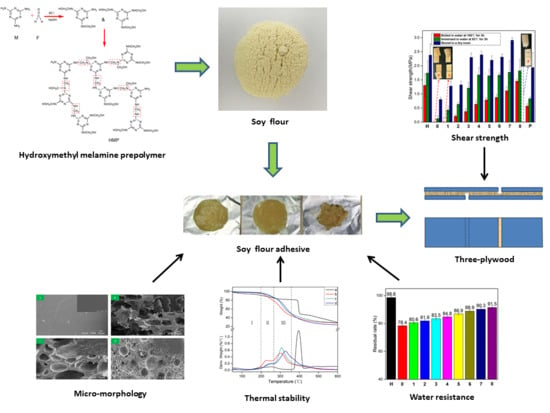
A High-Performance and Low-Cost Soy Flour Adhesive with a Hydroxymethyl Melamine Prepolymer
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
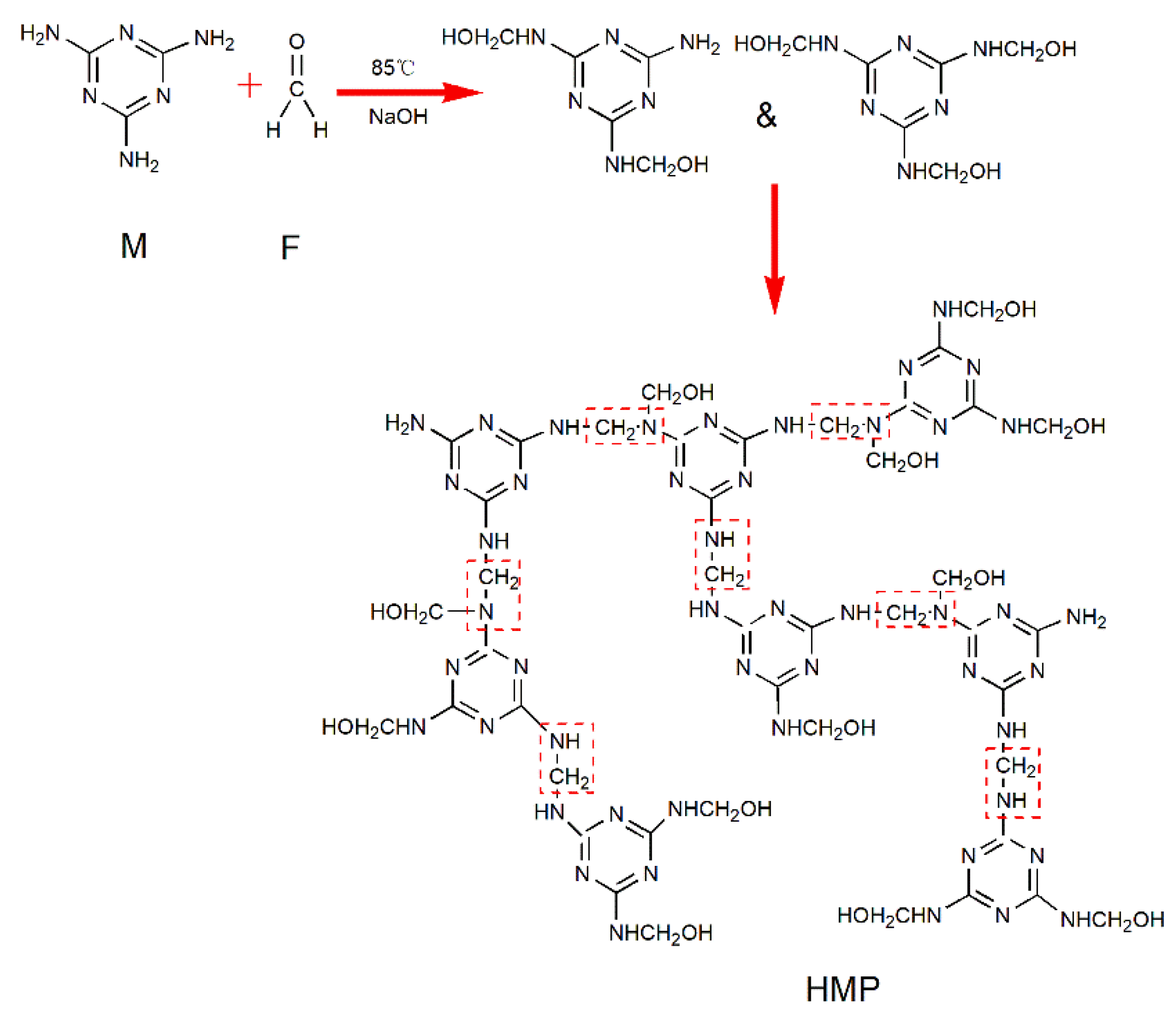
2.2. Preparation of HMP
2.3. Preparation of SF Adhesives
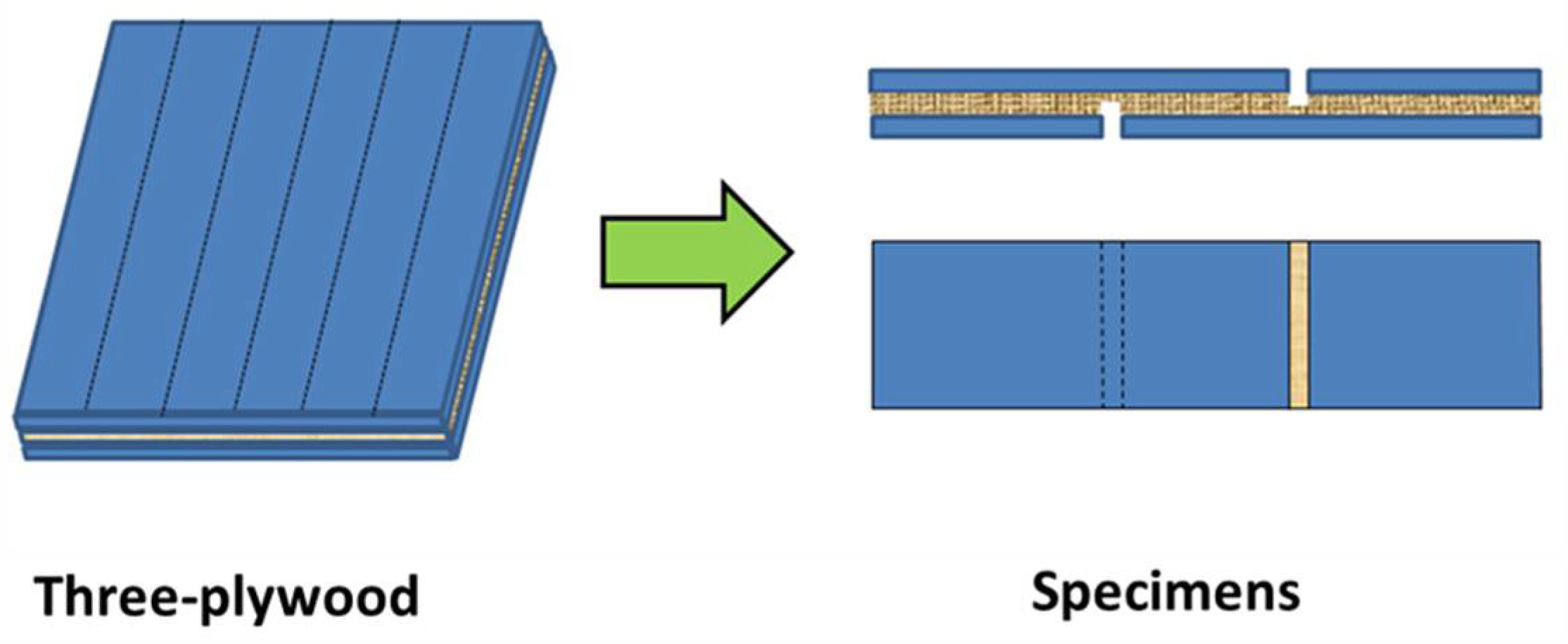
2.4. Preparation of Three-Plywood Samples
2.5. Characterization of Adhesive Samples
2.5.1. Shear Strength Measurement of Plywood
2.5.2. Residual Rate Test
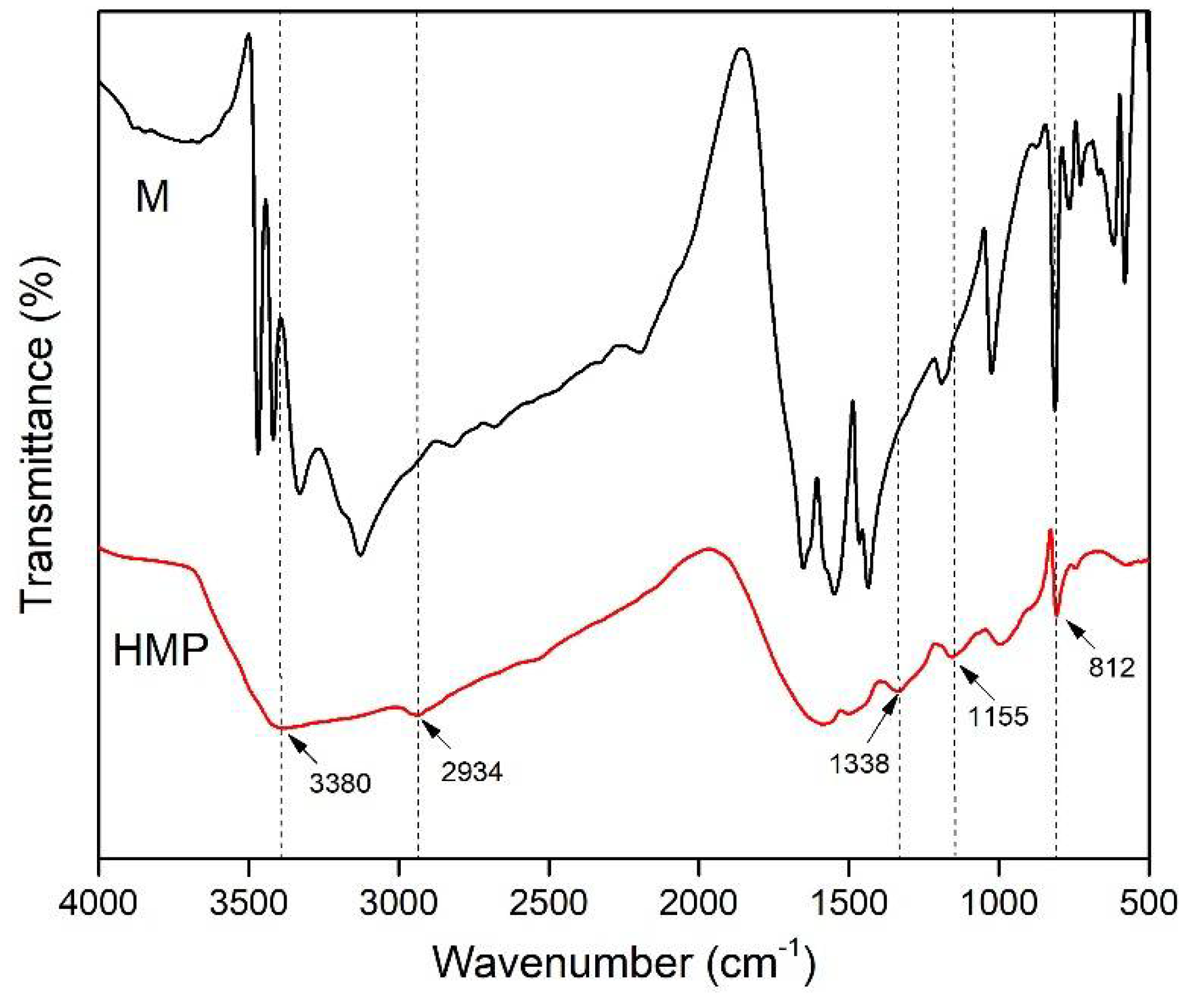
2.5.3. Fourier Transform Infrared (FTIR) Spectroscopy
2.5.4. Thermogravimetry (TGA) Test
2.5.5. Scanning Electron Microscopy (SEM) Analysis
3. Results and Discussion
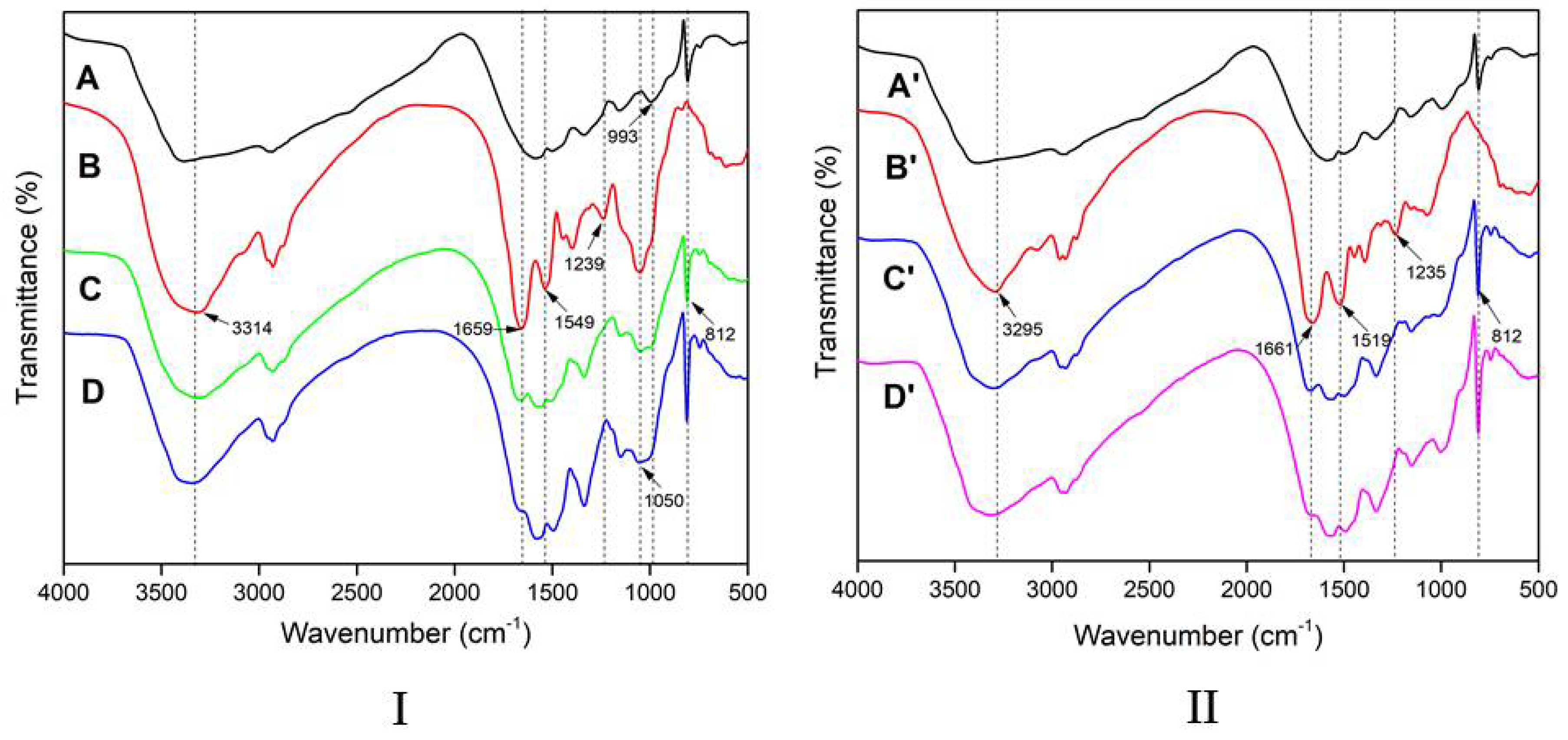
3.1. FTIR Spectra of M and HMP
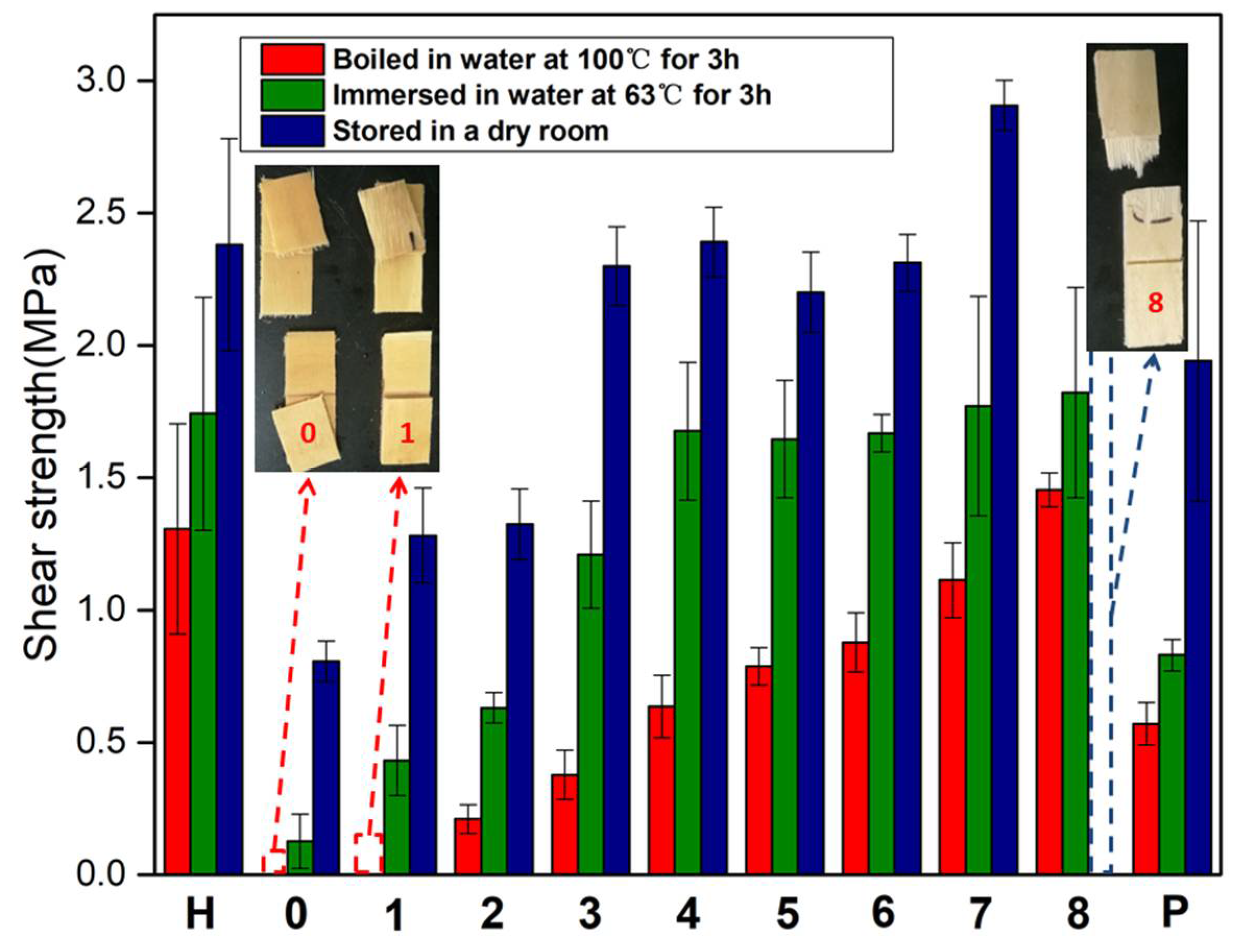
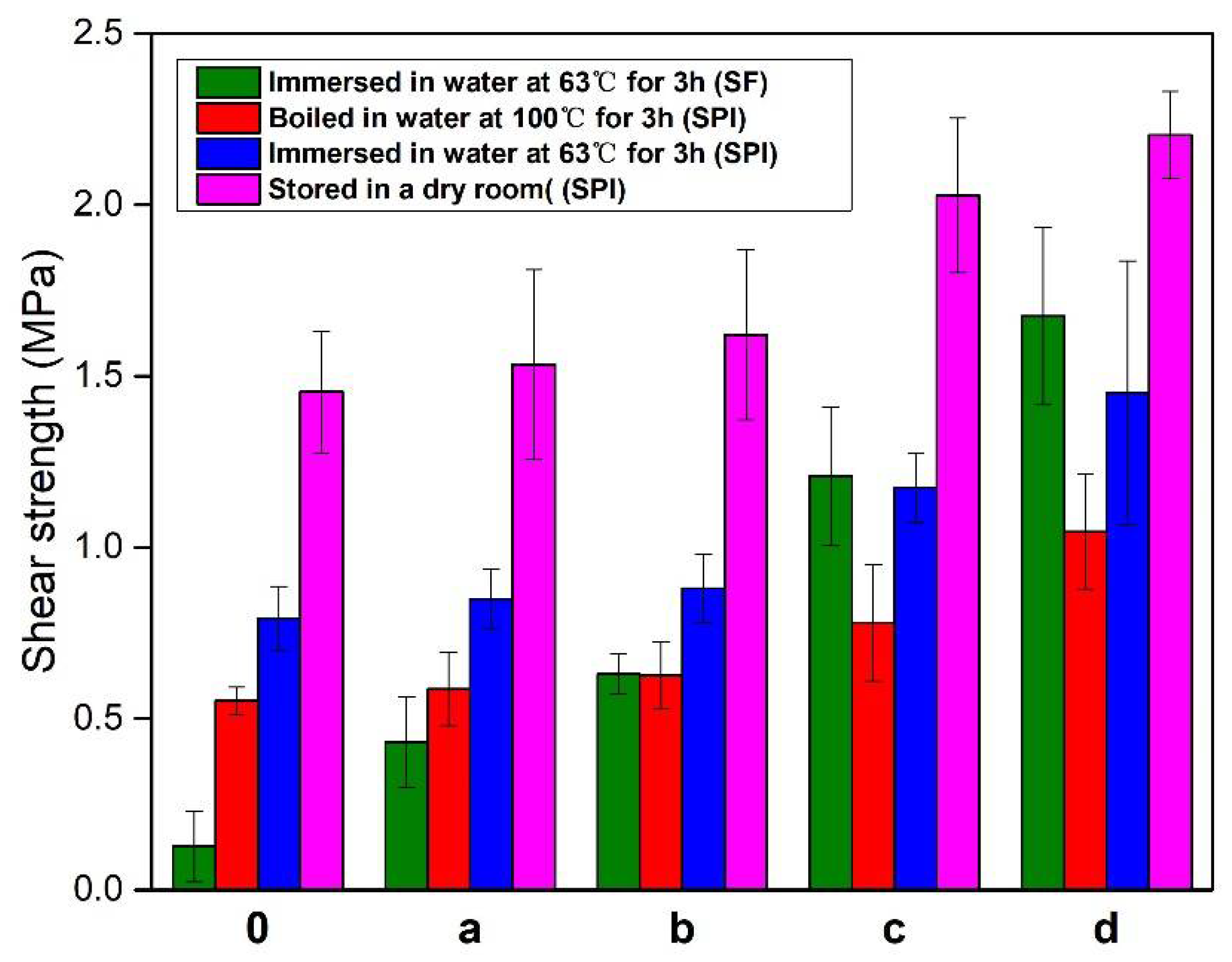
3.2. Tensile/Shear Strength Measurement
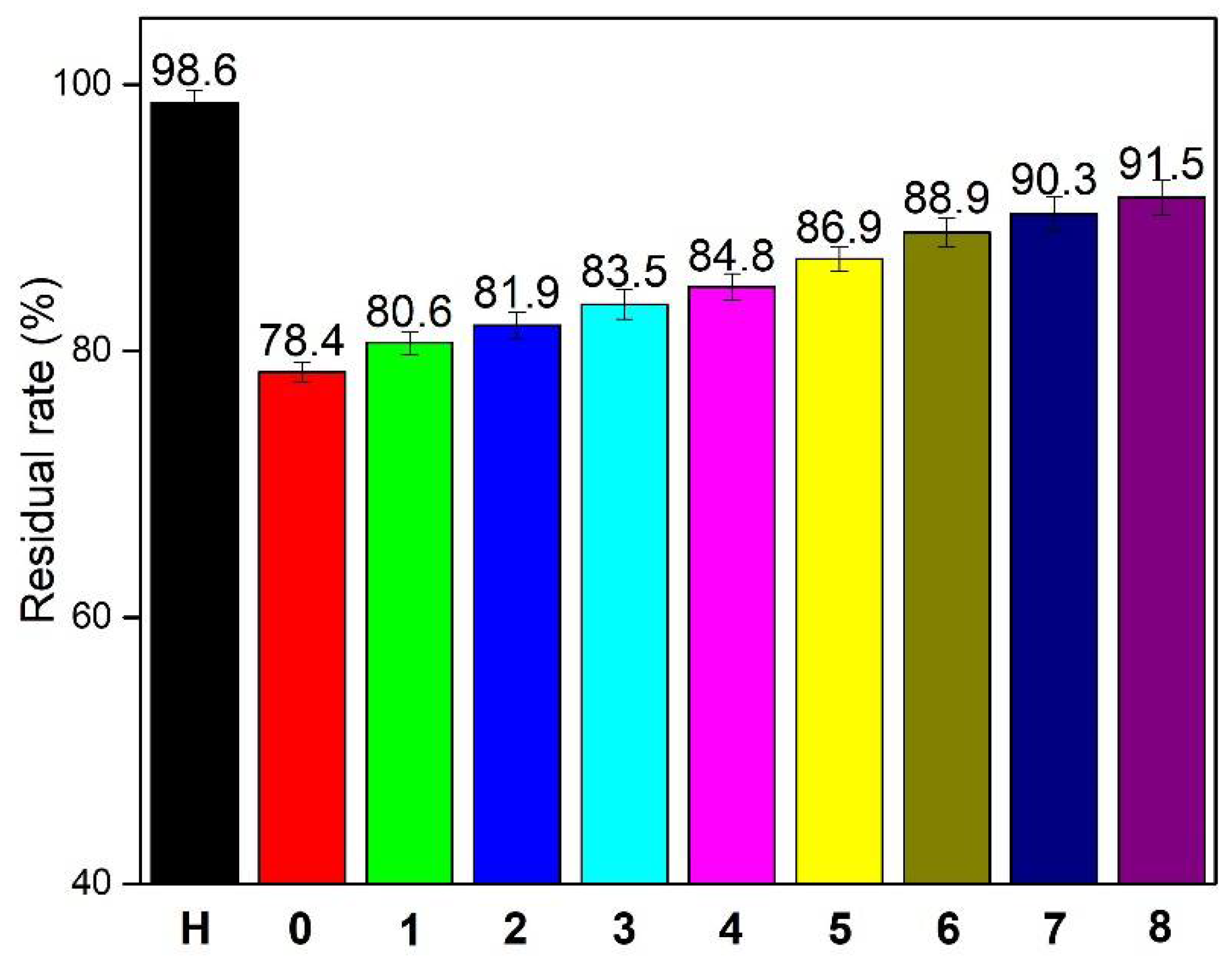
3.3. Residual Rate
3.4. FTIR Spectroscopic Analysis
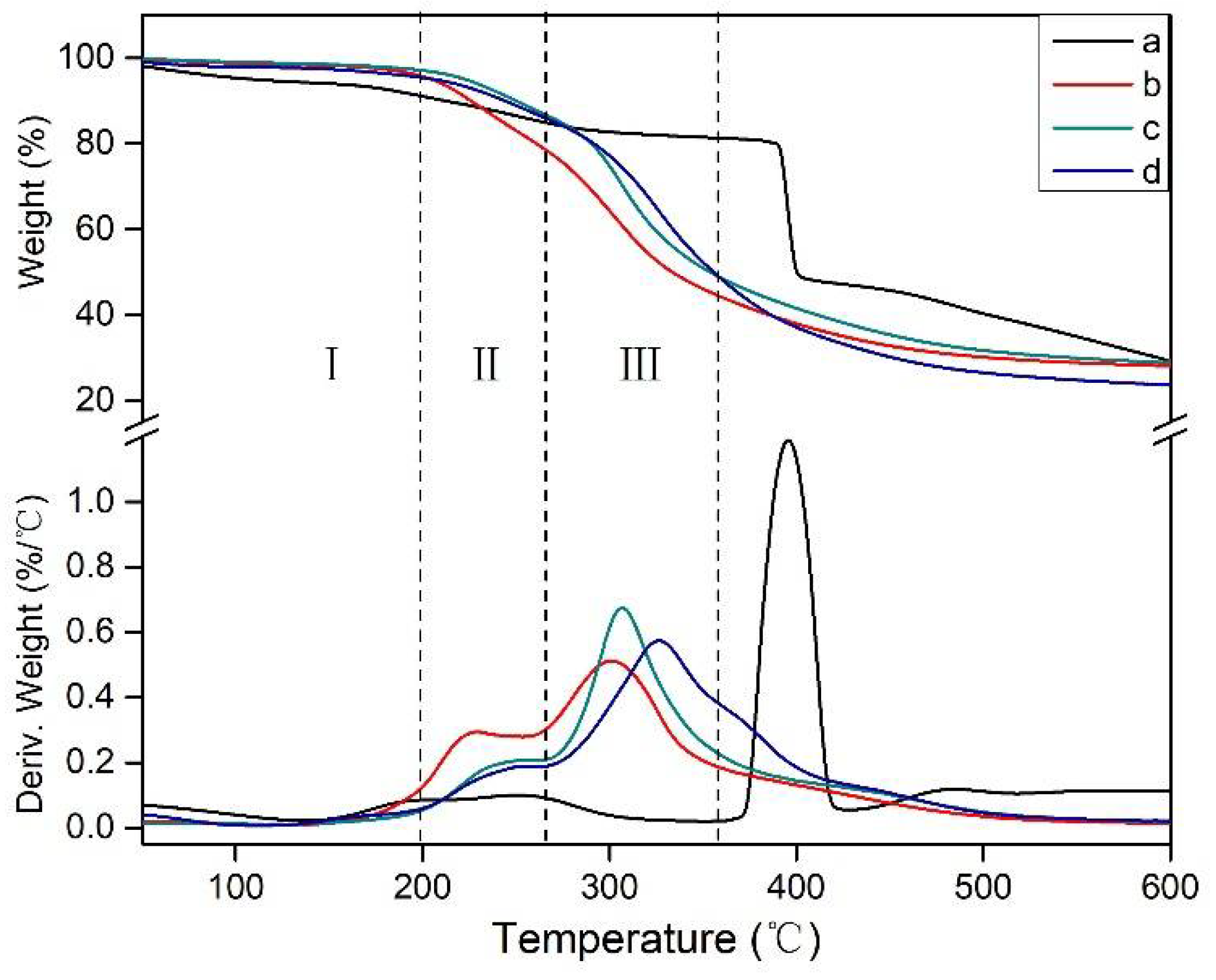
3.5. Thermogravimetric (TG) Analysis
3.6. SEM Analysis of Adhesives
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, C.; Zhang, Y.; Li, X.; Luo, J.; Gao, Q.; Li, J. “Green” bio-thermoset resins derived from soy protein isolate and condensed tannins. Ind. Crops Prod. 2017, 108, 363–370. [Google Scholar] [CrossRef]
- Wang, F.; Wang, J.; Chu, F.; Wang, C.; Jin, C.; Wang, S.; Pang, J. Combinations of soy protein and polyacrylate emulsions as wood adhesives. Int. J. Adhes. Adhes. 2018, 82, 160–165. [Google Scholar] [CrossRef]
