Preparation of Graphene-Perfluoroalkoxy Composite and Thermal and Mechanical Properties
Abstract
1. Introduction
2. Experiment
2.1. Materials
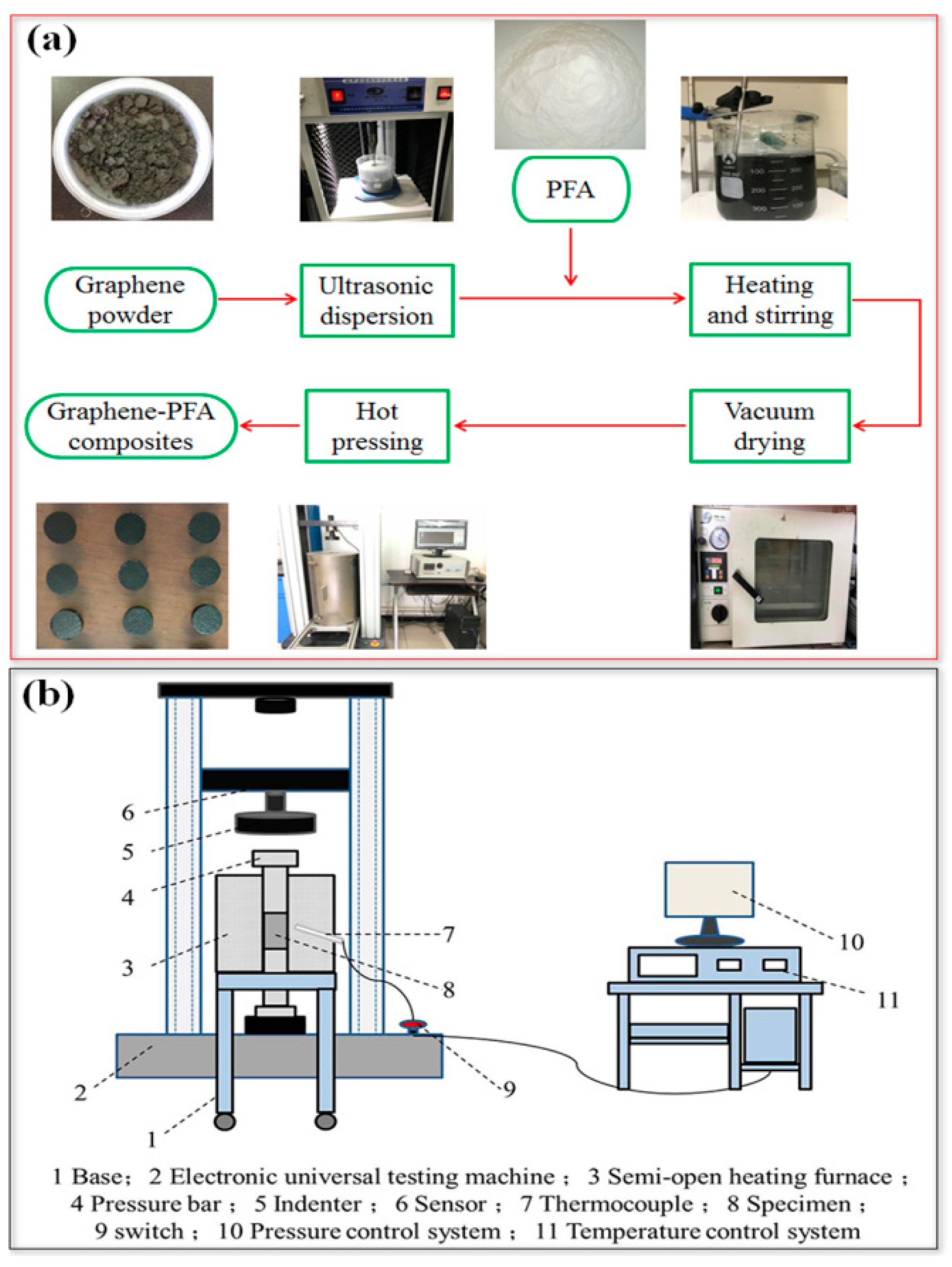
2.2. Dispersion Method
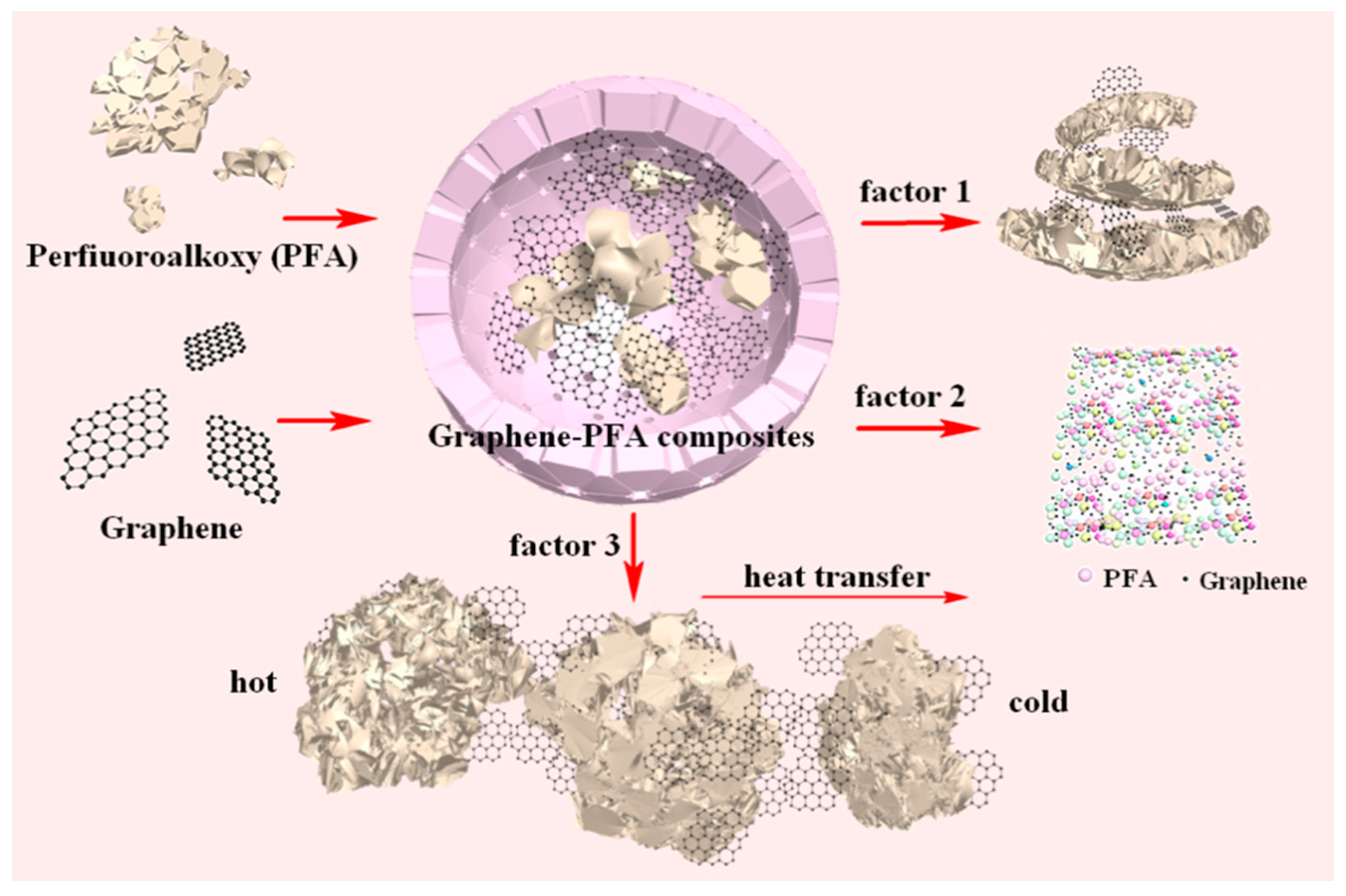
2.3. Preparation of Composite Materials
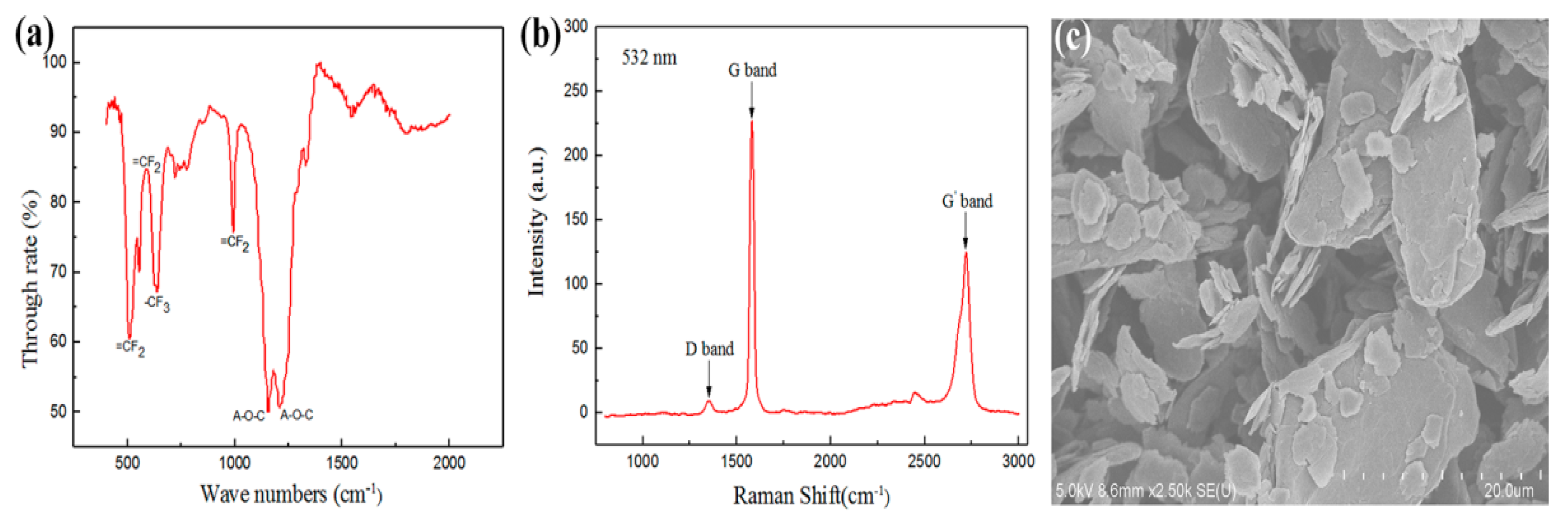
2.4. Composite Characterization
3. Results and Discussion
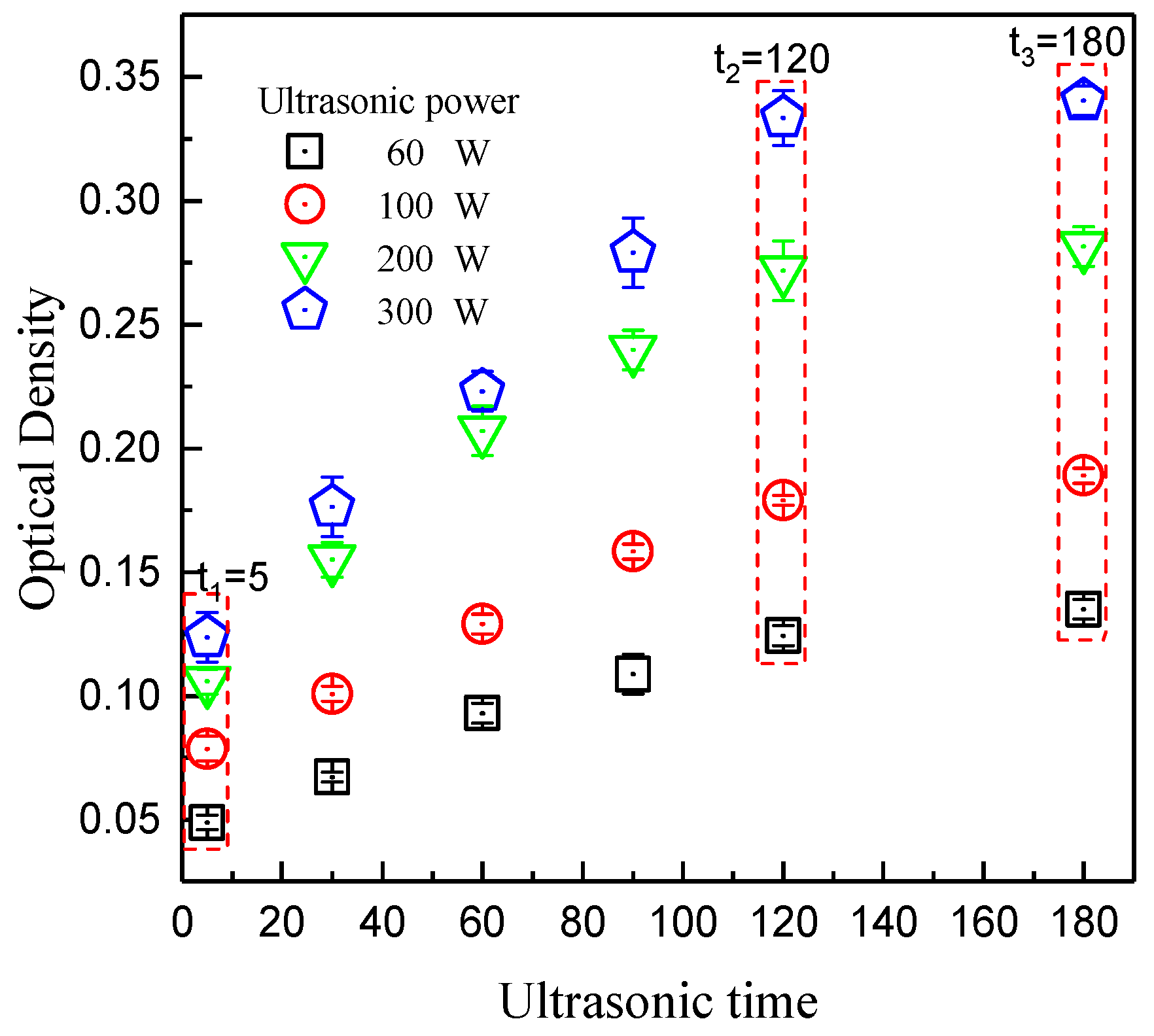
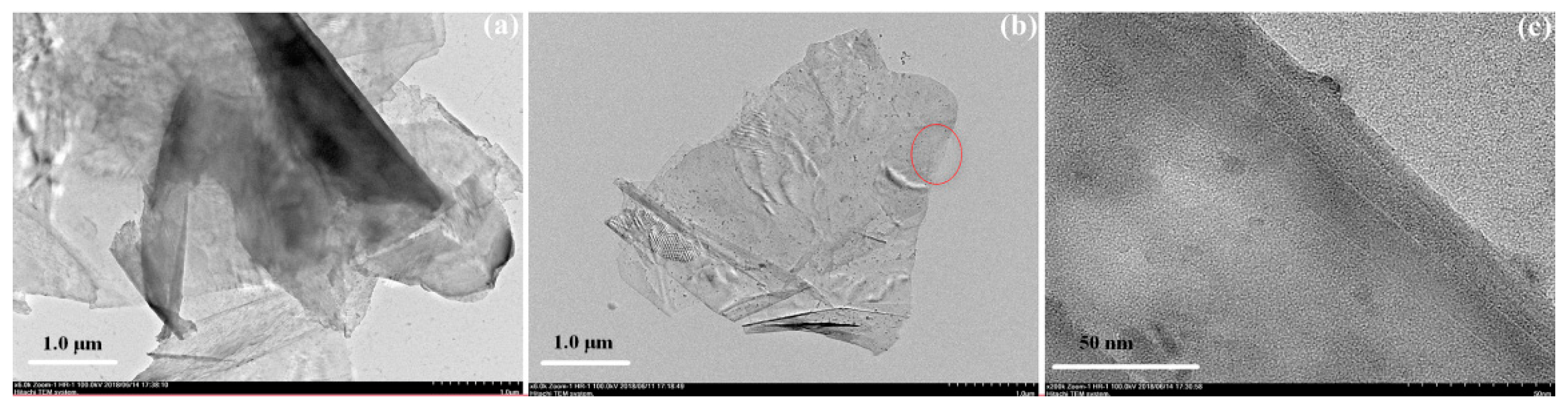
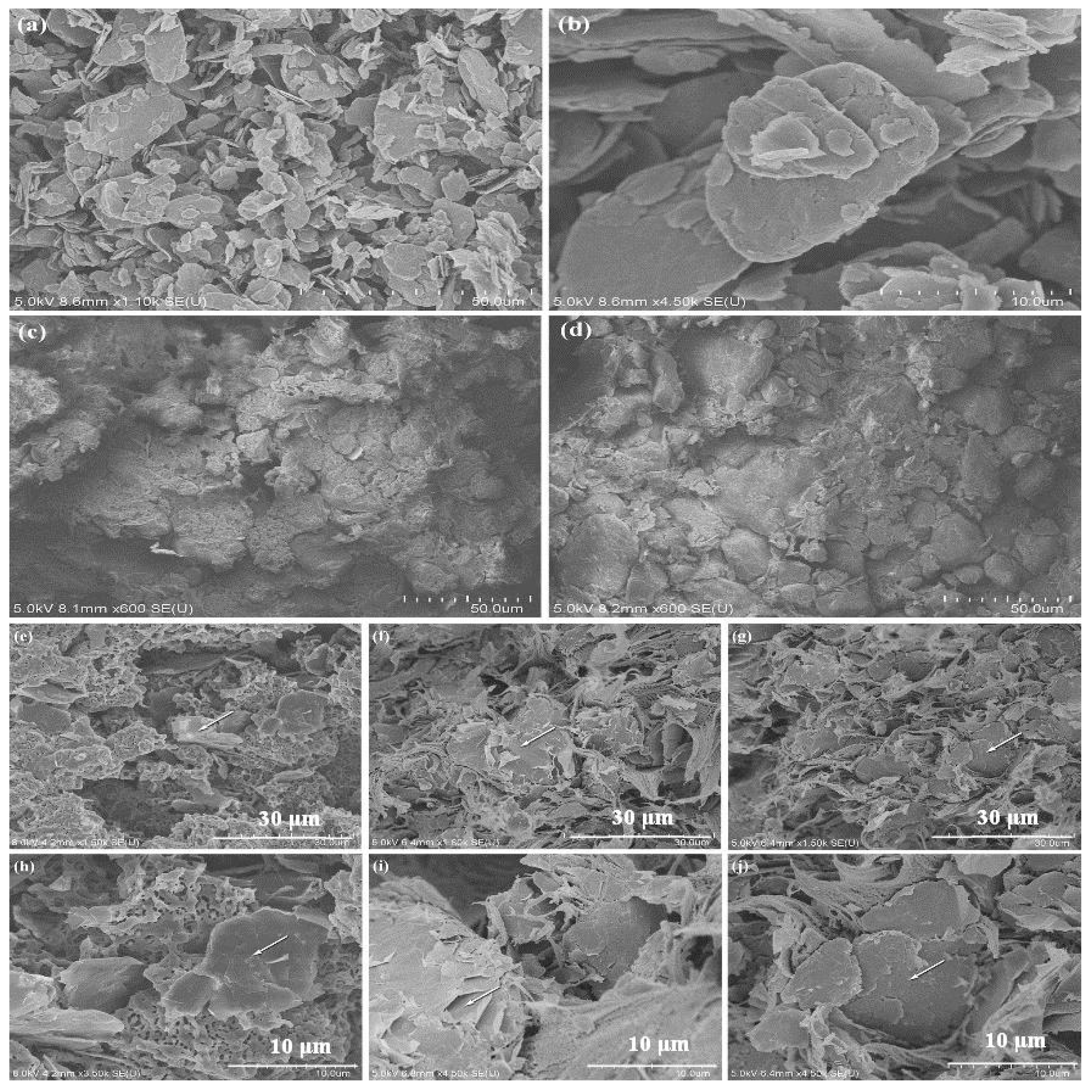
3.1. Dispersion of Graphene
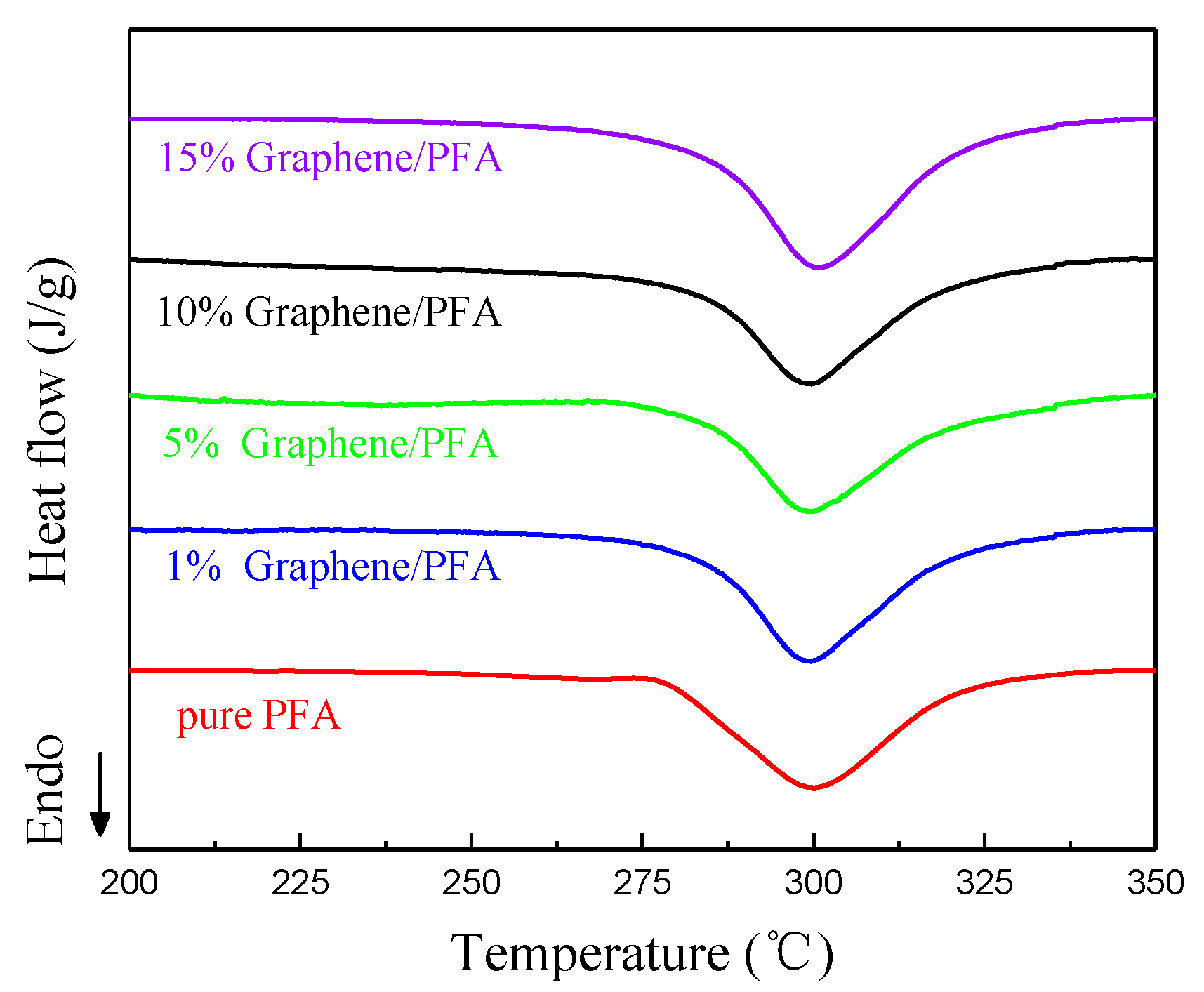
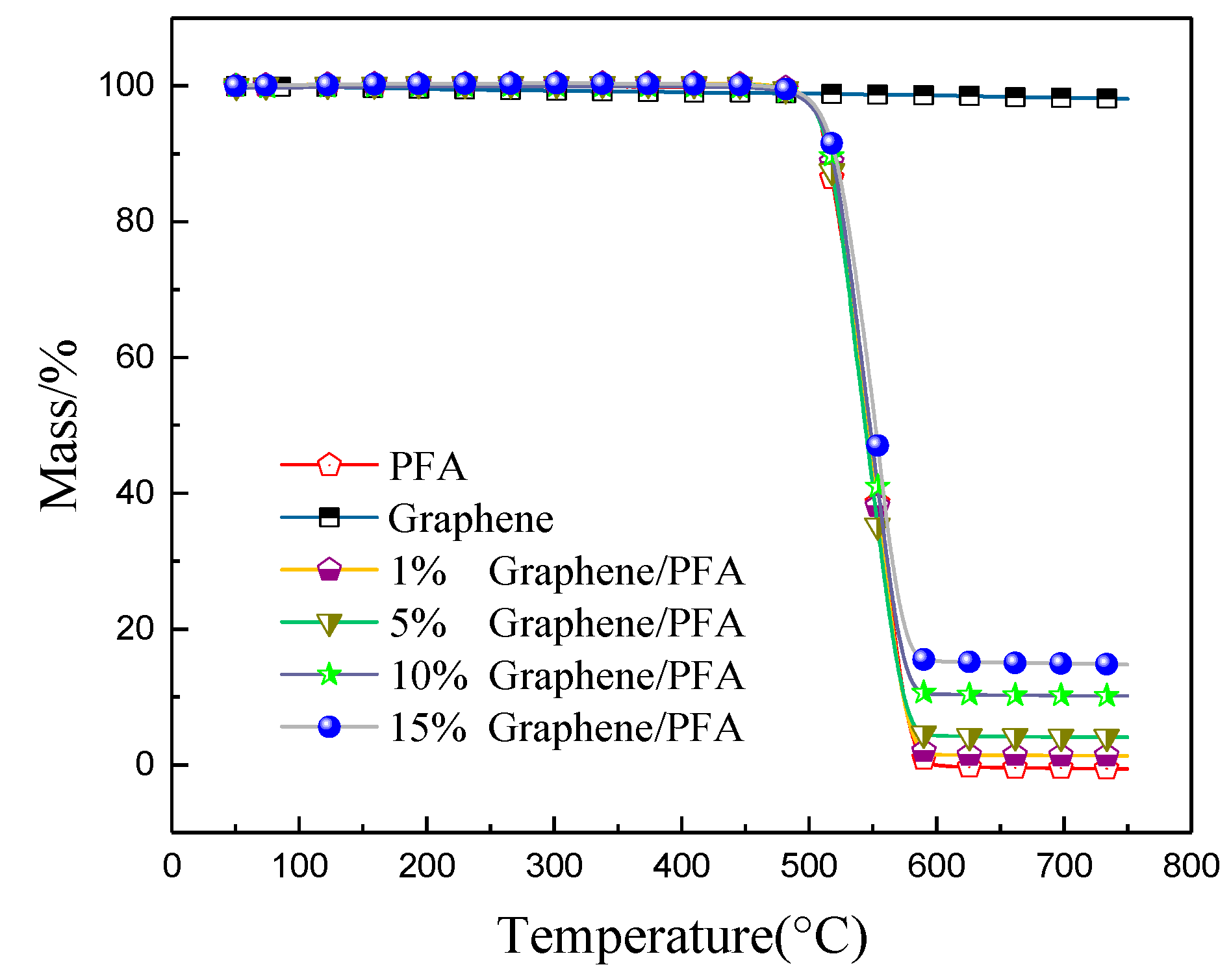
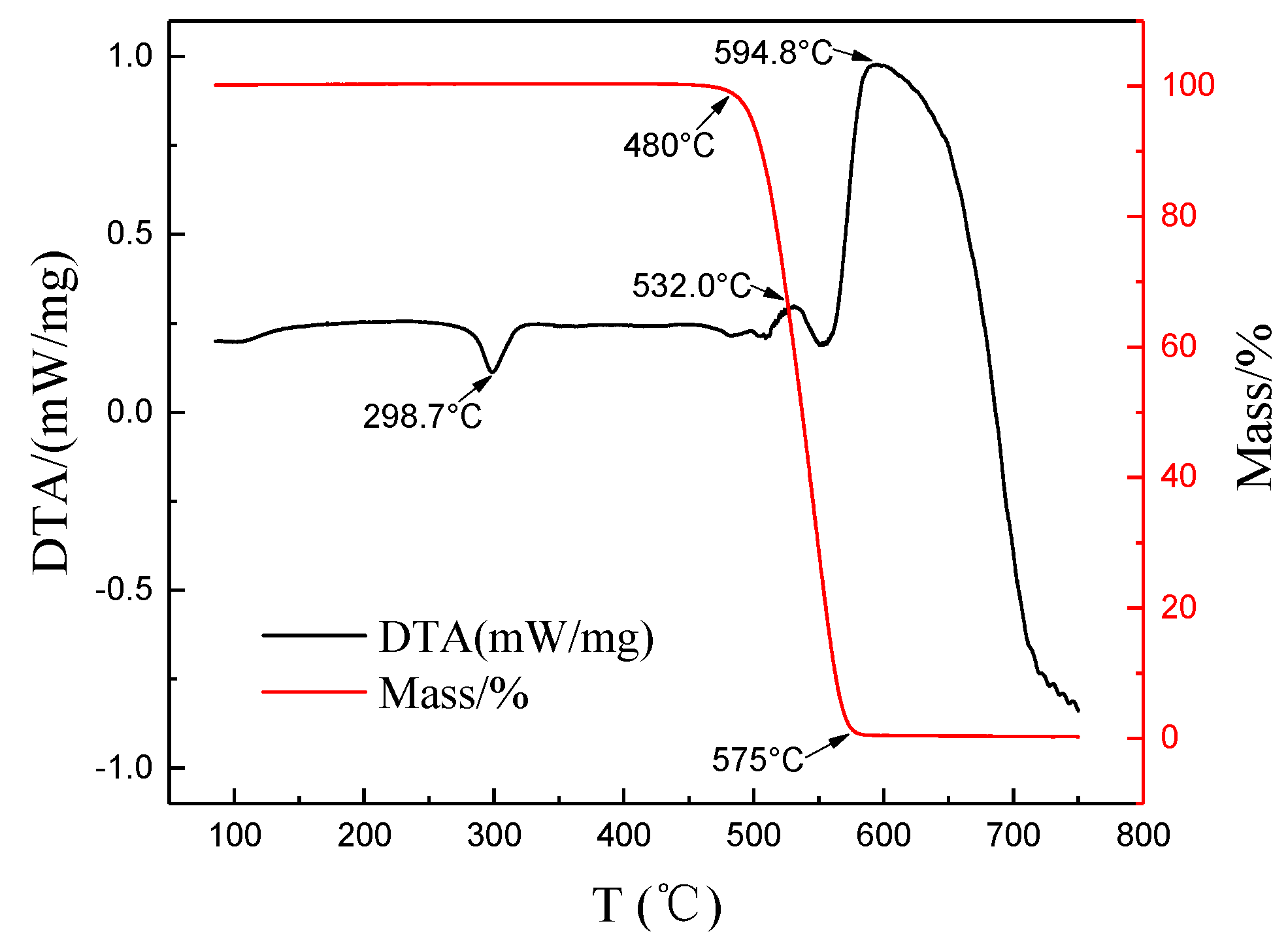
3.2. Thermal Decomposition Characteristics of Composite
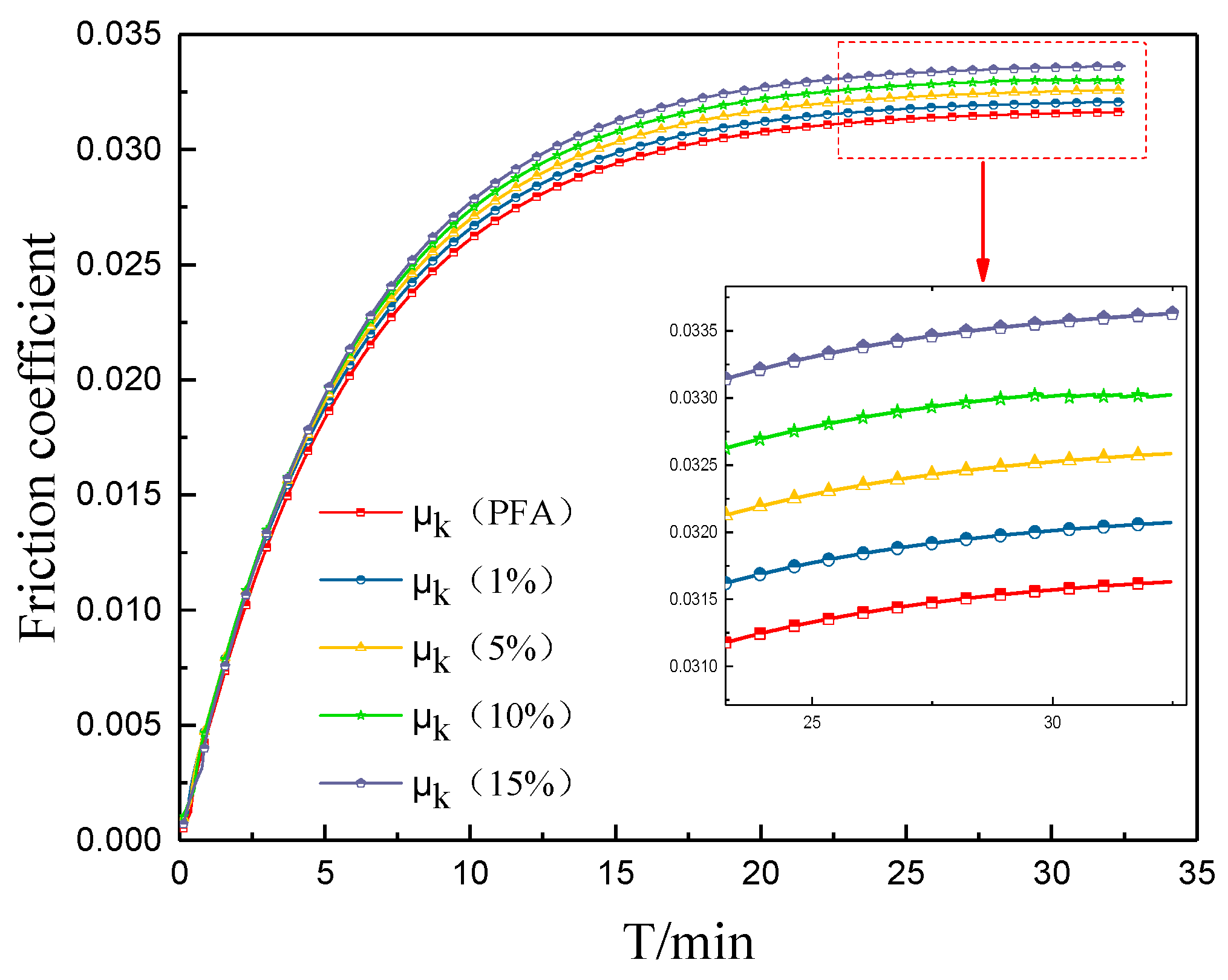
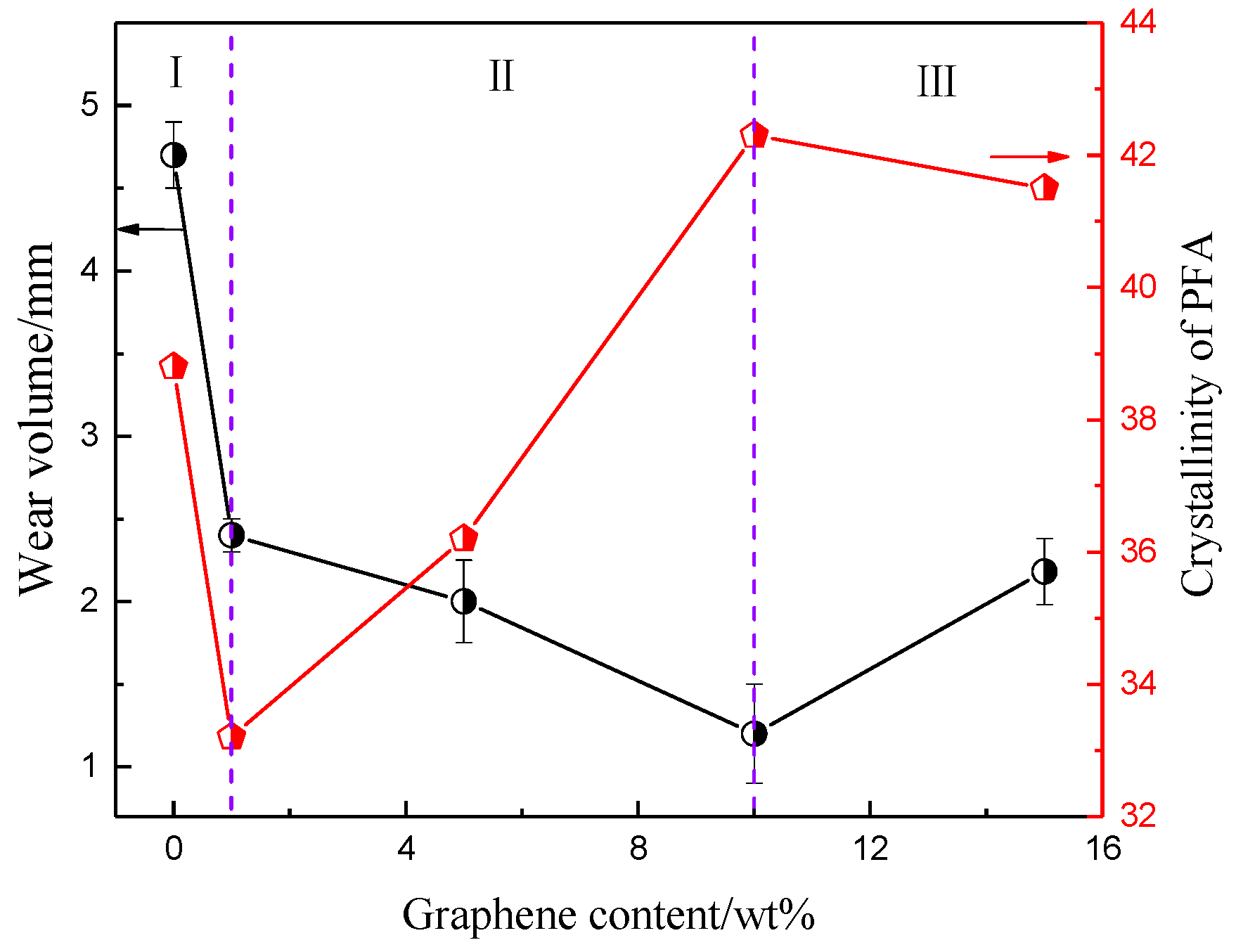
3.3. Abrasion Resistance
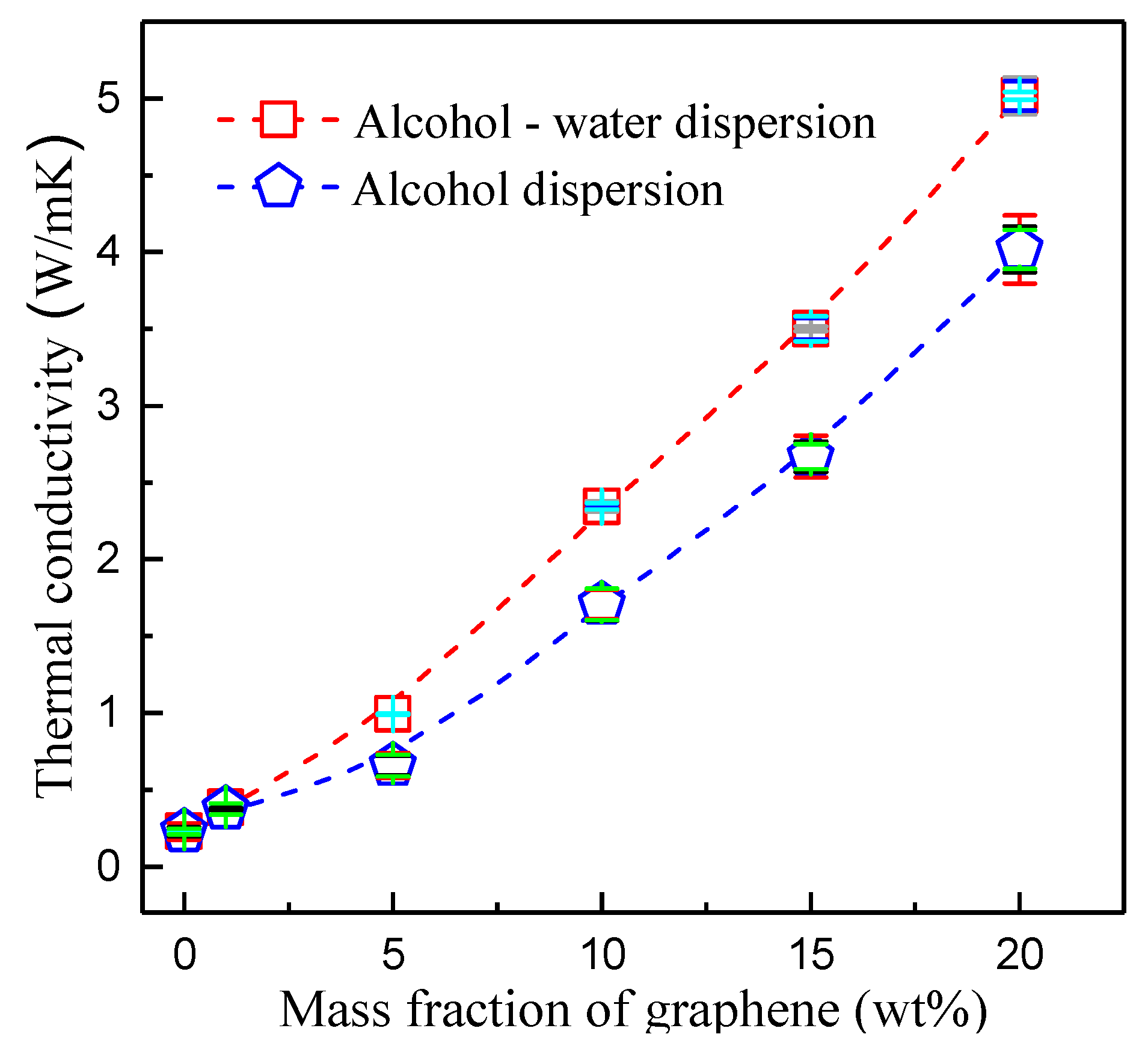
3.4. Thermal Conductivity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Qing, H.U.; Jiang, W.; Zhu, W.Z.; Yang, P. Application Analysis of Fluorine Plastic Heat Exchanger in the Field of Flue Gas Heat Recovery. Power Gener. Air Cond. 2016, 29, 1111–1116. [Google Scholar]
