Ternary Sulfur/Polyacrylonitrile/SiO2 Composite Cathodes for High-Performance Sulfur/Lithium Ion Full Batteries
Abstract
1. Introduction
2. Materials and Methods
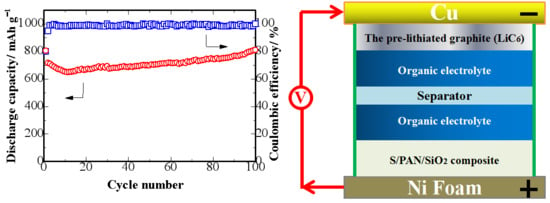
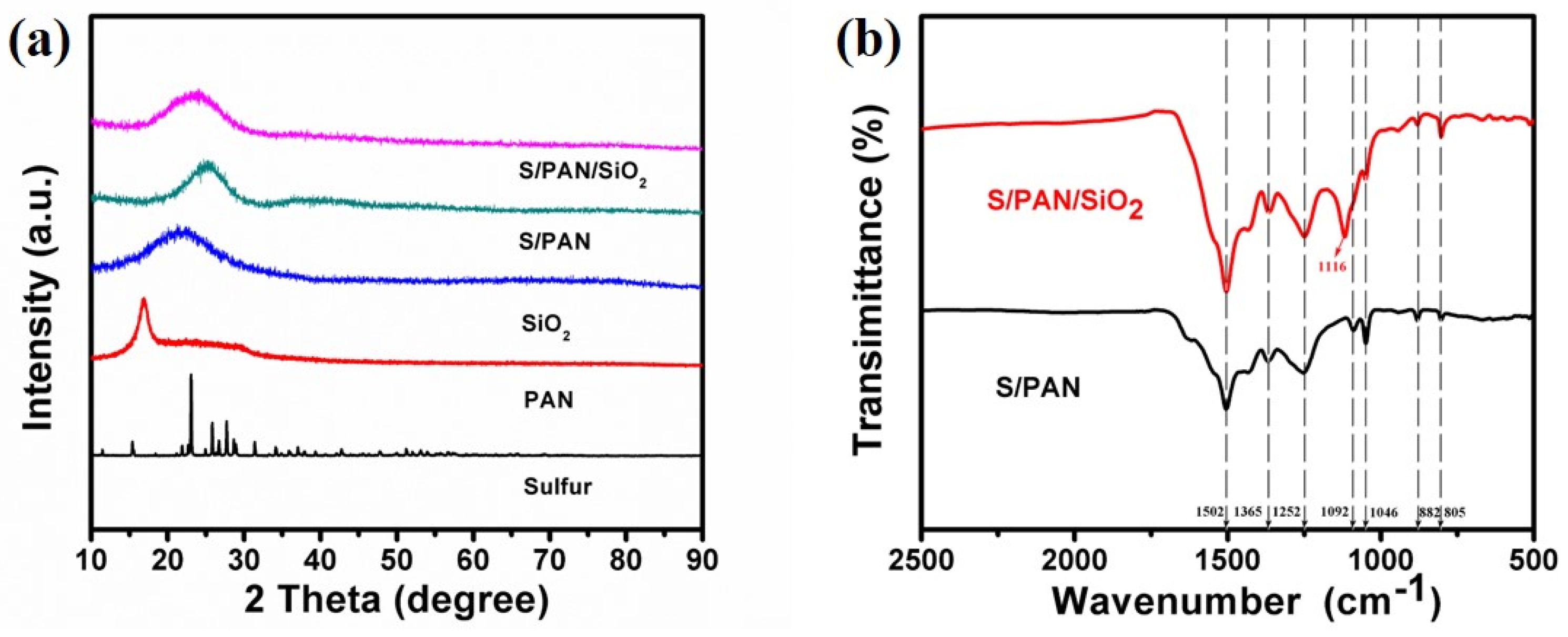
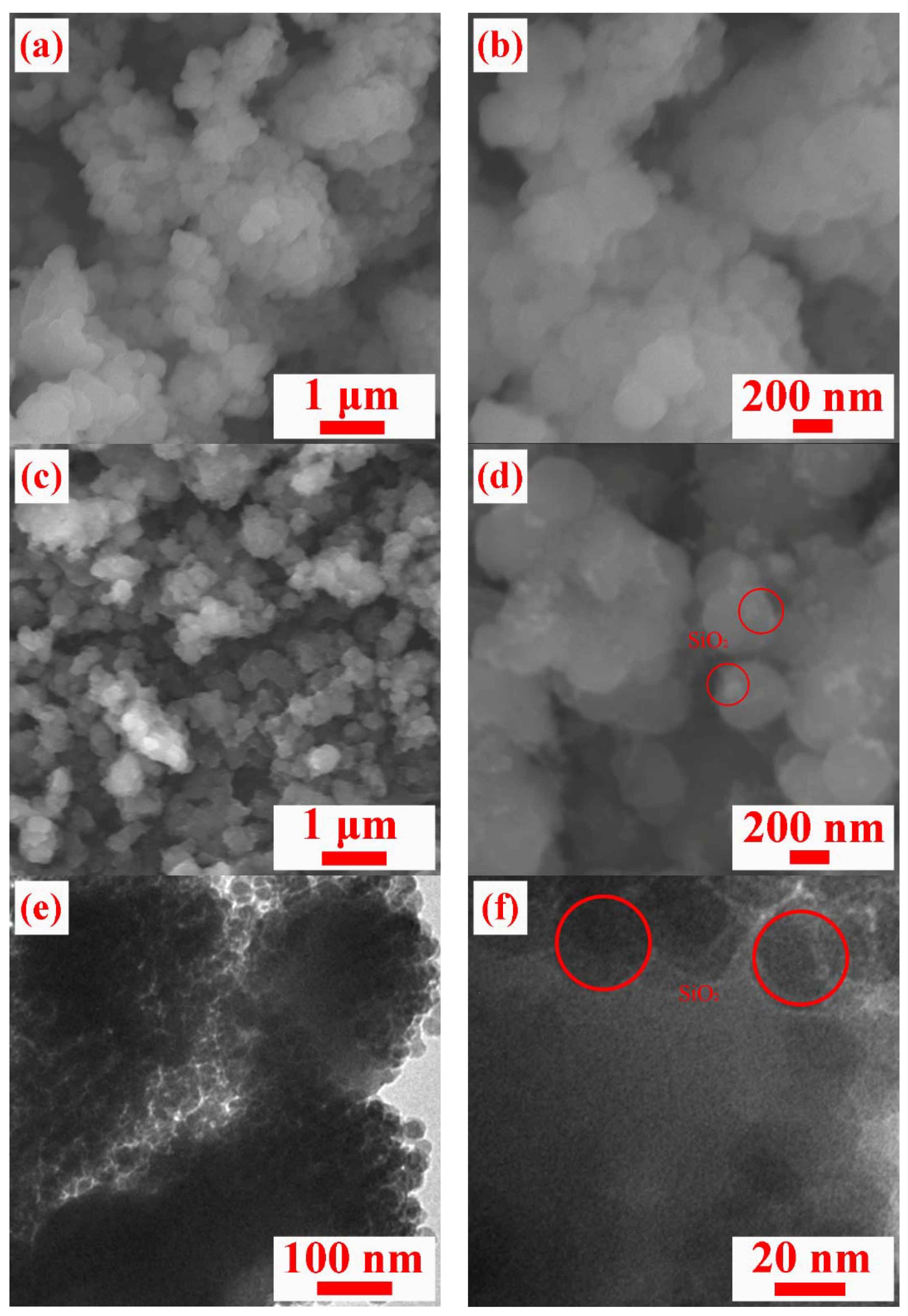
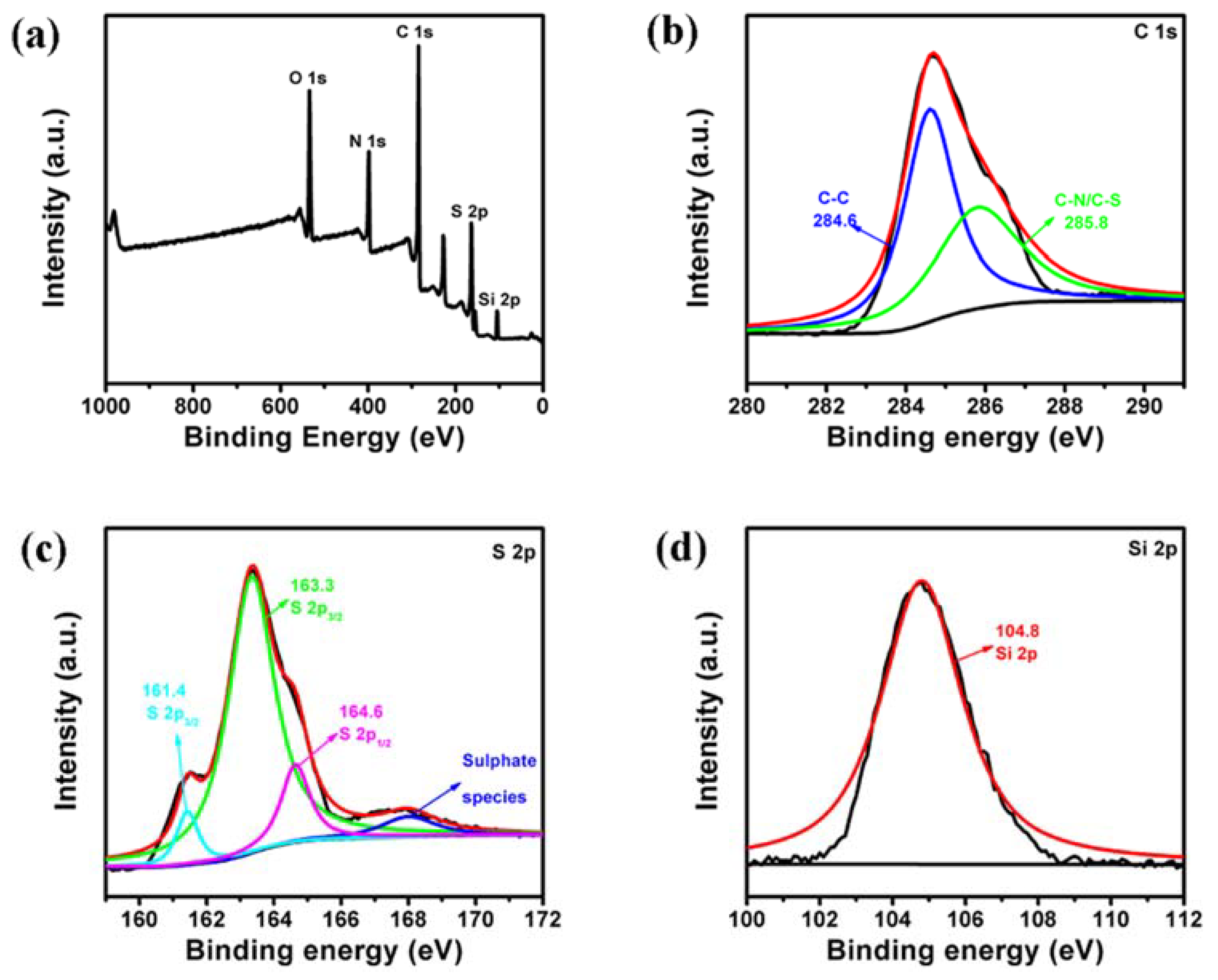
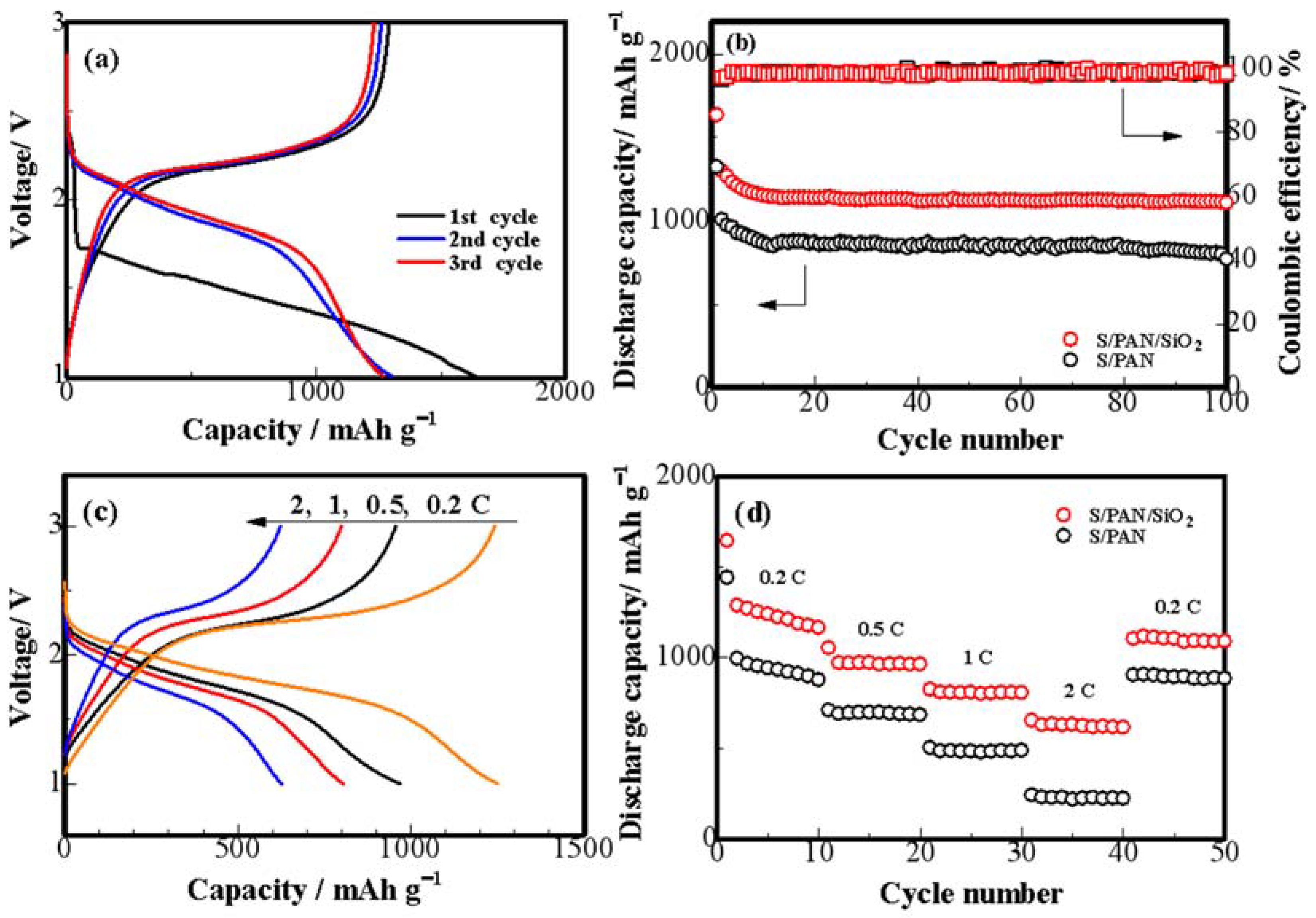
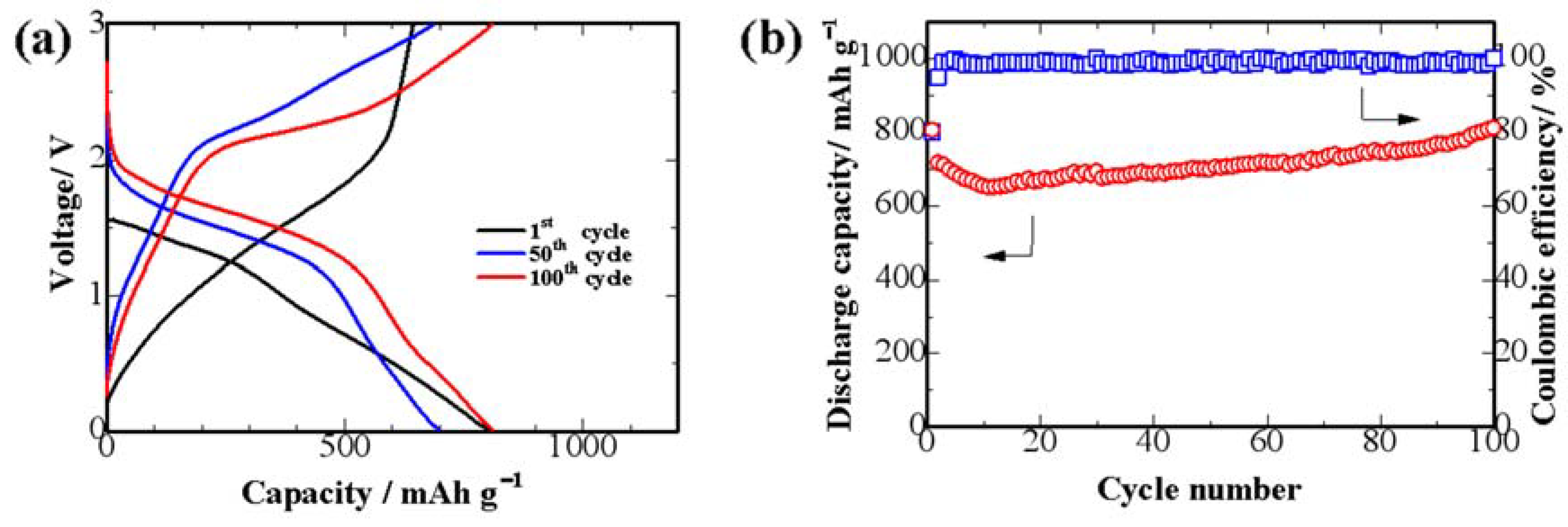
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Armand, M.; Tarascon, J.-M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.B.; Meng, S.J.; Xiong, B.Y.; Ji, D.X.; JetTseng, K. Enhanced online model identification and state of charge estimation for lithium-ion battery with a FBCRLS based observer. Appl. Energy 2016, 181, 332–341. [Google Scholar] [CrossRef]
- Li, H.L.; Wei, Y.Q.; Zhang, Y.G.; Zhang, C.W.; Wang, G.K.; Zhao, Y.; Yin, F.X.; Bakenov, Z. In situ sol-gel synthesis of ultrafine ZnO nanocrystals anchored on graphene as anode material for lithium-ion batteries. Ceram. Int. 2016, 42, 12371–12377. [Google Scholar] [CrossRef]
- Wei, Z.B.; Zhao, J.Y.; Ji, D.X.; JetTseng, K. A multi-timescale estimator for battery state of charge and capacity dual estimation based on an online identified model. Appl. Energy 2017, 204, 1264–1274. [Google Scholar] [CrossRef]
- Zhang, Y.G.; Li, Y.; Li, H.P.; Yin, F.X.; Zhao, Y.; Bakenov, Z. Synthesis of hierarchical MoS2 microspheres composed of nanosheets assembled via facile hydrothermal method as anode material for lithium-ion batteries. J. Nanopart. Res. 2016, 18, 63. [Google Scholar] [CrossRef]