- Mousavi, S.Y.; Huang, J.; Li, K. Investigation of poly(glycidyl methacrylate-co-styrene) as a curing agent for soy-based wood adhesives. Int. J. Adhes. Adhes. 2018, 82, 67–71. [Google Scholar] [CrossRef]
- Luo, J.; Luo, J.; Zhang, J.; Bai, Y.; Gao, Q.; Li, J.; Li, L. A New Flexible Soy-Based Adhesive Enhanced with Neopentyl Glycol Diglycidyl Ether: Properties and Application. Polymers 2016, 8, 346. [Google Scholar] [CrossRef]
- Ghahri, S.; Mohebby, B.; Pizzi, A.; Mirshokraie, A.; Mansouri, H.R. Improving Water Resistance of Soy-Based Adhesive by Vegetable Tannin. J. Polym. Environ. 2017, 26, 1881–1890. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, Y.; Li, X.; Luo, J.; Gao, Q.; Li, J. A high-performance bio-adhesive derived from soy protein isolate and condensed tannins. RSC Adv. 2017, 7, 21226–21233. [Google Scholar] [CrossRef]
- Li, X.; Li, J.; Luo, J.; Li, K.; Gao, Q.; Li, J. A Novel Eco-friendly Blood Meal-based Bio-adhesive: Preparation and Performance. J. Polym. Environ. 2017, 26, 607–615. [Google Scholar] [CrossRef]
- Fan, D.-B.; Qin, T.-F.; Chu, F.-X. A Soy Flour-Based Adhesive Reinforced by Low Addition of MUF Resin. J. Adhes. Sci. Technol. 2012, 25, 323–333. [Google Scholar] [CrossRef]
- He, Z. Bio-Based Wood Adhesives: Preparation, Characterization, and Testing; CRC Press, Taylor & Franics Group: Boca Raton, FL, USA, 2017. [Google Scholar]
- Wang, Z.; Kang, H.; Zhang, W.; Zhang, S.; Li, J. Improvement of Interfacial Adhesion by Bio-Inspired Catechol-Functionalized Soy Protein with Versatile Reactivity: Preparation of Fully Utilizable Soy-Based Film. Polymers 2017, 9, 95. [Google Scholar] [CrossRef]