- Pu, Y.N.; Chen, H.T.; Qian, X. A Study of New Type Plastic Heat Exchanger. J. Zhejiang Univ. Technol. 1986, 2, 38–42. [Google Scholar]
- Mühlenbein, H.; Schlierkamp-Voosen, D. Predictive Models for the Breeder Genetic Algorithm I. Continuous Parameter Optimization. Evol. Comput. 1993, 1, 25–49. [Google Scholar] [CrossRef]
- Hintzer, K.; Kaulbach, R.; Kloos, F.; Schwertfeger, W. Fluoropolymers from Tetrafluoroethylene and Perfluoro(Alkoxyalkyl Vinyl) Ether. U.S. Patent 7,060,772, 13 June 2006. [Google Scholar]
- Balandin, A.A. Thermal properties of graphene and nanostructured carbon materials. Nat. Mater. 2011, 10, 569. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Fina, A. Thermal conductivity of carbon nanotubes and their polymer nanocomposites: A review. Prog. Polym. Sci. 2011, 36, 914–944. [Google Scholar] [CrossRef]
- Zhao, X.; Koos, A.A.; Chu, B.T.T.; Johnston, C.; Grobert, N.; Grant, P.S. Spray deposited fluoropolymer/multi-walled carbon nanotube composite films with high dielectric permittivity at low percolation threshold. Carbon 2009, 47, 561–569. [Google Scholar] [CrossRef]
