Nanoparticles-Modified Chemical Sensor Fabricated on a Flexible Polymer Substrate for Cadmium(II) Detection
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
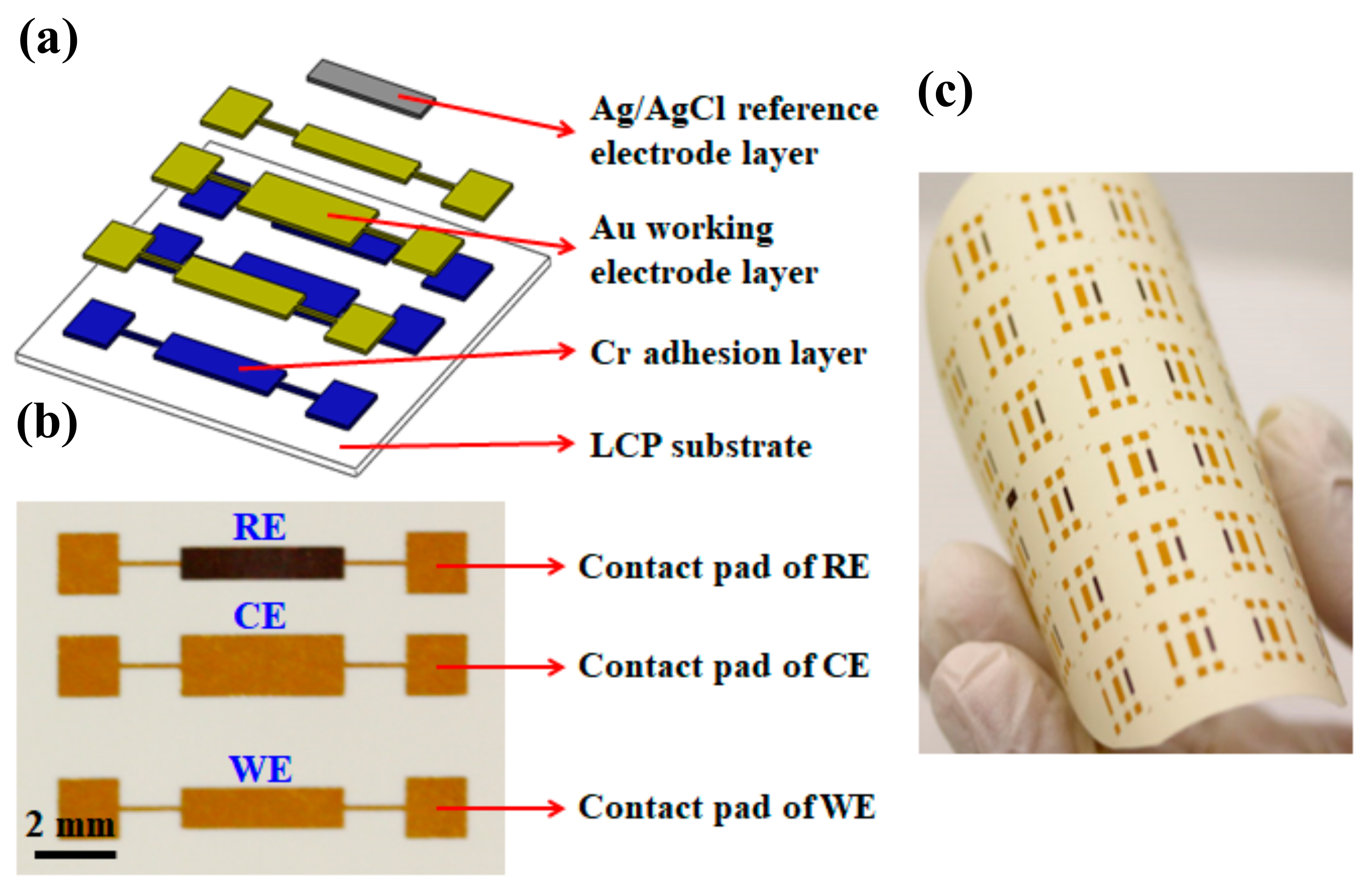