- Zhao, H.-J.; Wang, Y.; Yang, L.-L.; Yuan, L.-W.; Peng, D.-C. Relationship between phytoplankton and environmental factors in landscape water supplemented with reclaimed water. Ecol. Indic. 2015, 58, 113–121. [Google Scholar] [CrossRef]
- Wang, C.; Wu, J.; Bernard, G.M. Preparation and characterization of canola protein isolate–poly(glycidyl methacrylate) conjugates: A bio-based adhesive. Ind. Crops. Prod. 2014, 57, 124–131. [Google Scholar] [CrossRef]
- Luo, J.; Li, C.; Li, X.; Luo, J.; Gao, Q.; Li, J. A new soybean meal-based bioadhesive enhanced with 5,5-dimethyl hydantoin polyepoxide for the improved water resistance of plywood. RSC Adv. 2015, 5, 62957–62965. [Google Scholar] [CrossRef]
- Cheng, H.N.; Ford, C.; Dowd, M.K.; He, Z. Wood adhesive properties of cottonseed protein with denaturant additives. J. Adhes. Sci. Technol. 2017, 31, 2657–2666. [Google Scholar] [CrossRef]
- Li, X.; Chen, M.; Zhang, J.; Gao, Q.; Zhang, S.; Li, J. Physico-Chemical Properties of Soybean Meal-Based Adhesives Reinforced by Ethylene Glycol Diglycidyl Ether and Modified Nanocrystalline Cellulose. Polymers 2017, 9, 463. [Google Scholar] [CrossRef]
- Jang, Y.; Li, K. An All-Natural Adhesive for Bonding Wood. J. Am. Oil Chem. Soc. 2015, 92, 431–438. [Google Scholar] [CrossRef]
- Lawlor, C.M.; Riley, C.A.; Hildrew, D.M.; Guarisco, J.L. Respiratory failure after superior-based pharyngeal flap for velopharyngeal insufficiency: A rare complication. Int. J. Pediatr. Otorhinolaryngol. 2015, 79, 1155–1157. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhu, Y.; Yu, Y.; Song, J. Improve Performance of Soy Flour-Based Adhesive with a Lignin-Based Resin. Polymers 2017, 9, 261. [Google Scholar] [CrossRef]
