Construction of a Different Polymer Chain Structure to Study π-π Interaction between Polymer and Reduced Graphene Oxide
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of Polymers with Different Chain Structure
2.3. Preparation of RGO
2.4. Preparation of Polymer Nanocomposites
2.5. Characterization
3. Results and Discussion
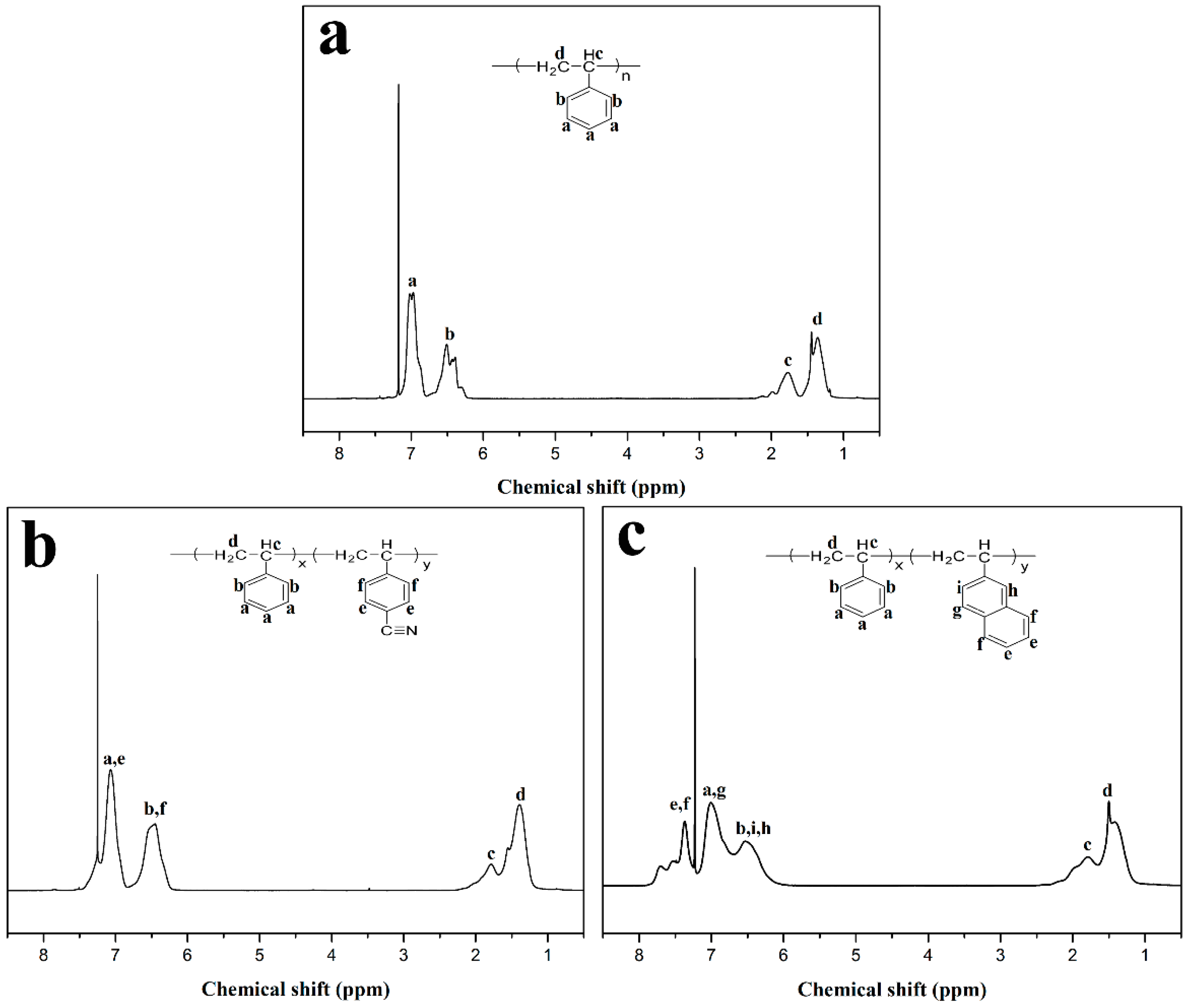
3.1. Structure Characterization of Polymers with Different Chain Structure
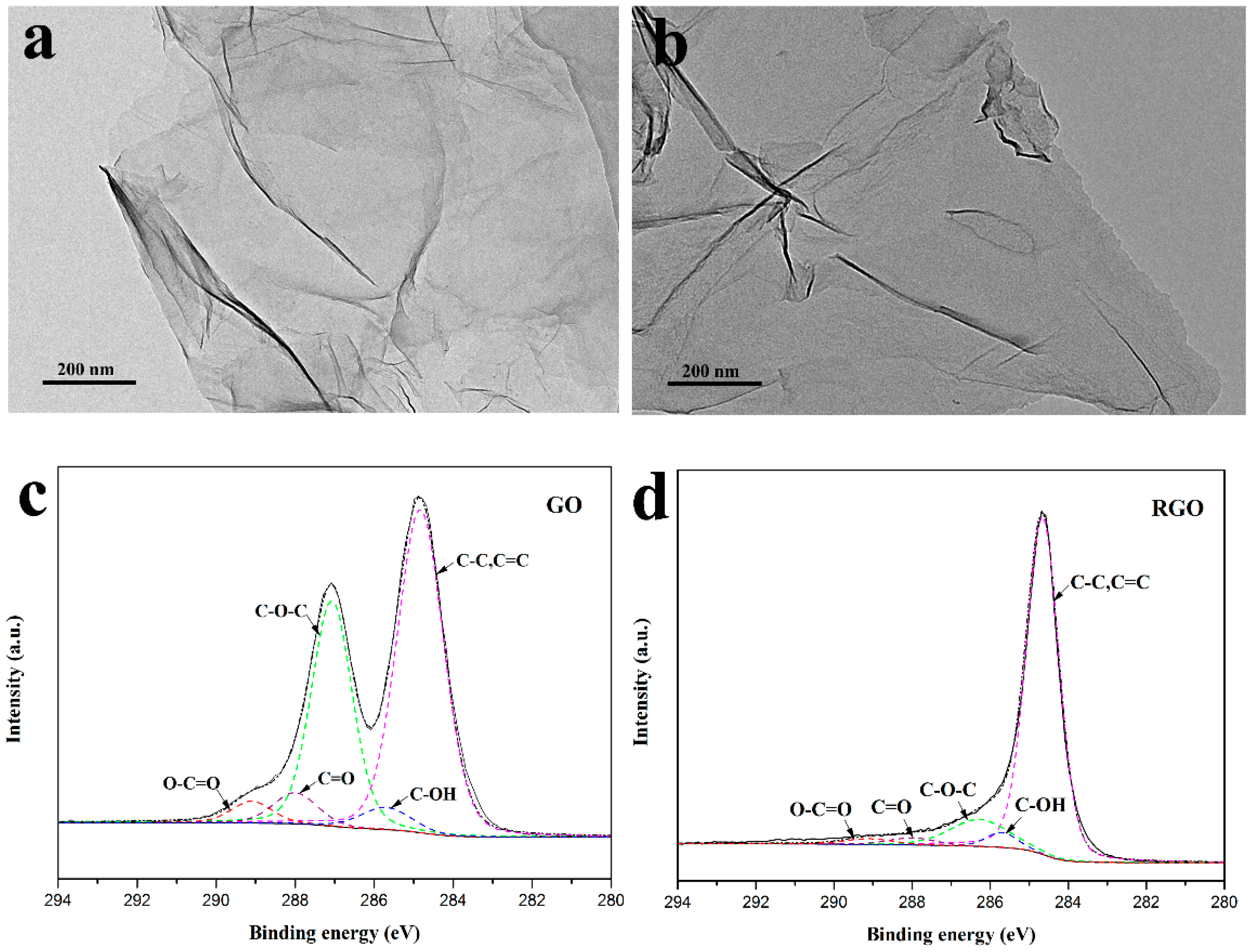
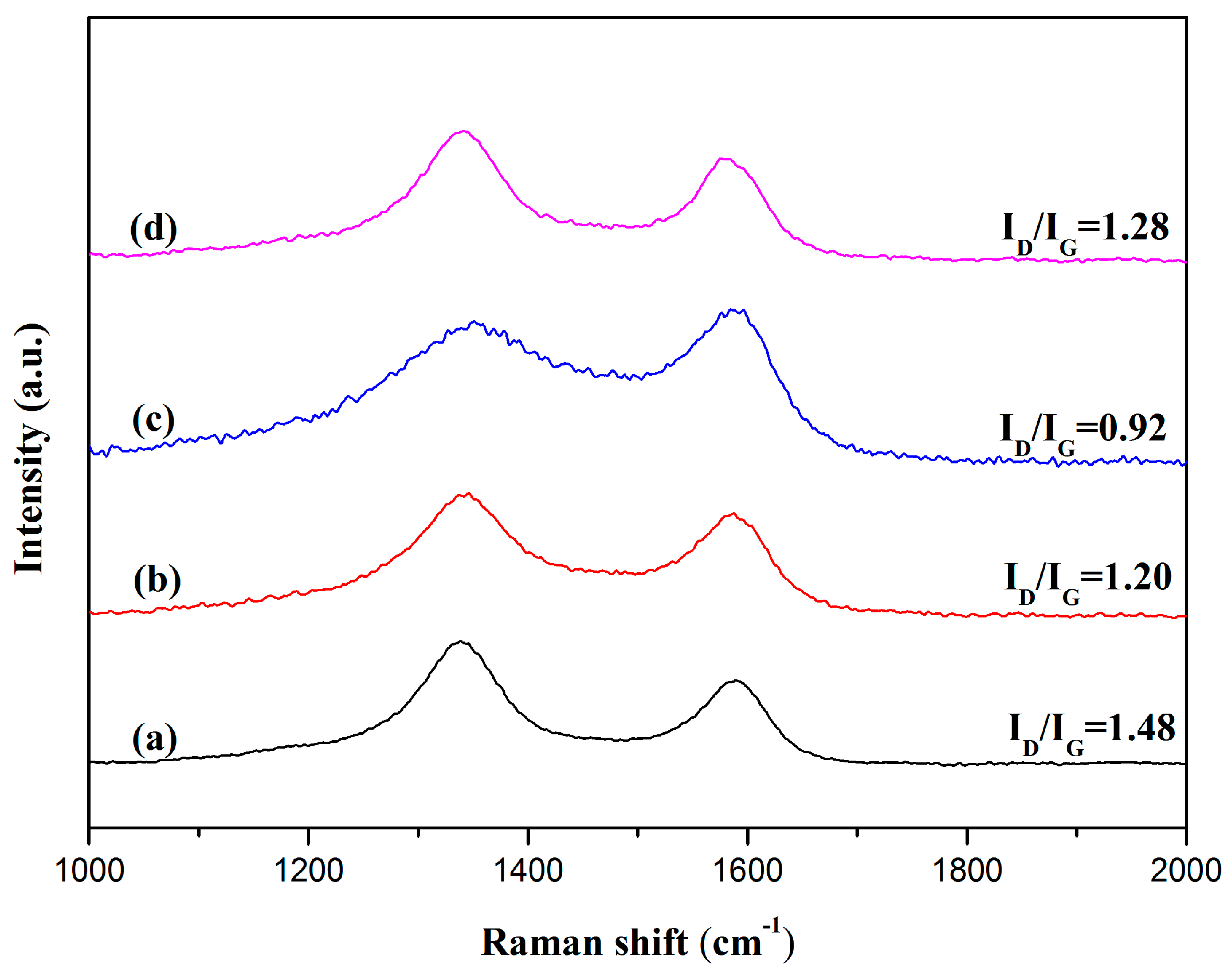
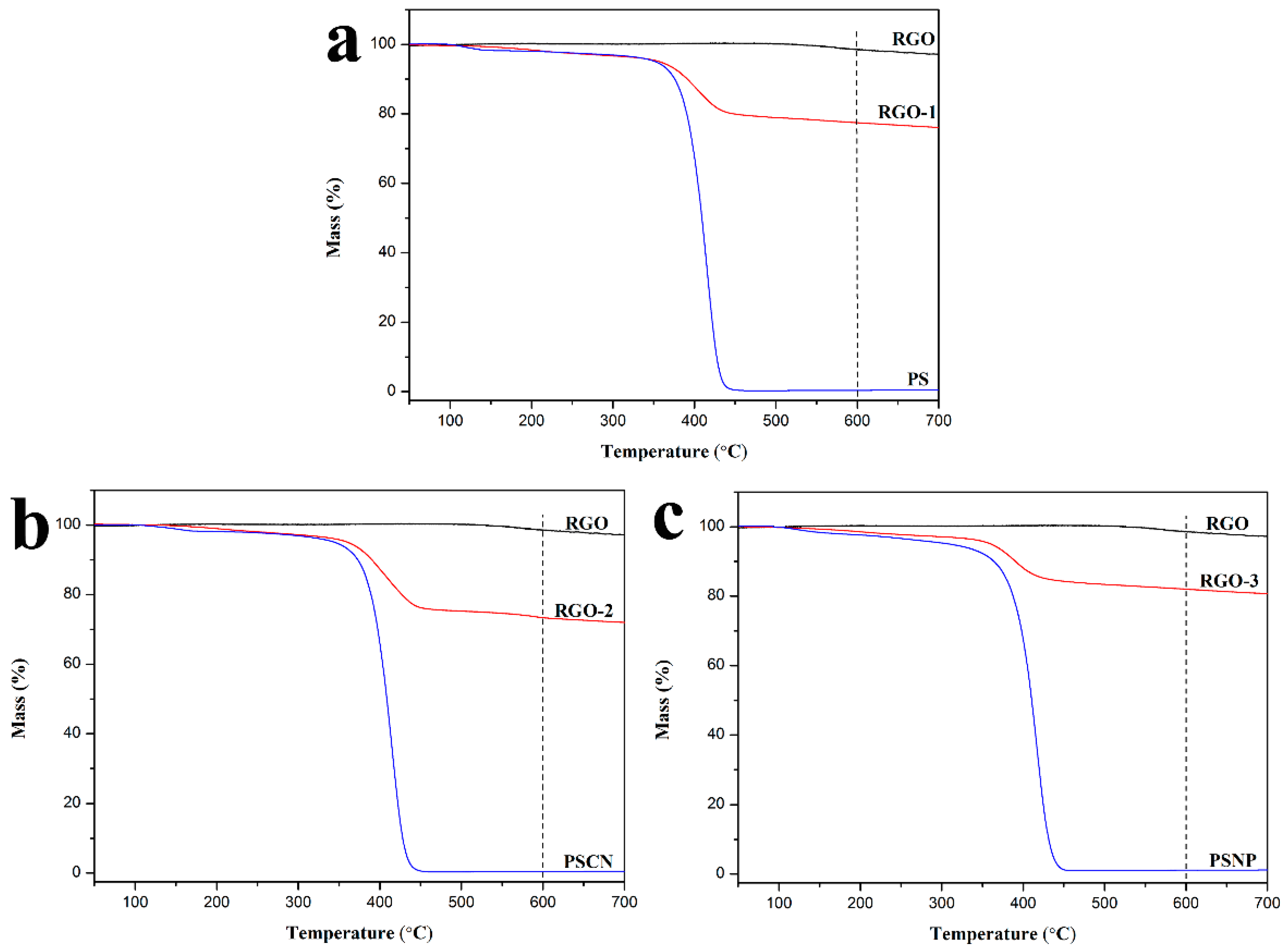
3.2. Characterization of RGO
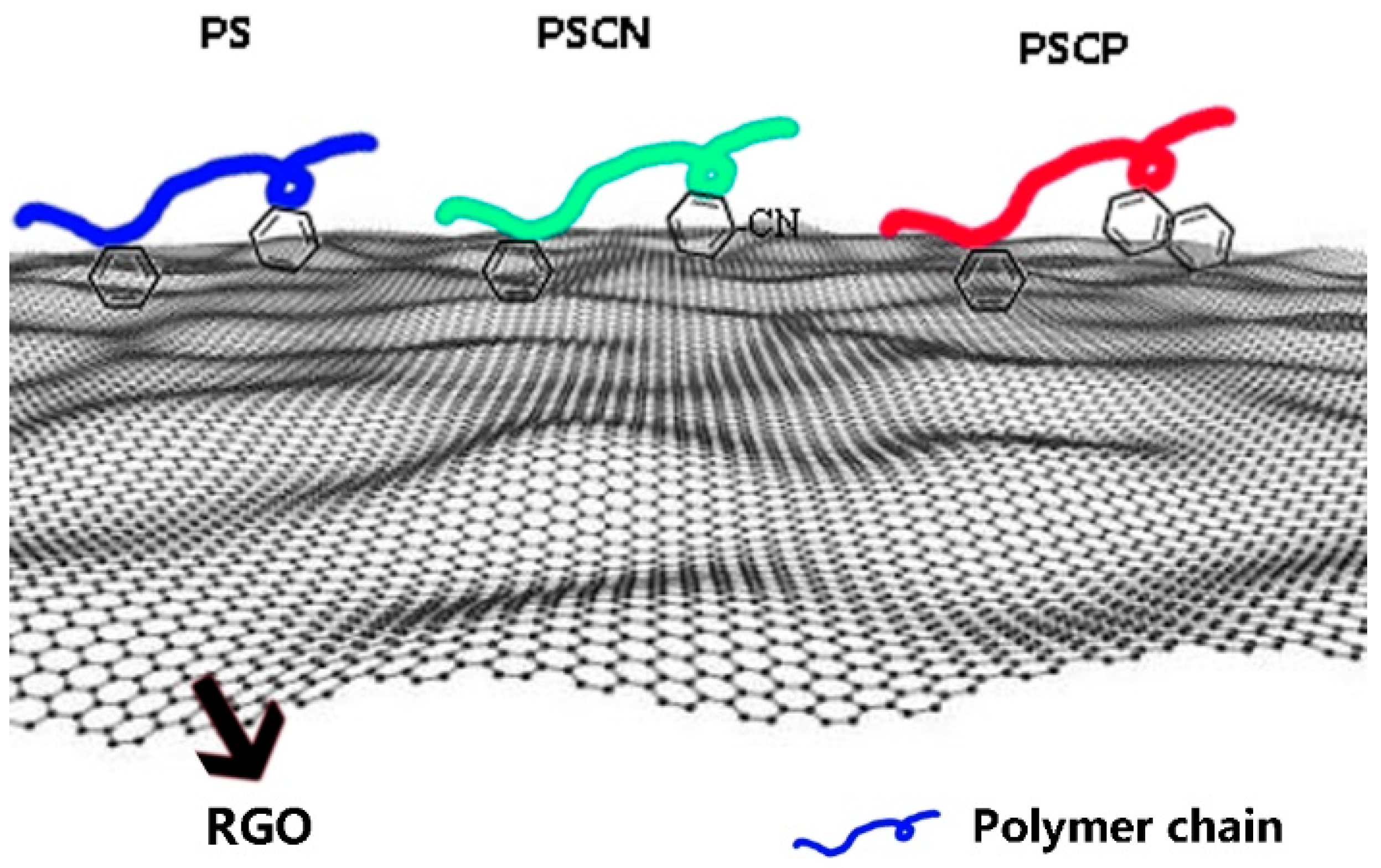
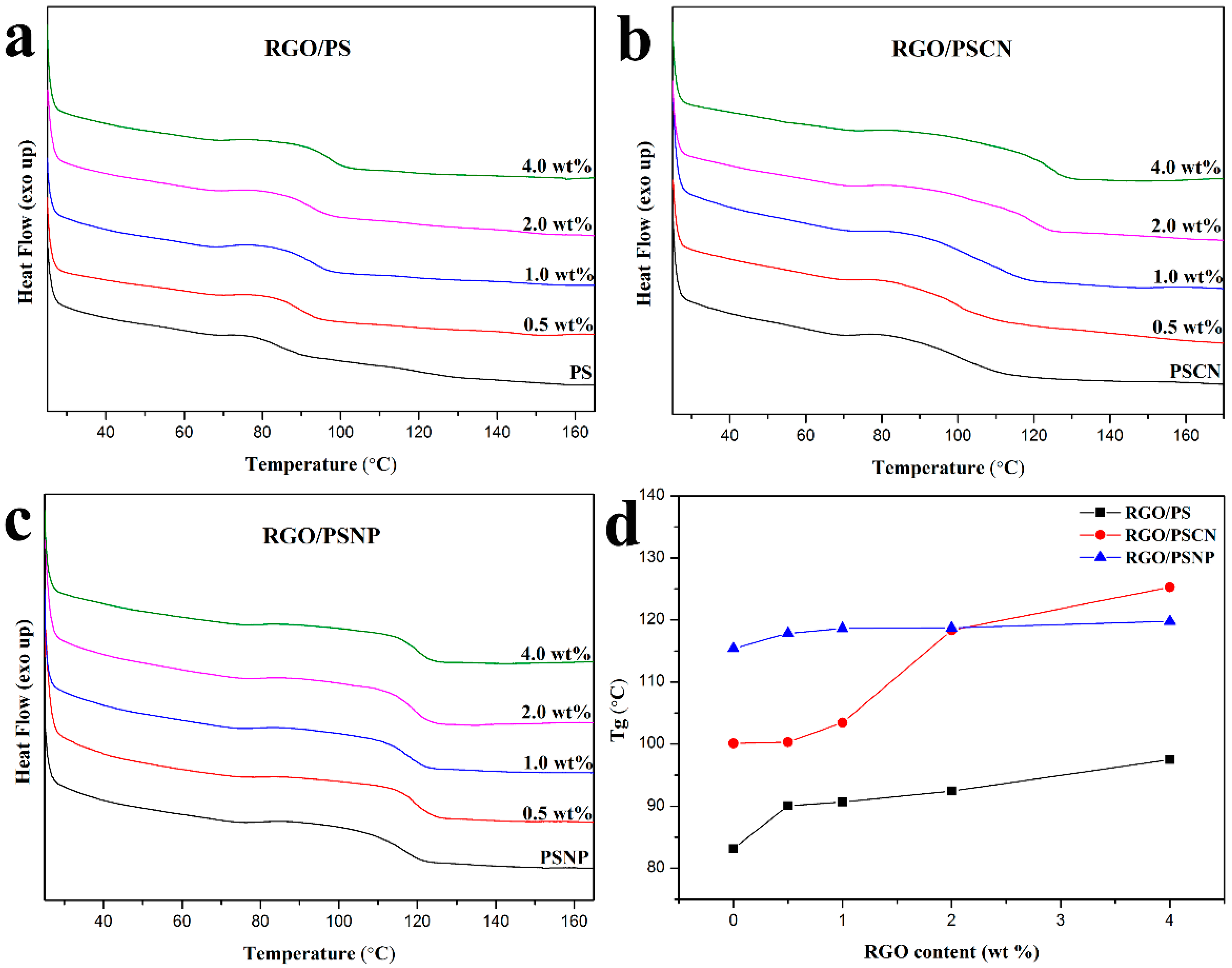
3.3. Interfacial Interaction between RGO and Polymers
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, S.; Liu, P.; Zhao, X.; Xu, J. Preparation of poly(vinyl alcohol)-grafted graphene oxide/poly(vinyl alcohol) nanocomposites via in-situ low-temperature emulsion polymerization and their thermal and mechanical characterization. Appl. Surf. Sci. 2017, 396, 1098–1107. [Google Scholar] [CrossRef]
- Vedhanarayanan, B.; Babu, B.; Shaijumon, M.M.; Ajayaghosh, A. Exfoliation of Reduced Graphene Oxide with Self-Assembled pi-Gelators for Improved Electrochemical Performance. ACS Appl. Mater. Interfaces 2017, 9, 19417–19426. [Google Scholar] [CrossRef] [PubMed]
- Teng, C.; Xie, D.; Wang, J.; Yang, Z.; Ren, G.; Zhu, Y. Ultrahigh conductive graphene paper based on ball-milling exfoliated graphene. Adv. Funct. Mater. 2017, 27, 1700240. [Google Scholar] [CrossRef]
- Smith, K.B.; Tomassone, M.S. Ultrathin hollow graphene oxide membranes for use as nanoparticle carriers. Langmuir 2017, 33, 3765–3775. [Google Scholar] [CrossRef] [PubMed]
