Sponge-Like Water De-/Ad-Sorption versus Solid-State Structural Transformation and Colour-Changing Behavior of an Entangled 3D Composite Supramolecuar Architecture, [Ni4(dpe)4(btc)2(Hbtc)(H2O)9]·3H2O
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Physical Techniques
2.2. Synthesis of [Ni4(dpe)4(btc)2(Hbtc)(H2O)9] 3H2O (1)
2.3. Crystallographic Data Collection and Refinements
2.4. In Situ X-ray Powder Diffraction
3. Results and Discussion
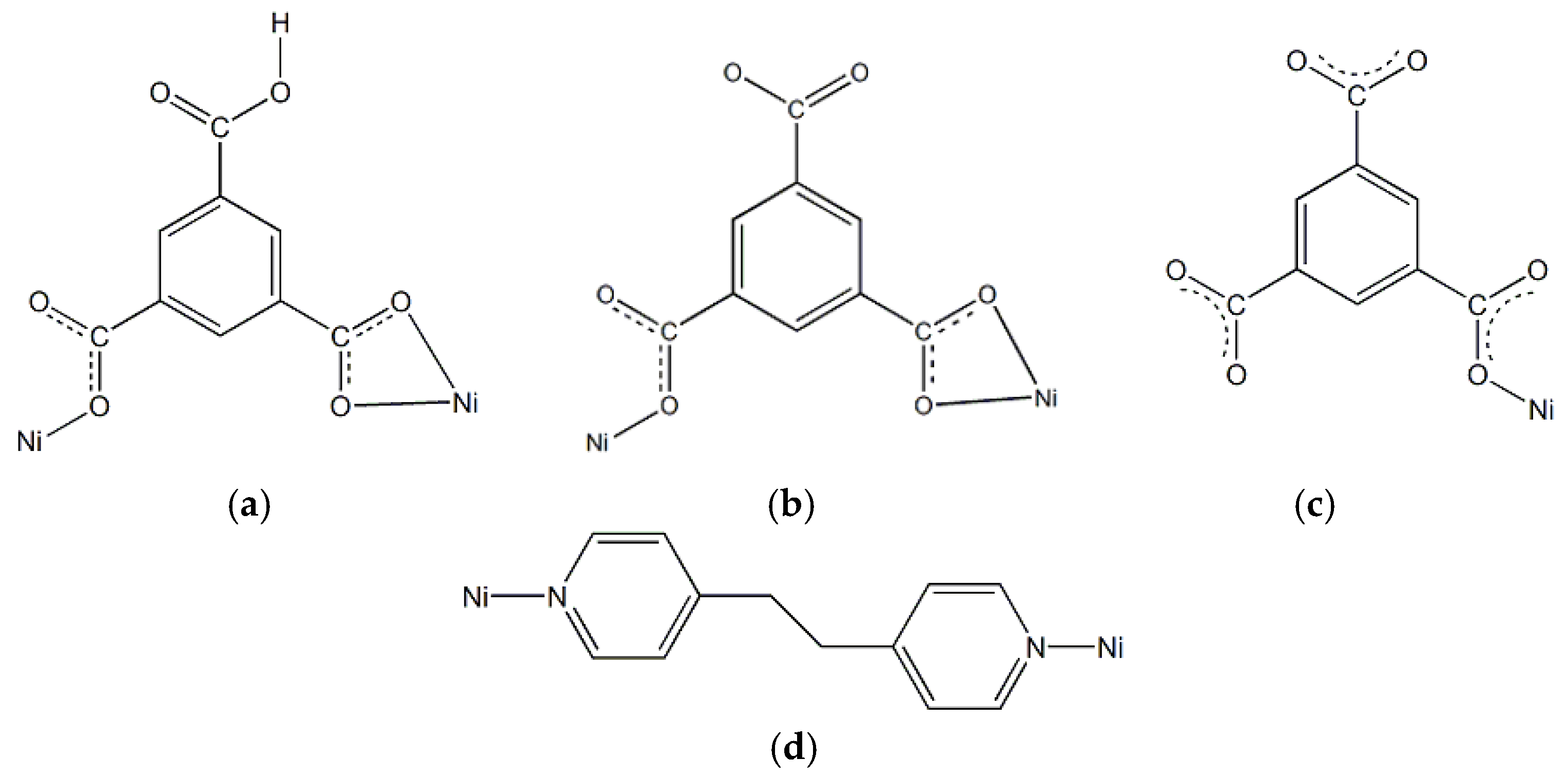
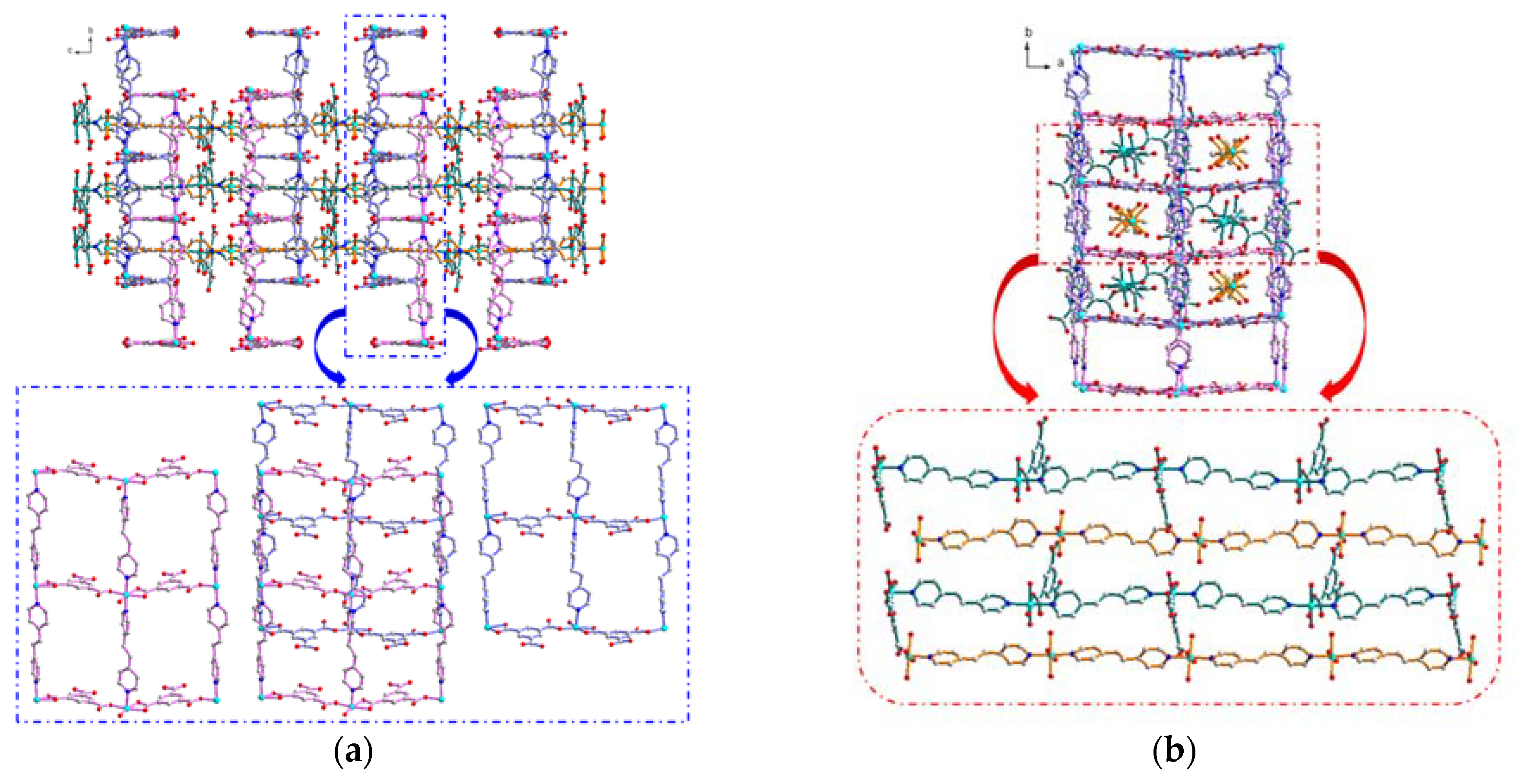
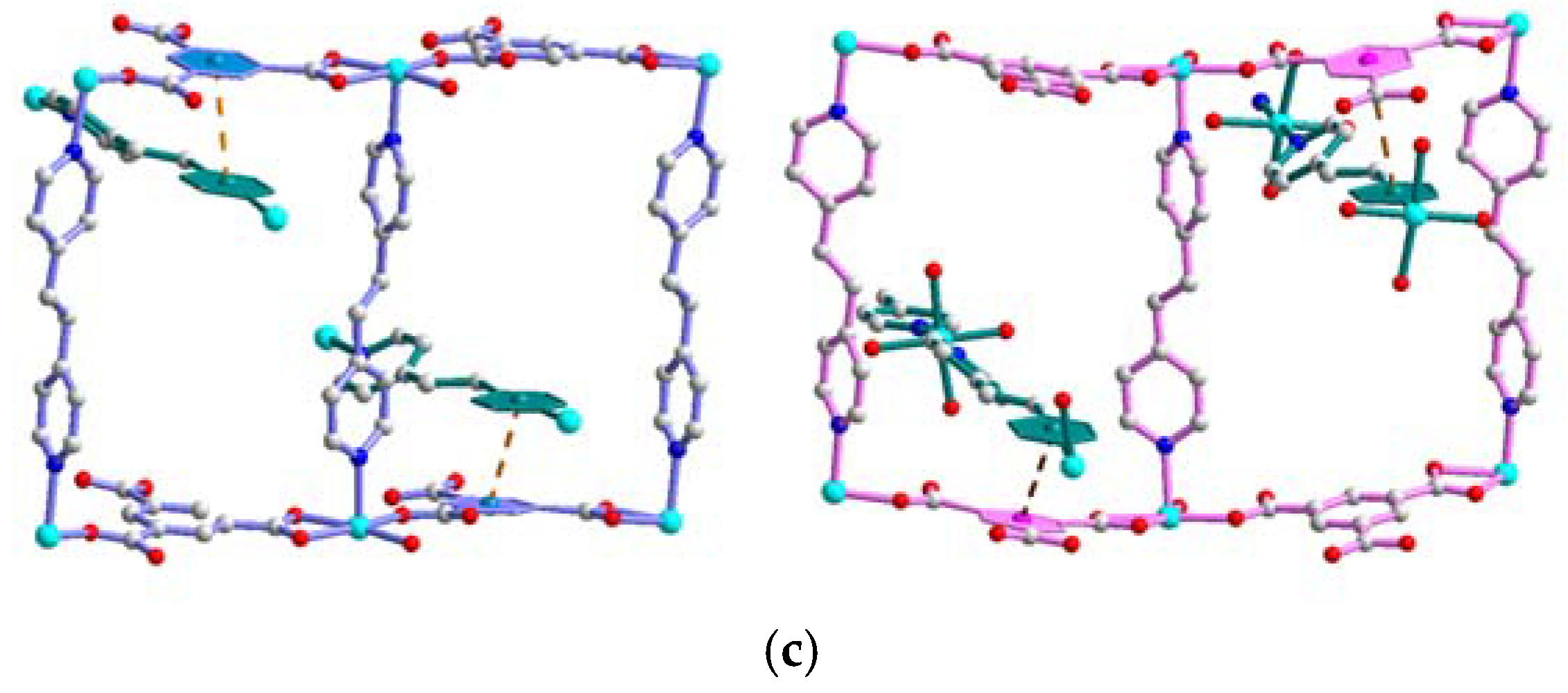
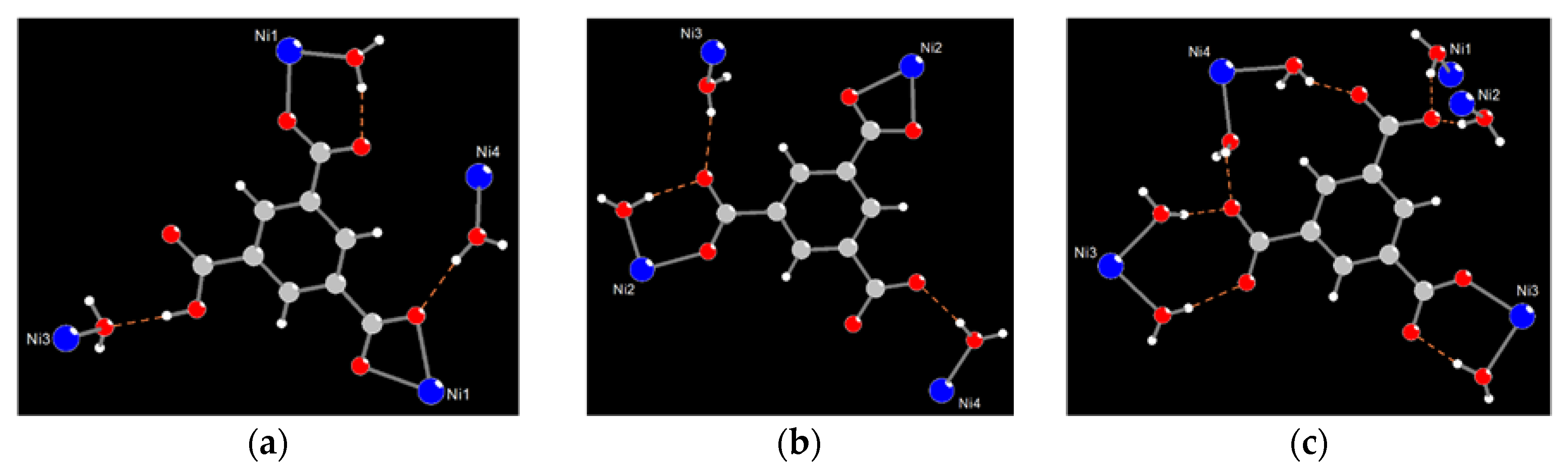
3.1. Synthesis and Structural Description of [Ni4(dpe)4(btc)2(Hbtc)(H2O)9]·3H2O (1)
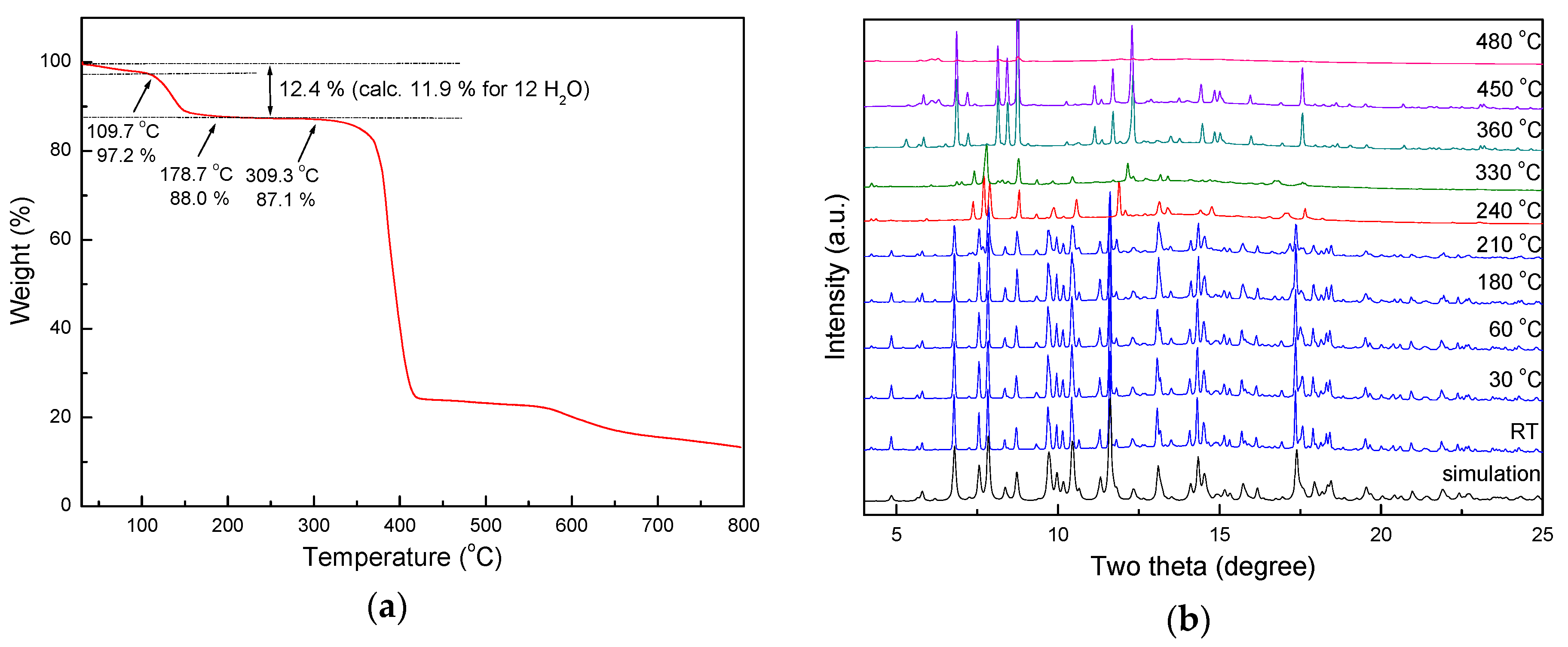
3.2. Thermal Stability of 1 by TG Analysis and In Situ Powder X-ray Diffraction Analyses
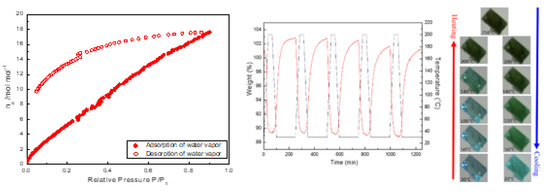
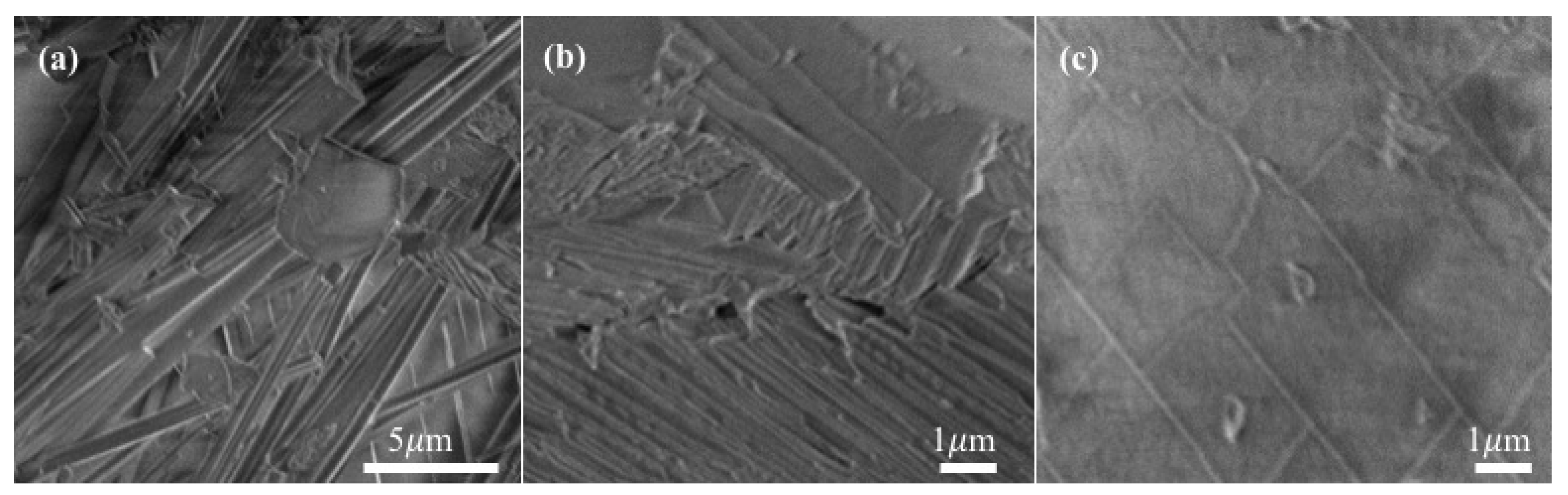
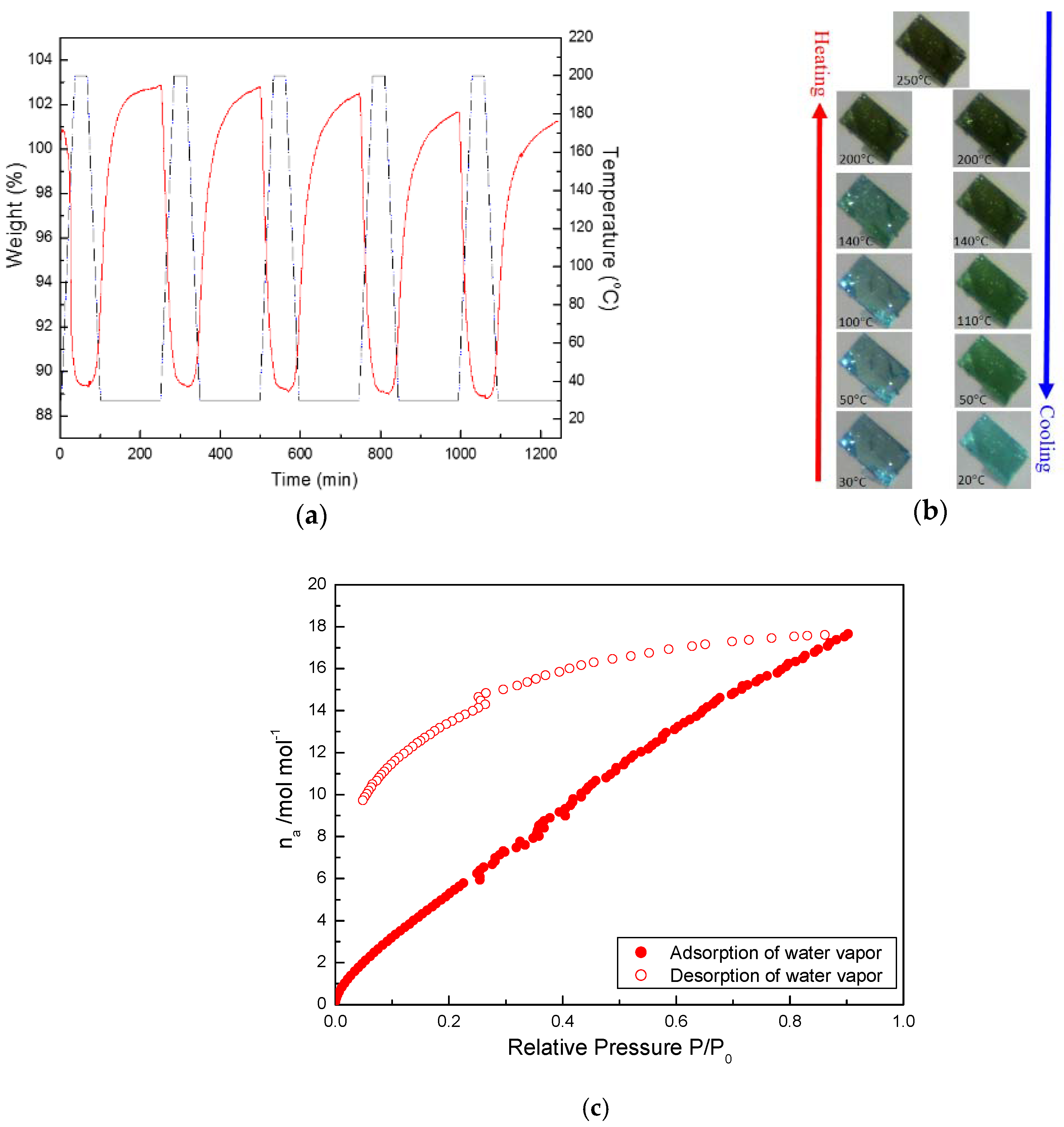
3.3. Reversible Water De-/Ad-Sorption Behavior Accompanying with Morphological Changes during the Thermal De/Re-Hydration Processes
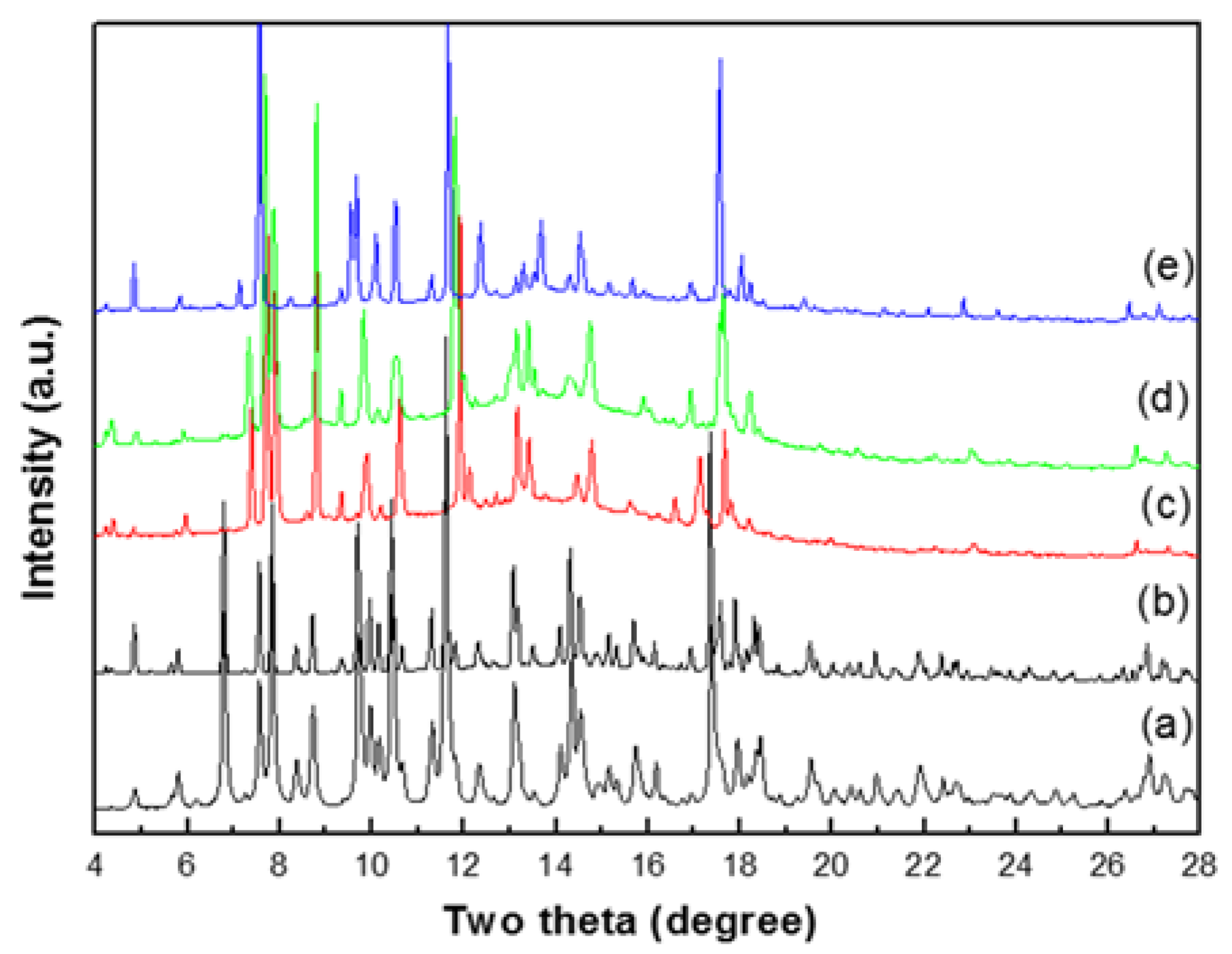
3.4. Solid-State Structural Transformation by Thermal De-/Re-Hydration Processes
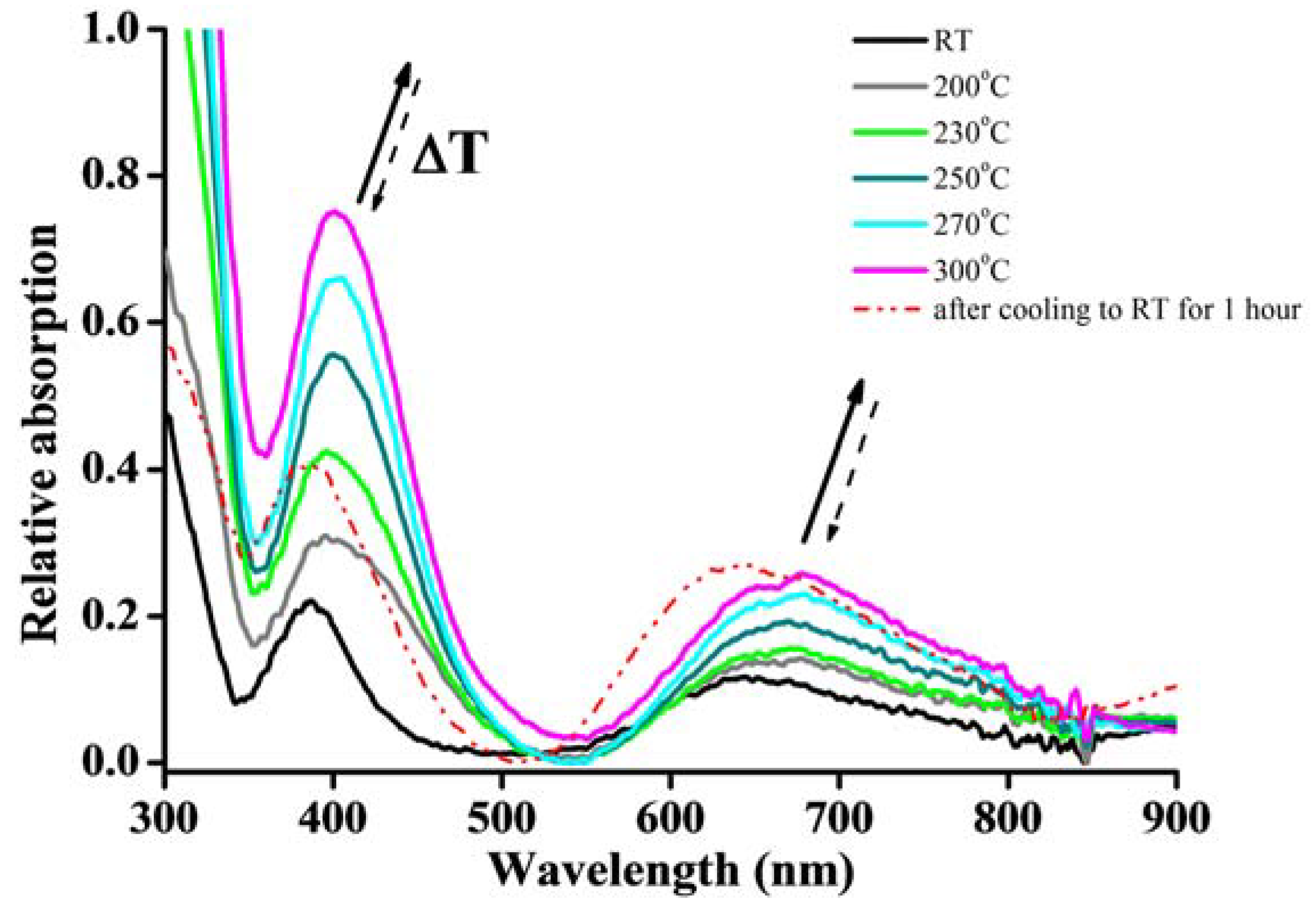
3.5. Colour-Changing Behavior and UV-vis Absorption Property of De-/Re-Hydration for Crystal 1
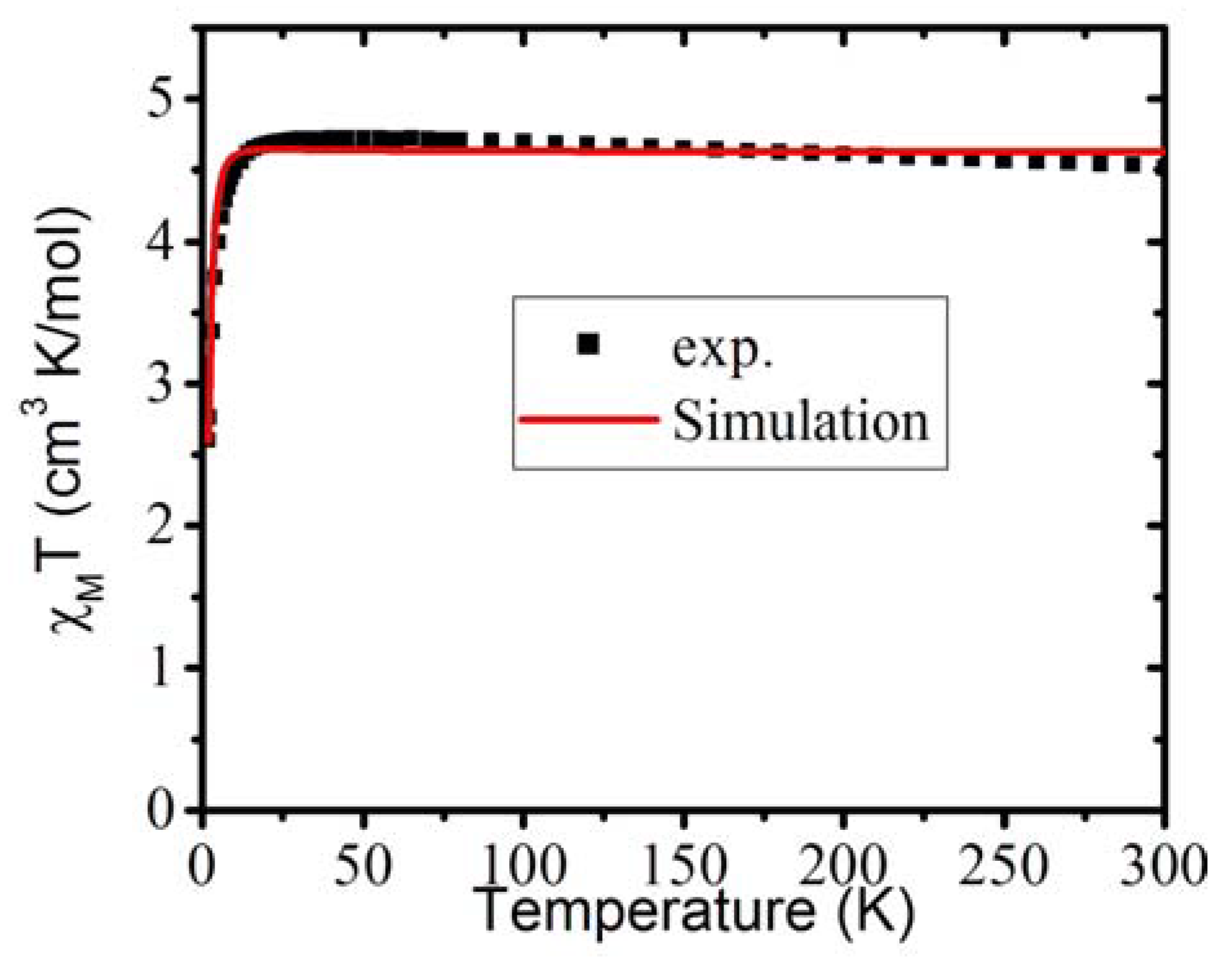
3.6. Magnetic Property of 1
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Batten, S.R.; Champness, N.R.; Chen, X.M.; Garcia-Martinez, J.; Kitagawa, S.; Öhrström, L.; O’keeffe, M.; Suh, M.P.; Reedijk, J. Terminology of metal–organic frameworks and coordination polymers. Pure Appl. Chem. 2013, 85, 1715–1724. [Google Scholar] [CrossRef]
- Batten, S.R.; Champness, N.R.; Chen, X.M.; Garcia-Martinez, J.; Kitagawa, S.; Öhrström, L.; O’keeffe, M.; Suh, M.P.; Reedijk, J. Coordination polymers, metal–organic frameworks and the need for terminology guidelines. CrystEngComm 2012, 14, 3001–3004. [Google Scholar] [CrossRef]
- O’Keeffe, M.; Peskov, M.A.; Ramsden, S.J.; Yaghi, O.M. The Reticular Chemistry Structure Resource (RCSR) Database of, and Symbols for, Crystal Nets. Acc. Chem. Res. 2008, 41, 1782–1789. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, S.; Mahata, P. Metal-organic framework structures—How closely are they related to classical inorganic structures? Chem. Soc. Rev. 2009, 38, 2304–2318. [Google Scholar] [CrossRef] [PubMed]
- Blatov, V.A.; O’Keeffe, M.; Proserpio, D.M. Vertex-, face-, point-, Schläfli-, and Delaney-symbols in nets, polyhedra and tilings: Recommended terminology. CrystEngComm 2010, 12, 44–48. [Google Scholar] [CrossRef]
- Alexandrov, E.V.; Blatov, V.A.; Kochetkov, A.V.; Proserpio, D.M. Underlying nets in three-periodic coordination polymers: Topology, taxonomy and prediction from a computer-aided analysis of the Cambridge Structural Database. CrystEngComm 2011, 13, 3947–3958. [Google Scholar] [CrossRef]
- Baburin, I.A.; Blatov, V.A.; Carlucci, L.; Ciani, G.; Proserpio, D.M. Interpenetrated Three-Dimensional Networks of Hydrogen-Bonded Organic Species: A Systematic Analysis of the Cambridge Structural Database. Cryst. Growth Des. 2008, 8, 519–539. [Google Scholar] [CrossRef]
- O’Keeffe, M.; Yaghi, O.M. Deconstructing the Crystal Structures of Metal–Organic Frameworks and Related Materials into Their Underlying Nets. Chem. Rev. 2012, 112, 675–702. [Google Scholar] [CrossRef] [PubMed]