- Luo, J.; Li, L.; Luo, J.; Li, X.; Li, K.; Gao, Q. A High Solid Content Bioadhesive Derived from Soybean Meal and Egg White: Preparation and Properties. J. Polym. Environ. 2016, 25, 948–959. [Google Scholar] [CrossRef]
- Yuan, C.; Luo, J.; Luo, J.; Gao, Q.; Li, J. A soybean meal-based wood adhesive improved by a diethylene glycol diglycidyl ether: Properties and performance. RSC Adv. 2016, 6, 74186–74194. [Google Scholar] [CrossRef]
- Li, H.; Li, C.; Gao, Q.; Zhang, S.; Li, J. Properties of soybean-flour-based adhesives enhanced by attapulgite and glycerol polyglycidyl ether. Ind. Crops Prod. 2014, 59, 35–40. [Google Scholar] [CrossRef]
- Guo, M.; Li, W.; Han, N.; Wang, J.; Su, J.; Li, J.; Zhang, X. Novel Dual-Component Microencapsulated Hydrophobic Amine and Microencapsulated Isocyanate Used for Self-Healing Anti-Corrosion Coating. Polymers 2018, 10, 319. [Google Scholar] [CrossRef]
- Banu, H.T.; Meenakshi, S. Synthesis of a novel quaternized form of melamine–formaldehyde resin for the removal of nitrate from water. J. Water Process Eng. 2017, 16, 81–89. [Google Scholar] [CrossRef]
- Zhu, P.; Gu, Z.; Hong, S.; Lian, H. Preparation and characterization of microencapsulated LDHs with melamine-formaldehyde resin and its flame retardant application in epoxy resin. Polym. Adv. Technol. 2018, 29, 2147–2160. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, Y.; Zhang, Y.; Tang, S.; Guo, C.; Wu, J.; Lau, R. Anionic and cationic dyes adsorption on porous poly-melamine-formaldehyde polymer. Chem. Eng. Res. Des. 2016, 114, 258–267. [Google Scholar] [CrossRef]