- Meng, X.; Liu, Y.; Huang, M.; Cao, J.P. Flexible perfluoroalkoxy films filled with carbon nanotubes and their electric heating property. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Eslami, H.; Mohammadzadeh, L.; Mehdipour, N. Anisotropic heat transport in nanoconfined polyamide-6,6 oligomers: Atomistic reverse nonequilibrium molecular dynamics simulation. J. Chem. Phys. 2012, 136, 064703. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Jiang, Z.; Zhang, X.; Gao, T.; Yue, K.; Zhang, X. Thermal property improvement of polytetrafluoroethylene nanocomposites with graphene nanoplatelets. RSC Adv. 2018, 8, 11367–11374. [Google Scholar] [CrossRef]
- Van Rooyen, L.J.; Bissett, H.; Khoathane, M.C.; Karger-Kocsis, J. Preparation of ptfe/graphene nanocomposites by compression moulding and free sintering: A guideline. J. Appl. Polym. Sci. 2016, 133, 43369. [Google Scholar] [CrossRef]
- Tanaka, K.; Kawakami, S. Effect of various fillers on the friction and wear of polytetrafluoroethylene-based composites. Wear 1982, 79, 221–234. [Google Scholar] [CrossRef]
- Takeichi, Y.; Wibowo, A.; Kawamura, M.; Uemura, M. Effect of morphology of carbon black fillers on the tribological properties of fibrillated ptfe. Wear 2008, 264, 308–315. [Google Scholar] [CrossRef]
- Sidebottoma, M.A.; Pitenisb, A.A.; Junkcd, C.P.; Kasprzakd, D.J.; Blackmand, G.S.; Burchd, H.E.; Harrise, K.L.; GregorySawyerbe, W.; Krick, B.A. Ultralow wear Perfluoroalkoxy (PFA) and alumina composites. Wear 2016, 362–363, 179–185. [Google Scholar] [CrossRef]
- Wang, K.; Hu, N.X.; Xu, G.; Qi, Y. Stable superhydrophobic composite coatings made from an aqueous dispersion of carbon nanotubes and a fluoropolymer. Carbon 2011, 49, 1769–1774. [Google Scholar] [CrossRef]
- Zhai, M.; Gong, Y.; Chen, X.; Xiao, T.; Zhang, G.; Xu, L.; Lia, H. Mass-producible hydrophobic perfluoroalkoxy/nano-silver coatings by suspension flame spraying for antifouling and drag reduction applications. Surf. Coat. Technol. 2017, 328, 115–120. [Google Scholar] [CrossRef]
