Synthesis of Polypyrrole Induced by [Fe(CN)6]3− and Redox Cycling of [Fe(CN)6]4−/[Fe(CN)6]3−
Abstract
:1. Introduction
2. Methodology
2.1. Reagents and Materials
2.2. Apparatus
2.3. Evaluation of Ppy Synthesis Initiated by [Fe(CN)6]3−
3. Results and Discussion
3.1. Polypyrrole Formation
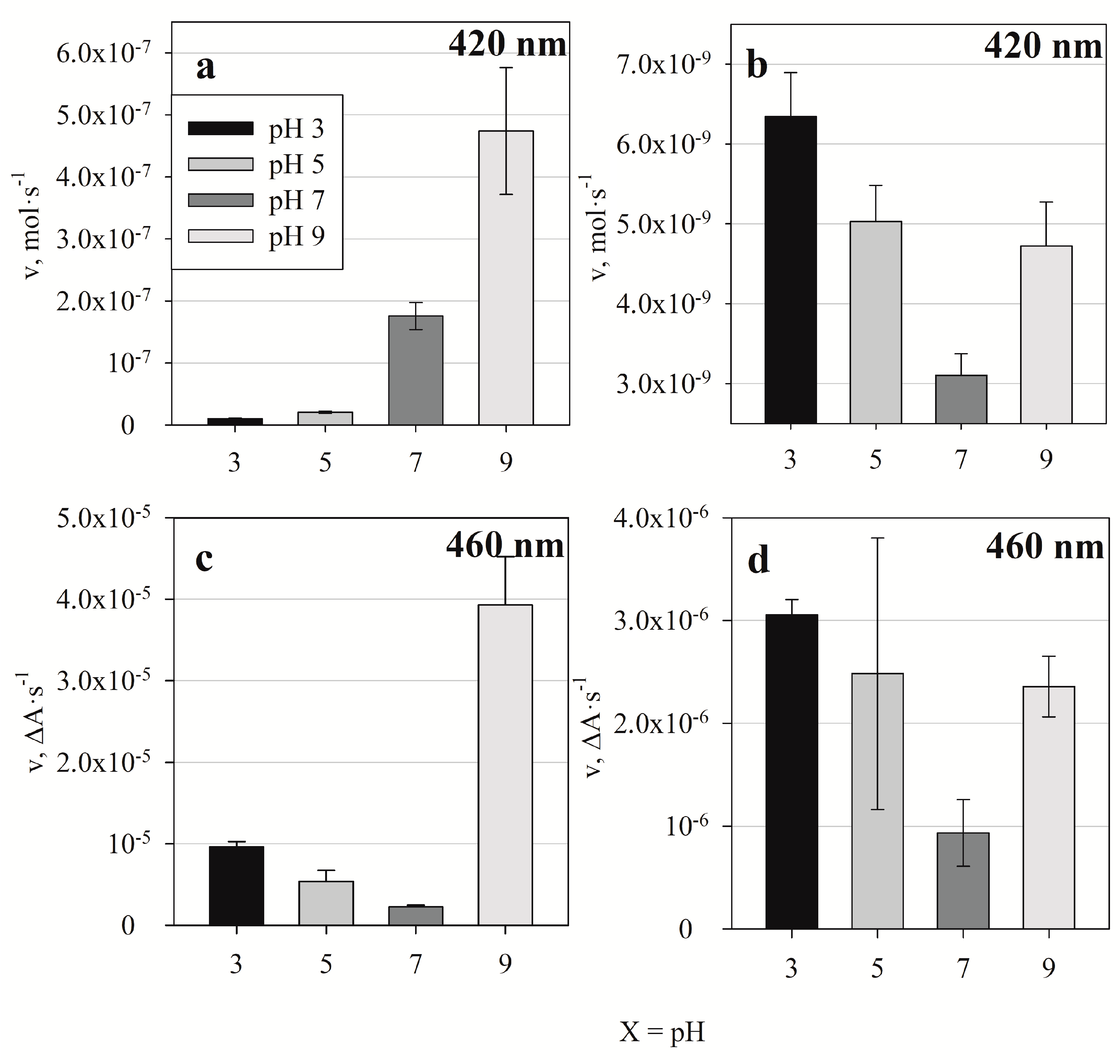
3.2. Spectrophotometric Evaluation of Ppy Formation Initiated by [Fe(CN)6]3−
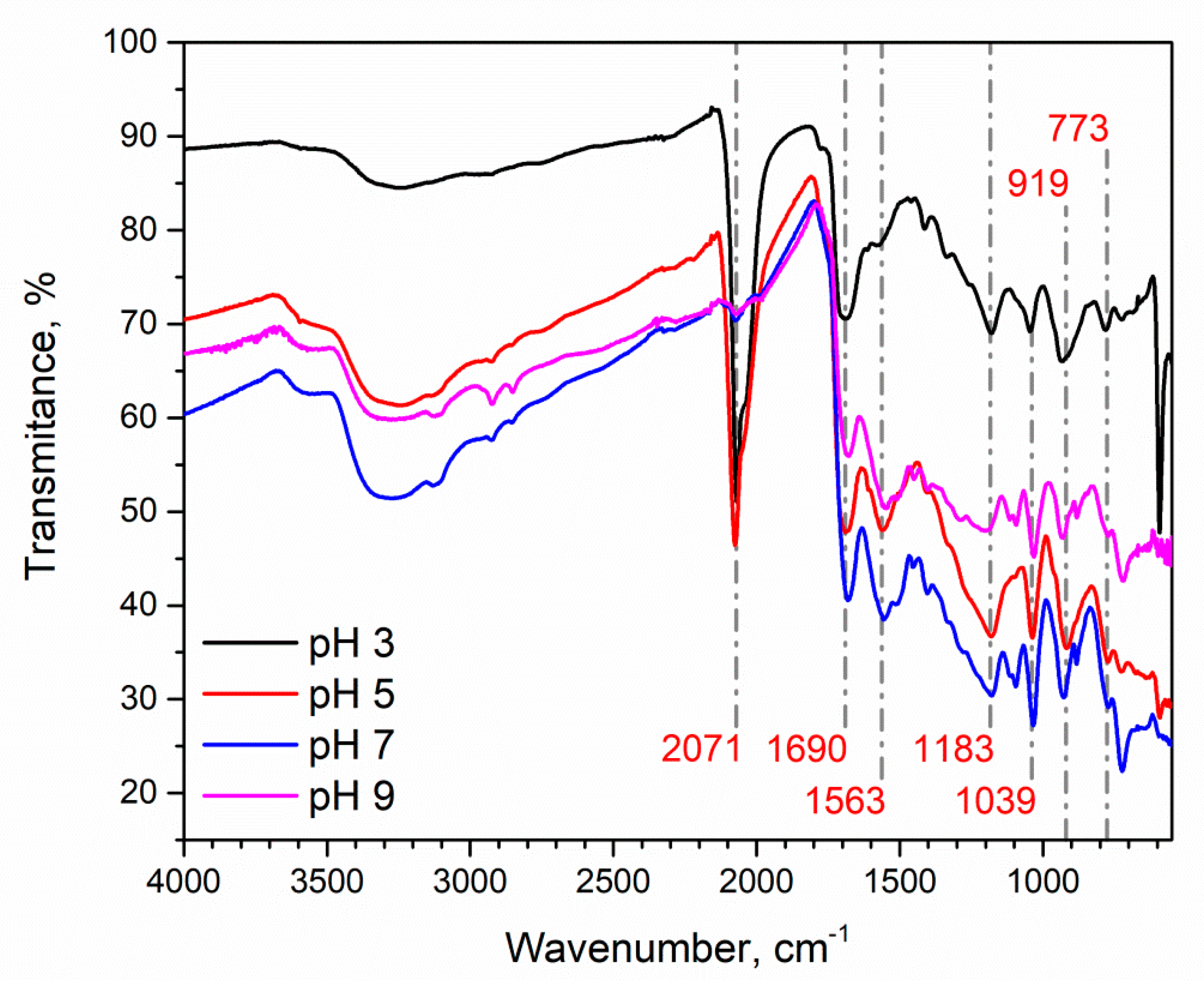
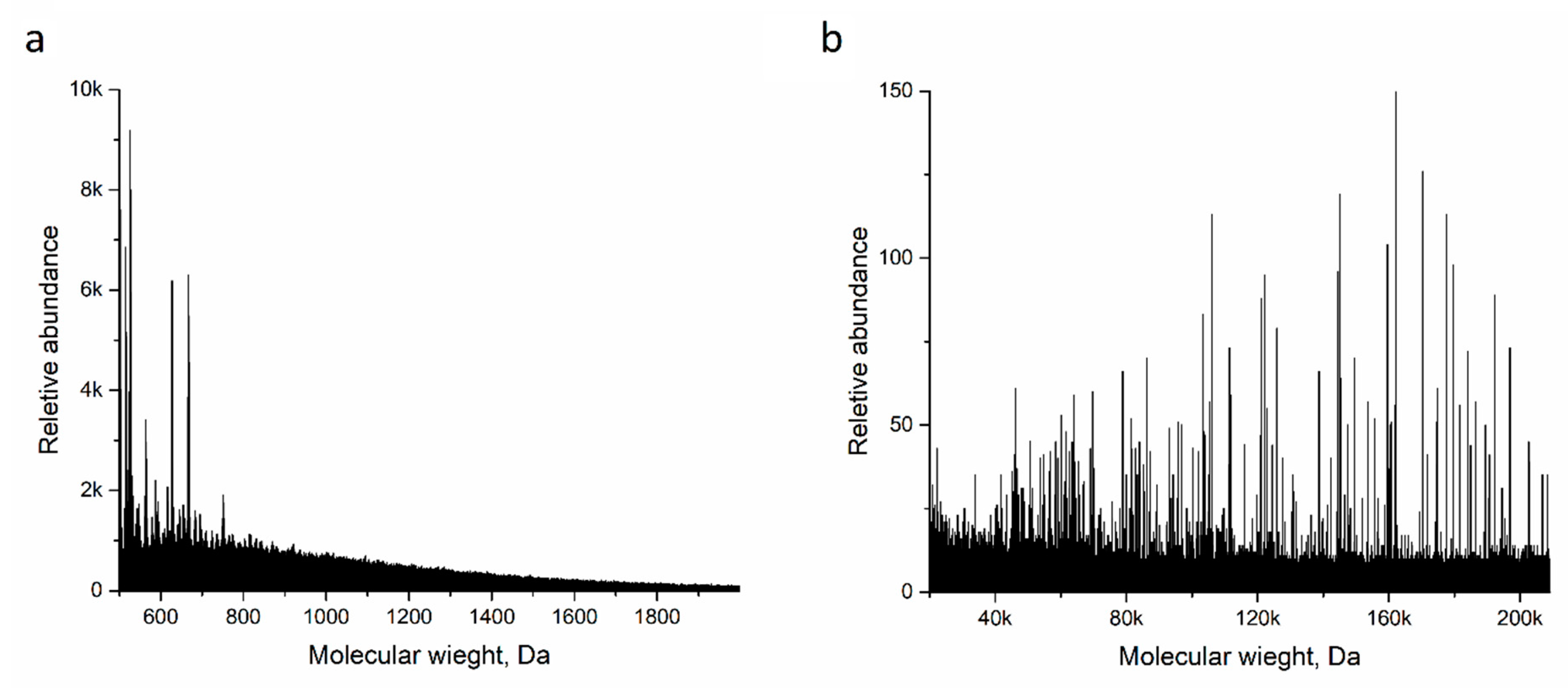
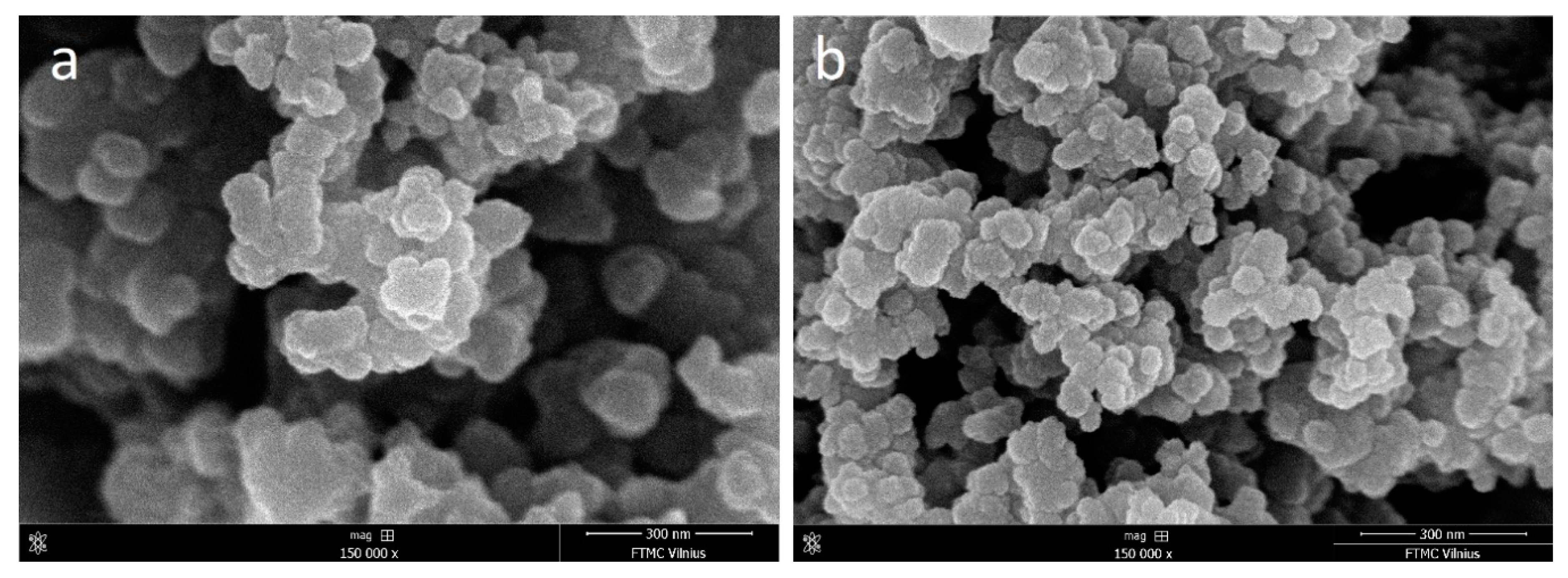
3.3. Ppy Doping in Acidic Medium and Characterization of Aggregates
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Das, T.K.; Prusty, S. Review on conducting polymers and their applications. Polym. Plast. Technol. Eng. 2012, 51, 1487–1500. [Google Scholar] [CrossRef]
- Ravichandran, R.; Sundarrajan, S.; Venugopal, J.R.; Mukherjee, S.; Ramakrishna, S. Applications of conducting polymers and their issues in biomedical engineering. J. R. Soc. Interface 2010, 7, S559–S579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, E.J.; Jang, K.S.; MacDiarmid, A.G. High molecular weight soluble polypyrrole. Synth. Met. 2001, 125, 267–272. [Google Scholar] [CrossRef]
- Njagi, J.; Andreescu, S. Stable enzyme biosensors based on chemically synthesized Au–polypyrrole nanocomposites. Biosens. Bioelectron. 2007, 23, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.H.; Ma, L.; Li, F.B.; Mai, H.J.; Lang, X.M.; Fan, S.S. A polypyrrole/anthraquinone-2,6-disulphonic disodium salt (PPy/AQDS)-modified anode to improve performance of microbial fuel cells. Biosens. Bioelectron. 2010, 25, 1516–1520. [Google Scholar] [CrossRef] [PubMed]
