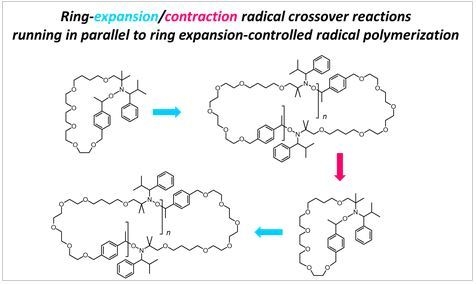
Ring-Expansion/Contraction Radical Crossover Reactions of Cyclic Alkoxyamines: A Mechanism for Ring Expansion-Controlled Radical Polymerization
Abstract
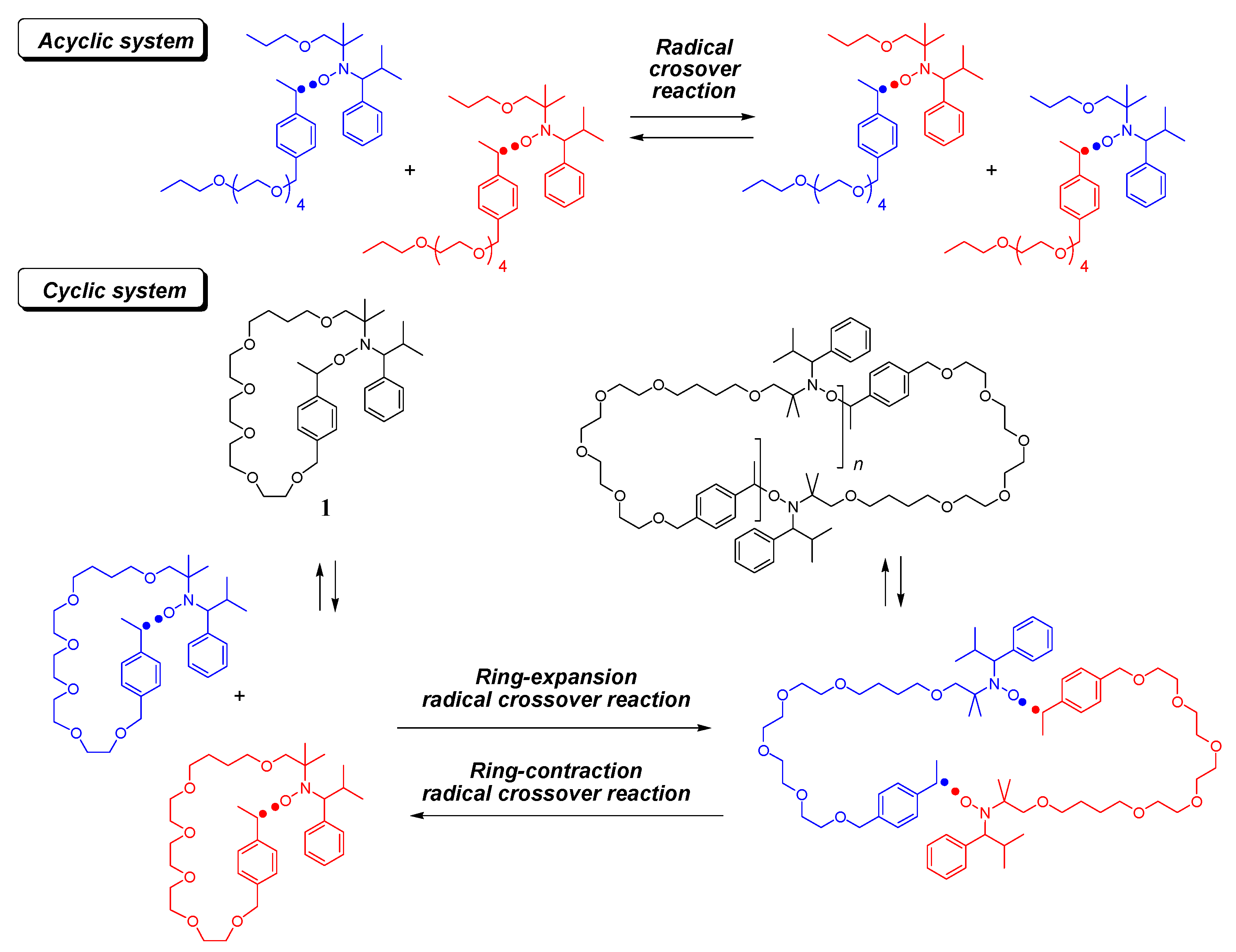
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
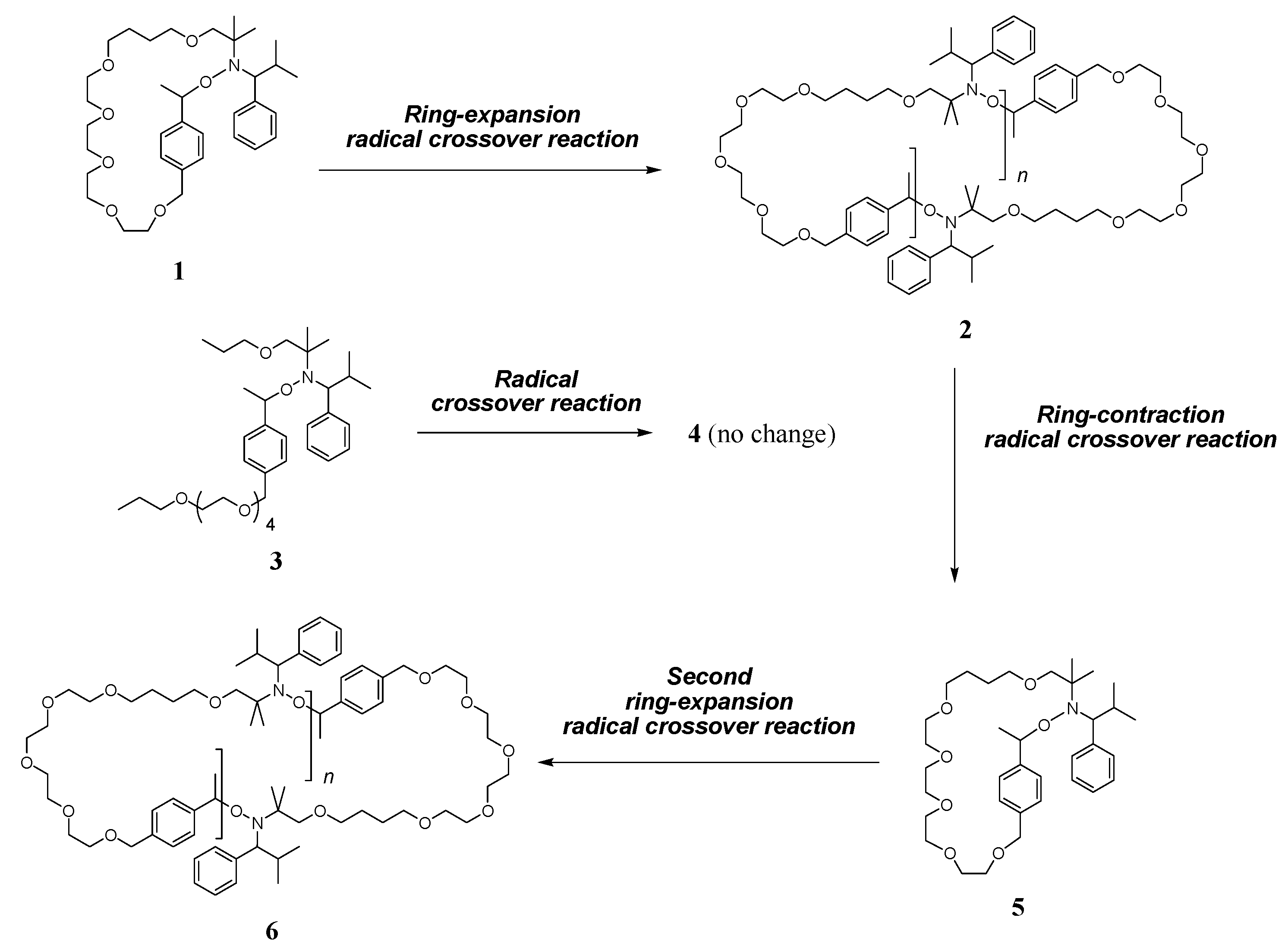
2.3. Ring-Expansion Radical Crossover Reaction (Synthesis of 2)
2.4. Ring-Contraction Radical Crossover Reaction (Synthesis of 5)
2.5. Second Ring-Expansion Radical Crossover Reaction (Synthesis of 6)
3. Results and Discussion
3.1. Ring-Expansion Radical Crossover Reaction
3.1.1. Temperature Effect
3.1.2. Time-Dependent Changes
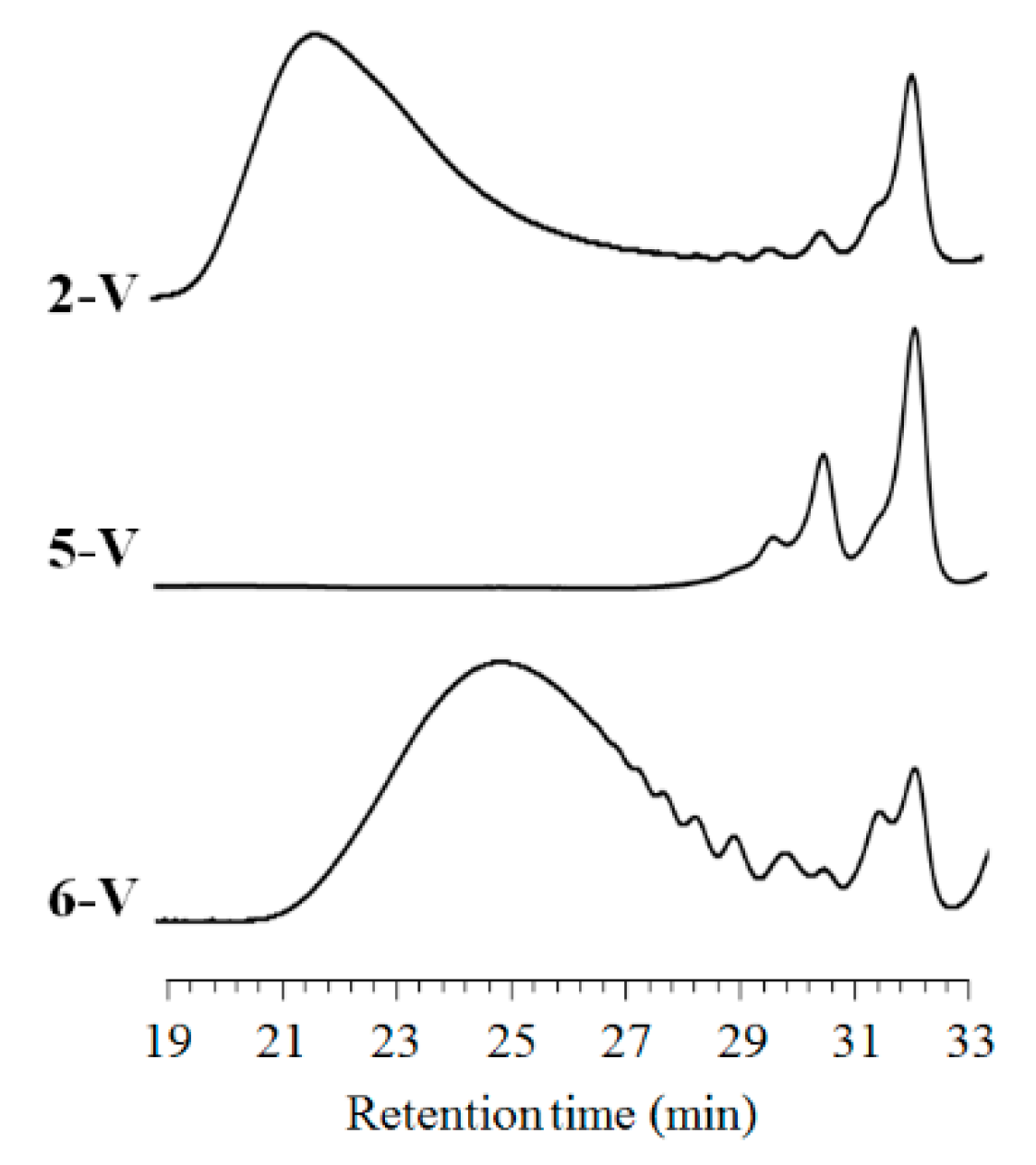
3.2. Ring-Contraction Radical Crossover Reaction
3.3. Second Ring-Expansion Radical Crossover Reaction
