Author Contributions
Conceptualization: S.S., D.G., and P.J.; methodology: S.S., D.G., and P.J.; validation: S.S., D.G.; investigation: S.S., D.G., N.Y., and P.J.; resources: P.J. and N.Y.; data curation: S.S. and D.G.; writing—original draft preparation: S.S., D.G., N.Y., and P.J.; writing—review and editing: S.S., D.G., and P.J.; visualization: S.S., D.G., and P.J.; supervision: D.G. and P.J.; project administration: P.J. and N.Y.
Figure 1.
Proton assignment for β-cyclodextrin (β-CD) and both pesticides (4-chlorophenoxyacetic acid (4-CPA) and 2,3,4,6-tetrachlorophenol (TCF)).
Figure 1.
Proton assignment for β-cyclodextrin (β-CD) and both pesticides (4-chlorophenoxyacetic acid (4-CPA) and 2,3,4,6-tetrachlorophenol (TCF)).
Figure 2.
X-ray powder diffraction (XRPD) analysis for nanosponges (NS) and NS–pesticide complexes.
Figure 2.
X-ray powder diffraction (XRPD) analysis for nanosponges (NS) and NS–pesticide complexes.
Figure 3.
Thermogravimetric analysis (TGA) analysis for nanosponges (NS), the NS–2,3,4,6-tetrachlorophenol (TCF) complex and the NS–4-chlorophenoxyacetic acid (4-CPA) complex.