- Shaygan Nia, A.; Binder, W.H. Graphene as initiator/catalyst in polymerization chemistry. Prog. Polym. Sci. 2017, 67, 48–76. [Google Scholar] [CrossRef]
- Ou, B.; Zhou, Z.; Liu, Q.; Liao, B.; Yi, S.; Ou, Y.; Zhang, X.; Li, D. Covalent functionalization of graphene with poly(methyl methacrylate) by atom transfer radical polymerization at room temperature. Polym. Chem. 2012, 3, 2768–2775. [Google Scholar] [CrossRef]
- Li, A.; Zhang, C.; Zhang, Y. Thermal conductivity of graphene-polymer composites: Mechanisms, properties, and applications. Polymers 2017, 9, 437. [Google Scholar]
- Rissanou, A.; Power, A.; Harmandaris, V. Structural and dynamical properties of polyethylene/graphene nanocomposites through molecular dynamics simulations. Polymers 2015, 7, 390–417. [Google Scholar] [CrossRef]
- Hu, K.; Gupta, M.K.; Kulkarni, D.D.; Tsukruk, V.V. Ultra-robust graphene oxide-silk fibroin nanocomposite membranes. Adv. Mater. 2013, 25, 2301–2307. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, N.; Sun, X.; Lin, X.; Shen, X.; Jia, J.; Zhang, B.; Tang, B.; Chan, M.; Kim, J.K. Highly aligned graphene/polymer nanocomposites with excellent dielectric properties for high-performance electromagnetic interference shielding. Adv. Mater. 2014, 26, 5480–5487. [Google Scholar] [CrossRef] [PubMed]
- Ding, P.; Su, S.; Song, N.; Tang, S.; Liu, Y.; Shi, L. Highly thermal conductive composites with polyamide-6 covalently-grafted graphene by an in situ polymerization and thermal reduction process. Carbon 2014, 66, 576–584. [Google Scholar] [CrossRef]
