Preparation of Novel Epoxy Resins Bearing Phthalazinone Moiety and Their Application as High-Temperature Adhesives
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Polymers
2.3. Characterization
3. Results and Discussion
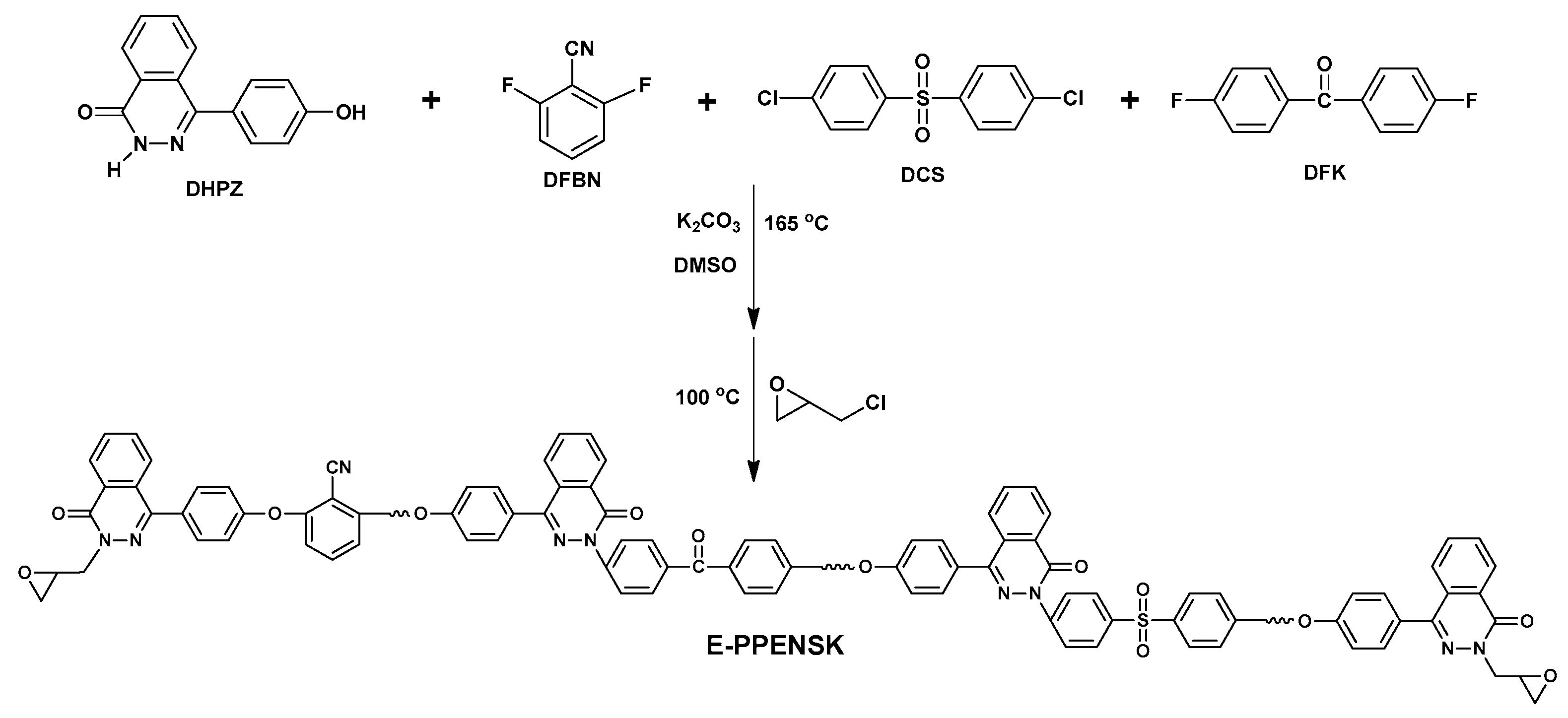
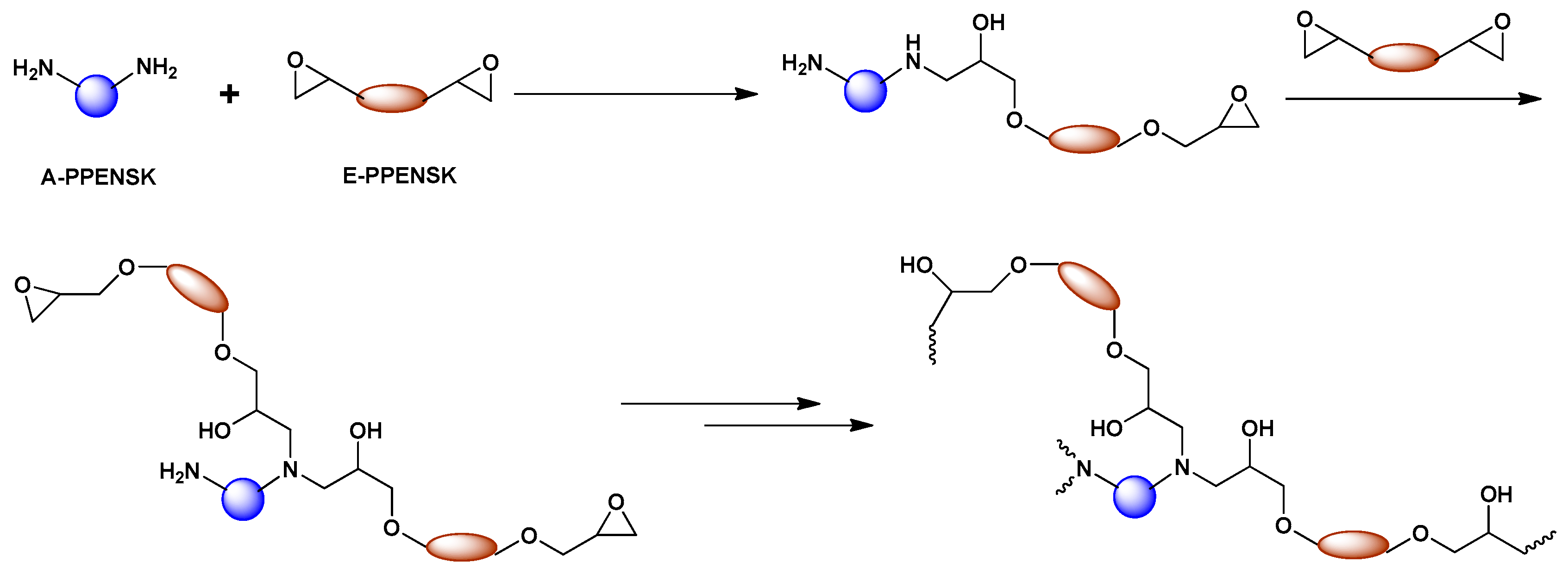
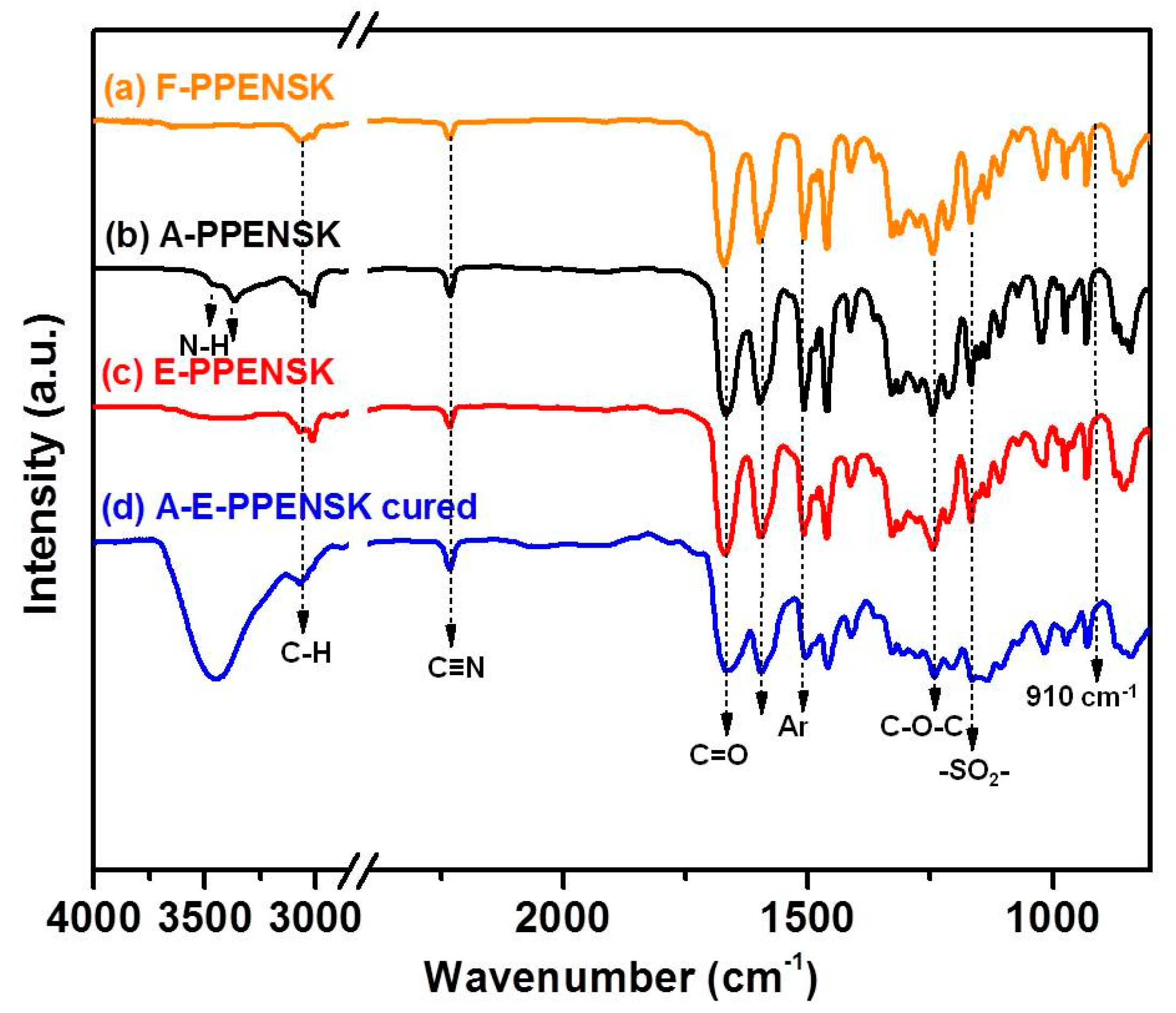
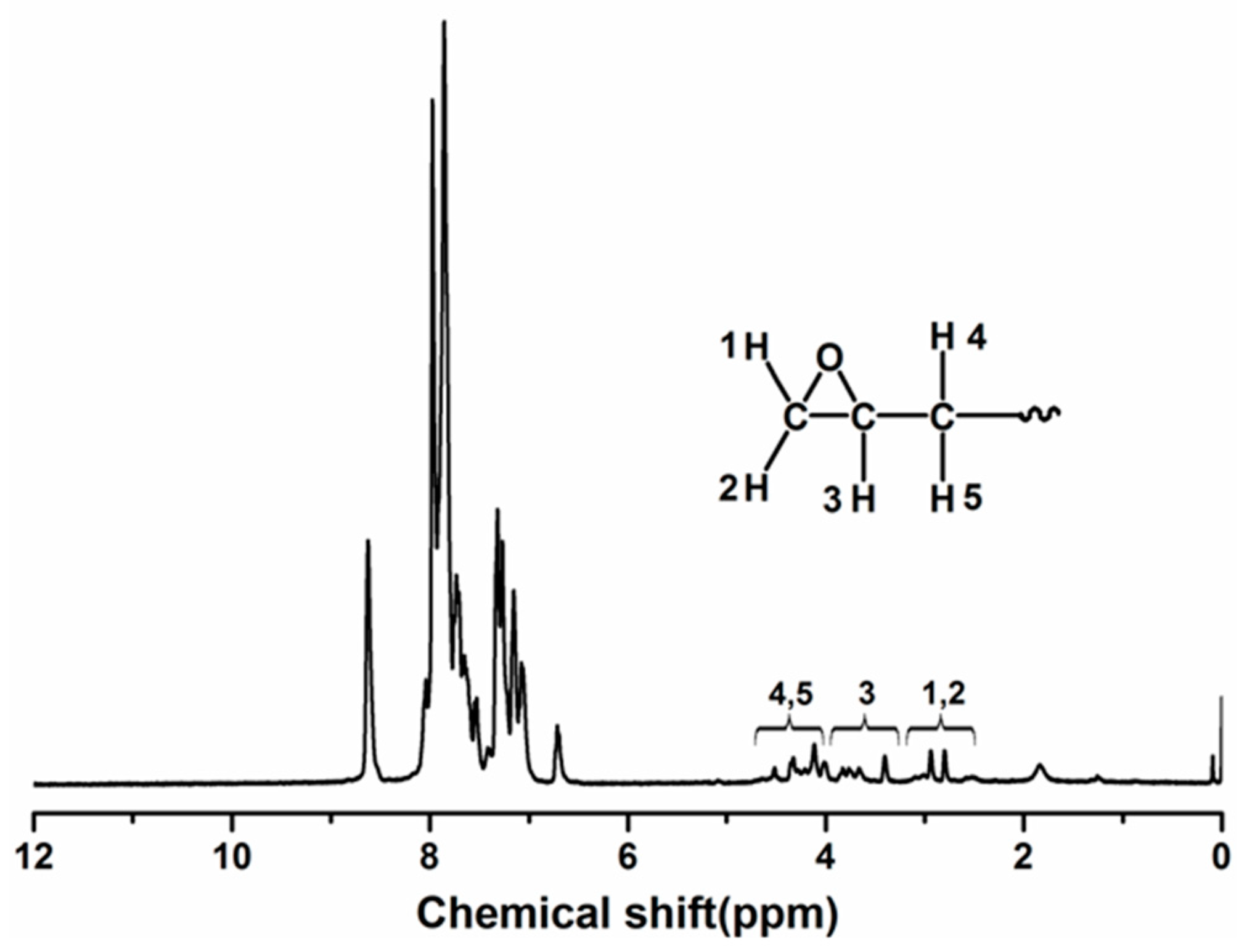
3.1. Synthesis of Epoxy Resin Based on Poly(Phthalazinone Ether Nitrile Sulfone Ketone)s
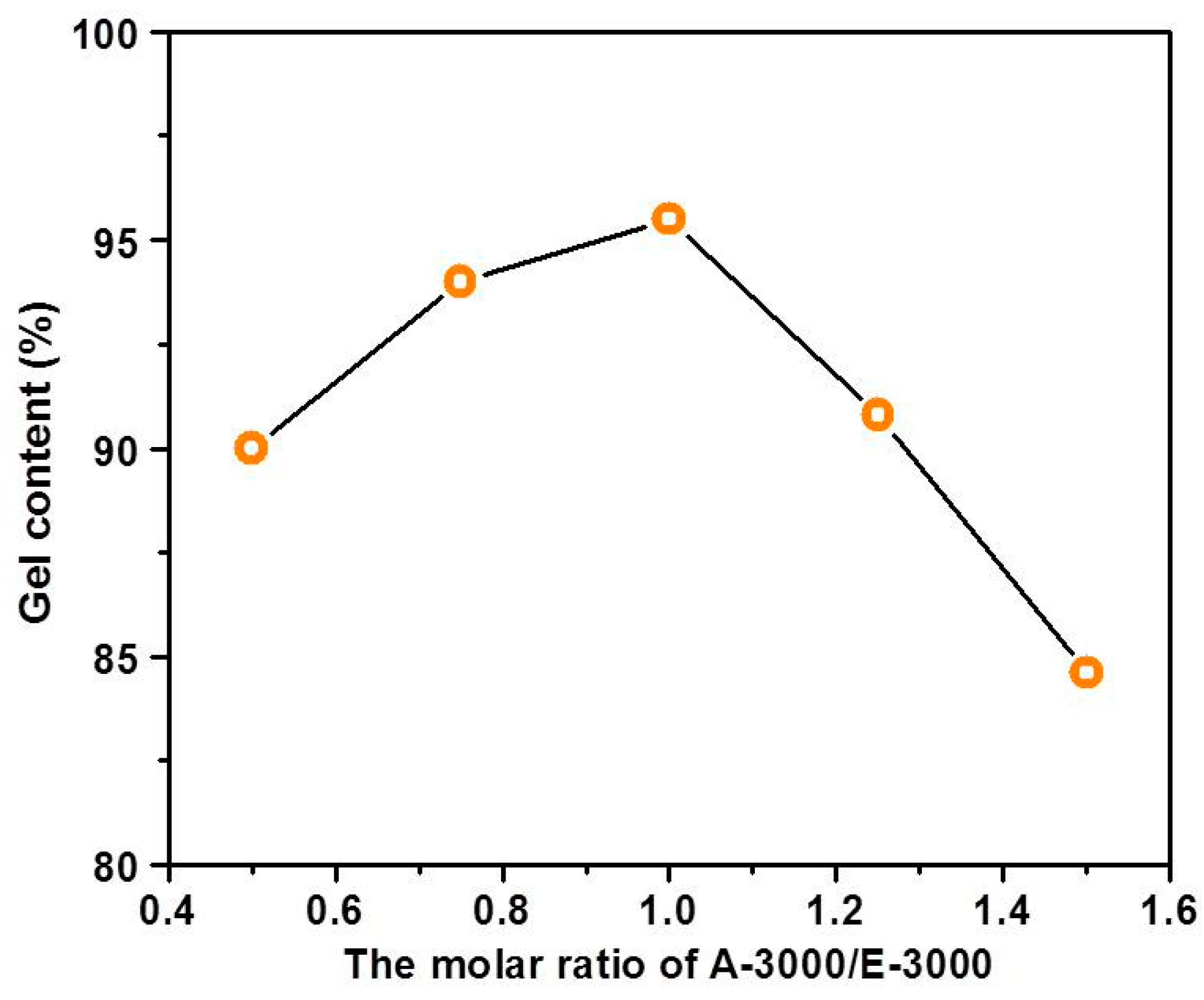
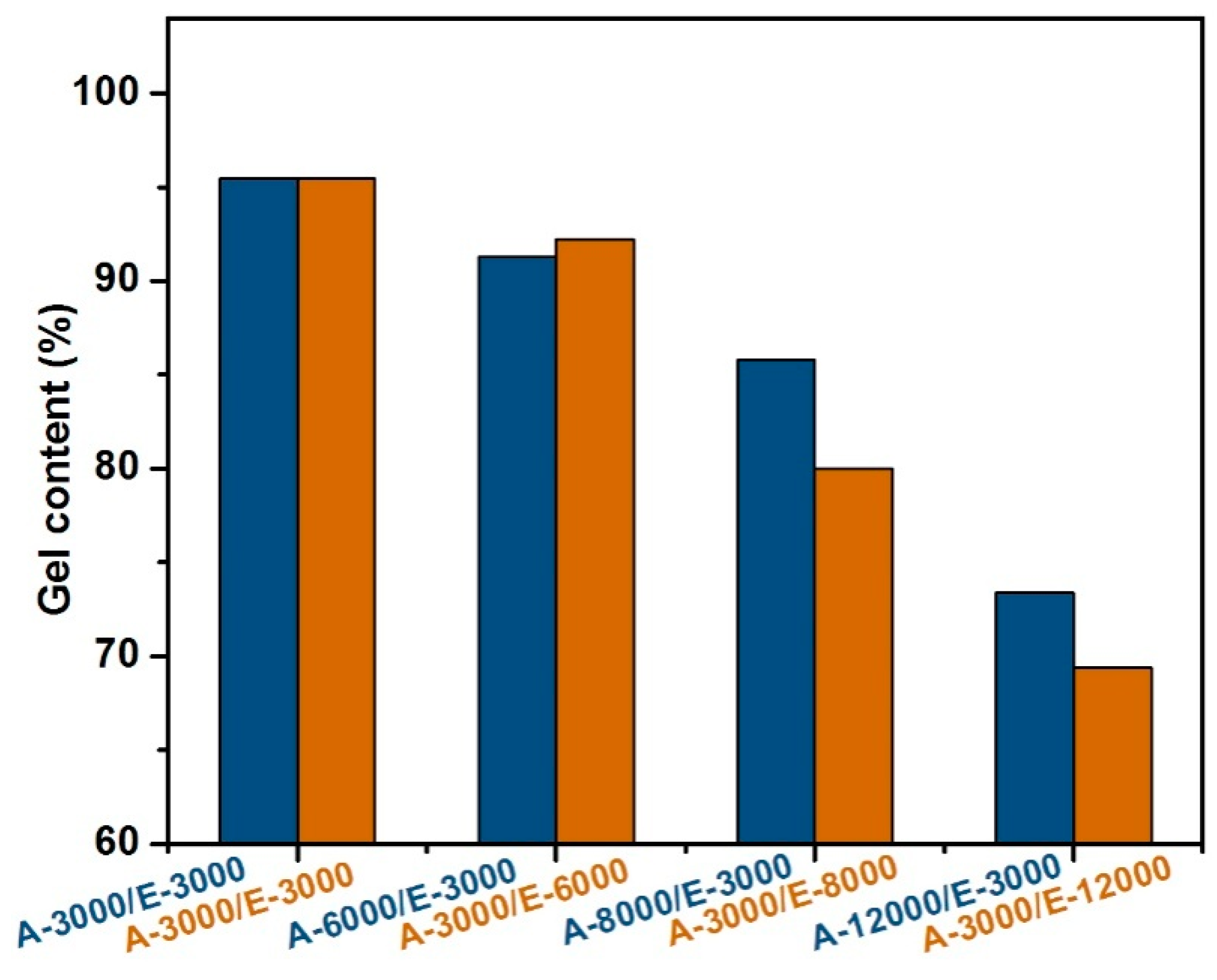
3.2. The Effect of Formula of Adhesive on Gel Content
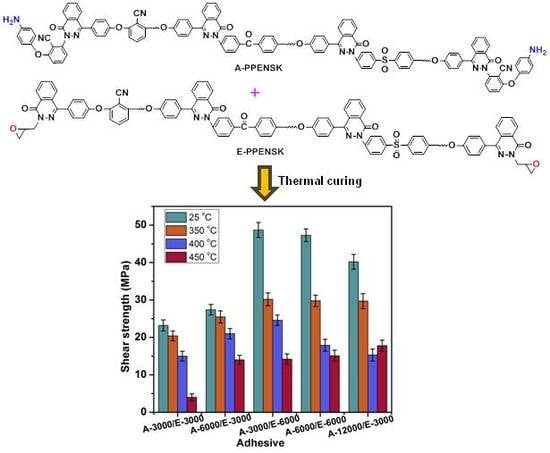
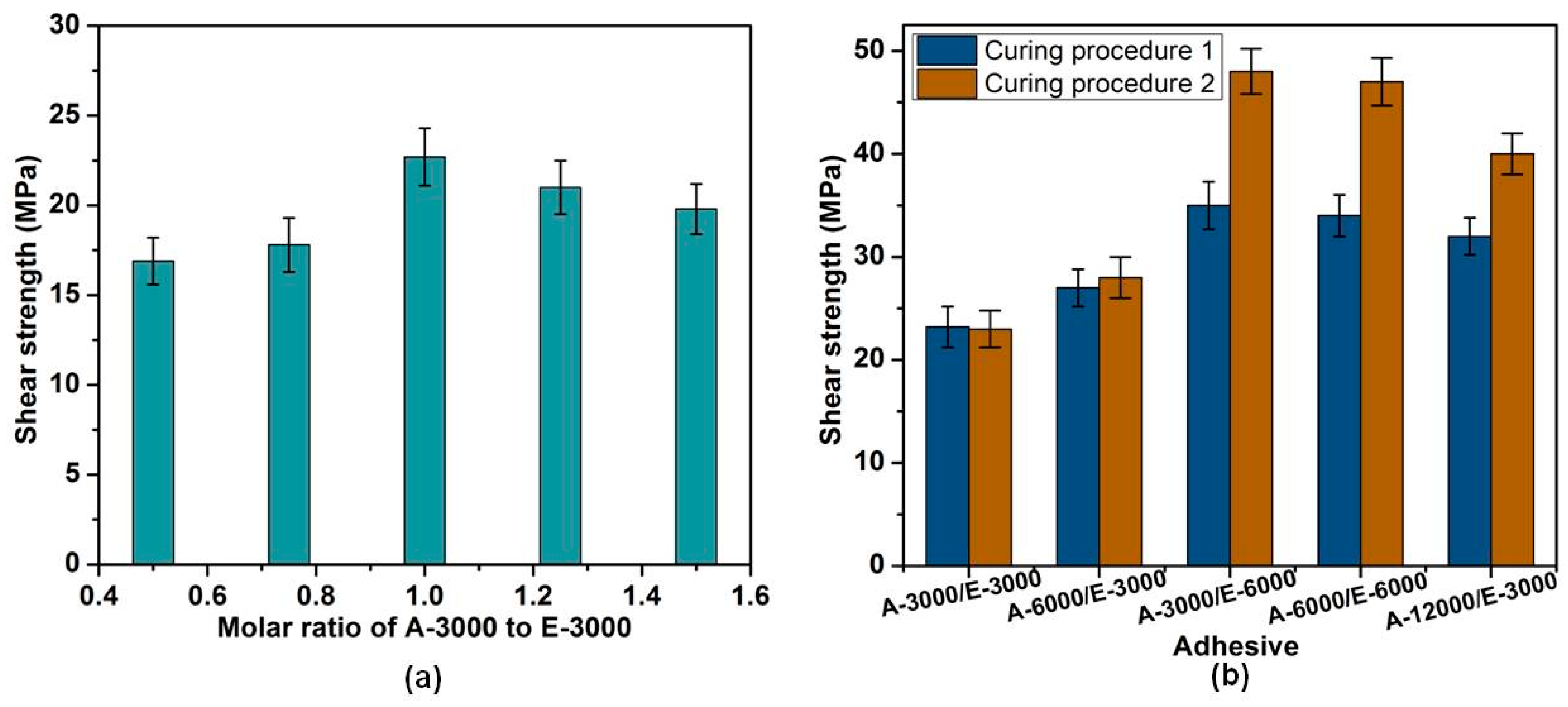
3.3. The Effects of Formula and Curing Procedure on Shear Strength
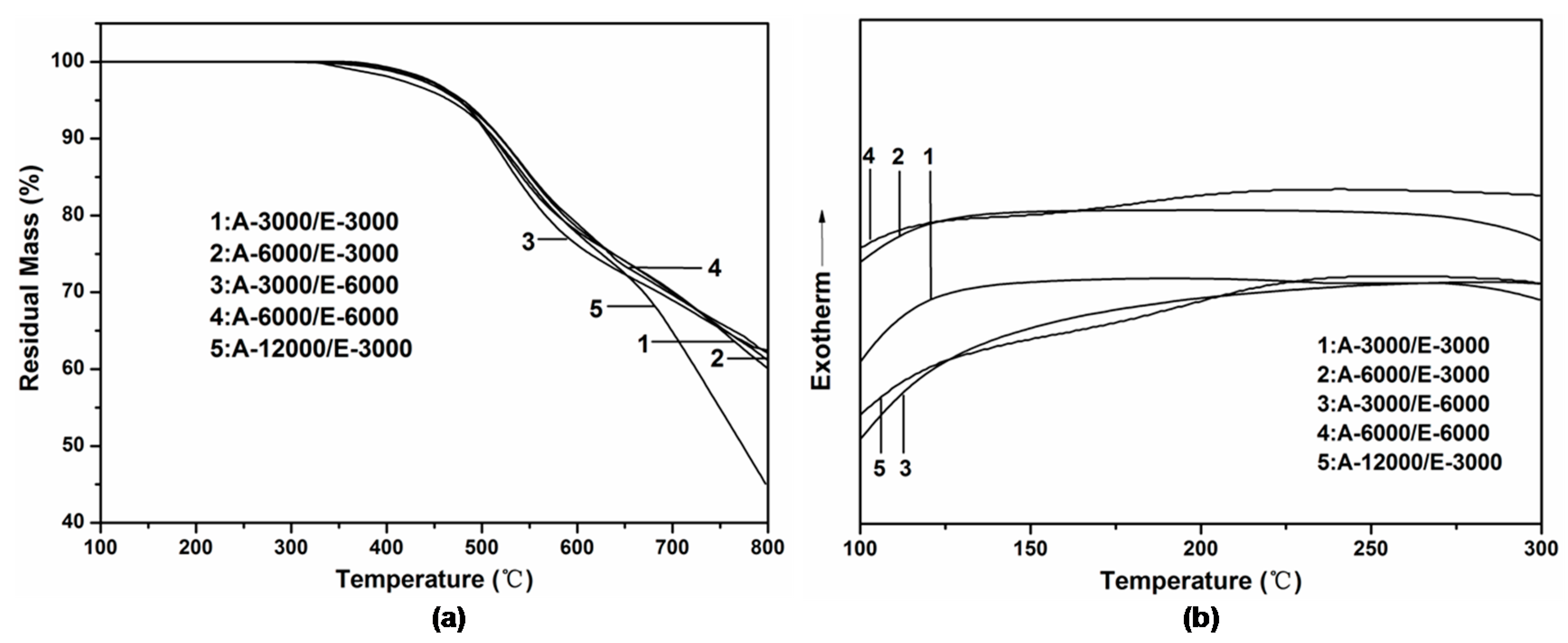
3.4. Thermal Stability of Adhesive
3.5. The Mechanical Properties after Treated under High Temperature
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Banea, M.D.; da Silva, L.F.M.; Campilho, R.D.S.G. The effect of adhesive thickness on the mechanical behavior of a structural polyurethane adhesive. J. Adhes. 2015, 90, 331–346. [Google Scholar] [CrossRef]
- Pethrick, R.A. Design and ageing of adhesives for structural adhesive bonding—A review. Proc. Inst. Mech. Eng. L-J. Mater. 2015, 229, 349–379. [Google Scholar] [CrossRef]