Figure 3.
Thermogravimetric analysis (TGA) analysis for nanosponges (NS), the NS–2,3,4,6-tetrachlorophenol (TCF) complex and the NS–4-chlorophenoxyacetic acid (4-CPA) complex.
Figure 4.
SEM image of nanosponges (NS) (a,b), NS–2,3,4,6-tetrachlorophenol (TCF) (c,d), and NS–4-chlorophenoxyacetic acid (4-CPA) (e,f), and energy dispersive X-ray spectroscopy (EDS) analysis of the NS–pesticide complex (g,h).
Figure 4.
SEM image of nanosponges (NS) (a,b), NS–2,3,4,6-tetrachlorophenol (TCF) (c,d), and NS–4-chlorophenoxyacetic acid (4-CPA) (e,f), and energy dispersive X-ray spectroscopy (EDS) analysis of the NS–pesticide complex (g,h).
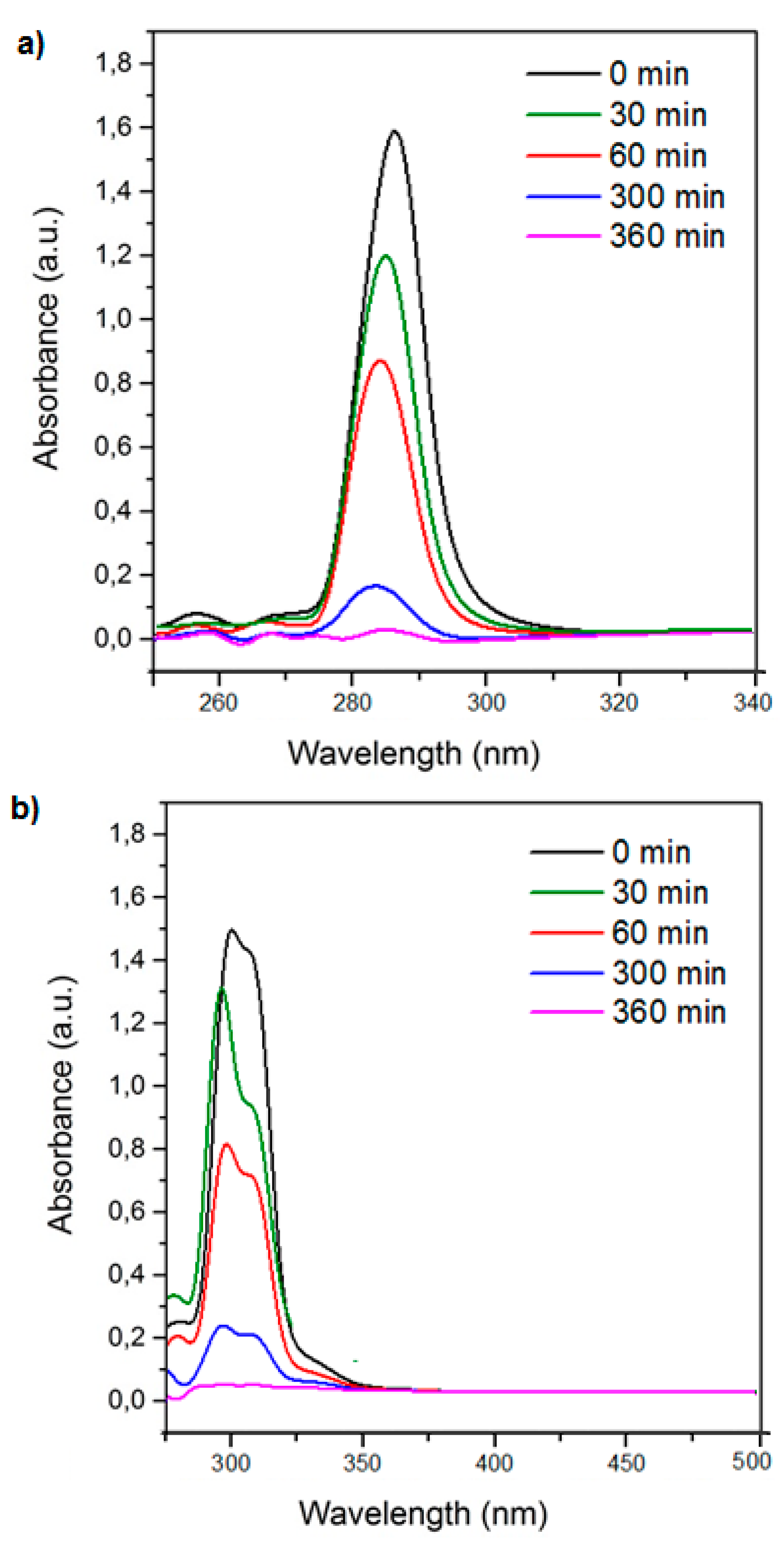
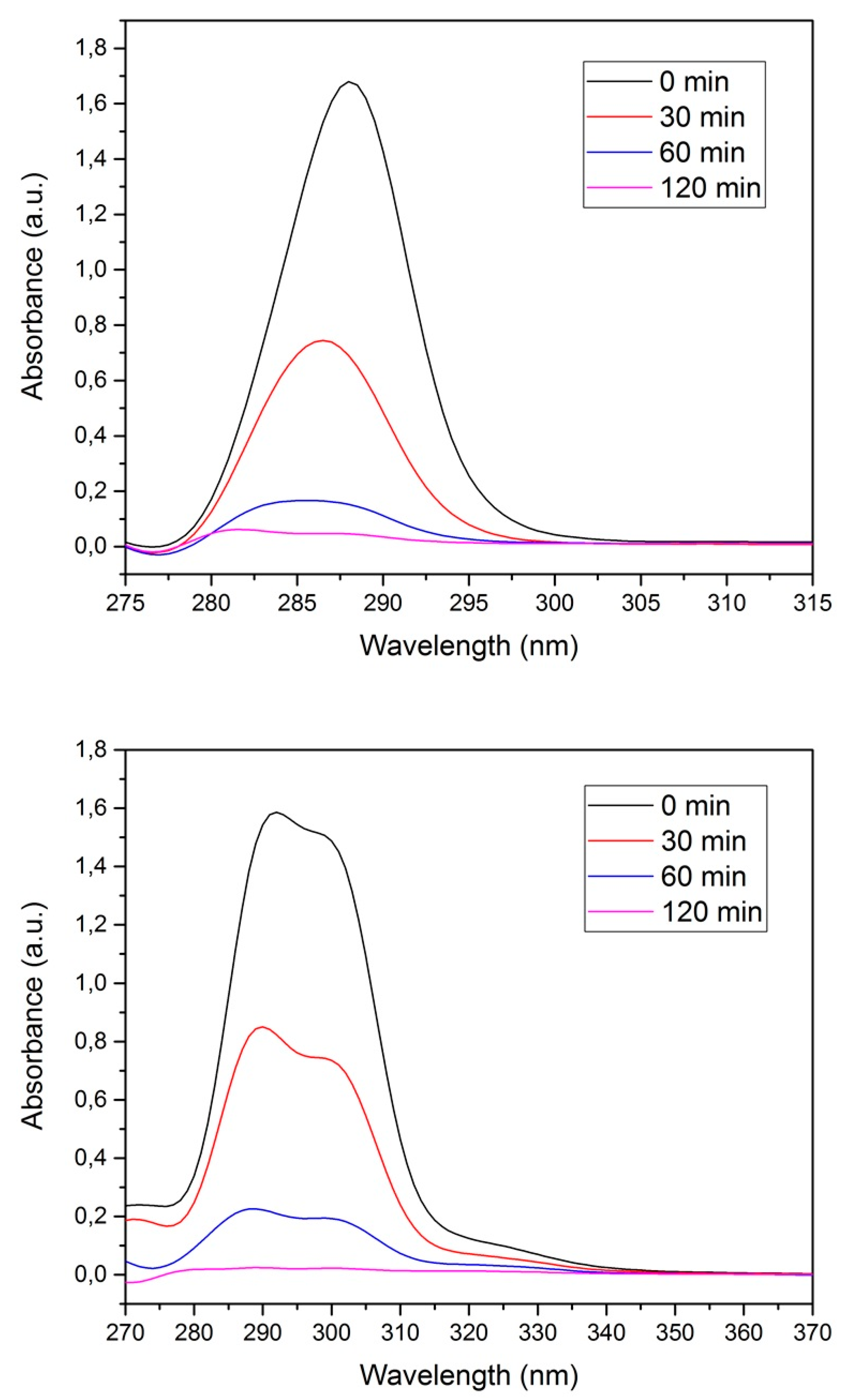
Figure 5.
UV–VIS spectra for 4-chlorophenoxyacetic acid (4-CPA) (a) and 2,3,4,6-tetrachlorophenol (TCF) (b) at different contact times.
Figure 5.
UV–VIS spectra for 4-chlorophenoxyacetic acid (4-CPA) (a) and 2,3,4,6-tetrachlorophenol (TCF) (b) at different contact times.