- Jiang, H.; Chen, L.; Chai, S.; Yao, X.; Chen, F.; Fu, Q. Facile fabrication of poly (tetrafluoroethylene)/graphene nanocomposite via electrostatic self-assembly approach. Compos. Sci. Technol. 2014, 103, 28–35. [Google Scholar] [CrossRef]
- Gong, J.; Liu, Z.; Yu, J.; Dai, D.; Dai, W.; Du, S.; Li, C.; Jiang, N.; Zhan, Z.; Lin, C.-T. Graphene woven fabric-reinforced polyimide films with enhanced and anisotropic thermal conductivity. Compos. Part A Appl. Sci. 2016, 87, 290–296. [Google Scholar] [CrossRef]
- Hofmann, D.; Thomann, R.; Mülhaupt, R. Thermoplastic SEBS elastomer nanocomposites reinforced with functionalized graphene dispersions. Macromol. Mater. Eng. 2018, 303, 1700324. [Google Scholar] [CrossRef]
- Díez-Pascual, A.; Luceño Sánchez, J.; Peña Capilla, R.; García Díaz, P. Recent developments in graphene/polymer nanocomposites for application in polymer solar cells. Polymers 2018, 10, 217. [Google Scholar] [CrossRef]
- Jun, Y.S.; Sy, S.; Ahn, W.; Zarrin, H.; Rasen, L.; Tjandra, R.; Amoli, B.M.; Zhao, B.; Chiu, G.; Yu, A. Highly conductive interconnected graphene foam based polymer composite. Carbon 2015, 95, 653–658. [Google Scholar] [CrossRef]
- Sekhavat Pour, Z.; Ghaemy, M. Polymer grafted graphene oxide: For improved dispersion in epoxy resin and enhancement of mechanical properties of nanocomposite. Compos. Sci. Technol. 2016, 136, 145–157. [Google Scholar] [CrossRef]
- Ye, X.; Gong, P.; Wang, J.; Wang, H.; Ren, S.; Yang, S. Fluorinated graphene reinforced polyimide films with the improved thermal and mechanical properties. Compos. Part A Appl. Sci. 2015, 75, 96–103. [Google Scholar] [CrossRef]