2.2. Fabrication of the Chemical Sensor
2.3. Packaging and Modification of the Chemical Sensor
2.4. Measurement Procedures
3. Results and Discussion
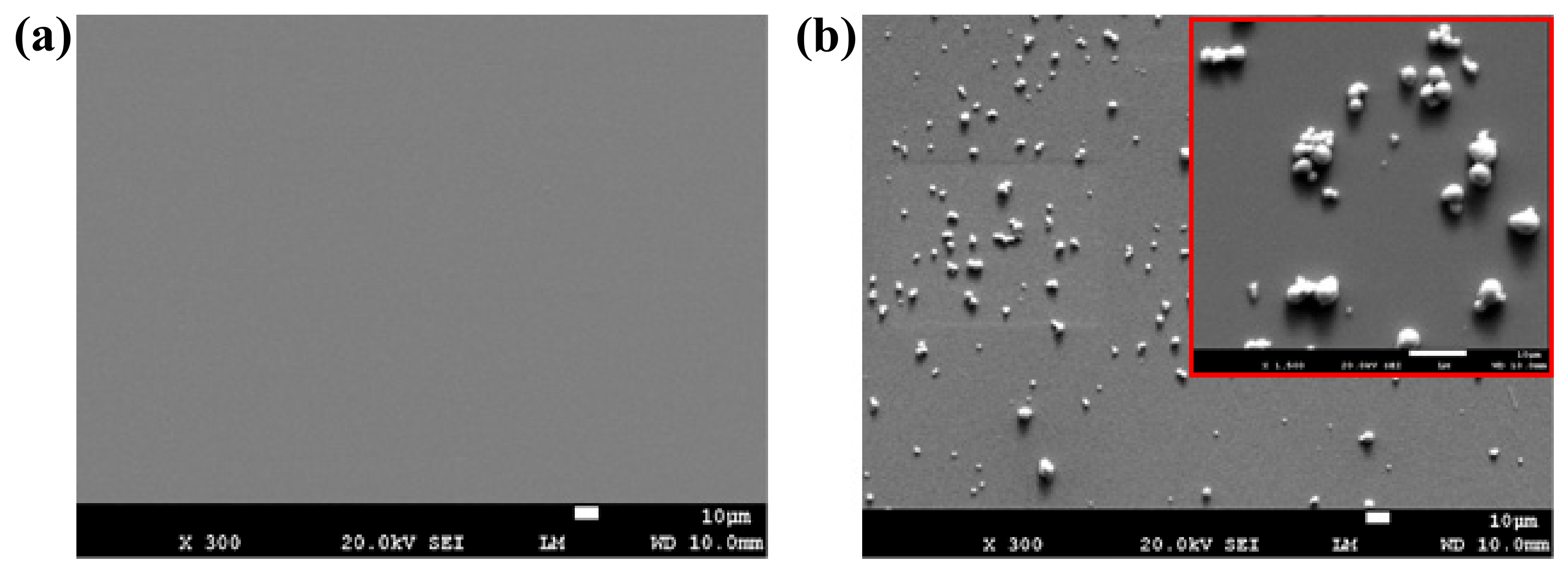