- Wei, Z.B.; Bhattarai, A.; Zou, C.F.; Meng, S.J.; Lim, T.M.; Skyllas-Kazacos, M. Real-time monitoring of capacity loss for vanadium redox flow battery. J. Power Sources 2018, 390, 261–269. [Google Scholar] [CrossRef]
- Kumaresan, K.; Mikhaylik, Y.; White, R.E. A Mathematical Model for a Lithium-Sulfur Cell. J. Electrochem. Soc. 2008, 155, A576–A582. [Google Scholar] [CrossRef]
- Doan, T.N.L.; Ghaznavi, M.; Konarov, A.; Zhang, Y.G.; Chen, P. Cyclability of sulfur/dehydrogenated polyacrylonitrile composite cathode in lithium–sulfur batteries. J. Solid State Electr. 2014, 18, 69–76. [Google Scholar] [CrossRef]
- Grixti, S.; Mukherjee, S.; Singh, C.V. Two-Dimensional Boron as an impressive Lithium-Sulphur battery cathode material. Energy Storage Mater. 2018, 3, 80–87. [Google Scholar] [CrossRef]
- Konarov, A.; Gosselink, D.; Doan, T.N.L.; Zhang, Y.G.; Zhao, Y.; Chen, P. Simple, scalable, and economical preparation of sulfur-PAN composite cathodes for Li/S batteries. J. Power Sources 2014, 259, 183–187. [Google Scholar] [CrossRef]
- Li, G.; Wang, X.L.; Seo, M.H.; Li, M.; Ma, L.; Yuan, Y.F.; Wu, T.P.; Yu, A.P.; Wang, S.; Lu, J.; et al. Chemisorption of polysulfides through redox reactions with organic molecules for lithium-sulfur batteries. Nat. Commun. 2018, 9, 705. [Google Scholar] [CrossRef] [PubMed]