- Thomassin, J.M.; Trifkovic, M.; Alkarmo, W.; Detrembleur, C.; Jérôme, C.; Macosko, C. Poly(methyl methacrylate)/graphene oxide nanocomposites by a precipitation polymerization process and their dielectric and rheological characterization. Macromolecules 2014, 47, 2149–2155. [Google Scholar] [CrossRef]
- Pan, B.; Zhang, S.; Li, W.; Zhao, J.; Liu, J.; Zhang, Y.; Zhang, Y. Tribological and mechanical investigation of MC nylon reinforced by modified graphene oxide. Wear 2012, 294–295, 395–401. [Google Scholar] [CrossRef]
- Araby, S.; Meng, Q.; Zhang, L.; Kang, H.; Majewski, P.; Tang, Y.; Ma, J. Electrically and thermally conductive elastomer/graphene nanocomposites by solution mixing. Polymer 2014, 55, 201–210. [Google Scholar] [CrossRef]
- Lim, M.Y.; Kim, H.J.; Baek, S.J.; Kim, K.Y.; Lee, S.S.; Lee, J.C. Improved strength and toughness of polyketone composites using extremely small amount of polyamide 6 grafted graphene oxides. Carbon 2014, 77, 366–378. [Google Scholar] [CrossRef]
- Kim, H.W.; Yoon, J.H.; Diederichsen, K.M.; Shin, J.E.; Yoo, B.M.; McCloskey, B.D.; Park, H.B. Exceptionally reinforced polymer nanocomposites via incorporated surface porosity on graphene oxide Ssheets. Macromol. Mater. Eng. 2017, 302, 1700039. [Google Scholar] [CrossRef]
- Wode, F.; Tzounis, L.; Kirsten, M.; Constantinou, M.; Georgopanos, P.; Rangou, S.; Zafeiropoulos, N.E.; Avgeropoulos, A.; Stamm, M. Selective localization of multi-wall carbon nanotubes in homopolymer blends and a diblock copolymer. Rheological orientation studies of the final nanocomposites. Polymer 2012, 53, 4438–4447. [Google Scholar] [CrossRef]