- Yin, D.; Ma, L.; Geng, W.; Zhang, B.; Zhang, Q. Microencapsulation of n-hexadecanol by in situpolymerization of melamine-formaldehyde resin in emulsion stabilized by styrene-maleic anhydride copolymer. Int. J. Energy Res. 2015, 39, 661–667. [Google Scholar] [CrossRef]
- Chen, N.; Zheng, P.; Zeng, Q.; Lin, Q. Characterization and Performance of Soy-Based Adhesives Cured with Epoxy Resin. Polymers 2017, 9, 514. [Google Scholar] [CrossRef]
- Li, J.; Luo, J.; Li, X.; Yi, Z.; Gao, Q.; Li, J. Soybean meal-based wood adhesive enhanced by ethylene glycol diglycidyl ether and diethylenetriamine. Ind. Crops Prod. 2015, 74, 613–618. [Google Scholar] [CrossRef]
- Zheng, P.; Lin, Q.; Li, F.; Ou, Y.; Chen, N. Development and characterization of a defatted soy flour-based bio-adhesive crosslinked by 1,2,3,4-butanetetracarboxylic acid. Int. J. Adhes. Adhes. 2017, 78, 148–154. [Google Scholar] [CrossRef]
- Luo, J.; Luo, J.; Bai, Y.; Gao, Q.; Li, J. A high performance soy protein-based bio-adhesive enhanced with a melamine/epichlorohydrin prepolymer and its application on plywood. RSC Adv. 2016, 6, 67669–67676. [Google Scholar] [CrossRef]
- Luo, J.; Li, X.; Zhang, H.; Gao, Q.; Li, J. Properties of a soybean meal-based plywood adhesive modified by a commercial epoxy resin. Int. J. Adhes. Adhes. 2016, 71, 99–104. [Google Scholar] [CrossRef]
- Luo, J.; Luo, J.; Li, X.; Li, K.; Gao, Q.; Li, J. Toughening improvement to a soybean meal-based bioadhesive using an interpenetrating acrylic emulsion network. J. Mater. Sci. 2016, 51, 9330–9341. [Google Scholar] [CrossRef]