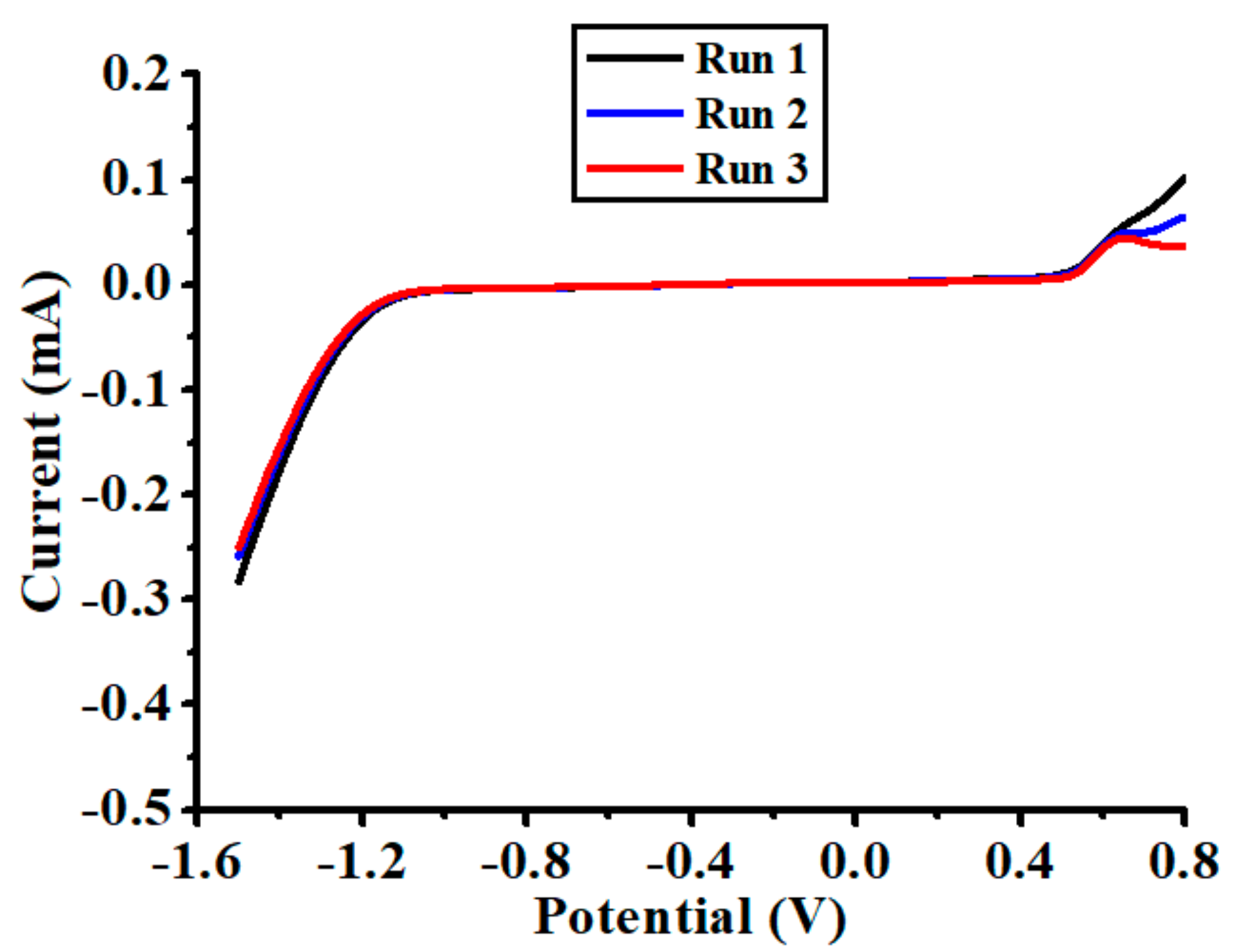
3.1. Preliminary Investigation of the Chemical Sensor
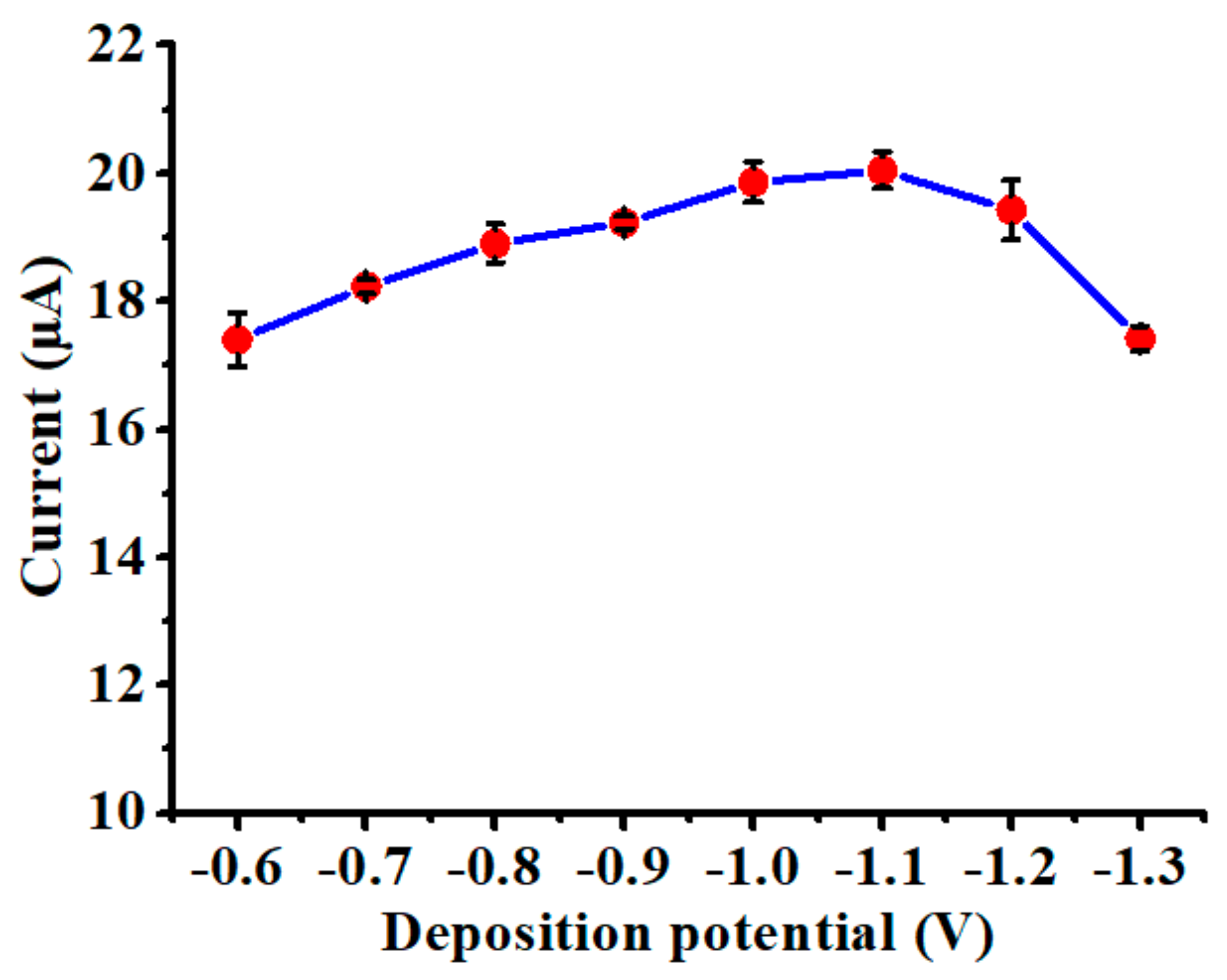
3.2. Influence of Deposition Potential
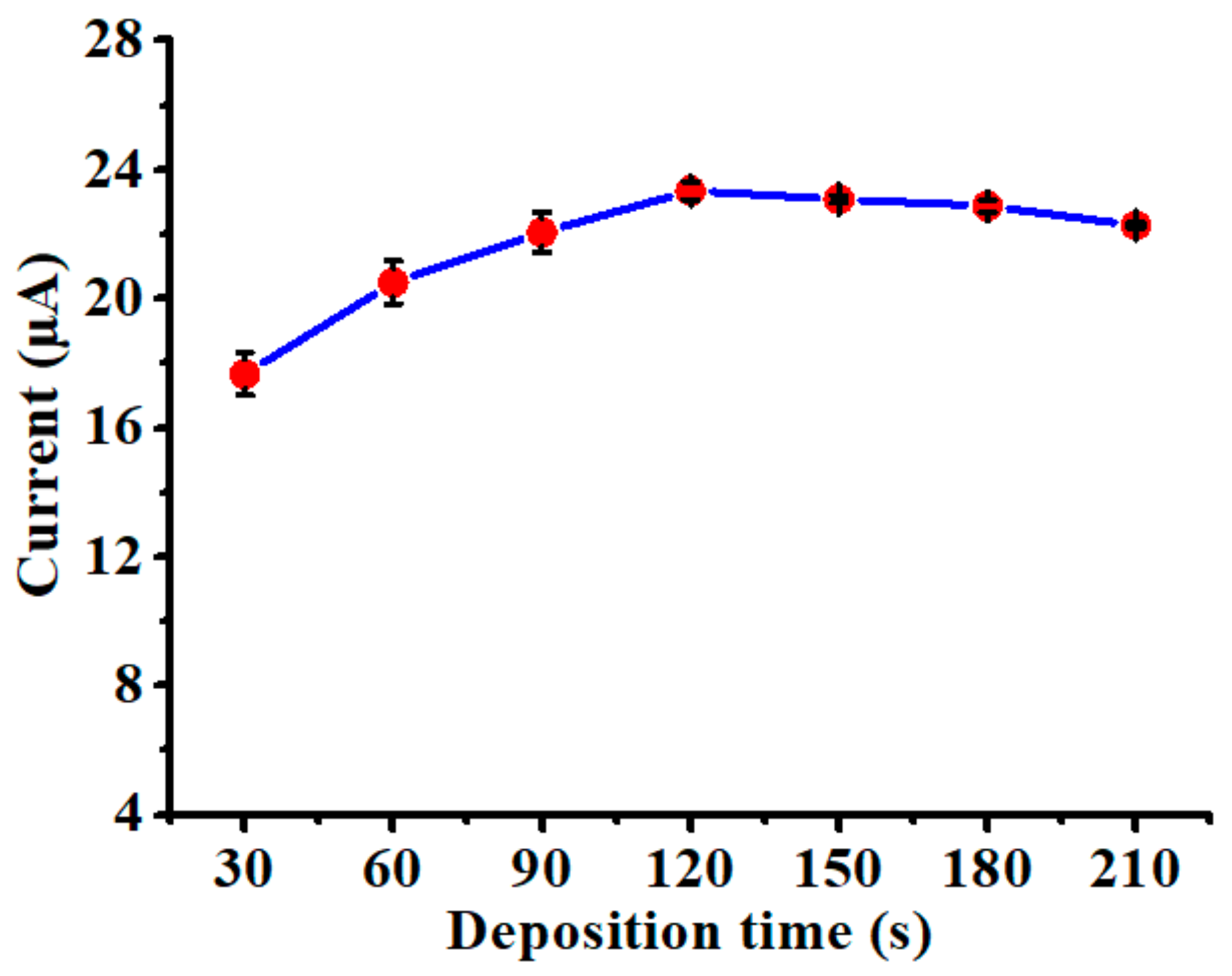