- Long, J.R.; Yaghi, O.M. The pervasive chemistry of metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1213–1314. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.C.; Long, J.R.; Yaghi, O.M. Introduction to Metal–Organic Frameworks. Chem. Rev. 2012, 112, 673–674. [Google Scholar] [CrossRef] [PubMed]
- Batten, S.R.; Neville, S.M.; Turner, D.R. Coordination Polymers: Design, Analysis and Application; Royal Society of Chemistry: Cambridge, UK, 2009. [Google Scholar]
- Farrusseng, D. Metal–Organic Frameworks Applications from Catalysis to Gas Storage; Wiley: Weinheim, Germany, 2011. [Google Scholar]
- Takamizawa, S.; Akatsuka, T.; Ueda, T. Gas-Conforming Transformability of an Ionic Single-Crystal HostConsisting of Discrete Charged Components. Angew. Chem. Int. Ed. 2008, 47, 1689–1692. [Google Scholar] [CrossRef] [PubMed]
- Takamizawa, S.; Kohara, M.; Akatsuka, T.; Miyake, R. Gas-adsorbing ability of tris-ethylenediamine metal complexes (M = Co(III), Cr(III), Rh(III), Ir(III)) as transformable ionic single crystal hosts. New J. Chem. 2008, 32, 1782–1787. [Google Scholar] [CrossRef]
- Morsali, A.; Masoomi, M.Y. Structures and properties of mercury(II) coordination polymers. Coord. Chem. Rev. 2009, 253, 1882–1905. [Google Scholar] [CrossRef]
- Masoomi, M.Y.; Morsali, A. Applications of metal-organic coordination polymers as precursors for preparation of nano-materials Authors. Coord. Chem. Rev. 2012, 256, 2921–2943. [Google Scholar] [CrossRef]
- Masoomi, M.Y.; Morsali, A. Morphological study and potential applications of nano metal–organic coordination polymer. RSC Adv. 2013, 3, 19191–19218. [Google Scholar] [CrossRef]
- Aslani, A.; Morsali, A.; Zeller, M. Dynamic crystal-to-crystal conversion of a 3D–3D coordination polymer by de- and re-hydration. Dalton Trans. 2008, 5173–5177. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.H.; Li, J.Y.; Chen, Q.; Han, Y.D.; Chang, Z.; Song, W.C.; Bu, X.H. [Co(en)3]1/3[In(ox)2] · 3.5H2O: A zeolitic metal-organic framework templated by Co(en)3Cl3. Microporous Mesoporous Mater. 2010, 132, 453–457. [Google Scholar] [CrossRef]
- Pan, Q.H.; Chen, Q.; Song, W.C.; Hu, T.L.; Bu, X.H. Template-directed synthesis of three new open-framework metal(II) oxalates using Co(III) complex as template. CrystEngComm 2010, 12, 4198–4204. [Google Scholar] [CrossRef]
- Bertani, R.; Sgarbossa, P.; Venzo, A.; Lelj, F.; Amati, M.; Resnati, G.; Pilati, T.; Metrangolo, P.; Terraneo, G. Halogen bonding in metal–organic–supramolecular networks. Coord. Chem. Rev. 2010, 254, 677–695. [Google Scholar] [CrossRef]
- Ingleson, M.J.; Bacsa, J.; Rosseinsky, M.J. Homochiral H-bonded proline based metal organic frameworks. Chem. Commun. 2007, 3036–3038. [Google Scholar] [CrossRef] [PubMed]
- Desiraju, G.R. C–H⋯O and other weak hydrogen bonds. From crystal engineering to virtual screening. Chem. Commun. 2005, 2995–3001. [Google Scholar] [CrossRef] [PubMed]
- García-Báez, E.V.; Martínez-Martínez, F.J.; Höpfl, H.; Padilla-Martínez, I.I. π-Stacking Interactions and CH···X (X = O, Aryl) Hydrogen Bonding as Directing Features of the Supramolecular Self-Association in 3-Carboxy and 3-Amido Coumarin Derivatives. Cryst. Growth Des. 2003, 3, 35–45. [Google Scholar] [CrossRef]
- Janiak, C. A critical account on π–π stacking in metal complexes with aromatic nitrogen-containing ligands. Dalton Trans. 2000, 3885–3896. [Google Scholar] [CrossRef]
- Claessens, C.G.; Stoddart, J.F. π–π INTERACTIONS IN SELF-ASSEMBLY. J. Phys. Org. Chem. 1997, 10, 254–272. [Google Scholar] [CrossRef]
