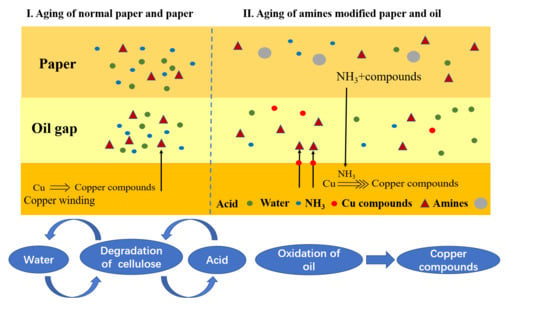
Influence of Amine Compounds on the Thermal Stability of Paper-Oil Insulation
Abstract
:1. Introduction
2. Thermal Aging Test of Paper-Oil Insulation
2.1. Preparation of Thermal Aging Samples
2.2. Determination of the Amine Compound Thermal Stabilizer
2.3. Thermal Aging Properties of Paper and Oil
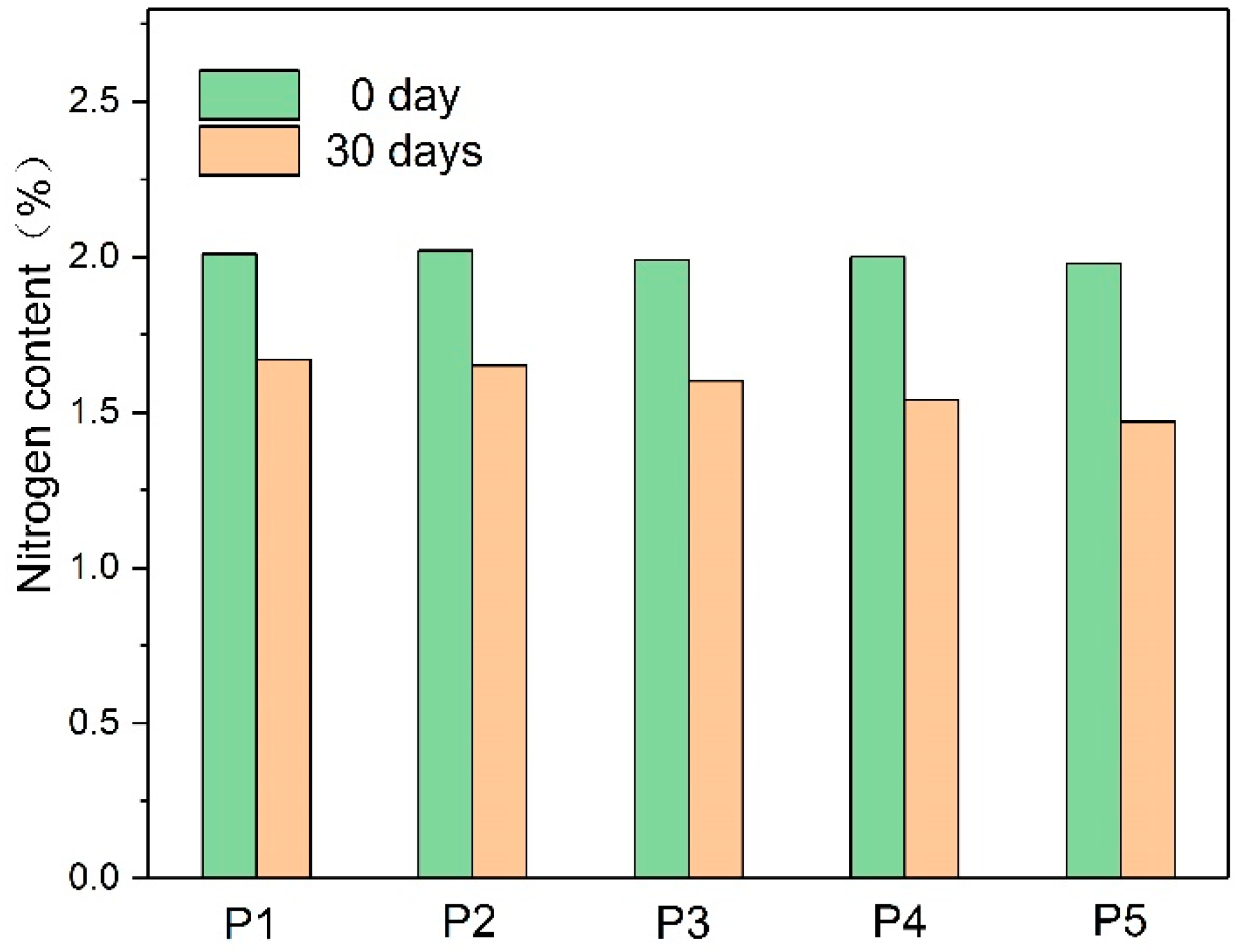
2.3.1. Nitrogen Content
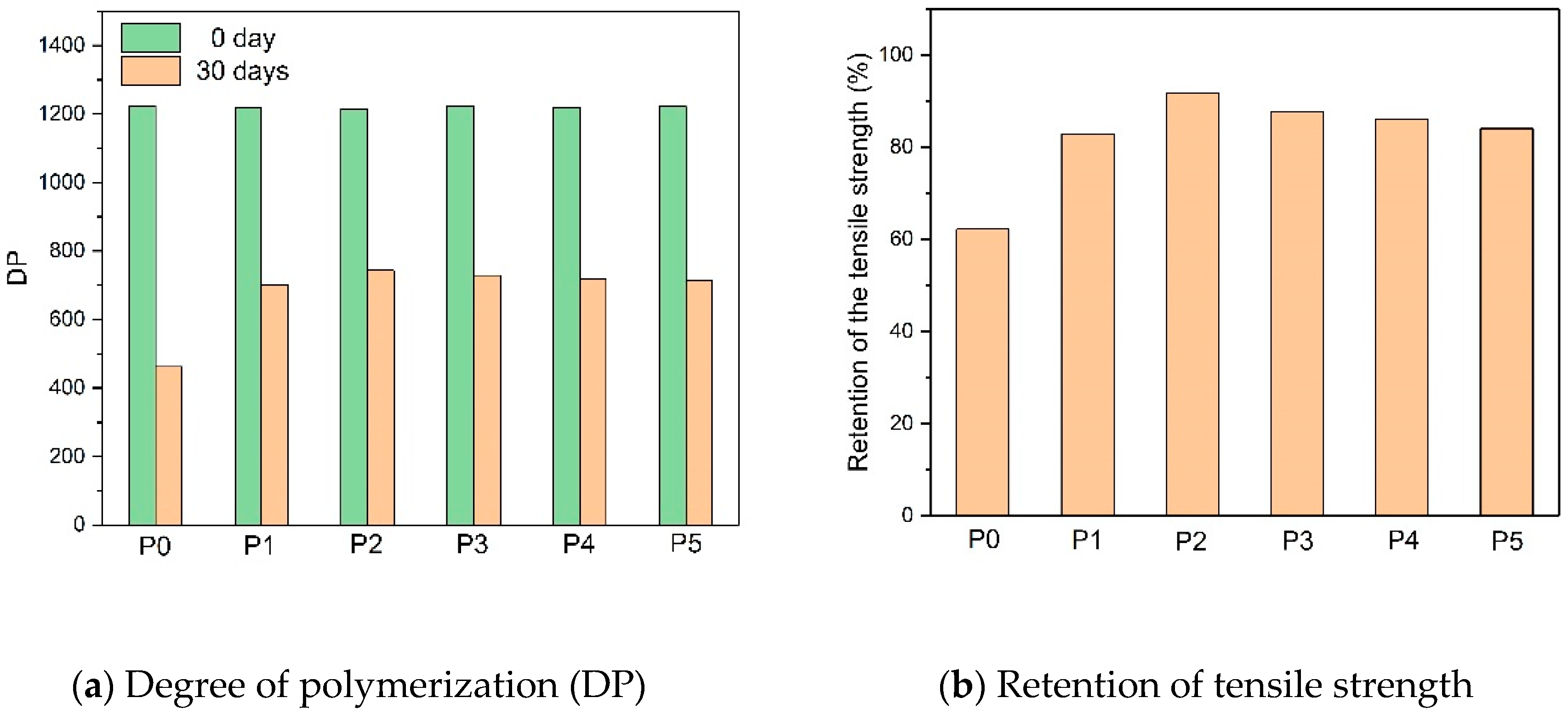
2.3.2. Tensile Strength and DP of the Insulating Paper
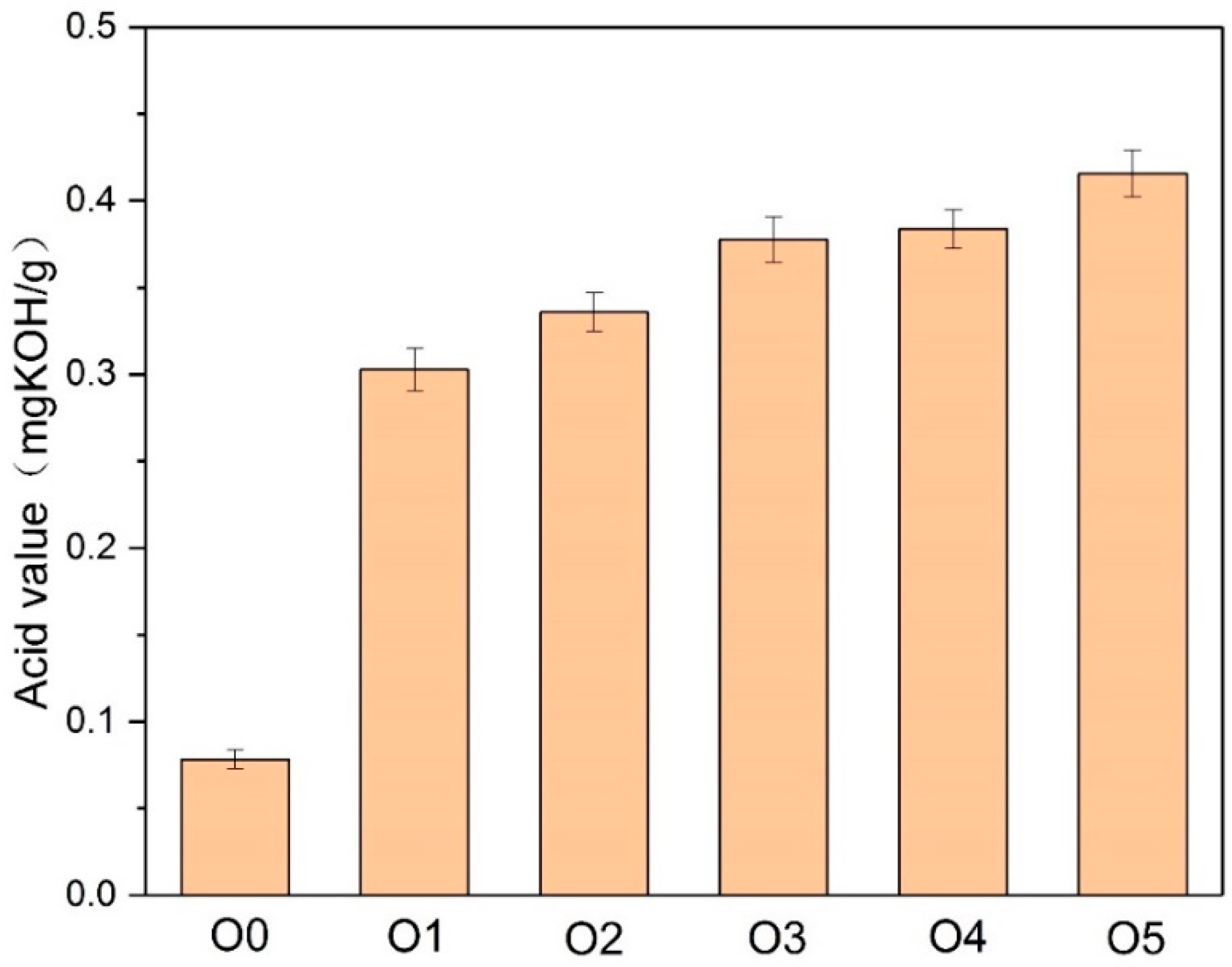