- Li, X.; Luo, J.; Gao, Q.; Li, J. A sepiolite-based united cross-linked network in a soybean meal-based wood adhesive and its performance. RSC Adv. 2016, 6, 45158–45165. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, Y.; Duan, X.; Tang, T.; Shen, Y.; Hu, B.; Liu, A.; Chen, H.; Li, C.; Liu, Y. Characterization and antioxidant activities of polysaccharides from thirteen boletus mushrooms. Int. J. Biol. Macromol. 2018, 113, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Ma, R.; Jiao, C.; Hao, L.; Wang, C.; Wu, Q.; Wang, Z. Magnetic mesoporous polymelamine-formaldehyde resin as an adsorbent for endocrine disrupting chemicals. Mikrochim. Acta 2017, 185, 19. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, Q.; Li, L.-S.; Xu, L. Removal of perfluorooctanoic acid from water with economical mesoporous melamine-formaldehyde resin microsphere. Chem. Eng. J. 2017, 320, 501–509. [Google Scholar] [CrossRef]
- Wu, X.; Shi, Z.; Tjandra, R.; Cousins, A.J.; Sy, S.; Yu, A.; Berry, R.M.; Tam, K.C. Nitrogen-enriched porous carbon nanorods templated by cellulose nanocrystals as high performance supercapacitor electrodes. J. Mater. Chem. A 2015, 3, 23768–23777. [Google Scholar] [CrossRef]
- Yuan, C.; Chen, M.; Luo, J.; Li, X.; Gao, Q.; Li, J. A novel water-based process produces eco-friendly bio-adhesive made from green cross-linked soybean soluble polysaccharide and soy protein. Carbohydr. Polym. 2017, 169, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Cui, S.W.; Gong, J.; Guo, Q.; Wang, Q.; Hua, Y. A soy protein-polysaccharides Maillard reaction product enhanced the physical stability of oil-in-water emulsions containing citral. Food Hydrocoll. 