- Anilkumar, K.M.; Jinisha, B.; Manoj, M.; Pradeep, V.S.; Jayalekshmi, S. Layered sulfur/PEDOT: PSS nano composite electrodes for lithium sulfur cell applications. Appl. Surf. Sci. 2018, 442, 556–564. [Google Scholar] [CrossRef]
- Yuan, G.H.; Yin, F.X.; Zhao, Y.; Bakenov, Z.; Wang, G.K.; Zhang, Y.G. Corn stalk-derived activated carbon with a stacking sheet-like structure as sulfur cathode supporter for lithium/sulfur batteries. Ionics 2016, 22, 63–69. [Google Scholar] [CrossRef]
- HyunKim, P.J.; Kim, K.; Pol, V.G. Towards highly stable lithium sulfur batteries: Surface functionalization of carbon nanotube scaffolds. Carbon 2018, 131, 175–183. [Google Scholar]
- Scholz, J.; Kayaalp, B.; Juhl, A.C.; Clemens, D.; Fröba, M.; Mascotto, S. Severe Loss of Confined Sulfur in Nano porous Carbon for Li-S Batteries under Wetting Conditions. ACS Energy Lett. 2018, 3, 387–392. [Google Scholar] [CrossRef]
- Zhang, Y.G.; Sun, L.C.; Li, H.P.; Tan, T.Z.; Li, J.D. Porous three-dimensional reduced graphene oxide for high-performance lithium-sulfur batteries. J. Alloys Compd. 2018, 739, 290–297. [Google Scholar] [CrossRef]
- Arava, L.M.R.; Gopalakrishnan, D.; Lee, A. Electrocatalytically Active Niobium Sulfide Modified Carbon Cloth for Lithium-Sulfur Batteries. J. Electrochem. Energy 2018, 15, 011005. [Google Scholar] [CrossRef]
- Tian, Y.; Sun, Z.H.; Zhang, Y.G.; Wang, X.; Bakenov, Z.; Yin, F.X. Micro-Spherical Sulfur/Graphene Oxide Composite via Spray Drying for High Performance Lithium Sulfur Batteries. Nanomaterials 2018, 8, 50. [Google Scholar] [CrossRef] [PubMed]