- Tzounis, L.; Pegel, S.; Zafeiropoulos, N.E.; Avgeropoulos, A.; Paipetis, A.S.; Stamm, M. Shear alignment of a poly(styrene-butadiene-styrene) triblock copolymer/MWCNT nanocomposite. Polymer 2017, 131, 1–9. [Google Scholar] [CrossRef]
- Liebscher, M.; Tzounis, L.; Pötschke, P.; Heinrich, G. Influence of the viscosity ratio in PC/SAN blends filled with MWCNTs on the morphological, electrical, and melt rheological properties. Polymer 2013, 54, 6801–6808. [Google Scholar] [CrossRef]
- Goldstein, A.; Venker, E.; Weng, C. Evidence appraisal: A scoping review, conceptual framework, and research agenda. J. Am. Med. Inform. Assoc. 2017, 24, 1192–1203. [Google Scholar] [CrossRef] [PubMed]
- Tzounis, L.; Hegde, M.; Liebscher, M.; Dingemans, T.; Pötschke, P.; Paipetis, A.S.; Zafeiropoulos, N.E.; Stamm, M. All-aromatic SWCNT-Polyetherimide nanocomposites for thermal energy harvesting applications. Compos. Sci. Technol. 2018, 156, 158–165. [Google Scholar] [CrossRef]
- Liebscher, M.; Gärtner, T.; Tzounis, L.; Mičušík, M.; Pötschke, P.; Stamm, M.; Heinrich, G.; Voit, B. Influence of the MWCNT surface functionalization on the thermoelectric properties of melt-mixed polycarbonate composites. Compos. Sci. Technol. 2014, 101, 133–138. [Google Scholar] [CrossRef]
- Tang, Z.; Lei, Y.; Guo, B.; Zhang, L.; Jia, D. The use of rhodamine B-decorated graphene as a reinforcement in polyvinyl alcohol composites. Polymer 2012, 53, 673–680. [Google Scholar] [CrossRef]
- Liu, J.; Tang, J.; Gooding, J.J. Strategies for chemical modification of graphene and applications of chemically modified graphene. J. Mater. Chem. 2012, 22, 12435–12452. [Google Scholar] [CrossRef]
