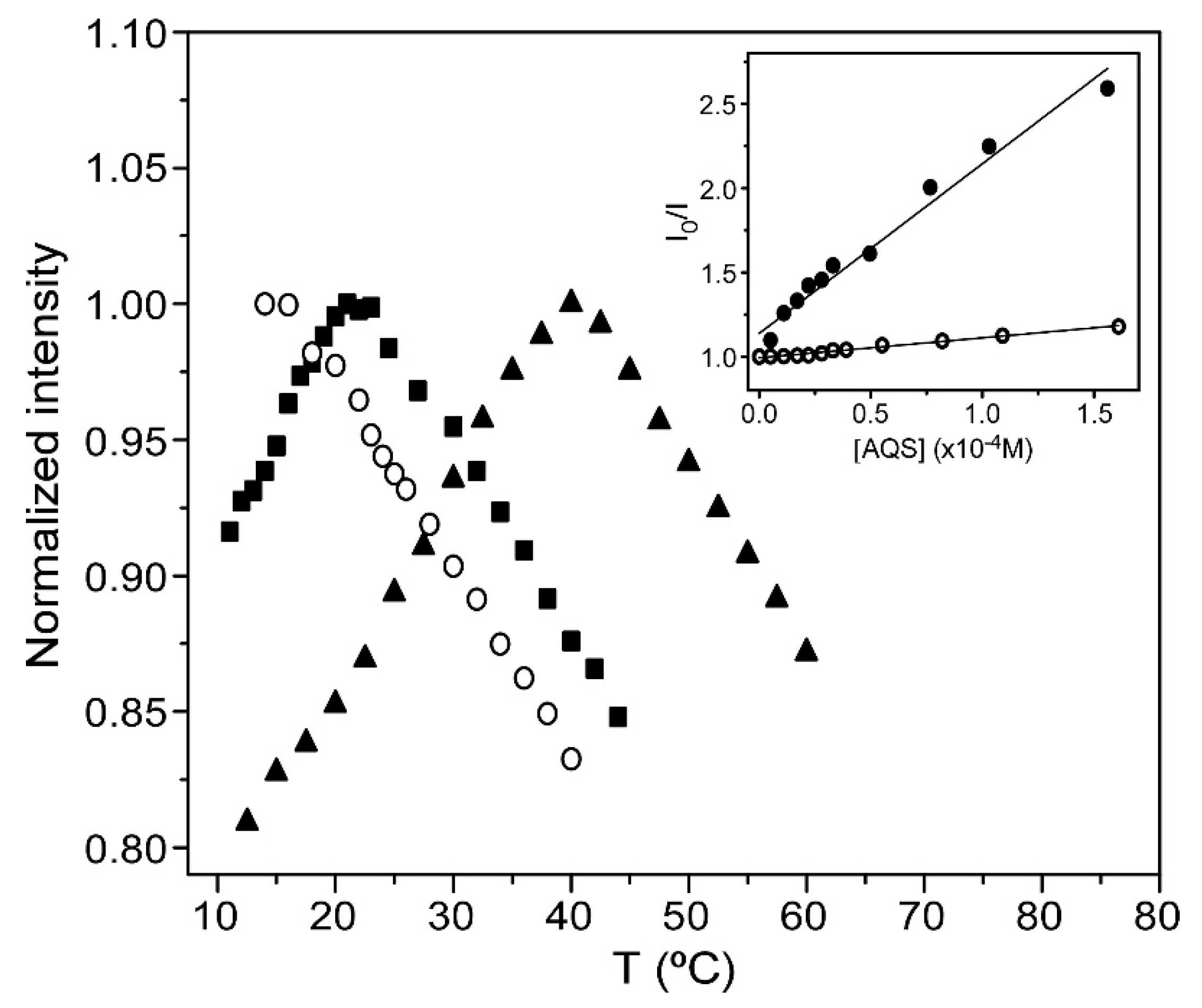
2.3.6. Fluorescence Quenching Experiments
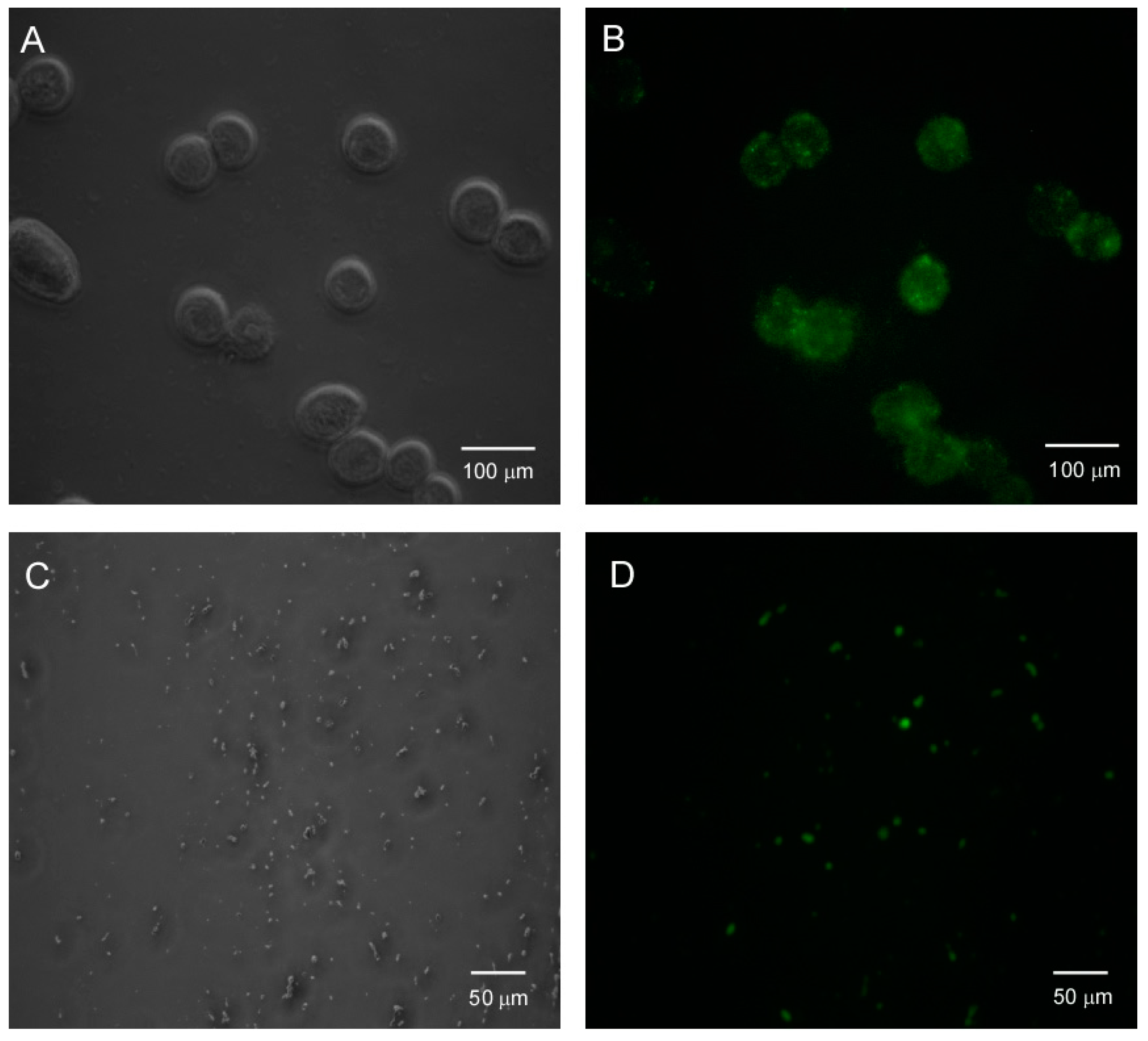
2.4. Bacterial and Mammalian Cell Imaging
3. Results and Discussion
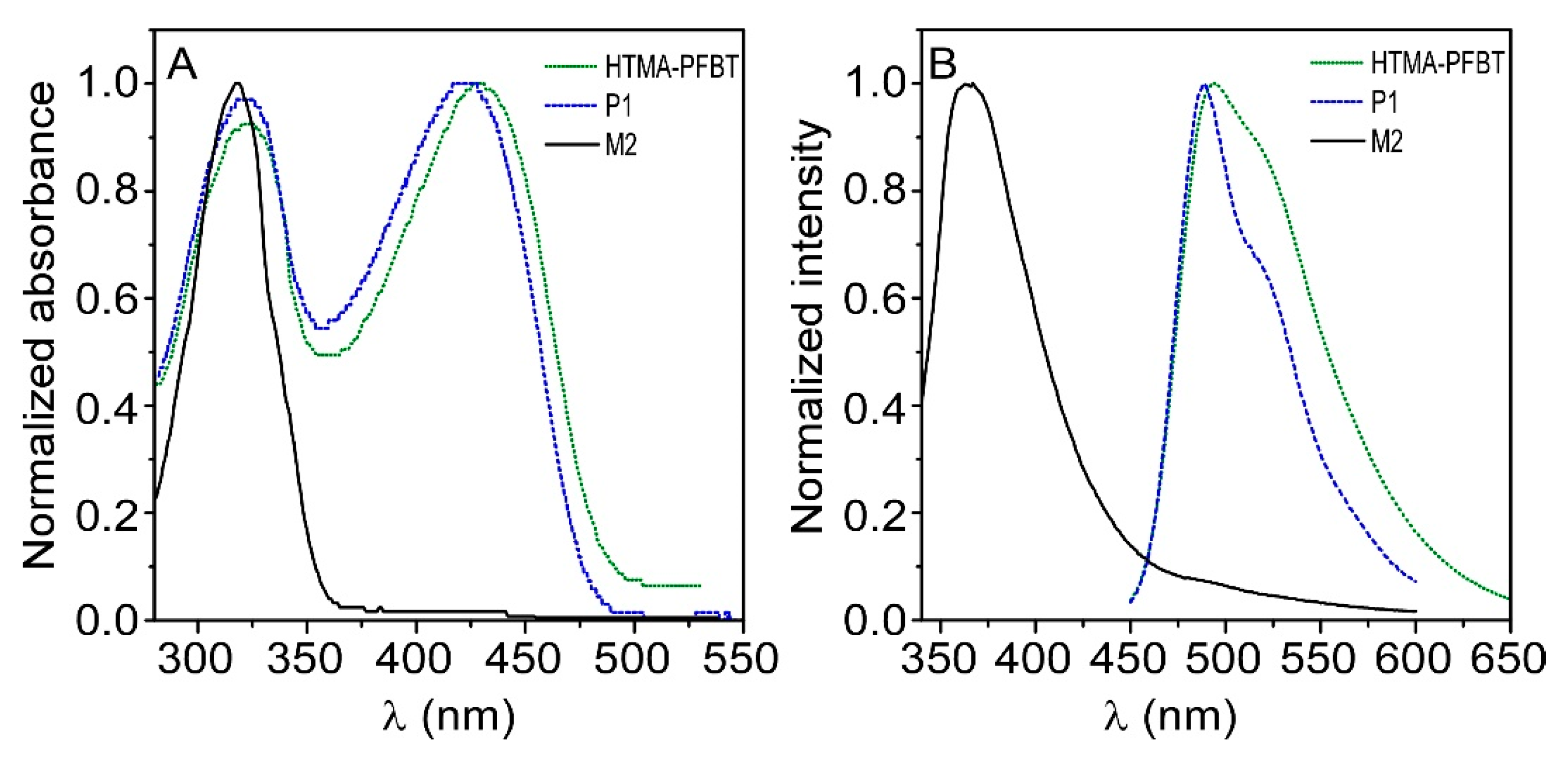
3.1. Synthesis and Characterization of HTMA-PFBT
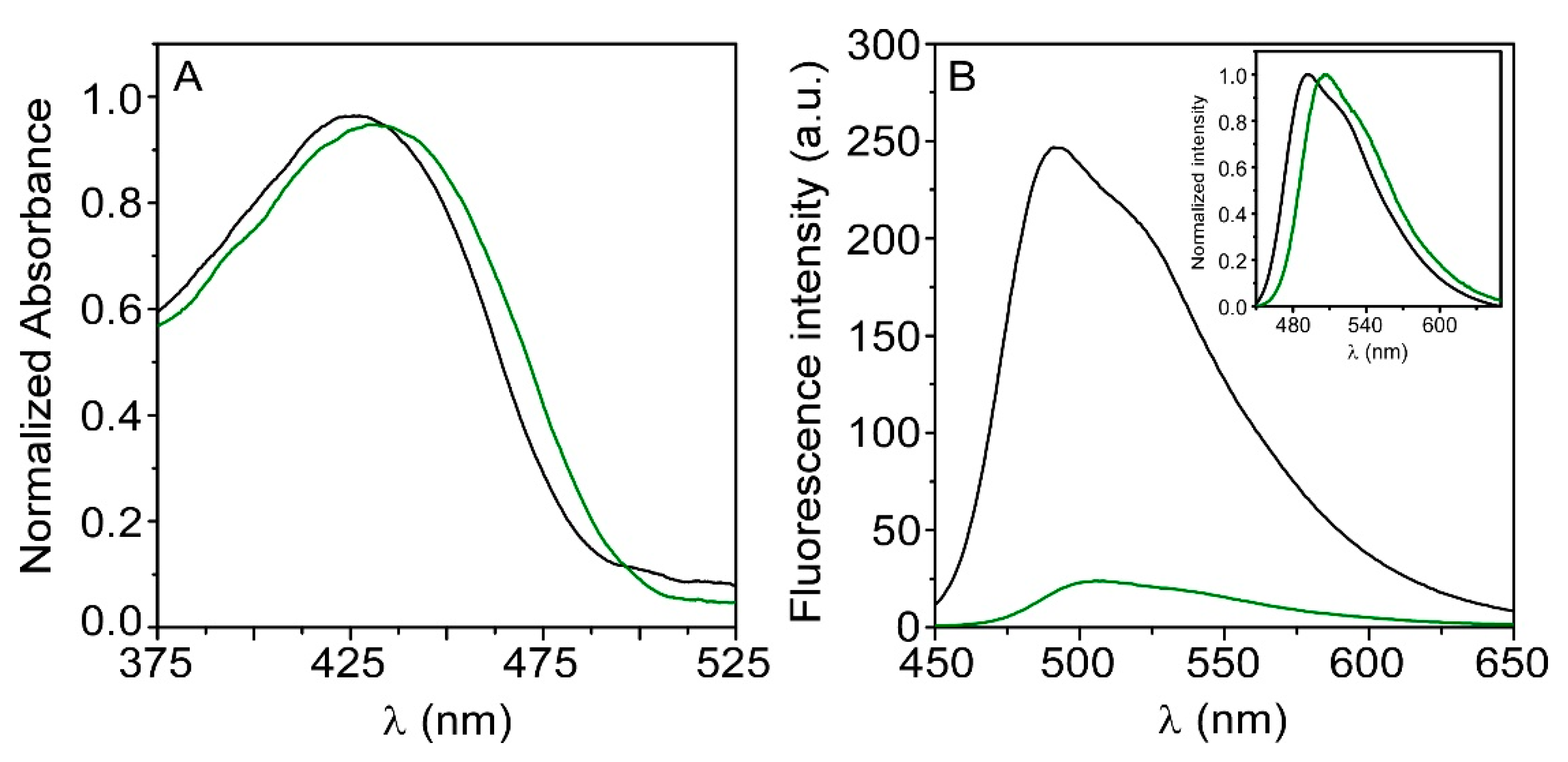
3.2. HTMA-PFBT in Aqueous Solvents
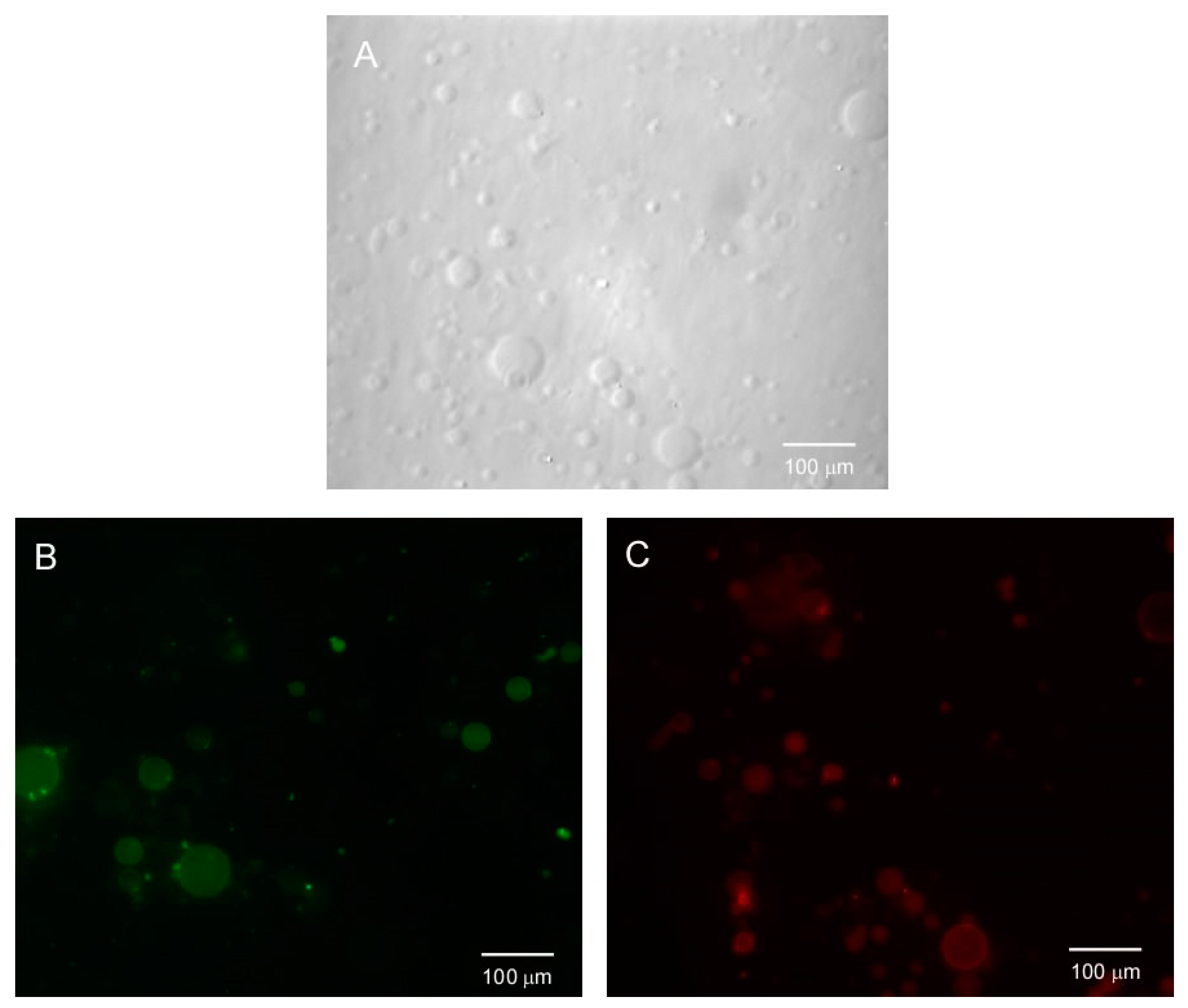
3.3. HTMA-PFBT in Model Membranes
3.4. Selectivity of HTMA-PFBT against Anionic and Zwitterionic Model Membranes
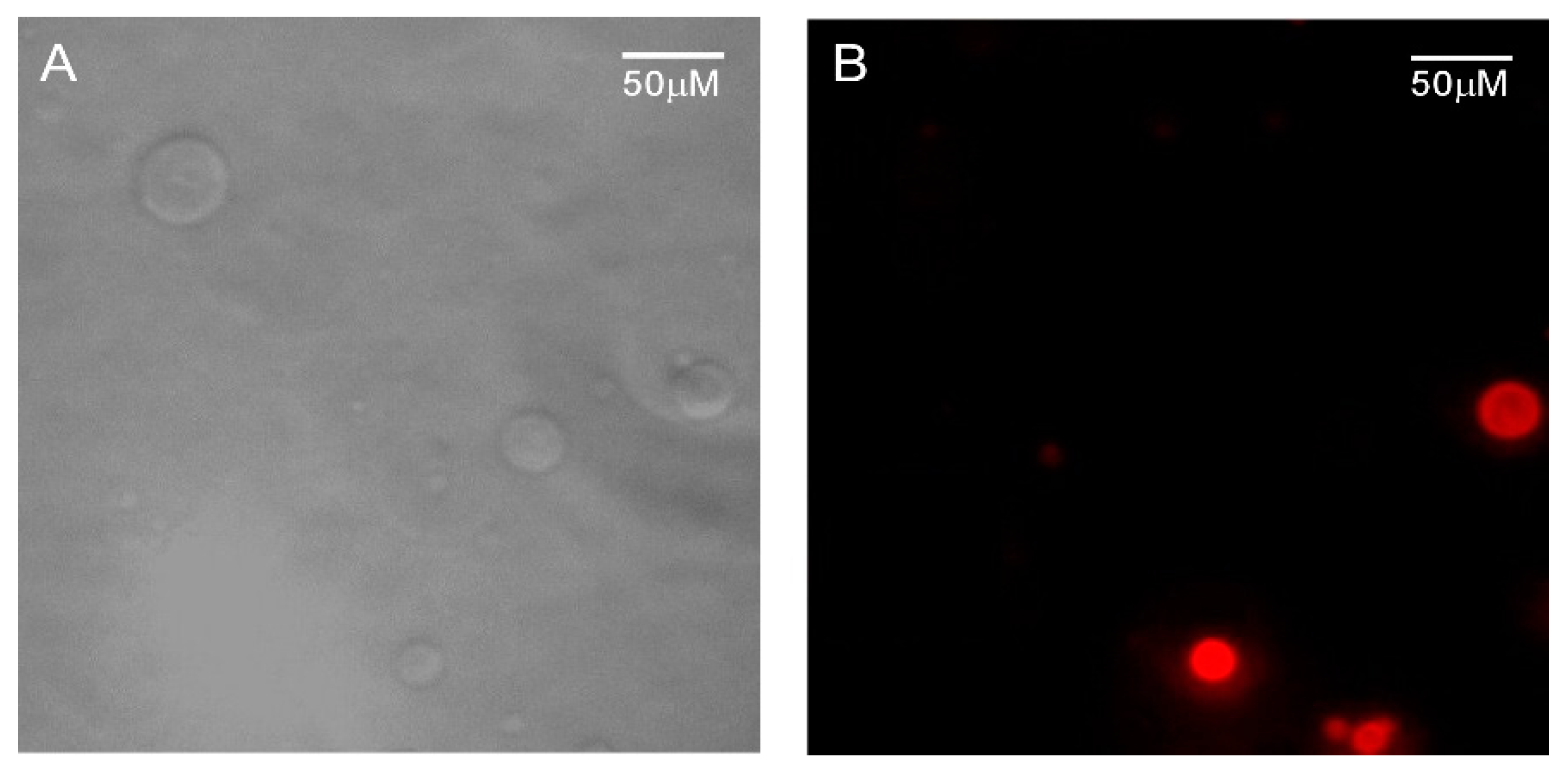
3.5. HTMA-PFBT as A Cell Membrane Marker
3.6. HTMA-PFBT as a Fluorescent Probe to Detect Lipid Phase Transitions
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wolfbeis, O.S. An overview of nanoparticles commonly used in fluorescent bioimaging. Chem. Soc. Rev. 2015, 44, 4743–4768. [Google Scholar] [CrossRef] [PubMed]
- Kolemen, S.; Akkaya, E.U. Reaction-based BODIPY probes for selective bio-imaging. Coord. Chem. Rev. 2018, 354, 121–134. [Google Scholar] [CrossRef]
- Klymchenko, A.S.; Kreder, R. Fluorescent probes for lipid rafts: From model membranes to living cells. Chem. Biol. 2014, 21, 97–113. [Google Scholar] [CrossRef] [PubMed]
- Terai, T.; Nagano, T. Fluorescent probes for bioimaging applications. Curr. Opin. Chem. Biol. 2008, 12, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Gerion, D. Fluorescent CdSe/ZnS nanocrystal-peptide conjugates for long-term, nontoxic imaging and nuclear targeting in living cells. Nano Lett. 2004, 4, 1827–1832. [Google Scholar] [CrossRef]
- Yang, W.; Pan, C.Y.; Liu, X.Q.; Wang, J. Multiple functional hyperbranched poly(amido amine) nanoparticles: Synthesis and application in cell imaging. Biomacromolecules 2011, 12, 1523–1531. [Google Scholar] [CrossRef] [PubMed]