- Jeong, T.-G.; Lee, Y.-S.; Cho, B.W.; Kim, Y.-T.; Jung, H.-G.; Chung, K.Y. Improved performance of dual-conducting polymer-coated sulfur composite with high sulfur utilization for lithium-sulfur batteries. J. Alloys Compd. 2018, 742, 868–876. [Google Scholar] [CrossRef]
- Zhang, Y.G.; Zhao, Y.; Yermukhambetova, A.; Bakenov, Z.; Chen, P. Ternary sulfur/polyacrylonitrile/Mg0.6Ni0.4O composite cathodes for high performance lithium/sulfur batteries. J. Mater. Chem. A 2013, 1, 295–301. [Google Scholar] [CrossRef]
- Li, F.; Kaiser, M.R.; Ma, J.; Guo, Z.P.; Liu, H.K.; Wang, J.Z. Free-standing sulfur-polypyrrole cathode in conjunction with polypyrrole-coated separator for flexible Li-S batteries. Energy Storage Mater. 2018, 13, 312–322. [Google Scholar] [CrossRef]
- Yin, F.X.; Liu, X.Y.; Zhang, Y.G.; Zhao, Y.; Menbayeva, A.; Bakenovd, Z.; Wang, X. Well-dispersed sulfur anchored on interconnected polypyrrole nanofiber network as high performance cathode for lithium-sulfur batteries. Solid State Sci. 2017, 66, 44–49. [Google Scholar] [CrossRef]
- Lee, F.; Tsaia, M.-C.; Lina, M.-H.; Nimaha, Y.L.; Hya, S.; Kuoa, C.Y.; Chenga, J.-H.; Ricka, J.; Sub, W.-N.; Hwanga, B.-J. Capacity retention of lithium sulfur batteries enhanced with nano-sized TiO2-embedded polyethylene oxide. J. Mater. Chem. A 2017, 5, 6708–6715. [Google Scholar] [CrossRef]
- Wang, J.L.; Yang, J.; Wan, C.R.; Du, K.; Xie, J.Y.; Xu, N.X. Sulfur Composite Cathode Materials for Rechargeable Lithium Batteries. Adv. Funct. Mater. 2003, 13, 487. [Google Scholar] [CrossRef]