- Shen, B.; Zhai, W.; Chen, C.; Lu, D.; Wang, J.; Zheng, W. Melt blending in situ enhances the interaction between polystyrene and graphene through pi-pi stacking. ACS Appl. Mater. Interfaces 2011, 3, 3103–3109. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xu, Y.; Cui, L.; Fu, A.; Yang, W.; Barrow, C.; Liu, J. Mechanical properties of graphene films enhanced by homo-telechelic functionalized polymer fillers via π–π stacking interactions. Compos. Part A Appl. Sci. 2015, 71, 1–8. [Google Scholar] [CrossRef]
- Liu, Y.; Hao, W.; Yao, H.; Li, S.; Wu, Y.; Zhu, J.; Jiang, L. Solution adsorption formation of a pi-conjugated polymer/graphene composite for high-performance field-effect transistors. Adv. Mater. 2018, 30, 1705377. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Cai, H.; Fang, X.; Zheng, J. The improved thermal oxidative stability of silicone rubber by incorporating reduced graphene oxide: Impact factors and action mechanism. Polym. Compos. 2016, 39, 1105–1115. [Google Scholar] [CrossRef]
- Arredondo, J.; Elizalde, L.E.; Le Droumaguet, B.; Grande, D. A new route toward imidazoline-functionalized porous polymeric materials from corresponding polystyrene-polylactide diblock copolymers. React. Funct. Polym. 2016, 104, 62–70. [Google Scholar] [CrossRef]
- Zeng, F.; Yang, M.; Zhang, J.; Varshney, S.K. Synthesis and characterization of block copolymers from 2-vinylnaphthalene by anionic polymerization. J. Polym. Sci. Polym. Chem. 2002, 40, 4387–4397. [Google Scholar] [CrossRef]
- Wan, X.; Zhang, G.; Ge, Z.; Narain, R.; Liu, S. Construction of polymer-protein bioconjugates with varying chain topologies: Polymer molecular weight and steric hindrance effects. Chem. Asian J. 2011, 6, 2835–2845. [Google Scholar] [CrossRef] [PubMed]