2.3.3. Acidity in Oil
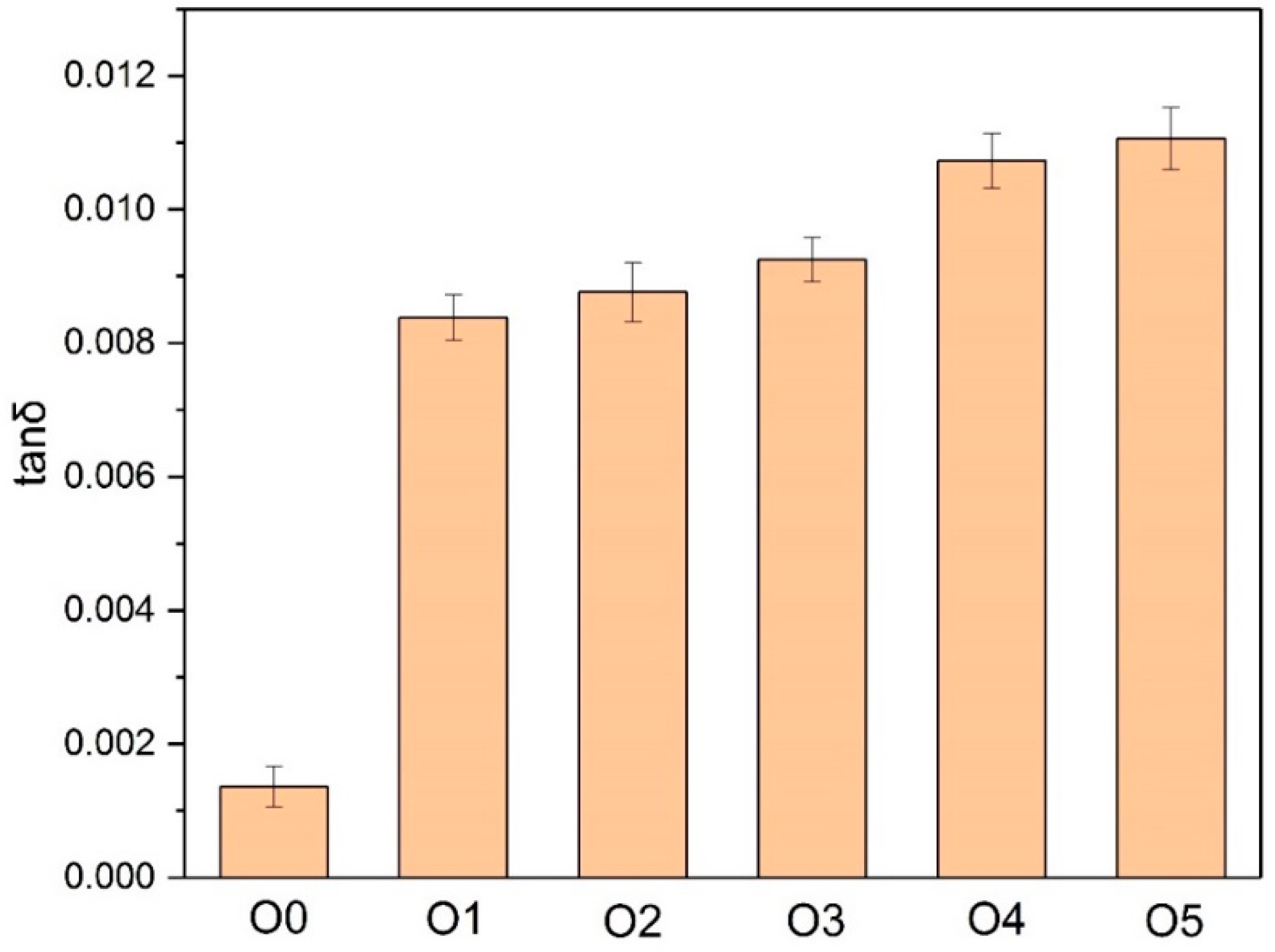
2.3.4. Dielectric Loss Factor of Oil
3. Results and Discussion

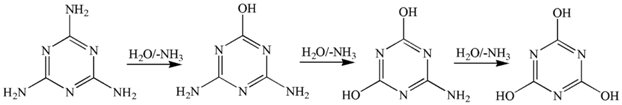

4. Conclusions
- The papers modified with melamine, DICY, and PAM (P2, P3, and P4) had better thermal stability, whereas the addition of an amine thermal stabilizer accelerated the thermal aging of the insulating oil. P2 (2.25 wt % melamine, 0.75 wt % DICY, and 0.2 wt % PAM) obtained the best thermal stability and had less impact on the insulating oil during thermal aging.