- Torres-Gomez, G.; Gomez-Romero, P. Conducting organic polymers with electroactive dopants. Synthesis and electrochemical properties of hexacyanoferrate-doped polypyrrole. Synth. Met. 1998, 98, 95–102. [Google Scholar] [CrossRef] [Green Version]
- Mazur, M.; Krywko-Cendrowska, A.; Krysinski, P.; Rogalski, J. Encapsulation of laccase in a conducting polymer matrix: A simple route towards polypyrrole microcontainers. Synth. Met. 2009, 159, 1731–1738. [Google Scholar] [CrossRef]
- Ramanaviciene, A.; Schuhmann, W.; Ramanavicius, A. AFM study of conducting polymer polypyrrole nanoparticles formed by redox enzyme—Glucose oxidase—Initiated polymerisation. Colloids Surf. B Biointerfaces 2006, 48, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Ramanavicius, A.; Kausaite, A.; Ramanaviciene, A.; Acaite, J.; Malinauskas, A. Redox enzyme—Glucose oxidase—Initiated synthesis of polypyrrole. Synth. Met. 2006, 156, 409–413. [Google Scholar] [CrossRef]
- Song, H.K.; Palmore, G.T.R. Conductive polypyrrole via enzyme catalysis. J. Phys. Chem. B 2005, 109, 19278–19287. [Google Scholar] [CrossRef] [PubMed]
- Riccardi, C.D.; Yamanaka, H.; Josowicz, M.; Kowalik, J.; Mizaikoff, B.; Kranz, C. Label-free DNA detection based on modified conducting polypyrrole films at microelectrodes. Anal. Chem. 2006, 78, 1139–1145. [Google Scholar] [CrossRef] [PubMed]
- Balint, R.; Cassidy, N.J.; Cartmell, S.H. Conductive polymers: Towards a smart biomaterial for tissue engineering. Acta Biomater. 2014, 10, 2341–2353. [Google Scholar] [CrossRef] [PubMed]
- Ansari, R. Polypyrrole Conducting Electroactive Polymers: Synthesis and Stability Studies. J. Chem. 2006, 3, 186–201. [Google Scholar] [CrossRef] [Green Version]
- Bjorklund, R.B. Kinetics of pyrrole polymerisation in aqueous iron chloride solution. J. Chem. Soc. Faraday Trans. Phys. Chem. Condens. Phases 1987, 83, 1507–1514. [Google Scholar] [CrossRef]