- Chandan, H.R.; Schiffman, J.D.; Balakrishna, R.G. Quantum dots as fluorescent probes: Synthesis, surface chemistry, energy transfer mechanisms and applications. Sens. Actuators B Chem. 2018, 258, 1191–1214. [Google Scholar]
- Hardman, R. A toxicologic review of quantum dots: Toxicity depends on physicochemical and environmental factors. Environ. Health Perspect. 2006, 114, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Houston, J.E.; Kraft, M.; Scherf, U.; Evans, R.C. Sequential detection of multiple phase transitions in model biological membranes using a red-emitting conjugated polyelectrolyte. Phys. Chem. Chem. Phys. 2016, 18, 12423–12427. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.H.; Wu, P.J. Semiconducting polymer nanoparticles as fluorescent probes for biological imaging and sensing. Part. Part. Syst. Charact. 2015, 32, 11–28. [Google Scholar] [CrossRef]
- Braeken, Y.; Cheruku, S.; Ethirajan, A.; Maes, W. Conjugated polymer nanoparticles for bioimaging. Materials 2017, 10, 1420. [Google Scholar] [CrossRef] [PubMed]
- Kahveci, Z.; Martínez-Tomé, M.J.; Mallavia, R.; Mateo, C.R. Use of the conjugated polyelectrolyte poly{[9,9-bis(6′-N,N,N-trimethylammonium)hexyl]fluorene-phenylene} bromide (HTMA-PFP) as a fluorescent membrane marker. Biomacromolecules 2013, 14, 1990–1998. [Google Scholar] [CrossRef] [PubMed]
- Shuang, L.; Jiang, X.F.; Xu, Q.H. Conjugated Polymers for Two-Photon Live Cell Imaging. In Conjugated Polymers for Biological and Biomedical Applications; Wiley-VCH: Weinheim, Germany, 2018. [Google Scholar]
- Jiang, H.; Taranekar, P.; Reynolds, J.R.; Schanze, K.S. Conjugated polyelectrolytes: Synthesis, photophysics and applications. Angew. Chem. Int. Ed. 2009, 48, 4300–4316. [Google Scholar] [CrossRef] [PubMed]