3.3. Influence of Deposition Time
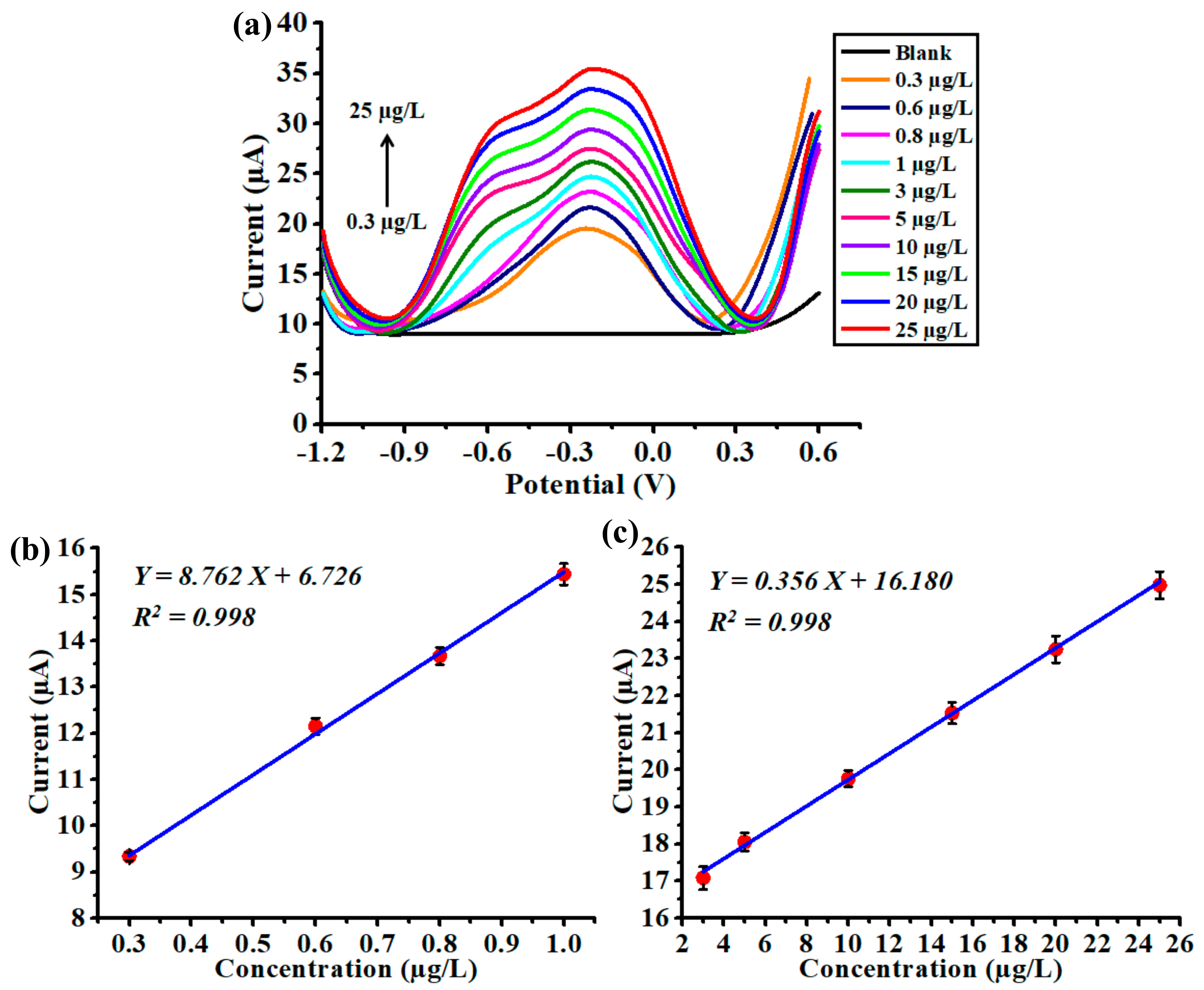
3.4. Analytical Performance of the Chemical Sensor
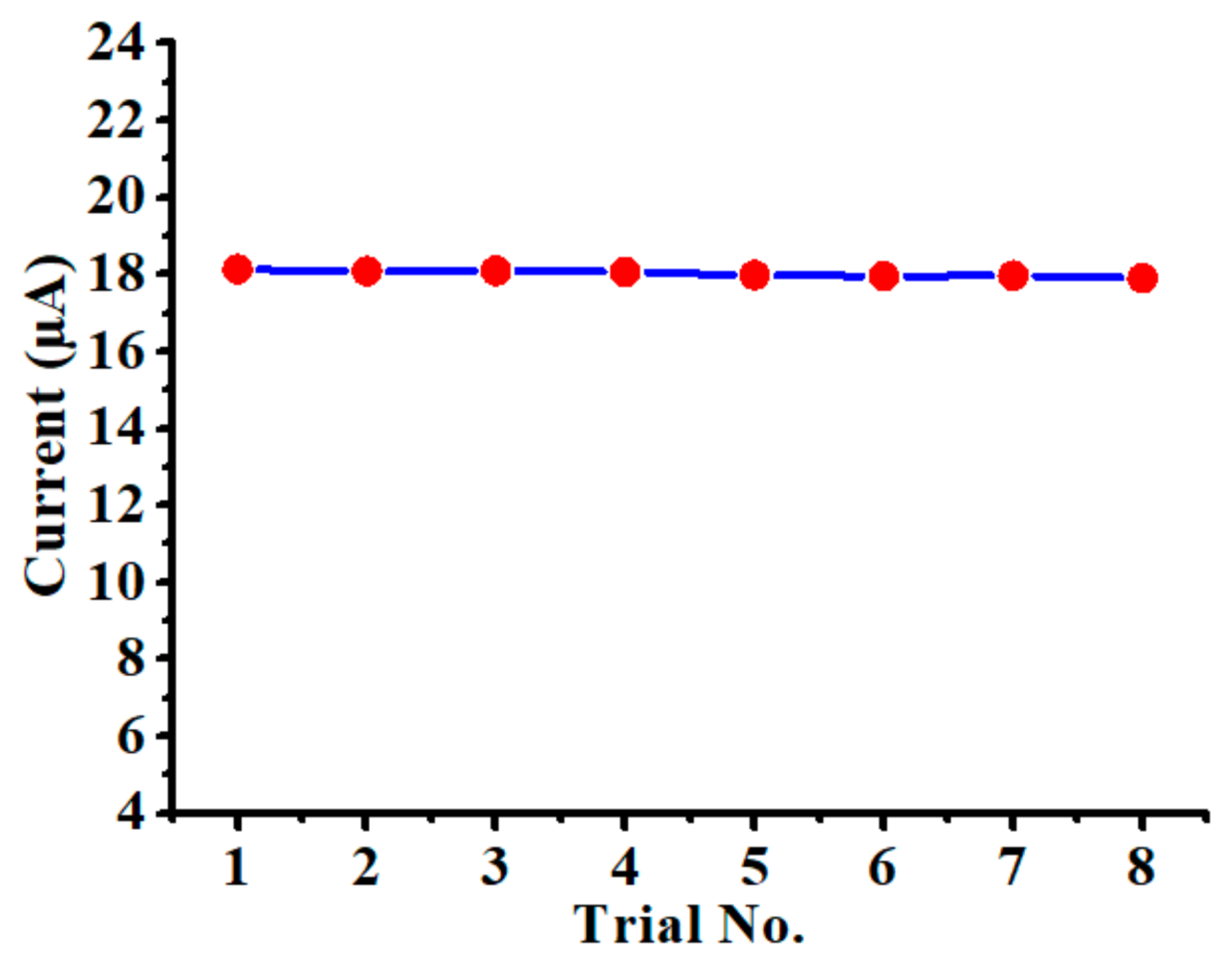
3.5. Evaluation of the Chemical Sensor
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Guidelines for Drinking-Water Quality, 4th ed.; World Health Organization: Geneva, Switzerland, 2011; pp. 327–328. ISBN 9789241548151. [Google Scholar]
- Elinder, C.G.; Jarup, L. Cadmium exposure and health risks: Recent findings. aMbio 1996, 25, 370–373. Available online: http://www.jstor.org/stable/4314494 (accessed on 14 May 2018).