- Tan, Y.; Ghandi, K. Kinetics and mechanism of pyrrole chemical polymerization. Synth. Met. 2013, 175, 183–191. [Google Scholar] [CrossRef]
- Ballav, N.; Biswas, M. Oxidative polymerization of aniline and pyrrole by isopolymetallates of vanadium. J. Appl. Polym. Sci. 2005, 96, 1483–1486. [Google Scholar] [CrossRef]
- Chen, J.H.; Huang, Z.P.; Wang, D.Z.; Yang, S.X.; Li, W.Z.; Wen, J.G.; Ren, Z.F. Electrochemical synthesis of polypyrrole films over each of well-aligned carbon nanotubes. Synth. Met. 2001, 125, 289–294. [Google Scholar] [CrossRef]
- Wei, Y.Y.; Li, L.; Yang, X.M.; Pan, G.L.; Yan, G.P.; Yu, X.H. One-Step UV-Induced Synthesis of Polypyrrole/Ag Nanocomposites at the Water/Ionic Liquid Interface. Nanoscale Res. Lett. 2010, 5, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Chougule, M.A.; Pawar, S.G.; Godse, P.R.; Mulik, R.N.; Sen, S.; Patil, V.B. Synthesis and Characterization of Polypyrrole (PPy) Thin Films. Soft Nanosci. Lett. 2011, 1, 6–10. [Google Scholar] [CrossRef]
- Vaitkuviene, A.; Kaseta, V.; Voronovic, J.; Ramanauskaite, G.; Biziuleviciene, G.; Ramanaviciene, A.; Ramanavicius, A. Evaluation of cytotoxicity of polypyrrole nanoparticles synthesized by oxidative polymerization. J. Hazard. Mater. 2013, 250, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Kausaite, A.; Ramanaviciene, A.; Ramanavicius, A. Polyaniline synthesis catalysed by glucose oxidase. Polymer 2009, 50, 1846–1851. [Google Scholar] [CrossRef]
- Krikstolaityte, V.; Kuliesius, J.; Ramanaviciene, A.; Mikoliunaite, L.; Kausaite-Minkstimiene, A.; Oztekin, Y.; Ramanavicius, A. Enzymatic polymerization of polythiophene by immobilized glucose oxidase. Polymer 2014, 55, 1613–1620. [Google Scholar] [CrossRef]