- Selvakumari, J.C.; Dhanalakshmi, J.; Padiyan, D.P. Effect of hydrogen peroxide and camellia sinensis extract on reduction of oxygen level in graphene oxide. Mater. Res. Express 2016, 3, 105011. [Google Scholar] [CrossRef]
- Chua, C.K.; Ambrosi, A.; Pumera, M. Graphene oxide reduction by standard industrial reducing agent: Thiourea dioxide. J. Mater. Chem. 2012, 22, 11054–11061. [Google Scholar] [CrossRef]
- Stobinski, L.; Lesiak, B.; Malolepszy, A.; Mazurkiewicz, M.; Mierzwa, B.; Zemek, J.; Jiricek, P.; Bieloshapka, I. Graphene oxide and reduced graphene oxide studied by the XRD, TEM and electron spectroscopy methods. J. Electron Spectrosc. 2014, 195, 145–154. [Google Scholar] [CrossRef]
- Jiang, S.-D.; Bai, Z.-M.; Tang, G.; Hu, Y.; Song, L. Fabrication and characterization of graphene oxide-reinforced poly(vinyl alcohol)-based hybrid composites by the sol–gel method. Compos. Sci. Technol. 2014, 102, 51–58. [Google Scholar] [CrossRef]
- Tzounis, L.; Kirsten, M.; Simon, F.; Mäder, E.; Stamm, M. The interphase microstructure and electrical properties of glass fibers covalently and non-covalently bonded with multiwall carbon nanotubes. Carbon 2014, 73, 310–324. [Google Scholar] [CrossRef]
- Tu, J.; Zhao, M.; Zhan, X.; Ruan, Z.; Zhang, H.-L.; Li, Q.; Li, Z. Functionalization of graphene by a TPE-containing polymer using nitrogen-based nucleophiles. Polym. Chem. 2016, 7, 4054–4062. [Google Scholar] [CrossRef]
- Turlakov, G.; Arias, E.; Moggio, I.; Jiménez-Barrera, R.M.; González-Morones, P.; Fernández, S.; Rodríguez, O.; Ávila-Orta, C.; Ziolo, R.F. Synthesis of reduced graphene oxide-poly(phenyleneethynylene) hybrids. A supramolecular and photophysical analyses. Polymer 2017, 122, 174–183. [Google Scholar] [CrossRef]