- Allen, M.J.; Tung, V.C.; Kaner, R.B. Honeycomb carbon: A review of graphene. Chem. Rev. 2010, 110, 132. [Google Scholar] [CrossRef] [PubMed]
- Walter, J.T. Surface Modified Expanded Polytetrafluoroethylene Devices and Methods of Producing the Same. U.S. Patent US6780497 B1, 5 August 2004. [Google Scholar]
- Fujimura, K.; Miyake, A. The effect of specific surface area of TiO2 on the thermal decomposition of ammonium perchlorate. J. Therm. Anal. Calorim. 2010, 99, 27–31. [Google Scholar] [CrossRef]
- Tkac, J.; Ruzgas, T. Dispersion of single walled carbon nanotubes. Comparison of different dispersing strategies for preparation of modified electrodes toward hydrogen peroxide detection. Electrochem. Commun. 2006, 8, 899–903. [Google Scholar] [CrossRef]
- Kim, Y.J.; Zhang, W.; Lee, H.R.; Kim, J.H. Surface Modification of Multi-walled Carbon Nanotubes for Enhancement of Dispersion and Electrochemical Properties. Korea Surf. Eng. Soc. 2008, 41, 194–198. [Google Scholar] [CrossRef]
- Ghosh, S.; Calizo, I.; Teweldebrhan, D.; Pokatilov, E.P. Extremely high thermal conductivity of graphene: Prospects for thermal management applications in nanoelectronic circuits. Appl. Phys. Lett. 2008, 92, 151911–151913. [Google Scholar] [CrossRef]