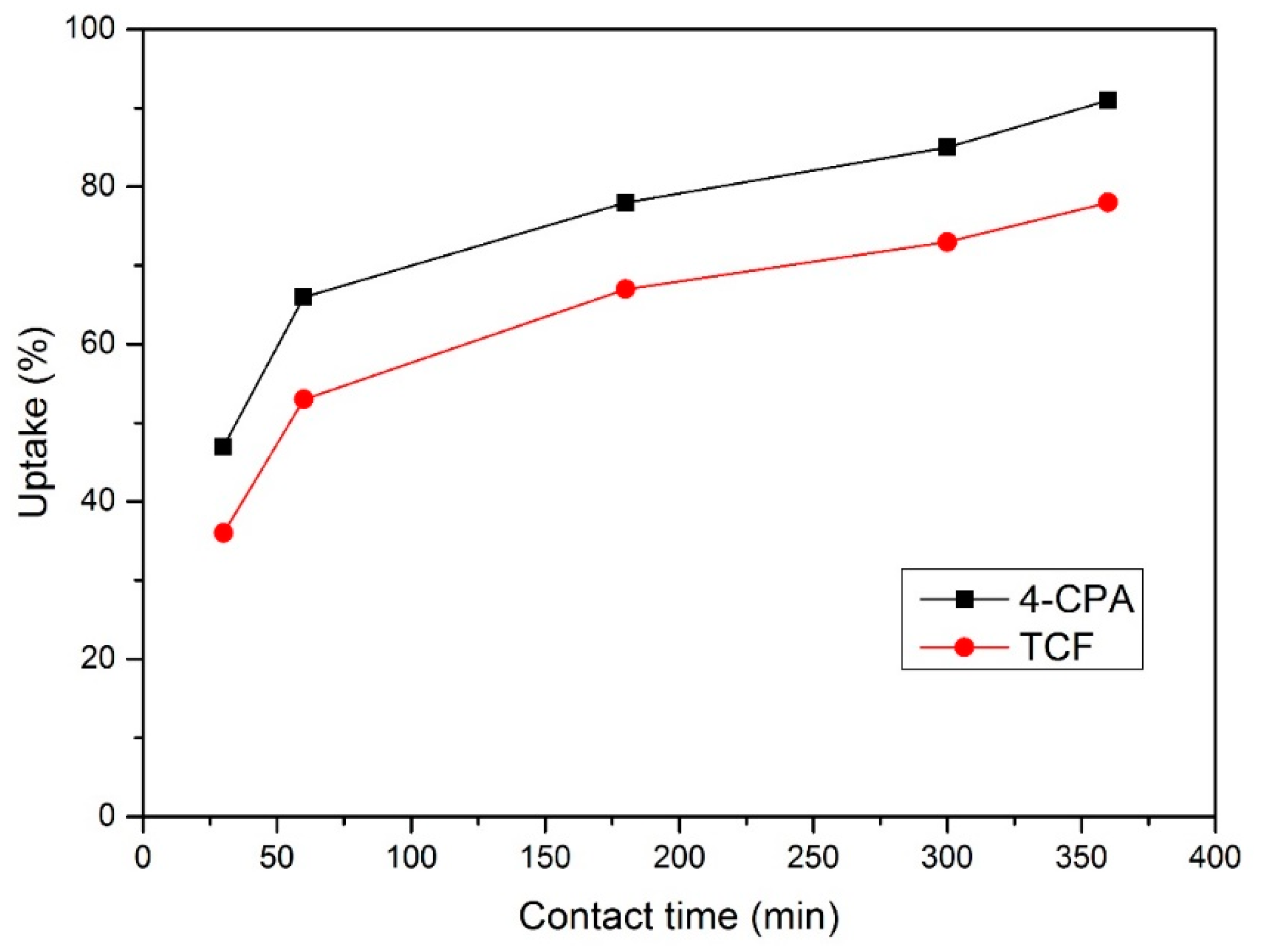
Figure 6.
Uptake percentage for each pesticide.
Figure 6.
Uptake percentage for each pesticide.
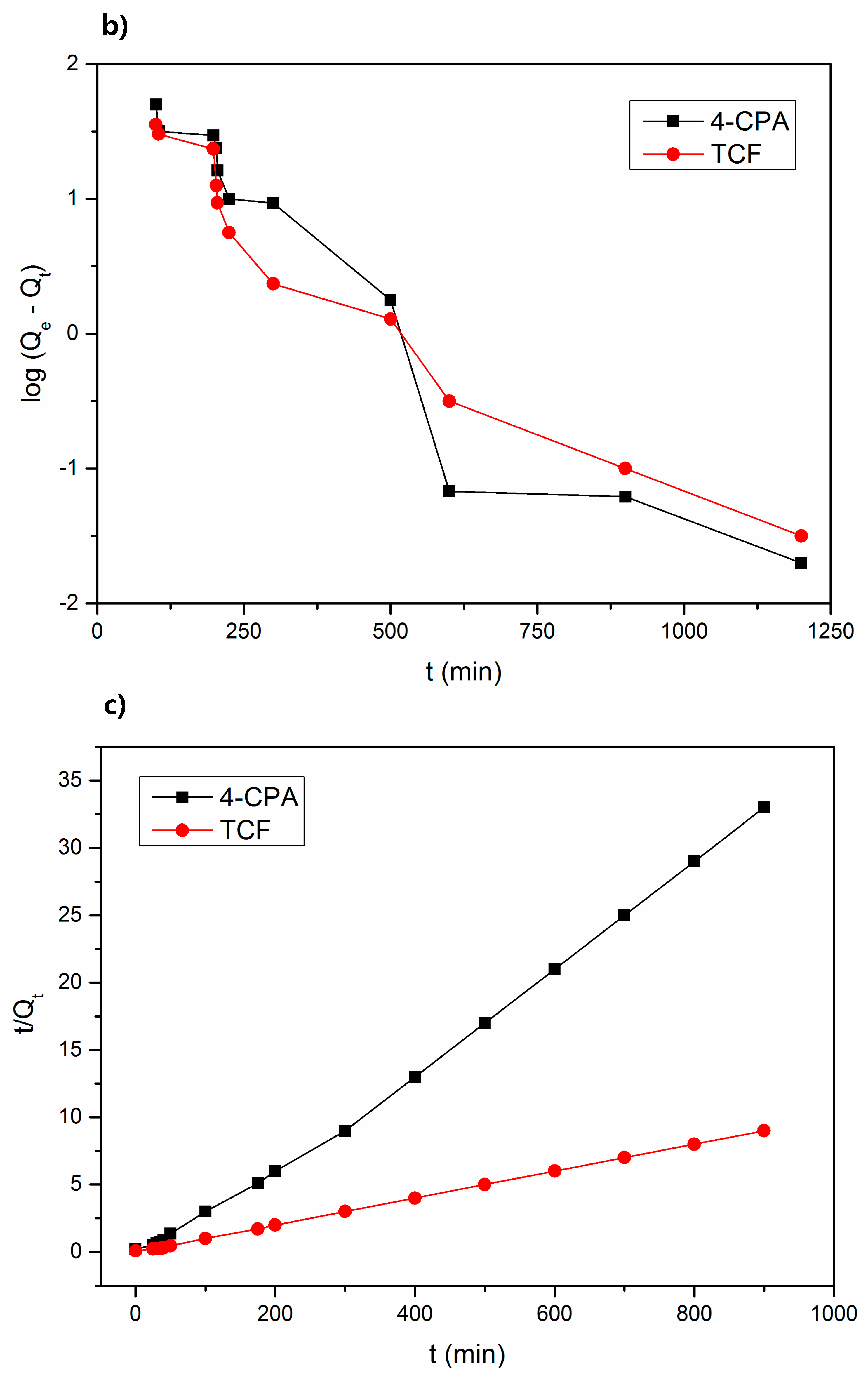
Figure 7.
Plots of the sorption kinetics (a), pseudo-first order kinetics (b), and pseudo-second order kinetics (c) for both pesticides.
Figure 7.