- Pu, K.Y.; Cai, L.; Liu, B. Design and synthesis of charge-transfer-based conjugated polyelectrolytes as multicolor light-up probes. Macromolecules 2009, 42, 5933–5940. [Google Scholar] [CrossRef]
- Hoogenboom, R.; Schubert, U.S. Microwave-assisted polymer synthesis: Recent developments in a rapidly expanding field of research. Macromol. Rapid Commun. 2007, 28, 368–386. [Google Scholar] [CrossRef]
- Ebner, C.; Bodner, T.; Stelzer, F.; Wiesbrock, F. One decade of microwave-assisted polymerizations: Quo vadis? Macromol. Rapid Commun. 2011, 32, 254–288. [Google Scholar] [CrossRef] [PubMed]
- Galbrecht, F.; Bünnagel, T.W.; Scherf, U.; Farrell, T. Microwave-assisted preparation of semiconducting polymers. Macromol. Rapid Commun. 2007, 28, 387–394. [Google Scholar] [CrossRef]
- Nehls, B.S.; Asawapirom, U.; Füldner, S.; Preis, E.; Farrell, T.; Scherf, U. Semiconducting polymers via microwave-assisted Suzuki and Stille cross-coupling reactions. Adv. Funct. Mater. 2004, 14, 352–356. [Google Scholar] [CrossRef]
- Nehls, B.S.; Füldner, S.; Preis, E.; Farrell, T.; Scherf, U. Microwave-assisted synthesis of 1,5- and 2,6-linked naphthylene-based ladder polymers. Macromolecules 2005, 38, 687–694. [Google Scholar] [CrossRef]
- Vázquez-Guilló, R.; Falco, A.; Martínez-Tomé, M.J.; Mateo, C.R.; Herrero, M.A.; Vázquez, E.; Mallavia, R. Advantageous microwave-assisted Suzuki polycondensation for the synthesis of aniline-fluorene alternate copolymers as molecular model with solvent sensing properties. Polymers 2018, 10, 215. [Google Scholar] [CrossRef]