- Li, L. Advances in Processing Technology, Modification and Application of Polytetrafluoroethylene. Contemp. ChemInd. 2010, 06, 723–725. [Google Scholar]
- Malard, L.M.; Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S. Raman spectroscopy in graphene. Phys. Rep. 2009, 473, 51–87. [Google Scholar] [CrossRef]
- Gao, G.D. Thermochemical Modification, Patterning and Raman Characterization of Graphene; Harbin Institute of Technology: Harbin, China, 2014. [Google Scholar]
- Li, K.W.; Hao, H.H.; Liu, J.B.; Wang, J. Raman Spectroscopic Characterization of Graphene Materials. Chem. Bull. 2017, 80, 236–240. [Google Scholar]
- Johnson, D.W.; Dobson, B.P.; Coleman, K.S. A manufacturing perspective on graphene dispersions. Curr. Opin. Colloid Interface Sci. 2015, 20, 367–382. [Google Scholar] [CrossRef]
- Kong, Y.; Hay, J.N. The enthalpy of fusion and degree of crystallinity of polymers as measured by DSC. Eur. Polym. J. 2003, 39, 1721–1727. [Google Scholar] [CrossRef]
- Makowiec, M.E.; Blanchet, T.A. Improved wear resistance of nanotube- and other carbon-filled PTFE composites. Wear 2017, 374–375, 77–85. [Google Scholar] [CrossRef]
- Xia, J.B.; Chen, W.X.; Li, F.; Han, G.; Wang, L.Y.; Tu, J.P.; Xu, Z.D. Study of the tribological properties of carbon nanotubes filled ptfe composites. J. Zhejiang Univ. 2003, 37, 471–474. [Google Scholar]
- Bijwe, J.; Tewari, U.S.; Vasudevan, P. Friction and wear studies of an internally lubricated polyetherimide composite. Lubr. Sci. 1989, 6, 179–202. [Google Scholar] [CrossRef]

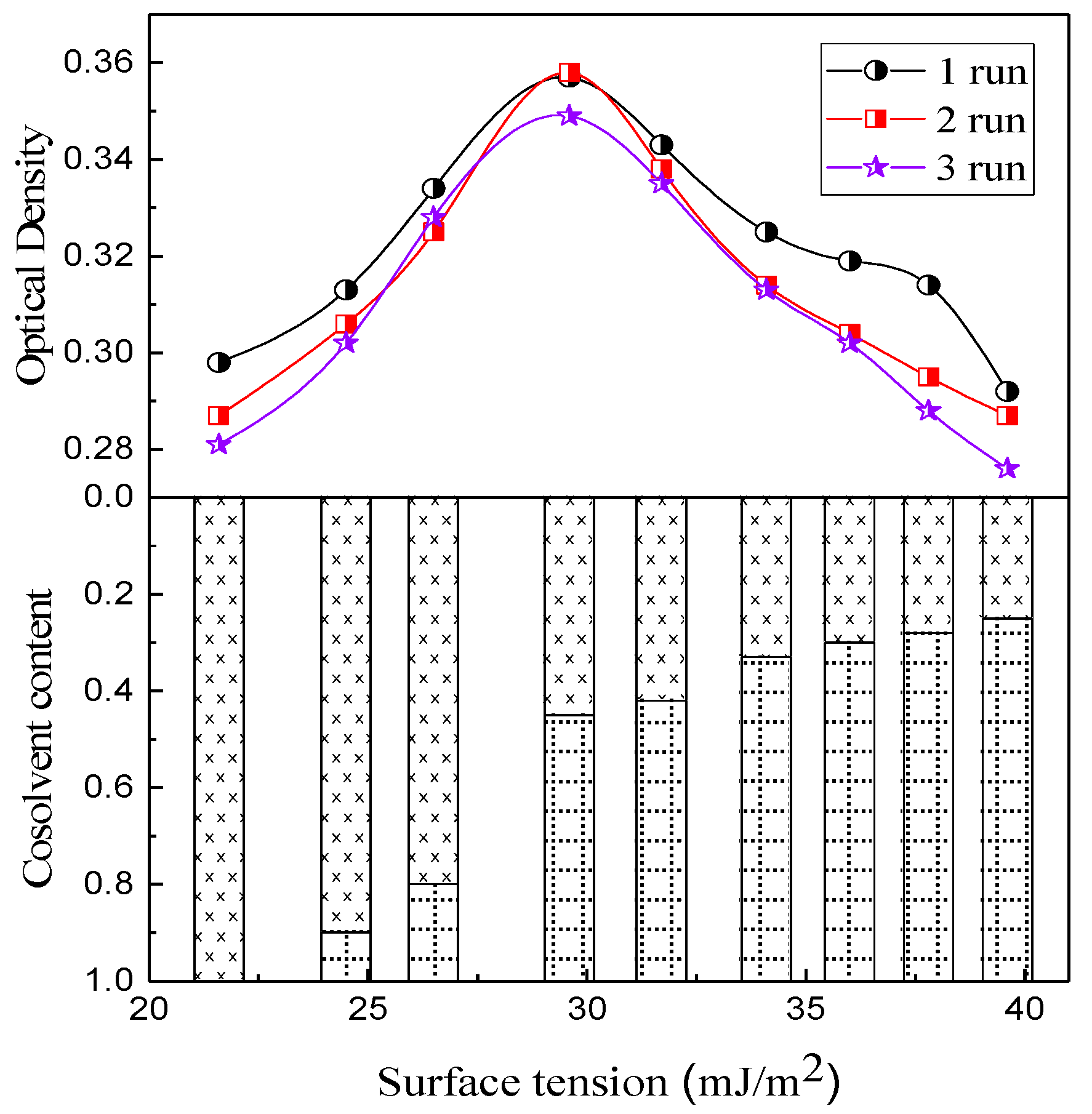
| Alcohol/Water (vol/vol) | Surface tension (γ) (mJ/m2) | Density (ρ) (kg/m3) |
|---|---|---|
| 100/0 | 21.6 | 789.0 |
| 90/10 | 24.5 | 809.9 |
| 45/55 | 29.6 | 903.9 |
| 30/70 | 36.0 | 935.3 |
| 20/80 | 42.7 | 956.2 |
| 10/90 | 51.2 | 977.1 |
| 0/100 | 73.1 | 998.0 |
| Sample | Melting temperature Tm (°C) | Melting enthalpy ΔHm (J/g) | Crystallinity degree Xc (×100%) |
|---|---|---|---|
| Pure PFA | 300.7 | 38.8 | 38.8/ΔH*m |
| 1 wt % Graphene | 299.6 | 32.9 | 33.2/ΔH*m |