- Ramanaviciene, A.; Kausaite, A.; Tautkus, S.; Ramanavicius, A. Biocompatibility of polypyrrole particles: An in vivo study in mice. J. Pharm. Pharmacol. 2007, 59, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Henry, M.C.; Hsueh, C.-C.; Timko, B.P.; Freund, M.S. Reaction of Pyrrole and Chlorauric acid A New Route to Composite Colloids. J. Electrochem. Soc. 2001, 148, D155–D162. [Google Scholar] [CrossRef]
- Ramanavičius, A.; Kaušaitė, A.; Ramanavičienė, A. Polypyrrole-coated glucose oxidase nanoparticles for biosensor design. Sens. Actuators B Chem. 2005, 111, 532–539. [Google Scholar] [CrossRef]
- Leonavicius, K.; Ramanaviciene, A.; Ramanavicius, A. Polymerization model for hydrogen peroxide initiated synthesis of polypyrrole nanoparticles. Langmuir 2011, 27, 10970–10976. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, D.; Endo, Y.; Takahashi, T.; Otake, K.; Shono, A. New method for the synthesis of polypyrrole particle using water/oil emulsion. J. Chem. Eng. Jpn. 2013, 46, 550–555. [Google Scholar] [CrossRef]
- Shanthala, V.S.; Shobha Devi, S.N.; Murugendrappa, M.V. Synthesis, characterization and DC conductivity studies of polypyrrole/copper zinc iron oxide nanocomposites. J. Asian Ceram. Soc. 2017, 5, 227–234. [Google Scholar] [CrossRef]
- Wang, J. Electrochemical glucose biosensors. Chem. Rev. 2008, 108, 814–825. [Google Scholar] [CrossRef] [PubMed]
- Rawson, F.J.; Downard, A.J.; Baronian, K.H. Electrochemical detection of intracellular and cell membrane redox systems in Saccharomyces cerevisiae. Sci. Rep. 2014, 4, 9. [Google Scholar] [CrossRef] [PubMed]
- Heiskanen, A.; Yakovleva, J.; Spegel, C.; Taboryski, R.; Koudelka-Hep, M.; Emneus, J.; Ruzgas, T. Amperometric monitoring of redox activity in living yeast cells: Comparison of menadione and menadione sodium bisulfite as electron transfer mediators. Electrochem. Commun. 2004, 6, 219–224. [Google Scholar] [CrossRef]