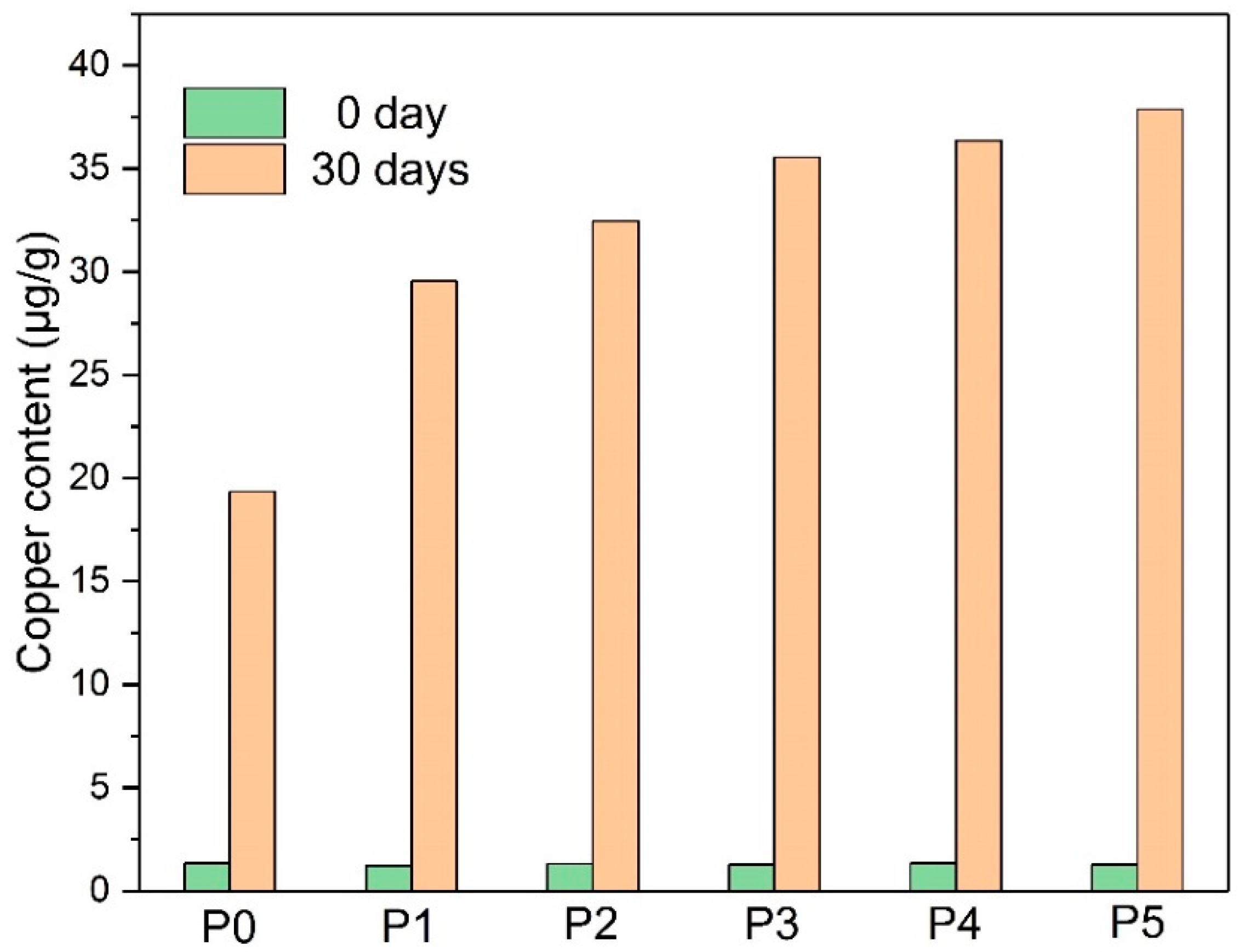
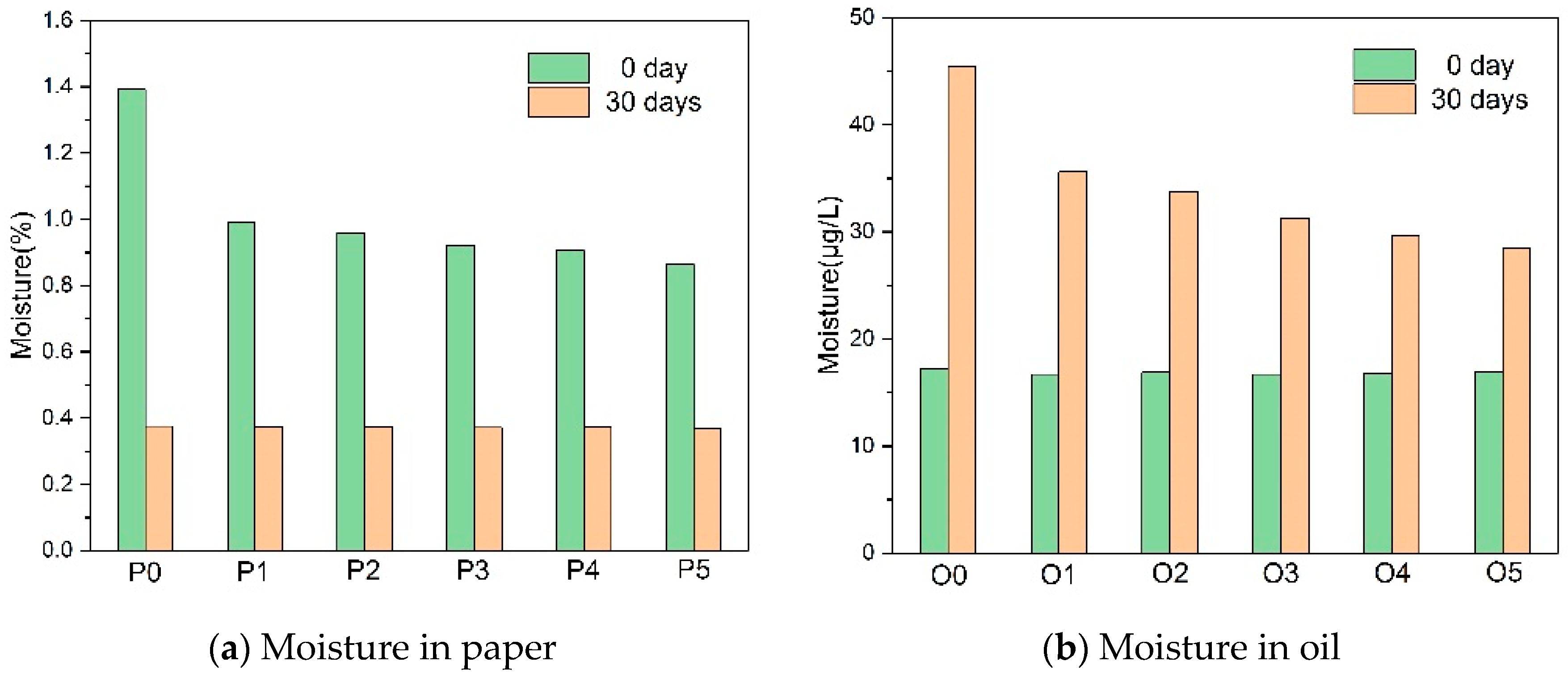
- The addition of the amine compounds inhibited cellulose degradation by depleting the acids and the water in the paper-oil systems while producing ammonia, which led to the increase of copper compounds in the insulating oil, thereby increasing the aging rate of the insulating oil.
- Melamine was the core thermal stabilizer among the three amine compounds in P2 due to its lower energy gap between both the water and the small molecular acid and less defects than PAM and DICY.