- Perumal, S.; Lee, H.M.; Cheong, I.W. A study of adhesion forces between vinyl monomers and graphene surfaces for non-covalent functionalization of graphene. Carbon 2016, 107, 74–76. [Google Scholar] [CrossRef]
- Teh, S.-L.; Linton, D.; Sumpter, B.; Dadmun, M.D. Controlling non-Covalent interactions to modulate the dispersion of fullerenes in polymer nanocomposites. Macromolecules 2011, 44, 7737–7745. [Google Scholar] [CrossRef]
| Samples | (×104) | (×104) | PDI |
|---|---|---|---|
| PS | 3.84 | 5.70 | 1.48 |
| PSCN | 4.06 | 6.35 | 1.56 |
| PSNP | 2.76 | 5.84 | 2.11 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, D.; Zhu, G.; Ding, Y.; Zheng, J. Construction of a Different Polymer Chain Structure to Study π-π Interaction between Polymer and Reduced Graphene Oxide. Polymers 2018, 10, 716. https://doi.org/10.3390/polym10070716
Zhao D, Zhu G, Ding Y, Zheng J. Construction of a Different Polymer Chain Structure to Study π-π Interaction between Polymer and Reduced Graphene Oxide. Polymers. 2018; 10(7):716. https://doi.org/10.3390/polym10070716
Chicago/Turabian StyleZhao, Dan, Guangda Zhu, Yong Ding, and Junping Zheng. 2018. "Construction of a Different Polymer Chain Structure to Study π-π Interaction between Polymer and Reduced Graphene Oxide" Polymers 10, no. 7: 716. https://doi.org/10.3390/polym10070716
APA StyleZhao, D., Zhu, G., Ding, Y., & Zheng, J. (2018). Construction of a Different Polymer Chain Structure to Study π-π Interaction between Polymer and Reduced Graphene Oxide. Polymers, 10(7), 716. https://doi.org/10.3390/polym10070716