- Heiskanen, A.; Coman, V.; Kostesha, N.; Sabourin, D.; Haslett, N.; Baronian, K.; Gorton, L.; Dufva, M.; Emneus, J. Bioelectrochemical probing of intracellular redox processes in living yeast cells-application of redox polymer wiring in a microfluidic environment. Anal. Bioanal. Chem. 2013, 405, 3847–3858. [Google Scholar] [CrossRef] [PubMed]
- Nagamine, K.; Takahashi, Y.; Ino, K.; Shiku, H.; Matsue, T. Influence of tip size on single yeast cell imaging using scanning electrochemical microscopy. Electroanalysis 2011, 23, 1168–1174. [Google Scholar] [CrossRef]
- Dong, S.; Lian, G. Redox reactions of Fe(CN)3−/4−6 in polypyrrole films: Accumulation and removal of cations. J. Electroanal. Chem. Interfacial Electrochem. 1990, 291, 23–39. [Google Scholar] [CrossRef]
- Michalska, A.; Ivaska, A.; Lewenstam, A. Modeling potentiometric sensitivity of conducting polymers. Anal. Chem. 1997, 69, 4060–4064. [Google Scholar] [CrossRef] [PubMed]
- Goel, S.; Mazumdar, N.A.; Gupta, A. Synthesis and characterization of polypyrrole nanofibers with different dopants. Polym. Adv. Technol. 2010, 21, 205–210. [Google Scholar] [CrossRef]
- Toshima, N.; Hara, S. Direct synthesis of conducting polymers from simple monomers. Prog. Polym. Sci. 1995, 20, 155–183. [Google Scholar] [CrossRef]
- Zagorska, M.; Pron, A.; Lefrant, S.; Kucharski, Z.; Suwalski, J.; Bernier, P. Synthesis and spectroscopic characterization of polypyrrole containing ferrous cyanide anions. Synth. Met. 1987, 18, 43–48. [Google Scholar] [CrossRef]
- Chakrabarti, M.H.; Roberts, E.P.L. Analysis of Mixtures of Ferrocyanide and Ferricyanide using UV-Visible Spectroscopy for Characterisation of a Novel Redox Flow Battery. J. Chem. Soc. Pak. 2008, 30, 817–823. [Google Scholar]