Plots of the sorption kinetics (a), pseudo-first order kinetics (b), and pseudo-second order kinetics (c) for both pesticides.
Figure 8.
Sorption isotherms for 4-chlorophenoxyacetic acid (4-CPA) (upper) and 2,3,4,6-tetrachlorophenol (TCF) (lower).
Figure 8.
Sorption isotherms for 4-chlorophenoxyacetic acid (4-CPA) (upper) and 2,3,4,6-tetrachlorophenol (TCF) (lower).
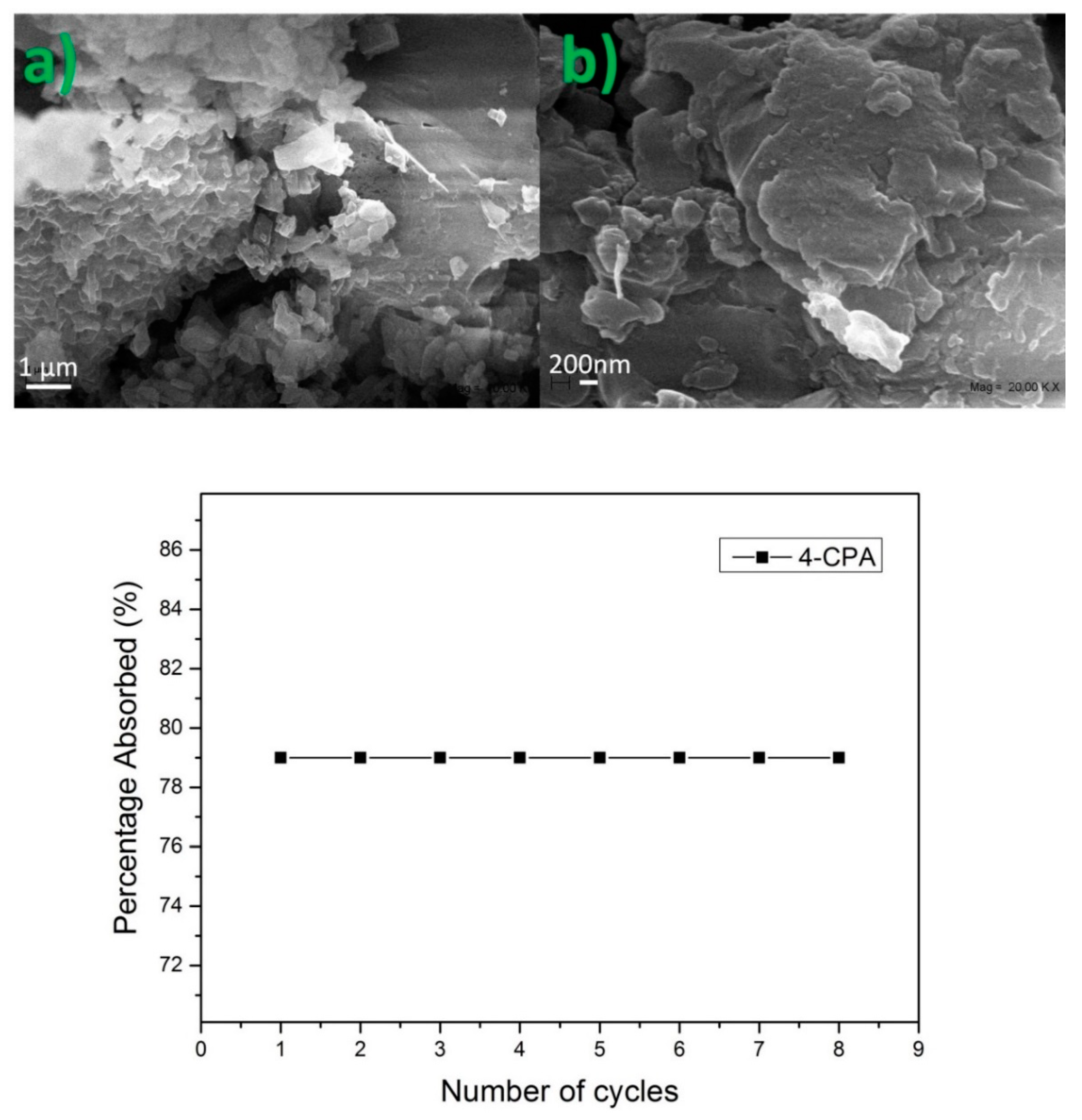
Figure 9.