- Guo, H.D.; Guo, X.M.; Batten, S.R.; Song, J.F.; Song, S.Y.; Dang, S.; Zhang, G.L.; Tang, J.K.; Zhang, H.J. Hydrothermal Synthesis, Structures, and Luminescent Properties of Seven d10 Metal–Organic Frameworks Based on 9,9-Dipropylfluorene-2,7-Dicarboxylic Acid (H2DFDA). Cryst. Growth Des. 2009, 9, 1394–1401. [Google Scholar] [CrossRef]
- Reger, D.L.; Horger, J.J.; Smith, M.D.; Long, G.J.; Grandjean, F. Homochiral, Helical Supramolecular Metal–Organic Frameworks Organized by Strong π···π Stacking Interactions: Single-Crystal to Single-Crystal Transformations in Closely Packed Solids. Inorg. Chem. 2011, 50, 686–704. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.C.; Ge, Y.Y.; Jia, H.Y.; Li, S.S.; Sun, F.; Zhang, L.G.; Cai, Y.P. Construction of three high-dimensional supramolecular networks from temperature-driven conformational isomers. CrystEngComm 2011, 13, 67–71. [Google Scholar] [CrossRef]
- Batten, S.R.; Hoskins, B.F.; Robson, R. Interdigitation, Interpenetration and Intercalation in Layered Cuprous Tricyanomethanide Derivatives. Chem. Eur. J. 2000, 6, 156–161. [Google Scholar] [CrossRef]
- Batten, S.R.; Robson, R. Interpenetrating Nets: Ordered, Periodic Entanglement. Angew. Chem. Int. Ed. 1998, 37, 1460–1494. [Google Scholar] [CrossRef]
- Wang, X.L.; Qin, C.; Wang, E.B.; Li, Y.G.; Su, Z.M.; Xu, L.; Carlucci, L. Entangled Coordination Networks with Inherent Features of Polycatenation, Polythreading, and Polyknotting. Angew. Chem. Int. Ed. 2005, 44, 5824–5827. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Liu, H.Y.; Liu, Y.Y.; Yang, J.; Liu, B.; Ma, J.F. An unprecedented 2D → 3D metal–organic polyrotaxane framework constructed from cadmium and a flexible star-like ligand. Chem. Commun. 2011, 47, 1818–1820. [Google Scholar] [CrossRef] [PubMed]
- Baburin, I.A.; Blatov, V.A.; Carlucci, L.; Ciani, G.; Proserpio, D.M. Interpenetrating metal-organic and inorganic 3D networks: A computer-aided systematic investigation. Part II [1]. Analysis of the Inorganic Crystal Structure Database (ICSD). J. Solid State Chem. 2005, 178, 2452–2474. [Google Scholar] [CrossRef]
- Carlucci, L.; Ciani, G.; Proserpio, D.M. Borromean links and other non-conventional links in ‘polycatenated’ coordination polymers: Re-examination of some puzzling networks. CrystEngComm 2003, 5, 269–279. [Google Scholar] [CrossRef]
- Batten, S.R.; Harris, A.R.; Jensen, P.; Murray, K.S.; Ziebell, A. Copper(I) dicyanamide coordination polymers: Ladders, sheets, layers, diamond-like networks and unusual interpenetration. J. Chem. Soc. Dalton Trans. 2000, 3829–3836. [Google Scholar] [CrossRef]
- Chuang, Y.C.; Ho, W.L.; Sheu, C.F.; Lee, G.H.; Wang, Y. Crystal engineering from a 1D chain to a 3D coordination polymer accompanied by a dramatic change in magnetic properties. Chem. Commun. 2012, 48, 10769–10771. [Google Scholar] [CrossRef] [PubMed]
- Zaman, M.B.; Smith, M.D.; Zur Loye, H.C. Two different one-dimensional structural motifs in the same coordination polymer: A novel interpenetration of infinite ladders by bundles of infinite chains. Chem. Commun. 2001, 2256–2257. [Google Scholar] [CrossRef]
- Mahmoudi, G.; Morsali, A. Novel rare case of 2D + 1D = 2D polycatenation Hg(II) coordination polymer. CrystEngComm 2009, 11, 50–51. [Google Scholar] [CrossRef]