- Marques, E.A.S.; da Silva, L.F.M.; Banea, M.D.; Carbas, R.J.C. Adhesive joints for low-and high-temperature use: An overview. J. Adhes. 2015, 91, 556–585. [Google Scholar] [CrossRef]
- Iabal, H.M.S.; Bhowmik, S.; Benedictus, R. Process optimization of solvent based polybenzimidazole adhesive for aerospace applications. Int. J. Adhes. Adhes. 2014, 48, 188–193. [Google Scholar] [CrossRef]
- Banea, M.D.; da Silva, L.F.M.; Campilho, R.D.S.G. Effect of temperature on the shear strength of aluminium single lap bonded joints for high temperature applications. J. Adhes. Sci. Technol. 2012, 28, 1367–1381. [Google Scholar] [CrossRef]
- Rudawska, A.; Haniecka, I.; Jaszek, M.; Stefaniuk, D. The influence of adhesive compounds biochemical modification on the mechanical properties of adhesive joints. Polymers 2018, 10, 344. [Google Scholar] [CrossRef]
- Wang, Y.Z.; Yuan, Y.C.; Zhao, Y.; Liu, S.M.; Zhao, J.Q. Flame-retarded epoxy resin with high glass transition temperature cured by DOPO-containing H-benzimidazole. High Perform. Polym. 2017, 29, 94–103. [Google Scholar] [CrossRef]
- Xu, Y.J.; Liao, G.X.; Gu, T.S.; Zheng, L.; Jian, X.G. Mechanical and morphological properties of epoxy resins modified by poly(phthalazinone ether sulfone ketone). J. Appl. Polym. Sci. 2008, 110, 2253–2260. [Google Scholar] [CrossRef]