SEM images of nanosponges (NS) after a repeated number of cycles (a,b). Percentage of 4-chlorophenoxyacetic acid (4-CPA) adsorbed after a repeated number of cycles.
Figure 9.
SEM images of nanosponges (NS) after a repeated number of cycles (a,b). Percentage of 4-chlorophenoxyacetic acid (4-CPA) adsorbed after a repeated number of cycles.
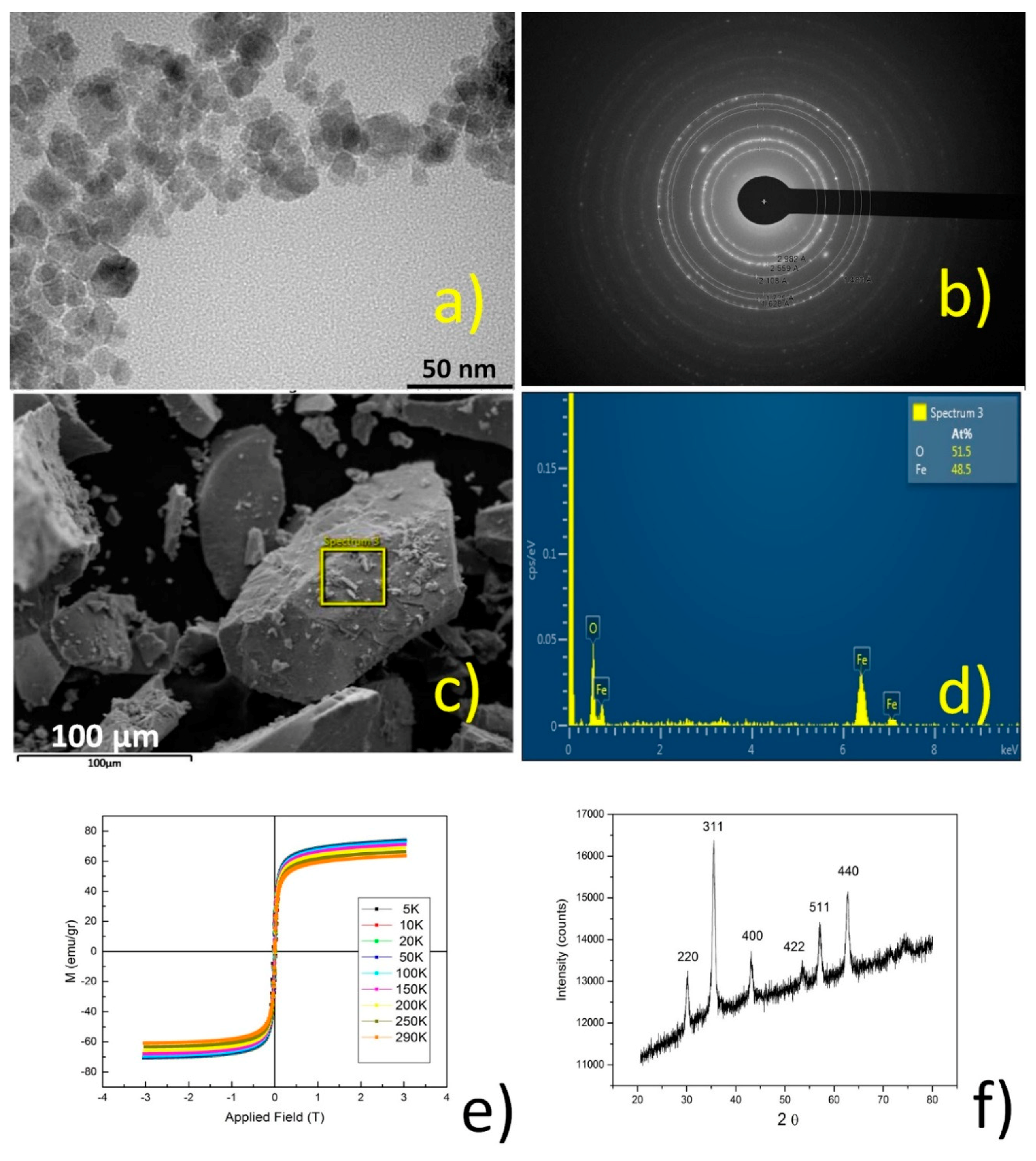
Figure 10.