2015, 48, 155–164. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Li, J.; Gao, Q. Investigating the use of peanut meal: A potential new resource for wood adhesives. RSC Adv. 2015, 5, 80136–80141. [Google Scholar] [CrossRef]
- Luo, J.; Luo, J.; Yuan, C.; Zhang, W.; Li, J.; Gao, Q.; Chen, H. An eco-friendly wood adhesive from soy protein and lignin: Performance properties. RSC Adv. 2015, 5, 100849–100855. [Google Scholar] [CrossRef]

| Sample | SF Adhesive Formulations | |||||
|---|---|---|---|---|---|---|
| SF (g) | WF (g) | Water (g) | HMP (g) | PAE (g) | ||
| H | HMP adhesive | - | 20 | 40 | 40 | - |
| 0 | SF adhesive | 25 | - | 75 | - | - |
| 1 | SF/3% HMP adhesive | 25 | - | 69 | 3 | - |
| 2 | SF/6% HMP adhesive | 25 | - | 66 | 6 | - |
| 3 | SF/9% HMP adhesive | 25 | - | 63 | 9 | - |
| 4 | SF/12% HMP adhesive | 25 | - | 60 | 12 | - |
| 5 | SF/15% HMP adhesive | 25 | - | 57 | 15 | - |
| 6 | SF/18% HMP adhesive | 25 | - | 54 | 18 | - |
| 7 | SF/21% HMP adhesive | 25 | - | 51 | 21 | - |
| 8 | SF/24% HMP adhesive | 25 | - | 48 | 24 | - |
| 8 | SF/24% HMP adhesive | 25 | - | 48 | 24 | - |
| P | SF/PAE adhesive | 25 | - | 25 | - | 50 (12.5%) |
| Sample | SPI Adhesive Formulations | |||
|---|---|---|---|---|
| SPI (g) | Water (g) | HMP (g) | ||
| 0 | SPI adhesive | 13.3 | 86.7 | - |
| a | SPI/3% HMP adhesive | 13.3 | 83.7 | 3 |
| b | SPI/6% HMP adhesive | 13.3 | 80.7 | 6 |
| c | SPI/9% HMP adhesive | 13.3 | 77.7 | 9 |
| d | SPI/12% HMP adhesive | 13.3 | 74.7 | 12 |
| Main Stages | Temperature Range | Changes in Thermal Degradation of the SF Adhesive |