- Hellstrom, L.; Elinder, C.G.; Dahlberg, B.; Lundberg, M.; Jarup, L.; Persson, B.; Axelson, O. Cadmium exposure and end-stage renal disease. Am. J. Kidney Dis. 2001, 38, 1001–1008. [Google Scholar] [CrossRef] [PubMed]
- Noonan, C.W.; Sarasua, S.M.; Campagna, D.; Kathman, S.J.; Lybarger, J.A.; Mueller, P.W. Effects of exposure to low levels of environmental cadmium on renal biomarkers. Environ. Health Perspect. 2002, 110, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Staessen, J.A.; Roels, H.A.; Emelianov, D.; Kuznetsova, T.; Thijs, L.; Vangronsveld, J.; Fagard, R. Environmental exposure to cadmium, forearm bone density, and risk of fractures: Prospective population study. Lancet 1999, 353, 1140–1144. [Google Scholar] [CrossRef]
- Wang, H.F.; Zhu, G.Y.; Shi, Y.X.; Weng, S.F.; Jin, T.Y.; Kong, Q.H.; Nordberg, G.F. Influence of environmental cadmium exposure on forearm bone density. J. Bone Miner. Res. 2003, 18, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Jarup, L.; Alfven, T. Low level cadmium exposure, renal and bone effects—The OSCAR study. BioMetals 2004, 17, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Chambers, R.C.; Laurent, G.J.; Westergren-Thorsson, G. Cadmium inhibits proteoglycan and procollagen production by cultured human lung fibroblasts. Am. J. Respir. Cell Mol. Biol. 1998, 19, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Croute, F.; Beau, B.; Arrabit, C.; Gaubin, Y.; Delmas, F.; Murat, J.C.; Soleilhavoup, J.P. Pattern of stress protein expression in human lung cell-line A549 after short- or long-term exposure to cadmium. Environ. Health Perspect. 2000, 108, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Shih, C.M.; Wu, J.S.; Ko, W.C.; Wang, L.F.; Wei, Y.H.; Liang, H.F.; Chen, Y.C.; Chen, C.T. Mitochondria-mediated caspase-independent apoptosis induced by cadmium in normal human lung cells. J. Cell. Biochem. 2003, 89, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Jothimuthu, P.; Wilson, R.A.; Herren, J.; Pei, X.; Kang, W.J.; Daniels, R.; Wong, H.; Beyette, F.; Heineman, W.R.; Papautsky, I. Zinc detection in serum by anodic stripping voltammetry on microfabricated bismuth electrodes. Electroanalysis 2013, 25, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Tseng, H.Y.; Adamik, V.; Parsons, J.; Lan, S.S.; Malfesi, S.; Lum, J.; Shannon, L.; Gray, B. Development of an electrochemical biosensor array for quantitative polymerase chain reaction utilizing three-metal printed circuit board technology. Sens. Actuators B 2014, 204, 459–466. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, H.; Williams, S.E.; Zhou, A.H. Microfabricated three-electrode on-chip PDMS device with a vibration motor for stripping voltammetric detection of heavy metal ions. Talanta 2015, 132, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, D.; Barus, C.; Giraud, W.; Calas, E.; Vanhove, E.; Laborde, A.; Launay, J.; Temple-Boyer, P.; Striebig, N.; Armengaud, M.; et al. Silicon-based electrochemical microdevices for silicate detection in seawater. Sens. Actuators B 2015, 211, 116–124. [Google Scholar] [CrossRef]
- Wang, N.; Kanhere, E.; Kottapalli, A.G.P.; Miao, J.M.; Triantafyllou, M.S. Flexible liquid crystal polymer-based electrochemical sensor for in-situ detection of zinc(II) in seawater. Microchim. Acta 2017, 184, 3007–3015. [Google Scholar] [CrossRef]