- Martínez-Tomé, M.J.; Esquembre, R.; Mallavia, R.; Mateo, C.R. Formation and characterization of stable fluorescent complexes between neutral conjugated polymers and cyclodextrins. J. Fluoresc. 2013, 23, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Zhan, R.; Liu, B. Benzothiadiazole-containing conjugated polyelectrolytes for biological sensing and imaging. Macromol. Chem. Phys. 2015, 216, 131–144. [Google Scholar] [CrossRef]
- Dias, F.B.; King, S.; Monkman, A.P.; Perepichka, I.I.; Kryuchkov, M.A.; Perepichka, I.F.; Bryce, M.R. Dipolar stabilization of emissive singlet charge transfer excited states in polyfluorene copolymers. J. Phys. Chem. B 2008, 112, 6557–6566. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Sun, H.; Yang, H.; Liu, S.; Jenkins, G.; Feng, W.; Li, F.; Zhao, Q.; Liu, B.; Huang, W. Cationic polyfluorenes with phosphorescent iridium(III) complexes for time-resolved luminescent biosensing and fluorescence lifetime imaging. Adv. Funct. Mater. 2013, 23, 3268–3276. [Google Scholar] [CrossRef]
- Liu, B.; Bazan, G.C. Synthesis of cationic conjugated polymers for use in label-free DNA microarrays. Nat. Protoc. 2006, 1, 1698–1702. [Google Scholar] [CrossRef] [PubMed]
- Pina, J.; Seixas De Melo, J.S.; Eckert, A.; Scherf, U. Unusual photophysical properties of conjugated, alternating indigo-fluorene copolymers. J. Mater. Chem. A 2015, 3, 6373–6382. [Google Scholar] [CrossRef]
- Mallavia, R.; Martinez-Peréz, D.; Chmelka, B.F.; Bazan, G.C. Blue fluorescent films based on poly-2,7-fluorene-phenylene derivatives. Bol. Soc. Esp. Ceram. Vidrio 2004, 43, 327–330. [Google Scholar] [CrossRef]
- Molina, R.; Gómez-Ruiz, S.; Montilla, F.; Salinas-Castillo, A.; Fernández-Arroyo, S.; Del Mar Ramos, M.; Micol, V.; Mallavia, R. Progress in the synthesis of poly(2,7-fluorene-alt-1,4-phenylene), PFP, via suzuki coupling. Macromolecules 2009, 42, 5471–5477. [Google Scholar] [CrossRef]