| 5 wt % Graphene | 299.7 | 34.4 | 36.2/ΔH*m |
| 10 wt % Graphene | 299.7 | 38.1 | 42.3/ΔH*m |
| 15 wt % Graphene | 300.6 | 35.3 | 41.5/ΔH*m |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Zuo, H.; Zhang, X.; Wang, J.; Guo, L.; Peng, X. Preparation of Graphene-Perfluoroalkoxy Composite and Thermal and Mechanical Properties. Polymers 2018, 10, 700. https://doi.org/10.3390/polym10070700
Zhang W, Zuo H, Zhang X, Wang J, Guo L, Peng X. Preparation of Graphene-Perfluoroalkoxy Composite and Thermal and Mechanical Properties. Polymers. 2018; 10(7):700. https://doi.org/10.3390/polym10070700
Chicago/Turabian StyleZhang, Wanlong, Haibin Zuo, Xinru Zhang, Jingsong Wang, Longfei Guo, and Xing Peng. 2018. "Preparation of Graphene-Perfluoroalkoxy Composite and Thermal and Mechanical Properties" Polymers 10, no. 7: 700. https://doi.org/10.3390/polym10070700
APA StyleZhang, W., Zuo, H., Zhang, X., Wang, J., Guo, L., & Peng, X. (2018). Preparation of Graphene-Perfluoroalkoxy Composite and Thermal and Mechanical Properties. Polymers, 10(7), 700. https://doi.org/10.3390/polym10070700