- Hagrman, D.; Hammond, R.P.; Haushalter, R.; Zubieta, J. Organic/Inorganic Composite Materials: Hydrothermal Syntheses and Structures of the One-, Two-, and Three-Dimensional Copper(II) Sulfate–Organodiamine Phases [Cu(H2O)3(4,4′-bipyridine)(SO4)]·2H2O, [Cu(bpe)2][Cu(bpe)(H2O)2(SO4)2]·2H2O, and [Cu(bpe)(H2O)(SO4)] (bpe = trans-1,2-Bis(4-pyridyl)ethylene). Chem. Mater. 1998, 10, 2091–2100. [Google Scholar]
- Du, M.; Jiang, X.J.; Zhao, X.J. Direction of unusual mixed-ligand metal–organic frameworks: A new type of 3-D polythreading involving 1-D and 2-D structural motifs and a 2-fold interpenetrating porous network. Chem. Commun. 2005, 5521–5523. [Google Scholar] [CrossRef] [PubMed]
- Carlucci, L.; Ciani, G.; Moret, M.; Proserpio, D.M.; Rizzato, S. Polymeric Layers Catenated by Ribbons of Rings in a Three-Dimensional Self-Assembled Architecture: A Nanoporous Network with Spongelike Behavior. Angew. Chem. Int. Ed. 2000, 39, 1506–1510. [Google Scholar] [CrossRef]
- Li, B.L.; Peng, Y.F.; Li, B.Z.; Zhang, Y. Supramolecular isomers in the same crystal: A new type of entanglement involving ribbons of rings and 2D (4,4) networks polycatenated in a 3D architecture. Chem. Commun. 2005, 2333–2335. [Google Scholar] [CrossRef] [PubMed]
- Biradha, K.; Fujita, M. A ‘three-in-one’ crystal of coordination networks. Chem. Commun. 2002, 1866–1867. [Google Scholar] [CrossRef]
- Wang, C.C.; Chung, W.C.; Lin, H.W.; Dai, S.C.; Shiu, J.S.; Lee, G.H.; Sheu, H.S.; Lee, W. Assembly of two Zinc(II)-squarate coordination polymers with noncovalent and covalent bonds derived from flexible ligands, 1,2-bis(4-pyridyl)ethane (dpe). CrystEngComm 2011, 13, 2130–2136. [Google Scholar] [CrossRef]
- Zheng, B.; Bai, J. An unprecedented nanoscale trilayered polythreading coordination array hierarchically formed from 2D square grid networks and induced by protonated 1,2-bis(4-pyridyl)ethane. CrystEngComm 2009, 11, 271–273. [Google Scholar] [CrossRef]
- Carlucci, L.; Ciani, G.; Proserpio, D.M. Three-dimensional architectures of intertwined planar coordination polymers: The first case of interpenetration involving two different bidimensional polymeric motifs. New J. Chem. 1998, 22, 1319–1321. [Google Scholar] [CrossRef]
- Shin, D.M.; Lee, I.S.; Chung, Y.K.; Lah, M.S. Coordination polymers based on square planar Co(II) node and linear spacer: Solvent-dependent pseudo-polymorphism and an unprecedented interpenetrating structure containing both 2D and 3D topological isomers. Chem. Commun. 2003, 1036–1037. [Google Scholar] [CrossRef]
- Ke, S.Y.; Chang, Y.F.; Wang, H.Y.; Yang, C.C.; Ni, C.W.; Lin, G.Y.; Chen, T.T.; Ho, M.L.; Lee, G.H.; Chuang, Y.C.; et al. Self-Assembly of Four Coordination Polymers in Three-Dimensional Entangled Architecture Showing Reversible Dynamic Solid-State Structural Transformation and Colour-Changing Behavior upon Thermal Dehydration and Rehydration. Cryst. Growth Des. 2014, 8, 4011–4018. [Google Scholar] [CrossRef]
- Majumder, A.; Shit, S.; Choudhury, C.R.; Batten, S.R.; Pilet, G.; Luneau, D.; Daro, N.; Sutter, J.P.; Chattopadhyay, N.; Mitra, S. Synthesis, structure and fluorescence of two novel manganese(II) and zinc(II)-1,3,5-benzene tricarboxylate coordination polymers: Extended 3D supramolecular architectures stabilised by hydrogen bonding. Inorg. Chim. Acta 2005, 358, 3855–3864. [Google Scholar] [CrossRef]
- Plater, M.J.; Foreman, M.R.; Coronado, E.; Gomez-Garcia, C.J.; Slawin, A.M.Z. Crystallisation of H3BTC, H3TPO or H2SDA with MII (M = Co, Mn or Zn) and 2,2′-bipyridyl: Design and control of co-ordination architecture, and magnetic properties (H3BTC = benzene-1,3,5-tricarboxylic acid, H3TPO = tris(4-carboxylphenyl)phosphine oxide, H2SDA = cis-stilbene-4,4′-dicarboxylic acid). J. Chem. Soc. Dalton Trans. 1999, 4209–4216. [Google Scholar] [CrossRef]