- Kahveci, Z.; Vázquez-Guilló, R.; Martínez-Tomé, M.J.; Mallavia, R.; Mateo, C.R. New Red-Emitting Conjugated Polyelectrolyte: Stabilization by Interaction with Biomolecules and Potential Use as Drug Carriers and Bioimaging Probes. ACS Appl. Mater. Interfaces 2016, 8, 1958–1969. [Google Scholar] [CrossRef] [PubMed]
- Mira, A.; Mateo, C.R.; Mallavia, R.; Falco, A. Poly(methyl vinyl ether-alt-maleic acid) and ethyl monoester as building polymers for drug-loadable electrospun nanofibers. Sci. Rep. 2017, 7, 17205. [Google Scholar] [CrossRef] [PubMed]
- Kahveci, Z.; Martínez-Tomé, M.J.; Mallavia, R.; Mateo, C.R. Fluorescent biosensor for phosphate determination based on immobilized polyfluorene-liposomal nanoparticles coupled with alkaline phosphatase. ACS Appl. Mater. Interfaces 2017, 9, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Hama, Y.; Urano, Y.; Koyama, Y.; Bernardo, M.; Choyke, P.L.; Kobayashi, H. A comparison of the emission efficiency of four common green fluorescence dyes after internalization into cancer cells. Bioconjugate Chem. 2006, 17, 1426–1431. [Google Scholar] [CrossRef] [PubMed]
- Jerome, K.R. Lennette’s Laboratory Diagnosis of Viral Infections, 4th ed.; Informa Healthcare USA: New York, NY, USA, 2016; p. 115. ISBN 9781420084955. [Google Scholar]
- Torres, I.; Carrillo, J.R.; Díaz-Ortiz, A.; Martín, R.; Gómez, M.V.; Stegemann, L.; Strassert, C.A.; Orduna, J.; Buendía, J.; Greciano, E.E.; et al. Self-assembly of T-shape 2 H-benzo [d] [1,2,3]-triazoles. Optical waveguide and photophysical properties. RSC Adv. 2016, 6, 36544–36553. [Google Scholar] [CrossRef]
- Angelova, M.I.; Dimitrov, D.S. Liposome electroformation. Faraday Discuss. Chem. Soc. 1986, 81, 303–311. [Google Scholar] [CrossRef]