- Auvergne, R.; Caillol, S.; David, G.; Boutevin, B.; Pascault, J.P. Biobased thermosetting epoxy: Present and future. Chem. Rev. 2014, 114, 1082–1115. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Krishnan, S.; Samal, S.K.; Mohanty, S.; Nayak, S.K. Toughening of petroleum based (DGEBA) epoxy resins with various renewable resources based flexible chains for high performance application: A review. Ind. Eng. Chem. Res. 2018, 57, 2711–2726. [Google Scholar] [CrossRef]
- Park, S.Y.; Choi, W.J.; Choi, C.H.; Choi, H.S. The effect of curing temperature on thermal, physical and mechanical characteristics of two types of adhesives for aerospace structures. J. Adhes. Sci. Technol. 2018, 32, 1200–1223. [Google Scholar] [CrossRef]
- Chen, N.R.; Zheng, P.T.; Zeng, Q.Z.; Lin, Q.J.; Rao, J.P. Characterization and performance of soy-based adhesives cured with epoxy resin. Polymers 2017, 9, 514. [Google Scholar] [CrossRef]
- Lahouar, M.A.; Caron, J.F.; Pinoteau, N.; Foret, G.; Benzarti, K. Mechanical behavior of adhesive anchors under high temperature exposure: Experimental investigation. Int. J. Adhes. Adhes. 2017, 78, 200–211. [Google Scholar] [CrossRef]
- Liu, R.; Wang, J.Y.; He, Q.Z.; Zong, L.S.; Jian, X.G. Interaction and properties of epoxy-amine system modified with poly(phthalazinone ether nitrile ketone). J. Appl. Polym. Sci. 2016, 133, 42938–42945. [Google Scholar] [CrossRef]