TEM (a); selected area electron diffraction (SAED) (b); SEM (c); EDS (d); magnetization saturation (VSM) (e); and XRPD (f) analysis for Fe3O4 nanoparticles.
Figure 10.
TEM (a); selected area electron diffraction (SAED) (b); SEM (c); EDS (d); magnetization saturation (VSM) (e); and XRPD (f) analysis for Fe3O4 nanoparticles.
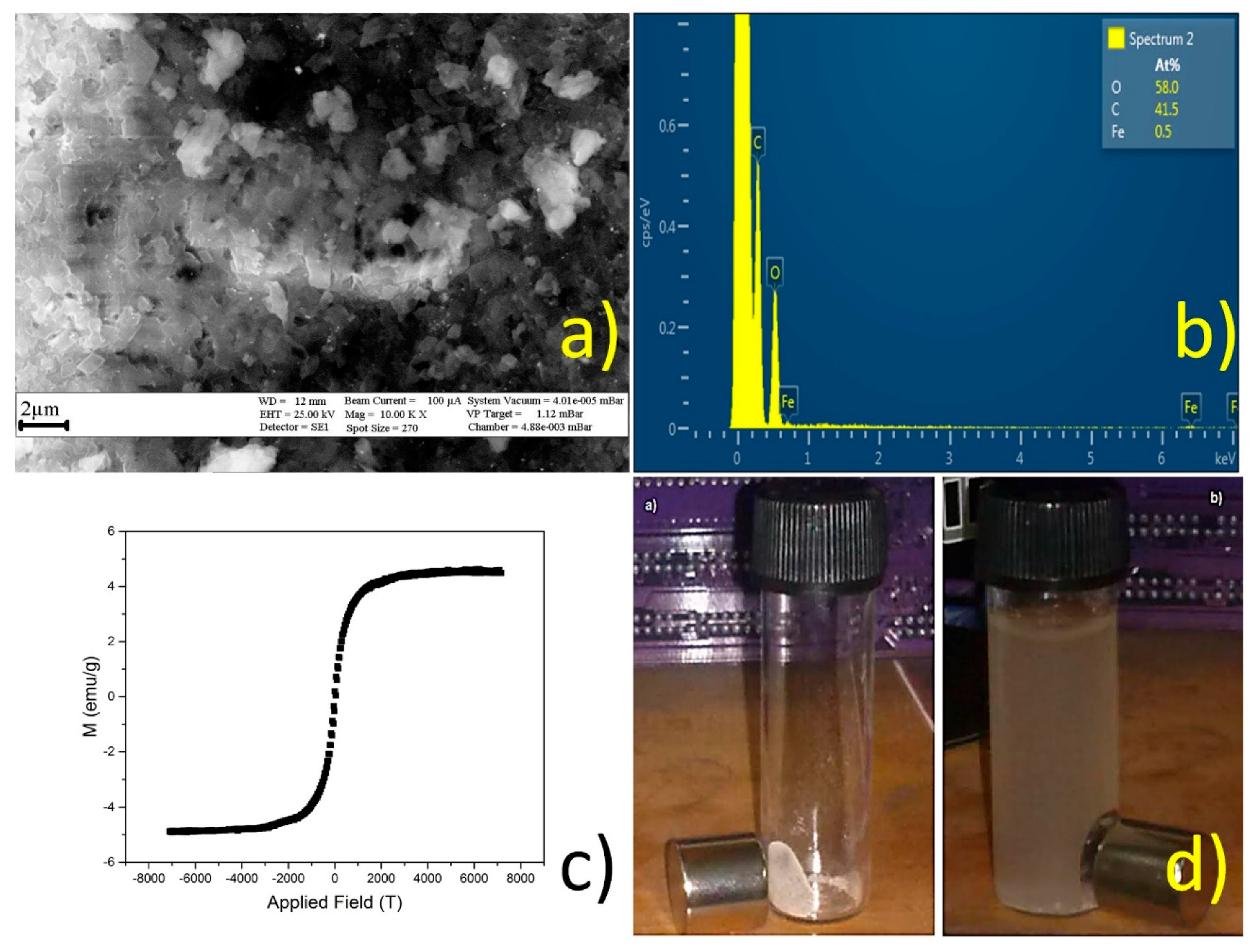
Figure 11.
SEM (a); EDS (b); VSM (c); and magnetic response of nanosponges (NS) decorated with magnetite nanoparticles (d).
Figure 11.
SEM (a); EDS (b); VSM (c); and magnetic response of nanosponges (NS) decorated with magnetite nanoparticles (d).
Figure 12.
UV–VIS spectra for 4-chlorophenoxyacetic acid (4-CPA) (upper) and 2,3,4,6-tetrachlorophenol (TCF) (lower) after contact with NS decorated with magnetite.
Figure 12.
UV–VIS spectra for 4-chlorophenoxyacetic acid (4-CPA) (upper) and 2,3,4,6-tetrachlorophenol (TCF) (lower) after contact with NS decorated with magnetite.