- Wang, X.F.; Engel, J.; Liu, C. Liquid crystal polymer (LCP) for MEMS: Processes and applications. J. Micromech. Microeng. 2003, 13, 628–633. [Google Scholar] [CrossRef]
- Wang, J. Stripping analysis at bismuth electrodes: A review. Electroanalysis 2005, 17, 1341–1346. [Google Scholar] [CrossRef]
- Hwang, G.H.; Han, W.K.; Hong, S.J.; Park, J.S.; Kang, S.G. Determination of trace amounts of lead and cadmium using a bismuth/glassy carbon composite electrode. Talanta 2009, 77, 1432–1436. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.J.; Kim, C.K.; Lee, M.K.; Rhee, C.K. Advanced use of nanobismuth/Nafion electrode for trace analyses of zinc, cadmium, and lead. J. Electrochem. Soc. 2010, 157, J241–J244. [Google Scholar] [CrossRef]
- Lien, C.H.; Chang, K.H.; Hu, C.C.; Wang, D.S.H. Optimizing bismuth-modified graphene-carbon nanotube composite-coated screen printed electrode for lead-ion sensing through the experimental design strategy. J. Electrochem. Soc. 2013, 160, B107–B112. [Google Scholar] [CrossRef]
- Yang, D.; Wang, L.; Chen, Z.L.; Megharaj, M.; Naidu, R. Anodic stripping voltammetric determination of traces of Pb(II) and Cd(II) using a glassy carbon electrode modified with bismuth nanoparticles. Microchim. Acta 2014, 181, 1199–1206. [Google Scholar] [CrossRef]
- Armstrong, K.C.; Tatum, C.E.; Dansby-Sparks, R.N.; Chambers, J.Q.; Xue, Z.L. Individual and simultaneous determination of lead, cadmium, and zinc by anodic stripping voltammetry at a bismuth bulk electrode. Talanta 2010, 82, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Saturno, J.; Valera, D.; Carrero, H.; Fernandez, L. Electroanalytical detection of Pb, Cd and traces of Cr at micro/nano-structured bismuth film electrodes. Sens. Actuators B 2011, 159, 92–96. [Google Scholar] [CrossRef]
- Dos Santos, V.B.; Fava, E.L.; de Miranda Curi, N.S.; Faria, R.C.; Fatibello-Filho, O. A thermostated electrochemical flow cell with a coupled bismuth film electrode for square-wave anodic stripping voltammetric determination of cadmium(II) and lead(II) in natural, wastewater and tap water samples. Talanta 2014, 126, 82–90. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, N.; Kanhere, E.; Miao, J.; Triantafyllou, M.S. Nanoparticles-Modified Chemical Sensor Fabricated on a Flexible Polymer Substrate for Cadmium(II) Detection. Polymers 2018, 10, 694. https://doi.org/10.3390/polym10070694
Wang N, Kanhere E, Miao J, Triantafyllou MS. Nanoparticles-Modified Chemical Sensor Fabricated on a Flexible Polymer Substrate for Cadmium(II) Detection. Polymers. 2018; 10(7):694. https://doi.org/10.3390/polym10070694
Chicago/Turabian StyleWang, Nan, Elgar Kanhere, Jianmin Miao, and Michael S. Triantafyllou. 2018. "Nanoparticles-Modified Chemical Sensor Fabricated on a Flexible Polymer Substrate for Cadmium(II) Detection" Polymers 10, no. 7: 694. https://doi.org/10.3390/polym10070694
APA StyleWang, N., Kanhere, E., Miao, J., & Triantafyllou, M. S. (2018). Nanoparticles-Modified Chemical Sensor Fabricated on a Flexible Polymer Substrate for Cadmium(II) Detection. Polymers, 10(7), 694. https://doi.org/10.3390/polym10070694