- Li, G.; Huang, Z.B.; Li, P.; Xin, C.L.; Jia, X.L.; Wang, B.H.; He, Y.D.; Ryu, S.; Yang, X.P. Curing kinetics and mechanism of polysulfone nanofibrous membranes toughened epoxy/amine systems using isothermal DSC and NIR. Thermochim. Acta 2010, 497, 27–34. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, Q.P.; Fox, B.L. Study on thermoplastic-modified multifunctional epoxies: Influence of heating rate on cure behavior and phase separation. Compos. Sci. Technol. 2009, 68, 1172–1179. [Google Scholar] [CrossRef]
- Fernandez, B.; Arbelaiz, A.; Diaz, E.; Mondragon, I. Influence of polyethersulfone modification of a tetrafunctional epoxy matrix on the fracture behavior of composite laminates based on woven carbon fibers. Polym. Compos. 2004, 25, 480–488. [Google Scholar] [CrossRef]
- Giannotti, M.I.; Bernal, C.R.; Oyanguren, P.A.; Galante, M.J. Morphology and fracture properties relationship of epoxy-diamine systems simultaneously modified with polysulfone and poly(ether imide). Polym. Eng. Sci. 2005, 45, 1312–1318. [Google Scholar] [CrossRef]
- Hourston, D.J.; Lane, J.M.; Zhang, H.X. Toughening of epoxy resins with thermoplastics: 3. An investigation into the effects of composition on the properties of epoxy resin blends. Polym. Int. 1997, 42, 349–355. [Google Scholar] [CrossRef]