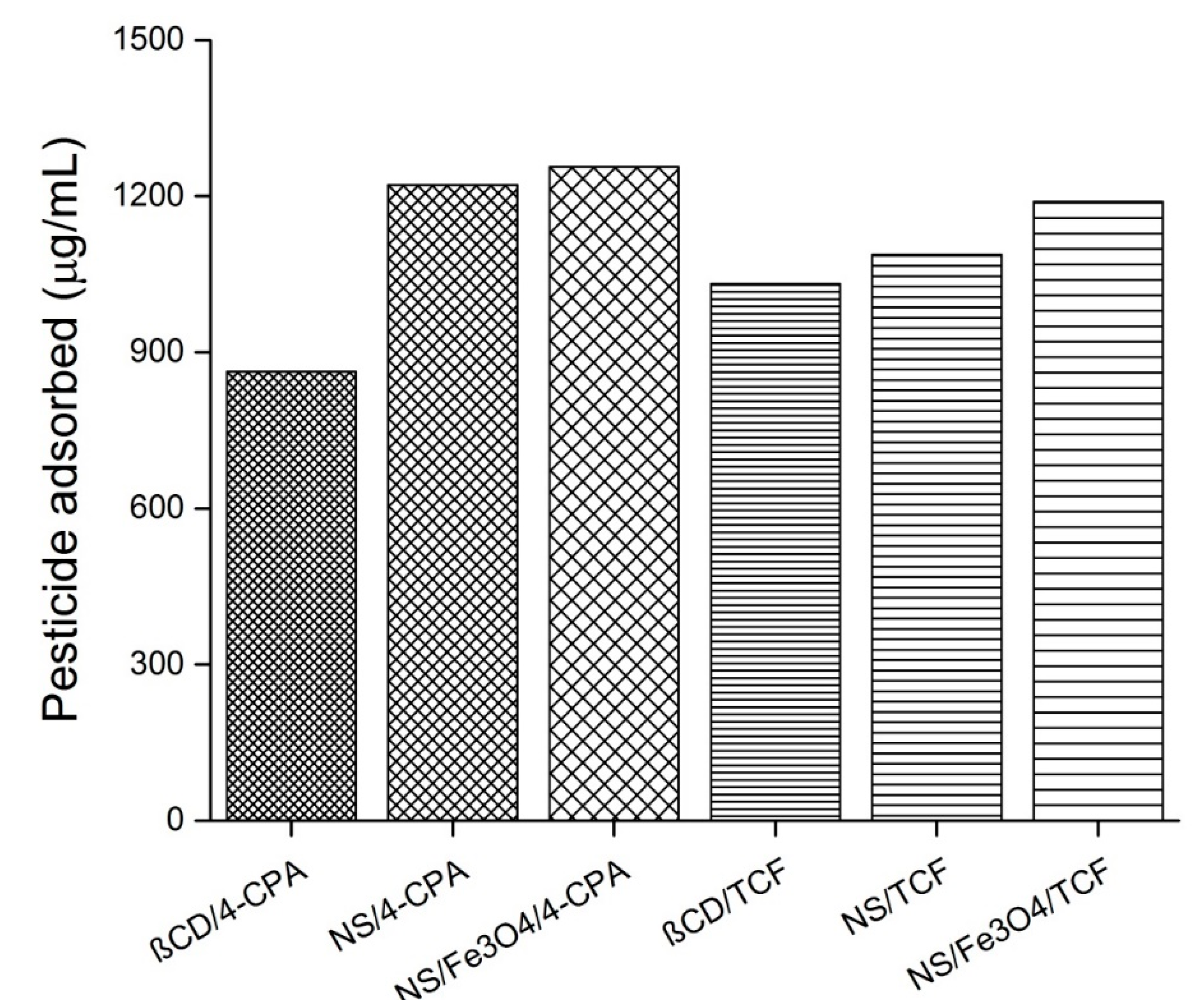
Figure 13.
Amount of pesticide adsorbed with β-Cyclodextrin (β-CD), nanosponges (NS), and Fe3O4/NS. Other abbreviations: 4-chlorophenoxyacetic acid (4-CPA), and 2,3,4,6-tetrachlorophenol (TCF).
Figure 13.
Amount of pesticide adsorbed with β-Cyclodextrin (β-CD), nanosponges (NS), and Fe3O4/NS. Other abbreviations: 4-chlorophenoxyacetic acid (4-CPA), and 2,3,4,6-tetrachlorophenol (TCF).
Table 1.
1H-NMR assignments and chemical shifts for pesticides and the nanosponge (NS)—4-chlorophenoxyacetic acid (4-CPA) complex.
Table 1.
1H-NMR assignments and chemical shifts for pesticides and the nanosponge (NS)—4-chlorophenoxyacetic acid (4-CPA) complex.
| H 4-CPA | δ 4-CPA (ppm) | δ NS–4-CPA (ppm) | Δδ (ppm) |
| OH’ | 13.035 | 12.926 | −0.106 |
| H’ 2 | 7.322 | 7.319 | −0.002 |
| H’ 3 | 6.937 | 6.934 | −0.004 |
| H’ 4 | 4.682 | 4.677 | −0.004 |
| H NS | δ NS (ppm) | δ NS–4-CPA (ppm) | Δδ (ppm) |
| OH 2 | 5.704 | 5.716 | 0.012 |
| OH 3 | 5.670 | 5.670 | 0.001 |
| OH 6 | 4.440 | 4.447 | 0.006 |
| H 1 | 4.827 | 4.829 | 0.002 |
| H 3 | 3.627 | 3.636 | 0.009 |
| H 5 | 3.572 | 3.636 | 0.003 |
| H 6 | 3.572 | 3.574 | 0.002 |
Table 2.