- Esquembre, R.; Pinto, S.N.; Poveda, J.A.; Prieto, M.; Mateo, C.R. Immobilization and characterization of giant unilamellar vesicles (GUVs) within porous silica glasses. Soft Matter 2012, 8, 408–417. [Google Scholar] [CrossRef]
- Coutinho, A.; Prieto, M. Self-association of the polyene antibiotic nystatin in dipalmitoylphosphatidylcholine vesicles: A time-resolved fluorescence study. Biophys. J. 1995, 69, 2541–2557. [Google Scholar] [CrossRef]
- Melo, M.N.; Castanho, M.A.R.B. Omiganan interaction with bacterial membranes and cell wall models. Assigning a biological role to saturation. Biochim. Biophys. Acta Biomembr. 2007, 1768, 1277–1290. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Dong, W.F.; Möhwald, H.; Krastev, R. Amplified fluorescence quenching of self-assembled polyelectrolyte—Dye nanoparticles in aqueous solution. Chem. Mater. 2008, 20, 1664–1666. [Google Scholar] [CrossRef]
- Chemburu, S.; Ji, E.; Casana, Y.; Wu, Y.; Buranda, T.; Schanze, K.S.; Lopez, G.P.; Whitten, D.G. Conjugated polyelectrolyte supported bead based assays for phospholipase A2 activity. J. Phys. Chem. B 2008, 112, 14492–14499. [Google Scholar] [CrossRef] [PubMed]
- Molina, R.; Gomez-Ruiz, S.; Montilla, F.; Salinas-Castillo, A.; Fernandez-Arroyo, S.; Ramos, M.; Micol, V.; Mallavia, R. Progress in the synthesis of poly(2,7-fluorene-alt-1,4-phenlynene), PFP, via Suzuki coupling. Macromolecules 2009, 42, 5417–5477. [Google Scholar] [CrossRef]
- Zhang, W.; Lu, P.; Wang, Z.; Ma, Y. Microwave-assisted suzuki coupling reaction for rapid synthesis of conjugated polymer-poly(9,9-dihexylfluorene)s as an example. J. Polym. Sci. Part. A 2013, 51, 1950–1955. [Google Scholar] [CrossRef]