- Appleby, C.A.; Morton, R.K. Lactic dehydrogenase and cytochrome b(2) of baker’s yeast. Purification and crystallization. Biochem. J. 1959, 71, 492–499. [Google Scholar] [CrossRef] [PubMed]
- De Winter, J.; Deshayes, G.; Boon, F.; Coulembier, O.; Dubois, P.; Gerbaux, P. MALDI-ToF analysis of polythiophene: Use of trans-2-[3-(4-t-butyl-phenyl)-2-methyl-2-propenylidene]malononitrile-DCTB-as matrix. J. Mass Spectrom. JMS 2011, 46, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Weng, B.; Shepherd, R.; Chen, J.; Wallace, G.G. Gemini surfactant doped polypyrrole nanodispersions: An inkjet printable formulation. J. Mater. Chem. 2011, 21, 1918–1924. [Google Scholar] [CrossRef]
- Wagner, B.A.; Venkataraman, S.; Buettner, G.R. The rate of oxygen utilization by cells. Free Radic. Biol. Med. 2011, 51, 700–712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, J.; Cooper, J.M. Method of determining oxygen concentrations in biological media, suitable for calibration of the oxygen electrode. Anal. Biochem. 1970, 33, 390–399. [Google Scholar] [CrossRef]
- Yan, H.; Kajita, M.; Toshima, N. Polymerization of Aniline Using Iron(III) Catalyst and Ozone, and Kinetics of Oxidation Reactions in the Catalytic System. Macromol. Mater. Eng. 2002, 287, 503–508. [Google Scholar] [CrossRef]
- Toshima, N.; Ihata, O. Catalytic synthesis of conductive polypyrrole using iron(III) catalyst and molecular oxygen. Synth. Met. 1996, 79, 165–172. [Google Scholar] [CrossRef]
- Dobson, K.D.; James McQuillan, A. An in situ IR spectroscopic investigation of adsorption of hexa- and penta-cyanoferrates to metal oxides from aqueous solution. Phys. Chem. Chem. Phys. 2000, 2, 5180–5188. [Google Scholar] [CrossRef]