- Plater, M.J.; Foreman, M.R.; Howie, R.A.; Skakle, J.M.; Coronado, E.; Gómez-García, C.J.; Gelbrich, T.; Hursthouse, M.B. Synthesis and characterisation of polymeric metal-ion carboxylates from benzene-1,3,5-tricarboxylic acid with Mn(II), Co(II) or Zn(II) and 2,2-bipyridyl, phenanthroline or a pyridyl-2-(1-methyl-1H-pyrazol-3-yl) derivative. Inorg. Chim. Acta 2001, 319, 159–175. [Google Scholar] [CrossRef]
- Du, M.; Jiang, X.J.; Zhao, X.J. Controllable Assembly of Metal-Directed Coordination Polymers under Diverse Conditions: A Case Study of the MII–H3tma/Bpt Mixed-Ligand System. Inorg. Chem. 2006, 45, 3998–4006. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.; Kim, D.; Shin, S.; Moon, D.; Cho, S.J.; Lah, M.S. Combinational Synthetic Approaches for Isoreticular and Polymorphic Metal–Organic Frameworks with Tuned Pore Geometries and Surface Properties. Chem. Mater. 2014, 26, 1711–1719. [Google Scholar] [CrossRef]
- Jeong, S.; Kim, D.; Song, X.; Choi, M.; Park, N.; Lah, M.S. Postsynthetic Exchanges of the Pillaring Ligand in Three-Dimensional Metal–Organic Frameworks. Chem. Mater. 2013, 25, 1047–1054. [Google Scholar] [CrossRef]
- Hu, J.S.; Huang, L.F.; Yao, X.Q.; Qin, L.; Li, Y.Z.; Guo, Z.J.; Zheng, H.G.; Xue, Z.L. Six New Metal–Organic Frameworks Based on Polycarboxylate Acids and V-shaped Imidazole-Based Synthon: Syntheses, Crystal Structures, and Properties. Inorg. Chem. 2011, 50, 2404–2414. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.Y.; Liu, S.X.; Xie, L.H.; Sun, C.Y.; Cao, J.F.; Ren, Y.H.; Feng, D.; Su, Z.M. Rational design microporous pillared-layer frameworks: Syntheses, structures and gas sorption properties. CrystEngComm 2009, 11, 177–182. [Google Scholar] [CrossRef]
- Gao, C.Y.; Liu, S.X.; Xie, L.H.; Ren, Y.H.; Cao, J.F.; Sun, C.Y. Design and construction of a microporous metal–organic framework based on the pillared-layer motif. CrystEngComm 2007, 9, 545–547. [Google Scholar] [CrossRef]
- Prior, T.J.; Rosseinsky, M.J. A dense coordination polymer bearing an extensive and highly intricate hydrogen bonding array. Chem. Commun. 2001, 1222–1223. [Google Scholar] [CrossRef]
- Prior, T.J.; Rosseinsky, M.J. A porous one-dimensional coordination polymer composed of edge-shared hexagonal supramolecular units. CrystEngComm 2000, 2, 128–133. [Google Scholar] [CrossRef]
- Wang, P.S.; Moorefield, C.N.; Panzer, M.; Newkome, G.R. Helical and polymeric nanostructures assembled from benzene tri- and tetracarboxylic acids associated with terpyridine copper(II) complexes. Chem. Commun. 2005, 465–467. [Google Scholar] [CrossRef] [PubMed]
- Pech, R.; Pickardt, J. catena-Triaqua-[mu]-[1,3,5-benzenetricarboxylato(2-)]-copper(II). Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1988, 44, 992–994. [Google Scholar] [CrossRef]
- Wang, Q.; Wu, M.J.; Wang, X.G.; Zhao, X.J. catena-Poly[[[aqua(5-amino-1H-1,2,4-triazole-κN4)zinc(II)]-μ-5-carboxybenzene-1,3-dicarboxylato] dihydrate]. Acta Crystallogr. 2006, 62, m2496–m2498. [Google Scholar]
- Zhou, Y.F.; Lou, B.Y.; Yuan, D.Q.; Xu, Y.Q.; Jiang, F.L.; Hong, M.C. pH-value-controlled assembly of photoluminescent zinc coordination polymers. Inorg. Chim. Acta 2005, 358, 3057–3064. [Google Scholar] [CrossRef]
- Yaghi, O.M.; Li, G.M.; Li, H.L. Crystal Growth of Extended Solids by Nonaqueous Gel Diffusion. Chem. Mater. 1997, 9, 1074–1076. [Google Scholar] [CrossRef]