- Yermukhambetova, A.; Bakenov, Z.; Zhang, Y.G.; Darr, J.A.; Brett, D.J.L.; Shearing, P.R. Examining the effect of nanosized Mg0.6Ni0.4O and Al2O3 additives on S/polyaniline cathodes for lithium–sulphur batteries. J. Eelctroanal. Chem. 2016, 780, 407–415. [Google Scholar] [CrossRef]
- Zhang, Y.G.; Bakenov, Z.; Zhao, Y.; Konarov, A.; Doan, T.N.L.; Sun, K.E.K.; Yermukhambetova, A.; Chen, P. Effect of nanosized Mg0.6Ni0.4O prepared by self-propagating high temperature synthesis on sulfur cathode performance in Li/S batteries. Powder Technol. 2013, 235, 248–255. [Google Scholar] [CrossRef]
- Agostini, M.; Scrosati, B.; Hassoun, J. An Advanced Lithium-Ion Sulfur Battery for High Energy Storage. Adv. Eng. Mater. 2015, 5, 1500481. [Google Scholar] [CrossRef]
- Wang, L.; Fu, Y.B.; Battaglia, V.S.; Liu, G. SBR-PVDF based binder for the application of SLMP in graphite anodes. Rsc. Adv. 2013, 3, 15022–15027. [Google Scholar] [CrossRef]
- Wang, Z.H.; Fu, Y.B.; Zhang, Z.C.; Yuan, S.W.; Amine, K.; Battaglia, V.; Liu, G. Application of Stabilized Lithium Metal Powder (SLMP®) in graphite anode—A high efficient prelithiation method for lithium-ion batteries. J. Power Sources 2014, 260, 57–61. [Google Scholar] [CrossRef]