- Chen, H.M.; Lv, R.G.; Liu, P.; Wang, H.Y.; Huang, Z.Y.; Huang, T.; Li, T.S. An investigation of cure and thermal stability of poly(amide-amidic acid) modified tetraglycidyl 4,4′-diaminodiphenylmethane/4,4′-diaminodiphenylsulfone. J. Appl. Polym. Sci. 2013, 128, 1592–1600. [Google Scholar] [CrossRef]
- Zhong, Z.K.; Zheng, S.X.; Huang, J.Y.; Cheng, X.G.; Guo, Q.P.; Wei, J. Phase behavior and mechanical properties of epoxy resin containing phenolphthalein poly(ether ether ketone). Polymer 1998, 39, 1075–1080. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, M.; Dang, G.D.; Li, Y.; An, X.F.; Chen, C.H.; Yi, X.S. Toughening of epoxy resin by poly(ether ether ketone) with pendant fluorocarbon groups. J. Appl. Polym. Sci. 2011, 122, 1758–1765. [Google Scholar] [CrossRef]
- Liu, R.; Wang, J.Y.; Li, J.L.; Jian, X.G. An investigation of epoxy/thermoplastic blends based on addition of a novel coply(aryl ether nitrile) containing phthalazinone and biphenyl moieties. Polym. Int. 2015, 64, 1786–1793. [Google Scholar] [CrossRef]
- Xu, Y.J.; Fu, X.J.; Liao, G.X.; Zhou, H.X.; Jian, X.G. Preparation, morphology and thermo-mechanical properties of epoxy resins modified by co-poly(phthalazinone ether sulfone). High Perform. Polym. 2011, 23, 248–254. [Google Scholar] [CrossRef]