- Shi, X.; Zhu, G.S.; Fang, Q.R.; Wu, G.; Tian, G.; Wang, R.W.; Zhang, D.L.; Xue, M.; Qiu, S.L. Novel Supramolecular Frameworks Self-Assembled from One-Dimensional Polymeric Coordination Chains. Eur. J. Inorg. Chem. 2004, 2004, 185–191. [Google Scholar] [CrossRef]
- Riou-Cavellec, M.; Albinet, C.; Greneche, J.M.; Ferey, G. Study of the iron/trimesic acid system for the hydrothermal synthesis of hybrid materials. J. Mater. Chem. 2001, 11, 3166–3171. [Google Scholar] [CrossRef]
- Ding, B.B.; Weng, Y.Q.; Mao, Z.W.; Lam, C.K.; Chen, X.M.; Ye, B.H. Pillared-Layer Microporous Metal–Organic Frameworks Constructed by Robust Hydrogen Bonds. Synthesis, Characterization, and Magnetic and Adsorption Properties of 2,2′-Biimidazole and Carboxylate Complexes. Inorg. Chem. 2005, 44, 8836–8845. [Google Scholar] [CrossRef] [PubMed]
- Yaghi, O.M.; Li, H.L.; Groy, T.L. Construction of Porous Solids from Hydrogen-Bonded Metal Complexes of 1,3,5-Benzenetricarboxylic Acid. J. Am. Chem. Soc. 1996, 118, 9096–9101. [Google Scholar] [CrossRef]
- Yaghi, O.M.; Li, G.; Li, H.L. Selective binding and removal of guests in a microporous metal–organic framework. Nature 1995, 378, 703–706. [Google Scholar] [CrossRef]
- Daiguebonne, C.; Deluzet, A.; Camara, M.; Boubekeur, K.; Audebrand, N.; Gerault, Y.; Baux, C.; Guillou, O. Lanthanide-Based Molecular Materials: Gel Medium Induced Polymorphism. Cryst. Growth Des. 2003, 3, 1015–1020. [Google Scholar] [CrossRef]
- Choi, K.Y.; Chun, K.M.; Suh, I.H. Synthesis and characterization of one-dimensional nickel(II) macrocyclic complexes with bridging organic ligands. Polyhedron 2001, 20, 57–65. [Google Scholar] [CrossRef]
- Wang, X.; Le, M.; Lin, H.Y.; Luan, J.; Liu, G.C.; Sui, F.F.; Chang, Z.H. Assembly, structures, photophysical properties and photocatalytic activities of a series of coordination polymers constructed from semi-rigid bis-pyridyl-bis-amide and benzenetricarboxylic acid. Inorg. Chem. Front. 2015, 2, 373–387. [Google Scholar] [CrossRef]
- Tomar, K.; Rajak, R.; Sanda, S.; Konar, S. Synthesis and Characterization of Polyhedral-Based Metal–Organic Frameworks Using a Flexible Bipyrazole Ligand: Topological Analysis and Sorption Property Studies. Cryst. Growth Des. 2015, 15, 2732–2741. [Google Scholar] [CrossRef]
- Habib, H.A.; Sanchiz, J.; Janiak, C. Mixed-ligand coordination polymers from 1,2-bis(1,2,4-triazol-4-yl)ethane and benzene-1,3,5-tricarboxylate: Trinuclear nickel or zinc secondary building units for three-dimensional networks with crystal-to-crystal transformation upon dehydration. Dalton Trans. 2008, 1734–1744. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, P.; Prior, T.J.; Cussen, E.J.; Claridge, J.B.; Rosseinsky, M.J. Permanent Microporosity and Enantioselective Sorption in a Chiral Open Framework. J. Am. Chem. Soc. 2004, 126, 6106–6114. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Xiao, B.; Fletcher, A.J.; Thomas, K.M.; Bradshaw, D.; Rosseinsky, M.J. Hysteretic adsorption and desorption of hydrogen by nanoporous metal-organic frameworks. Science 2004, 306, 1012–1015. [Google Scholar] [CrossRef] [PubMed]
- Prior, T.J.; Bradshaw, D.; Teat, S.J.; Rosseinsky, M.J. Designed layer assembly: A three-dimensional framework with 74% extra-framework volume by connection of infinite two-dimensional sheets. Chem. Commun. 2003, 500–501. [Google Scholar] [CrossRef]