Author Contributions
Funding
Conflicts of Interest
References
- Wang, S.; Zhang, G.; Wei, J.; Huang, X.; Chen, Y. Experimental study of the aged states effects on pressboard PDC characteristics. Proc. CSEE 2011, 31, 177–183. [Google Scholar]
- Liao, R.J.; Yang, L.J.; Li, J.; Grzybowski, S. Aging condition assessment of transformer paper-oil insulation model based on partial discharge analysis. IEEE Trans. Dielectr. Electr. Insul. 2011, 18, 303–311. [Google Scholar] [CrossRef]
- Yang, L.J.; Liao, R.J.; Sun, H.G.; Sun, C.X.; Li, J. Contrasting analysis and investigation on properties and products of paper-oil during thermal aging process. Proc. CSEE 2008, 28, 53–58. [Google Scholar]
- Saha, T.K.; Darveniza, M.; Yao, Z.T.; Hill, D.J.T. Investigating the effects of oxidation and thermal degradation on electrical and chemical properties of power transformers insulation. IEEE Trans. Power Deliv. 1999, 14, 1359–1367. [Google Scholar] [CrossRef]
- Liu, R. Cellulose Chemistry; Science Press: Beijing, China, 1985. [Google Scholar]
- Wei, S.; Fan, T.D.; Wen, C.; Kai, W.U.; Cheng, Y.H. Residual breakdown voltage prediction of simple paper-oil insulation model. High Volt. Eng. 2011, 37, 910–915. [Google Scholar]
- Han, H.H.; Yang, D.W.; Su, W.; Zhe-Wen, L.I.; Hai-Ru, L.I.; Zhuo, R. Insulation aging characteristics of transformer paper-oil by multi-parameter regression analysis. High Volt. Eng. 2010, 36, 2937–2941. [Google Scholar]
- Ese, M.H.G.; Liland, K.B.; Lundgaard, L.E. Oxidation of paper insulation in transformers. IEEE Trans. Dielectr. Electr. Insul. 2010, 17, 939–946. [Google Scholar] [CrossRef]
- Yang, L.; Deng, B.; Liao, R.; Sun, C.; Zhu, M. Influence of vegetable oil on the thermal aging rate of kraft paper and its mechanism. IEEE Trans. Dielectr. Electr. Insul. 2011, 18, 692–700. [Google Scholar] [CrossRef]
- Kalia, S.; Kaith, B.S.; Kaur, I. Pretreatments of natural fibers and their application as reinforcing material in polymer composites—A review. Polym. Eng. Sci. 2009, 49, 1253–1272. [Google Scholar] [CrossRef]
- Yang, L.; Liao, R.; Sun, C.; Sun, H. In Study on the influence of natural ester on thermal ageing characteristics of paper-oil in power transformer. In Proceedings of the International Conference on High Voltage Engineering and Application, Chongqing, China, 9–12 Novemebr 2008; pp. 437–440. [Google Scholar] [CrossRef]
- Bomben, S.; Sedding, H.G.; Rizzetto, S. Assessment of station cable condition using wireless telemetry and diagnostic tests. Electr. Insul. Mag. IEEE 2002, 18, 24–29. [Google Scholar] [CrossRef]
- Darveniza, M.; Saha, T.K.; Hill, D.J.T.; Le, T.T. Investigations into effective methods for assessing the condition of insulation in aged power transformers. IEEE Trans. Power Deliv. 1998, 13, 1214–1223. [Google Scholar] [CrossRef]
- Stone, G.C. The statistics of aging models and practical reality. IEEE Trans. Electr. Insul. 1993, 28, 716–728. [Google Scholar] [CrossRef]
- Mcnutt, W.J. Insulation thermal life considerations for transformer loading guides. IEEE Trans. Power Deliv. 1992, 7, 392–401. [Google Scholar] [CrossRef]
- Oommen, T.V.; Prevost, T.A. Cellulose insulation in oil-filled power transformers: Part II maintaining insulation integrity and life. Electr. Insul. Mag. IEEE 2006, 22, 5–14. [Google Scholar] [CrossRef]