- Yoshida, S.; Hay, A.S. Synthesis of all aromatic phthalazinone-containing polymers by a novel N-C coupling reaction. Macromolecules 1995, 28, 2579–2581. [Google Scholar] [CrossRef]
- Meng, Y.Z.; Hay, A.S.; Jian, X.G.; Tong, S.C. Synthesis novel poly(phthalazinone ether sulfone ketone)s and improvement of their melt flow properties. J. Appl. Polym. Sci. 1997, 66, 1425–1432. [Google Scholar] [CrossRef]
- Li, G.H.; Wang, J.Y.; Yu, G.P.; Jian, X.G.; Wang, L.H.; Zhao, M.S. Synthesis and characterization of partly fluorinated poly(phthalazinone ether)s crosslinked by allyl group for passive optical wave-guide. Polymer 2010, 51, 1524–1529. [Google Scholar] [CrossRef]
- Song, Y.; Wang, J.Y.; Li, G.H.; Sun, Q.M.; Jian, X.G.; Teng, J.; Zhang, H.B. Synthesis, characterization and optical properties of cross-linkable poly(phthalazinone ether ketone sulfone). Polymer 2008, 49, 724–731. [Google Scholar] [CrossRef]
- Smith, I.T. The mechanism of the crosslinking of epoxide resins by amines. Polymer 1961, 2, 95–108. [Google Scholar] [CrossRef]
- Wang, J.Y.; Liao, G.X.; Liu, C.; Jian, X.G. Poly(ether imide)s derived from phthalazinone-containing dianhydrides. J. Polym. Sci. A Polym. Chem. 2004, 42, 6089–6097. [Google Scholar] [CrossRef]
- Patil, D.M.; Phalak, G.A.; Mhaske, S.T. Synthesis of bio-based epoxy resin from gallic acid with various epoxy equivalent weights and its effects on coating properties. J. Coat. Technol. Res. 2017, 14, 355–365. [Google Scholar] [CrossRef]