- Lu, T.B.; Xiang, H.; Luck, R.L.; Jiang, L.; Mao, Z.W.; Ji, L.N. [NiL]3[BTC]2·14H2O[L = 3,10-bis(2-ethyl)-1,3,5,8,10,12-hexaazacyclotetradecane, BTC = 1,3,5-benzenetricarboxylate]: Synthesis, structure and unique selective guest molecule absorption properties. New J. Chem. 2002, 26, 969–971. [Google Scholar] [CrossRef]
- Lu, T.B.; Xiang, H.; Luck, R.L.; Mao, Z.W.; Wang, D.; Chen, C.; Ji, L.N. A two-dimensional coordination polymer with a brick wall structure and hydrophobic channels: Synthesis and structure of a macrocyclic nickel(II) complex with 1,3,5-benzenetricarboxylate. CrystEngComm 2001, 3, 168–169. [Google Scholar] [CrossRef]
- Kepert, C.J.; Prior, T.J.; Rosseinsky, M.J. A Versatile Family of Interconvertible Microporous Chiral Molecular Frameworks: The First Example of Ligand Control of Network Chirality. J. Am. Chem. Soc. 2000, 122, 5158–5168. [Google Scholar] [CrossRef]
- Kepert, C.J.; Rosseinsky, M.J. A porous chiral framework of coordinated 1,3,5-benzenetricarboxylate: Quadruple interpenetration of the (10,3)-a network. Chem. Commun. 1998, 31–32. [Google Scholar] [CrossRef]
- Choi, H.J.; Suh, M.P. Self-Assembly of Molecular Brick Wall and Molecular Honeycomb from Nickel(II) Macrocycle and 1,3,5-Benzenetricarboxylate: Guest-Dependent Host Structures. J. Am. Chem. Soc. 1998, 120, 10622–10628. [Google Scholar] [CrossRef]
- Holmes, K.E.; Kelly, P.F.; Elsegood, M.R.J. Honeycombs, herringbones and brick-walls; three-fold guest-dependent variation in copper trimesate complexes bearing sulfimide ligands. Dalton Trans. 2004, 3488–3494. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.W.; Min, K.S.; Suh, M.P. A Hybrid Consisting of Coordination Polymer and Noncovalent Organic Networks: A Highly Ordered 2-D Phenol Network Assembled by Edge-to-Face π–π Interactions. Inorg. Chem. 2002, 41, 2151–2157. [Google Scholar] [CrossRef] [PubMed]
- Oshio, H.; Ichida, H. Control of Intramolecular Magnetic Interaction by the Spin Polarization of d.pi. Spin to p.pi. Orbital of an Organic Bridging Ligand Hiroki Oshio, and Hikaru Ichida. J. Phys. Chem. 1995, 99, 3294–3302. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, Y.B.; Chen, S.M.; Li, Z.J.; Cheng, J.K.; Yao, Y.G. A Polar Luminescent Zn Polymer Containing an Unusual Noninterpenetrated utp Net. Inorg. Chem. 2006, 45, 3161–3163. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Shi, X.; Fang, Q.R.; Tian, G.; Wang, L.F.; Zhu, G.S.; Addison, A.W.; Wei, Y.; Qiu, S.L. Triethylammonium benzene-1,3,5-tricarboxylato(pyridine)zinc(II): A two-dimensional undulating mesh network. Inorg. Chem. Commun. 2003, 6, 402–404. [Google Scholar] [CrossRef]
- Li, X.J.; Sun, D.F.; Cao, R.; Sun, Y.Q.; Wang, Y.Q.; Bi, W.H.; Gao, S.Y.; Hong, M.C. [Zn2(H2O)3(2,2′-bipy)2(btc)][Zn(H2O)(2,2′-bipy)(btc)]·8H2O: A novel zinc–carboxylate complex consisting of independently cationic and anionic chains. Inorg. Chem. Commun. 2003, 6, 908–911. [Google Scholar] [CrossRef]
- Smith, G.; Reddy, A.N.; Byriel, K.A.; Kennard, C.H.L. Preparation and crystal structures of the silver(I) carboxylates [Ag2{C6H4(CO2)2}(NH3)2], [NH4][Ag5{C6H3(CO2)3}2(NH3)2(H2O)2]·H2O and [NH4][Ag{C4H2N2(CO2)2}]. J. Chem. Soc. Dalton Trans. 1995, 3565–3570. [Google Scholar] [CrossRef]
- Gomez-Lor, B.; Gutierrez-Puebla, E.; Iglesias, M.; Monge, M.A.; Ruiz-Valero, C.; Snejko, N. Novel 2D and 3D Indium Metal-Organic Frameworks: Topology and Catalytic Properties. Chem. Mater. 2005, 17, 2568–2573. [Google Scholar] [CrossRef]
- SMART V 4.043 Software for CCD Detector System; Siemens Analytical Instruments Division: Madison, WI, USA, 1995.