- Lundgaard, L.E.; Hansen, W.; Linhjell, D.; Painter, T.J. Aging of oil-impregnated paper in power transformers. IEEE Trans. Power Deliv. 2004, 19, 230–239. [Google Scholar] [CrossRef]
- Zhang, F.Z.; Liao, R.J.; Yang, L.J.; Yuan, Y.; Gong, C.Y.; Shuang, Z. Influence of thermal stabilisers on oil during thermal aging process. High Volt. Eng. 2011, 37, 2437–2442. [Google Scholar]
- Liao, R.; Zhang, F.; Yuan, Y.; Zhang, S.; Bai, G.; LIU, T. Preparation of a dicyandiamide-contained novel insulation paper and an experiment on the thermal aging characteristics of relevant oil-paper insulations. J. Chongqing Univ. 2013, 694, 198–208. [Google Scholar]
- Sun, H.; Law, C.K. Chemical reactivity analysis of the CO + OHh and CO + HO2 reactions. J. Mol. Struct. Theochem. 2008, 862, 138–147. [Google Scholar] [CrossRef]
- Prevost, T.A.; Oommen, T.V. Cellulose insulation in oil-filled power transformers: Part I—History and development. IEEE Electr. Insul. Mag. 2006, 22, 28–35. [Google Scholar] [CrossRef]
- Prevost, T.A. Thermally upgraded insulation in transformers. In Proceedings of the Electrical Insulation Conference and Electrical Manufacturing Expo, Indianapolis, IN, USA, 23–26 October 2005; pp. 120–125. [Google Scholar] [CrossRef]
- Jian, H.; Liao, R.J.; Yang, L.J.; Liang, S.W.; Chao, T. Analysis on the generation rules of both acids and copper products of transformer paper-oil insulation under thermal aging. High Volt. Eng. 2010, 36, 2160–2166. [Google Scholar]
- Hathaway, B.J.; Tomlinson, A.A.G. Copper(ii) ammonia complexes. Coord. Chem. Rev. 1970, 5, 1–43. [Google Scholar] [CrossRef]
- Chattaraj, P.K. Chemical Reactivity and Selectivity: Local HSAB Principle versus Frontier Orbital Theory. J. Phys. Chem. A 2001, 105, 511–513. [Google Scholar] [CrossRef]
- Emsley, A.; Xiao, X.; Heywood, R.; Ali, M. Degradation of cellulosic insulation in power transformers. Part 3: Effects of oxygen and water on ageing in oil. IET 2000, 147, 115–119. [Google Scholar] [CrossRef]


| Group | Content |
|---|---|
| S0 | -- |
| S1 | 3 wt % melamine + 0.2 wt % PAM |
| S2 | 2.25 wt % melamine + 0.75 wt % DICY + 0.2 wt % PAM |
| S3 | 1.5 wt % melamine + 1.5 wt % DICY + 0.2 wt % PAM |
| S4 | 0.75 wt % melamine + 2.25 wt % DICY + 0.2 wt % PAM |
| S5 | 3 wt % DICY + 0.2 wt % PAM |
| EGap | ||||
|---|---|---|---|---|
| β-d-glucopyranose | DICY | Melamine | PAM | |
| Water | 6.68584 | 6.13073 | 6.21782 | 5.90284 |
| Formic acid | 4.88445 | 4.74839 | 4.43546 | 4.12607 |
| Acetic acid | 4.57424 | 4.43818 | 4.12525 | 3.81104 |
| Levulinic acid | 4.28852 | 4.15246 | 3.83953 | 3.52481 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, N.; Liao, R.; Xiang, M.; Mo, Y.; Yuan, Y. Influence of Amine Compounds on the Thermal Stability of Paper-Oil Insulation. Polymers 2018, 10, 891. https://doi.org/10.3390/polym10080891
Liang N, Liao R, Xiang M, Mo Y, Yuan Y. Influence of Amine Compounds on the Thermal Stability of Paper-Oil Insulation. Polymers. 2018; 10(8):891. https://doi.org/10.3390/polym10080891
Chicago/Turabian StyleLiang, Ningchuan, Ruijin Liao, Min Xiang, Yang Mo, and Yuan Yuan. 2018. "Influence of Amine Compounds on the Thermal Stability of Paper-Oil Insulation" Polymers 10, no. 8: 891. https://doi.org/10.3390/polym10080891
APA StyleLiang, N., Liao, R., Xiang, M., Mo, Y., & Yuan, Y. (2018). Influence of Amine Compounds on the Thermal Stability of Paper-Oil Insulation. Polymers, 10(8), 891. https://doi.org/10.3390/polym10080891