- Kahveci, Z.; Martínez-Tomé, M.J.; Esquembre, R.; Mallavia, R.; Mateo, C.R. Selective interaction of a cationic polyfluorene with model lipid membranes: Anionic versus zwitterionic lipids. Materials 2014, 7, 2120–2140. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Tome, M.J.; Esquembre, R.; Mallavia, R.; Mateo, C.R. Formation of complexes between the conjugated polyelectrolyte poly{[9,9-bis(6′-N,N,N-trimethylammonium) hexyl]fluorene-phenylene} bromide (HTMA-PFP) and human serum albumin. Biomacromolecules 2010, 11, 1494–1501. [Google Scholar] [CrossRef] [PubMed]
- Kahveci, Z.; Vázquez-Guilló, R.; Mira, A.; Martinez, L.; Falcó, A.; Mallavia, R.; Mateo, C.R. Selective recognition and imaging of bacterial model membranes over mammalian ones by using cationic conjugated polyelectrolytes. Analyst 2016, 141, 6287–6296. [Google Scholar] [CrossRef] [PubMed]
- Van Meer, G.; Voelker, D.R.; Feigenson, G.W. Membrane lipids: Where they are and how they behave. Nat. Rev. Mol. Cell Biol. 2008, 9, 112–124. [Google Scholar] [CrossRef] [PubMed]
- Reyes Mateo, C.; Tauc, P.; Brochon, J.C. Pressure effects on the physical properties of lipid bilayers detected by trans-parinaric acid fluorescence decay. Biophys. J. 1993, 65, 2248–2260. [Google Scholar] [CrossRef]
- Kuc, M.; Cieslik-Boczula, K.; Rospenk, M. Anesthetic-dependent changes in the chain-melting phase transition of DPPG liposomes studied using near-infrared spectroscopy supported by PCA. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 186, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Traiphol, R.; Sanguansat, P.; Srikhirin, T.; Kerdcharoen, T.; Osotchan, T. Spectroscopic study of photophysical change in collapsed coils of conjugated polymers: Effects of solvent and temperature. Macromolecules 2006, 39, 1165–1172. [Google Scholar] [CrossRef]



| - | Absorbance | Emission | d (nm) a | |||
|---|---|---|---|---|---|---|