- Evers, S.; Nazar, L.F. New approaches for high energy density lithium–sulfur battery cathodes. Acc. Chem. Res. 2012, 46, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, X.M.; He, Y.S.; Liu, N.; Tan, T.Z.; Liang, C.Y. Biomass Derived Nitrogen-Doped Highly Porous Carbon Material with a Hierarchical Porous Structure for High-Performance Lithium/Sulfur Batteries. Materials 2017, 10, 1158. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.Q.; Liu, Y.G.; Wang, W.K.; Wang, A.B.; Hu, B.W.; Shen, M.; Gao, T.; Zhao, P.C.; Yang, Y.S. A new insight into the lithium storage mechanism of sulfurized polyacrylonitrile with no soluble intermediates. Energy Storage Mater. 2018, 14, 272–278. [Google Scholar] [CrossRef]
- Xia, H.Y.; Yin, Z.C.; Zheng, F.C.; Zhang, Y.G. Facile synthesis of SiO2/C composites as anode materials for lithium-ion batteries. Mater. Lett. 2017, 205, 83–86. [Google Scholar] [CrossRef]
- Fanous, J.; Wegner, M.; Grimminger, J.; Andresen, A.; Buchmeiser, M.R. Structure-Related Electrochemistry of Sulfur-Poly (acrylonitrile) Composite Cathode Materials for Rechargeable Lithium Batteries. Chem. Mater. 2011, 23, 5024–5028. [Google Scholar] [CrossRef]
- Jiang, Y.; Mu, D.B.; Chen, S.; Wu, B.R.; Zhao, Z.K.; Wu, Y.Z.; Ding, Z.P.; Wu, F. Hollow silica spheres with facile carbon modification as an anode material for lithium-ion batteries. J. Alloys Compd. 2018, 744, 7–14. [Google Scholar] [CrossRef]
- Back, C.K.; Kim, T.J.; Choi, N.S. Activated natural porous silicate for a highly promising SiOx nanostructure finely impregnated with carbon nanofiber as a high performance anode material for lithium-ion batteries. J. Mater. Chem. A 2014, 2, 13648–13654. [Google Scholar] [CrossRef]