1H-NMR assignments and chemical shifts for pesticides and NS–2,3,4,6-tetrachlorophenol (TCF) complex.
Table 2.
1H-NMR assignments and chemical shifts for pesticides and NS–2,3,4,6-tetrachlorophenol (TCF) complex.
| H TCF | δ TCF (ppm) | δ NS–TCF (ppm) | Δδ (ppm) |
| H 1 | 7.215 | 7.136 | −0.079 |
| H 2 | 5.948 | 5.678 | −0.270 |
| H NS | δ NS (ppm) | δ NS–TCF (ppm) | Δδ (ppm) |
| OH 2 | 5.704 | 5.704 | 0.000 |
| OH 3 | 5.670 | 5.667 | 0.003 |
| OH 6 | 4.440 | 4.441 | −0.001 |
| H 1 | 4.827 | 4.825 | 0.002 |
| H 3 | 3.627 | 3.625 | 0.002 |
| H 5 | 3.572 | 3.570 | 0.002 |
| H 6 | 3.655 | 3.653 | 0.002 |
Table 3.
Molar attenuation for 4-chlorophenoxyacetic acid (4-CPA) and 2,3,4,6-tetrachlorophenol (TCF).
Table 3.
Molar attenuation for 4-chlorophenoxyacetic acid (4-CPA) and 2,3,4,6-tetrachlorophenol (TCF).
| Pesticide | ε (mM/cm) |
|---|
| 4-CPA | 10.10 |
| TCF | 8.27 |
Table 4.
Ce and Qe values for both pesticides and at different contact times.
Table 4.
Ce and Qe values for both pesticides and at different contact times.
| Pesticide | Contact Time (Min) | Ce (mM) | Qe (mmol/g) | Uptake |
|---|
| 4-CPA | 30 | 5.33 × 10−3 | 1.63 × 10−3 | 47% |
| 4-CPA | 60 | 3.41 × 10−3 | 2.33 × 10−3 | 66% |
| 4-CPA | 180 | 2.21 × 10−3 | 2.73 × 10−3 | 78% |
| 4-CPA | 300 | 1.53 × 10−3 | 2.95 × 10−3 | 85% |
| 4-CPA | 360 | 9.03 × 10−4 | 3.19 × 10−3 | 91% |
| TCF | 30 | 6.41 × 10−3 | 1.27 × 10−4 | 36% |
| TCF | 60 | 4.67 × 10−3 | 1.87 × 10−3 | 53% |
| TCF | 180 | 3.35 × 10−3 | 2.33 × 10−3 | 67% |
| TCF | 300 | 2.70 × 10−3 | 2.57 × 10−3 | 73% |
| TCF | 360 | 2.21 × 10−3 | 2.73 × 10−3 | 78% |
Table 5.
Ce and Qe values obtained for two sorbents: nanosponges (NS) and granular activated carbon (GAC).
Table 5.
Ce and Qe values obtained for two sorbents: nanosponges (NS) and granular activated carbon (GAC).
| Complex | Ce (mM) | Qe (mmol/g) | Uptake |
|---|
| GAC–4-CPA | 2.67 × 10−3 | 2.55 × 10−3 | 73% |
| GAC–TCF | 3.35 × 10−3 | 2.33 × 10−3 | 67% |
| NS–4-CPA | 9.03 × 10−4 | 3.19 × 10−3 | 91% |
| NS–TCF | 2.21 × 10−3 | 2.73 × 10−3 | 78% |
Table 6.
Values obtained for K1 and K2.
Table 6.
Values obtained for K1 and K2.
| Guest Molecule | Concentration (mg/L) | K1 (min−1) | K2 (mg/g min) | R2 (K1) | R2 (K2) |
|---|
| 4-CPA | 150 | 4.45 × 10−2 | 3.66 × 10−3 | 0.967 | 0.996 |
| TCF | 150 | 3.55 × 10−2 | 1.57 × 10−3 | 0.953 | 0.993 |
Table 7.
Qm values for both pesticides.
Table 7.
Qm values for both pesticides.
| Guest Molecule | Isotherm | ns | Qm (mmol/g) | r2 |
|---|
| 4-CPA | Sips | 0.78 | 0.671 | 0.965 |
| TCF | Sips | 0.81 | 0.077 | 0.966 |
Table 8.
Ce and Qe values for both pesticides and at different contact times with Fe3O4/NS.
Table 8.
Ce and Qe values for both pesticides and at different contact times with Fe3O4/NS.
| Pesticide | Contact Time (Min) | Ce (mM) | Qe (mmol/g) | Uptake |
|---|
| 4-CPA | 30 | 4.75 × 10−3 | 1.85 × 10−3 | 53% |
| 4-CPA | 60 | 2.81 × 10−3 | 2.53 × 10−3 | 71% |
| 4-CPA | 120 | 8.89 × 10−4 | 3.19 × 10−3 | 90% |
| TCF | 30 | 5.98 × 10−3 | 1.41 × 10−3 | 40% |
| TCF | 60 | 3.97 × 10−3 | 2,11 × 10−3 | 61% |
| TCF | 120 | 2.13 × 10−3 | 2.75 × 10−3 | 79% |