| λmax (nm) | λmax (nm) ΦF BW (nm) | HTMA-PFBT + HTMA-PFBT | ||||
| ethanol | 425 | 491 | 0.22 | 78 | not detected | not detected |
| buffer | 431 | 508 | 0.02 | 76 | not detected | 207 ± 4 |
| PC 1 mM | - | 496 | 0.45 | 65 | 158 ± 1 | 163 ± 1 |
| PG 1 mM | - | 495 | 0.47 | 64 | 140 ± 1 | 140 ± 1 |
| System | KP | KSV (M−1) |
|---|---|---|
| buffer | - | (7.2 ± 0.4) × 105 |
| PG | (9.4 ± 2.2) × 105 | (2.6 ± 0.3) × 103 |
| PC | (8.9 ± 3.2) × 103 | (1.4 ± 0.1) × 104 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vázquez-Guilló, R.; Martínez-Tomé, M.J.; Kahveci, Z.; Torres, I.; Falco, A.; Mallavia, R.; Mateo, C.R. Synthesis and Characterization of a Novel Green Cationic Polyfluorene and Its Potential Use as a Fluorescent Membrane Probe. Polymers 2018, 10, 938. https://doi.org/10.3390/polym10090938
Vázquez-Guilló R, Martínez-Tomé MJ, Kahveci Z, Torres I, Falco A, Mallavia R, Mateo CR. Synthesis and Characterization of a Novel Green Cationic Polyfluorene and Its Potential Use as a Fluorescent Membrane Probe. Polymers. 2018; 10(9):938. https://doi.org/10.3390/polym10090938
Chicago/Turabian StyleVázquez-Guilló, Rebeca, María José Martínez-Tomé, Zehra Kahveci, Ivan Torres, Alberto Falco, Ricardo Mallavia, and C. Reyes Mateo. 2018. "Synthesis and Characterization of a Novel Green Cationic Polyfluorene and Its Potential Use as a Fluorescent Membrane Probe" Polymers 10, no. 9: 938. https://doi.org/10.3390/polym10090938
APA StyleVázquez-Guilló, R., Martínez-Tomé, M. J., Kahveci, Z., Torres, I., Falco, A., Mallavia, R., & Mateo, C. R. (2018). Synthesis and Characterization of a Novel Green Cationic Polyfluorene and Its Potential Use as a Fluorescent Membrane Probe. Polymers, 10(9), 938. https://doi.org/10.3390/polym10090938