|---|---|---|
| First stage | 50–200 °C | A post-reaction stage, which is attributed to the possible reaction of the system under thermal action and produced vapor and gases, leading to a mass loss in the adhesive. The adhesive showed no degradation of the soy protein and other major components. |
| Second stage | 200–270 °C | An initial degradation stage, which is mainly considered to the weight loss of the degradation of small molecules and the break of some unstable chemical bonds. |
| Third stage | 270–360 °C | The degradation of the skeleton structure of the adhesive, which results from the thermal degradation of the cross-linked network structure in the adhesives. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Zhang, Y.; Chen, M.; Gao, Q.; Li, J. A High-Performance and Low-Cost Soy Flour Adhesive with a Hydroxymethyl Melamine Prepolymer. Polymers 2018, 10, 909. https://doi.org/10.3390/polym10080909
Zhang M, Zhang Y, Chen M, Gao Q, Li J. A High-Performance and Low-Cost Soy Flour Adhesive with a Hydroxymethyl Melamine Prepolymer. Polymers. 2018; 10(8):909. https://doi.org/10.3390/polym10080909
Chicago/Turabian StyleZhang, Meng, Yi Zhang, Mingsong Chen, Qiang Gao, and Jianzhang Li. 2018. "A High-Performance and Low-Cost Soy Flour Adhesive with a Hydroxymethyl Melamine Prepolymer" Polymers 10, no. 8: 909. https://doi.org/10.3390/polym10080909
APA StyleZhang, M., Zhang, Y., Chen, M., Gao, Q., & Li, J. (2018). A High-Performance and Low-Cost Soy Flour Adhesive with a Hydroxymethyl Melamine Prepolymer. Polymers, 10(8), 909. https://doi.org/10.3390/polym10080909