- Janvier, M.; Hollande, L.; Jaufurally, A.S.; Pernes, M.; Menard, R.; Grimaldi, M.; Beaugrand, J.; Balaguer, P.; Ducrot, P.; Allais, F. Syringaresinol: A renewable and safer alternative to bisphenol A for epoxy-amine resins. ChemSusChem 2017, 10, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Dutta, A.; Vanderklok, A.; Tekalur, S.A. High strain mechanical behavior of seashell-mimetic composites: Analytical model formulation and validation. Mech. Mater. 2012, 55, 102–111. [Google Scholar] [CrossRef]
- Dutta, A.; Tekalur, S.A. Crack tortuousity in the nacreous layer-topological dependence and biomimetic design guideline. Int. J. Solids Struct. 2014, 51, 325–335. [Google Scholar] [CrossRef]
- Marques, E.A.S.; Campilho, R.D.S.G.; Silva, L.F.M. Geometrical study of mixed adhesive joints for high-temperature applications. J. Adhes. Sci. Technol. 2016, 30, 691–707. [Google Scholar] [CrossRef]
- Progar, D.J.; Clair, T.L. Adhesive evaluation for new forms of LARCTM-TPI. J. Adhes. 1994, 47, 67–82. [Google Scholar] [CrossRef]
- Lu, X.C.; Cao, G.; Niu, Z.F.; Pan, Q.M. Viscoelastic and adhesive properties of single-component thermo-resistant acrylic pressure sensitive adhesives. J. Appl. Polym. Sci. 2014, 131, 40086–40095. [Google Scholar] [CrossRef]