- Jiao, M.L.; Liu, K.L.; Shi, Z.Q.; Wang, C.Y. SiO2/carbon composite microspheres with hollow core-shell structure as a high stability electrode for lithium ion batteries. Chemelectrochem 2016, 4, 542–549. [Google Scholar] [CrossRef]
- Zhang, Y.G.; Zhao, Y.; Bakenov, Z.; Konarov, A.; Chen, P. Preparation of novel network nanostructured sulfur composite cathode with enhanced stable cycle performance. J. Power Sources 2014, 270, 326–331. [Google Scholar] [CrossRef]
- Zhang, Y.G.; Zhao, Y.; Bakenov, Z.; Babaa, M.-R.; Konarov, A.; Ding, C.; Chen, P. Effect of graphene on sulfur/polyacrylonitrile nanocomposite cathode in high performance lithium/sulfur batteries. J. Electrochem. Soc. 2013, 160, 1194–1198. [Google Scholar] [CrossRef]
- Shi, L.; Liu, Y.G.; Wang, W.K.; Wang, A.B.; Jin, Z.Q.; Wu, F.; Yang, Y.S. High-safety lithium-ion sulfur battery with sulfurized polyacrylonitrile cathode, prelithiated SiOx/C anode and carbonate-based electrolyte. J. Alloys Compd. 2017, 723, 974–982. [Google Scholar] [CrossRef]
- Zeng, P.; Han, Y.M.; Duan, X.B.; Jia, G.C.; Huang, L.W.; Chen, Y.G. A stable graphite electrode in superconcentrated LiTFSI-DME/DOL electrolyte and its application in lithium-sulfur full battery. Mater. Res. Bull. 2017, 95, 61–70. [Google Scholar] [CrossRef]
| Cathodes | Sulfur Loading (wt %) | Current Density | Initial Discharge Capacity (mAh/g) | Discharge Capacity (mAh/g) (After nth Cycle) | References |
|---|---|---|---|---|---|
| S/DPAN | 48 | 0.2 C | 1550 | 1050 (80) | [10] |