- Idemura, S.; Suzuki, E.; Ono, Y. Electronic state of iron complexes in the interlayer of hydrotalcite-like materials. Clays Clay Miner. 1989, 37, 553–557. [Google Scholar] [CrossRef]
- Zou, Y.J.; Cheng, J.; Xiang, C.L.; Chu, H.L.; Qiu, S.J.; Xu, F.; Sun, L.X.; Zheng, L.J. Preparation, characterization of polypyrrole encapsulated Prussian blue nanocomposite and its application for biosensing. Int. J. Electrochem. Sci. 2015, 10, 4626–4636. [Google Scholar]
- Boutry, C.M.; Gerber-Hörler, I.; Hierold, C. Electrically conducting biodegradable polymer composites (polylactide-polypyrrole and polycaprolactone-polypyrrole) for passive resonant circuits. Polym. Eng. Sci. 2013, 53, 1196–1208. [Google Scholar] [CrossRef]
- Wu, K.J.; Odom, R.W. Peer Reviewed: Characterizing Synthetic Polymers by MALDI MS. Anal. Chem. 1998, 70, 456A–461A. [Google Scholar] [CrossRef] [PubMed]


| Sample No. | Compositions | Description of Result | ||
|---|---|---|---|---|
| Fe(CN)6]3− | [Fe(CN)6]4− | Pyrrole | ||
| 1 | 0.04 M | - | 0.5 M | Ppy was formed instantly |
| 2 | - | 0.04 M | 0.5 M | Ppy distinctly appeared only after 1–2 days |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andriukonis, E.; Ramanaviciene, A.; Ramanavicius, A. Synthesis of Polypyrrole Induced by [Fe(CN)6]3− and Redox Cycling of [Fe(CN)6]4−/[Fe(CN)6]3−. Polymers 2018, 10, 749. https://doi.org/10.3390/polym10070749
Andriukonis E, Ramanaviciene A, Ramanavicius A. Synthesis of Polypyrrole Induced by [Fe(CN)6]3− and Redox Cycling of [Fe(CN)6]4−/[Fe(CN)6]3−. Polymers. 2018; 10(7):749. https://doi.org/10.3390/polym10070749
Chicago/Turabian StyleAndriukonis, Eivydas, Almira Ramanaviciene, and Arunas Ramanavicius. 2018. "Synthesis of Polypyrrole Induced by [Fe(CN)6]3− and Redox Cycling of [Fe(CN)6]4−/[Fe(CN)6]3−" Polymers 10, no. 7: 749. https://doi.org/10.3390/polym10070749