| Polymers | Solvents b | |||||||
|---|---|---|---|---|---|---|---|---|
| NMP | DMF | DMSO | DMAc | THF | CHCl3 | Sulfolane | Acetone | |
| A-PPENSK | ++ | ++ | + | ++ | - | ++ | + | - |
| E-PPENSK | ++ | ++ | + | ++ | - | ++ | + | - |
| Cured A-E-PPENSK | - | - | - | - | - | - | - | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Wang, J.; Qi, Y.; Zhang, F.; Weng, Z.; Jian, X. Preparation of Novel Epoxy Resins Bearing Phthalazinone Moiety and Their Application as High-Temperature Adhesives. Polymers 2018, 10, 708. https://doi.org/10.3390/polym10070708
Wang L, Wang J, Qi Y, Zhang F, Weng Z, Jian X. Preparation of Novel Epoxy Resins Bearing Phthalazinone Moiety and Their Application as High-Temperature Adhesives. Polymers. 2018; 10(7):708. https://doi.org/10.3390/polym10070708
Chicago/Turabian StyleWang, Liwei, Jinyan Wang, Yu Qi, Fengfeng Zhang, Zhihuan Weng, and Xigao Jian. 2018. "Preparation of Novel Epoxy Resins Bearing Phthalazinone Moiety and Their Application as High-Temperature Adhesives" Polymers 10, no. 7: 708. https://doi.org/10.3390/polym10070708
APA StyleWang, L., Wang, J., Qi, Y., Zhang, F., Weng, Z., & Jian, X. (2018). Preparation of Novel Epoxy Resins Bearing Phthalazinone Moiety and Their Application as High-Temperature Adhesives. Polymers, 10(7), 708. https://doi.org/10.3390/polym10070708