| S/PAN/Mg0.6Ni0.4O | 38.5 | 0.1 C | 1540 | 1200 (100) | [20] |
| S@pPAN | 37.64 | 200 mA/g | 2200 | 1700 (100) | [32] |
| S@pPAN | 40.9 | 0.5 C | 1510 | 1100 (100) | [38] |
| S/PAN/Graphene | 47.3 | 0.1 C | 719 | 612 (10) | [39] |
| S@pPAN // Prelithiated SiOx/C | 87 | 0.36 C | 850 | 600 (100) | [40] |
| MesoC/Sulfur // Prelithiated Graphite | - | 0.1 C | 608 | 405 (105) | [41] |
| S/PAN/SiO2 // Prelithiated Graphite | 45 | 0.2 C | 804 | 810 (100) | This work |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Y.; Shan, Z.; Tan, T.; Chen, Z.; Zhang, Y. Ternary Sulfur/Polyacrylonitrile/SiO2 Composite Cathodes for High-Performance Sulfur/Lithium Ion Full Batteries. Polymers 2018, 10, 930. https://doi.org/10.3390/polym10080930
He Y, Shan Z, Tan T, Chen Z, Zhang Y. Ternary Sulfur/Polyacrylonitrile/SiO2 Composite Cathodes for High-Performance Sulfur/Lithium Ion Full Batteries. Polymers. 2018; 10(8):930. https://doi.org/10.3390/polym10080930
Chicago/Turabian StyleHe, Yusen, Zhenzhen Shan, Taizhe Tan, Zhihong Chen, and Yongguang Zhang. 2018. "Ternary Sulfur/Polyacrylonitrile/SiO2 Composite Cathodes for High-Performance Sulfur/Lithium Ion Full Batteries" Polymers 10, no. 8: 930. https://doi.org/10.3390/polym10080930
APA StyleHe, Y., Shan, Z., Tan, T., Chen, Z., & Zhang, Y. (2018). Ternary Sulfur/Polyacrylonitrile/SiO2 Composite Cathodes for High-Performance Sulfur/Lithium Ion Full Batteries. Polymers, 10(8), 930. https://doi.org/10.3390/polym10080930