- SAINT V 4.035 Software for CCD Detector System; Siemens Analytical Instruments Division: Madison, WI, USA, 1995.
- Sheldrick, G.M. Program for the Refinement of Crystal Structures; University of Göttingen: Göttingen, Germany, 1993. [Google Scholar]
- SHELXTL 5.03 (PC-Version), Program Liberary for Structure Solution and Molecular Graphics; Siemens Analytical Instruments Division: Madison, WI, USA, 1995.
- Hammersley, A.P.; Svensson, S.O.; Hanfland, M.; Fitch, A.N.; Häusermann, D. Two-dimensional detector software: From real detector to idealised image or two-theta scan. High Press. Res. 1996, 14, 235–248. [Google Scholar] [CrossRef]
- Wang, C.C.; Yang, C.C.; Yeh, C.T.; Cheng, K.Y.; Chang, P.C.; Ho, M.L.; Lee, G.H.; Shih, W.J.; Sheu, H.S. Reversible Solid-State Structural Transformation of a 1D–2D Coordination Polymer by Thermal De/Rehydration Processes. Inorg. Chem. 2011, 50, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Wang, Z.J.; Wang, T.; Zheng, H.G.; Chen, J.X. Coligand-directed synthesis of five Co(II)/Ni(II) coordination polymers with a neutral tetradentate ligand: Syntheses, crystal structures, and properties. Dalton Trans. 2014, 43, 12528–12535. [Google Scholar] [CrossRef] [PubMed]
- Taşdemir, E.; Özbek, F.E.; Sertçelik, M.; Hökelek, T.; Çelik, R.Ç.; Necefoğlu, H. Supramolecular complexes of Co(II), Ni(II) and Zn(II) p-hydroxybenzoates with caffeine: Synthesis, spectral characterization and crystal structure. J. Mol. Struct. 2016, 1119, 472–478. [Google Scholar] [CrossRef]
- Kharadi, G.J. Thermal and Microbicidal Study of Coordination Polymers: Transition Metal with 8HQMIT Ligand. Synth. React. Inorg. Met.-Org. Nano-Met. Chem. 2014, 44, 1001–1010. [Google Scholar] [CrossRef]
- Borras-Almenar, J.J.; Clemente-Juan, J.M.; Coronado, E.; Tsukerblat, B.S. High-nuclearity magnetic clusters: generalized spin Hamiltonian and its use for the calculation of the energy levels, bulk magnetic properties, and inelastic neutron scattering spectra. Inorg. Chem. 1999, 38, 6081–6088. [Google Scholar] [CrossRef] [PubMed]
- Borras-Almenar, J.J.; Clemente-Juan, J.M.; Coronado, E.; Tsukerblat, B.S. MAGPACK 1 A package to calculate the energy levels, bulk magnetic properties, and inelastic neutron scattering spectra of high nuclearity spin clusters. J. Comput. Chem. 2001, 22, 985–991. [Google Scholar] [CrossRef]
- Shin, J.W.; Kim, D.W.; Moon, D. Syntheses and characterization of various supramolecular compounds from the self-assembly of nickel (II) hexaaza macrocyclic complex with carboxylic acid derivatives. Polyhedron 2016, 105, 62–70. [Google Scholar] [CrossRef]
- Bi, J.H.; Li, M.; Yao, Z.L. Synthesis and Magnetic Properties of [Ni3(BTC)2(H2O)12] n (BTC= 1, 3, 5-Benzenetricarboxylate). Asian J. Chem. 2011, 23, 5181. [Google Scholar]
- Cortijo, M.; Herrero, S.; Jiménez-Aparicio, R.; Matesanz, E. Modulation of the Magnetic Properties of Two-Dimensional Compounds [NiX2 (N–N)] by Tailoring Their Crystal Structure. Inorg. Chem. 2013, 52, 7087–7093. [Google Scholar] [CrossRef] [PubMed]
| Empirical Formula | C75H82N8Ni4O30 | Formula Mass (g mol−1) | 1810.33 |
|---|---|---|---|
| crystal system | Orthorhombic | space group | Pna21 |
| a/Å | 20.3669(9) | α (°) | 90 |
| b/Å | 13.6680(6) | β (°) | 90 |
| c/Å | 27.1571(12) | γ (°) | 90 |
| V/Å3 | 7559.9(6) | Z | 4 |
| Dcalcd (g cm−3) | 1.591 | θ range (deg.) | 1.49–27.50 |
| μ/mm–1 | 1.075 | T (K) | 150(2) |
| total no. of data collected | 56,835 | no. of unique data | 13,812 |
| R1, wR21 (I > 2σ(I)) | 0.0636, 0.1226 | R1, wR21 (all data) | 0.0856, 0.1324 |
| GOF 2 | 1.072 | refine params | 1050 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.-C.; Ke, S.-Y.; Chen, K.-T.; Sun, N.-K.; Liu, W.-F.; Ho, M.-L.; Lu, B.-J.; Hsieh, Y.-T.; Chuang, Y.-C.; Lee, G.-H.; et al. Sponge-Like Water De-/Ad-Sorption versus Solid-State Structural Transformation and Colour-Changing Behavior of an Entangled 3D Composite Supramolecuar Architecture, [Ni4(dpe)4(btc)2(Hbtc)(H2O)9]·3H2O. Polymers 2018, 10, 1014. https://doi.org/10.3390/polym10091014
Wang C-C, Ke S-Y, Chen K-T, Sun N-K, Liu W-F, Ho M-L, Lu B-J, Hsieh Y-T, Chuang Y-C, Lee G-H, et al. Sponge-Like Water De-/Ad-Sorption versus Solid-State Structural Transformation and Colour-Changing Behavior of an Entangled 3D Composite Supramolecuar Architecture, [Ni4(dpe)4(btc)2(Hbtc)(H2O)9]·3H2O. Polymers. 2018; 10(9):1014. https://doi.org/10.3390/polym10091014
Chicago/Turabian StyleWang, Chih-Chieh, Szu-Yu Ke, Kuan-Ting Chen, Ning-Kuei Sun, Wei-Fang Liu, Mei-Lin Ho, Bing-Jyun Lu, Yi-Ting Hsieh, Yu-Chun Chuang, Gene-Hsiang Lee, and et al. 2018. "Sponge-Like Water De-/Ad-Sorption versus Solid-State Structural Transformation and Colour-Changing Behavior of an Entangled 3D Composite Supramolecuar Architecture, [Ni4(dpe)4(btc)2(Hbtc)(H2O)9]·3H2O" Polymers 10, no. 9: 1014. https://doi.org/10.3390/polym10091014
APA StyleWang, C.-C., Ke, S.-Y., Chen, K.-T., Sun, N.-K., Liu, W.-F., Ho, M.-L., Lu, B.-J., Hsieh, Y.-T., Chuang, Y.-C., Lee, G.-H., Huang, S.-Y., & Yang, E.-C. (2018). Sponge-Like Water De-/Ad-Sorption versus Solid-State Structural Transformation and Colour-Changing Behavior of an Entangled 3D Composite Supramolecuar Architecture, [Ni4(dpe)4(btc)2(Hbtc)(H2O)9]·3H2O. Polymers, 10(9), 1014. https://doi.org/10.3390/polym10091014