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Oike, H.; Hamada, M.; Eguchi, S.; Danda, Y.; Tezuka, Y. Novel synthesis of single- and double-cyclic polystyrenes by electrostatic self-assembly and covalent fixation with telechelics having cyclic ammonium salt groups. Macromolecules 2001, 34, 2776–2782. [Google Scholar] [CrossRef]
- Bielawski, C.W.; Benitez, D.; Grubbs, R.H. An “endless” route to cyclic polymers. Science 2002, 297, 2041–2044. [Google Scholar] [CrossRef] [PubMed]
- Bielawski, C.W.; Benitez, D.; Grubbs, R.H. Synthesis of Cyclic Polybutadiene via Ring-Opening Metathesis Polymerization: The Importance of Removing Trace Linear Contaminants. J. Am. Chem. Soc. 2003, 125, 8424–8425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Culkin, D.A.; Jeong, W.H.; Csihony, S.; Gomez, E.D.; Balsara, N.R.; Hedrick, J.L.; Waymouth, R.M. Zwitterionic polymerization of lactide to cyclic poly(lactide) by using N-heterocyclic carbene organocatalysts. Angew. Chem. Int. Ed. 2007, 46, 2627–2630. [Google Scholar] [CrossRef] [PubMed]
- Schappacher, M.; Deffieux, A. Atomic Force Microscopy Imaging and Dilute Solution Properties of Cyclic and Linear Polystyrene Combs. J. Am. Chem. Soc. 2008, 130, 14684–14689. [Google Scholar] [CrossRef] [PubMed]
- Schappacher, M.; Deffieux, A. Synthesis of Macrocyclic Copolymer Brushes and Their Self-Assembly into Supramolecular Tubes. Science 2008, 319, 1512–1515. [Google Scholar] [CrossRef] [PubMed]
- Jeong, W.; Shin, E.J.; Culkin, D.A.; Hedrick, J.L.; Waymouth, R.M. Zwitterionic Polymerization: A Kinetic Strategy for the Controlled Synthesis of Cyclic Polylactide. J. Am. Chem. Soc. 2009, 131, 4884–4891. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Boydston, A.J.; Grubbs, R.H. Synthesis and Direct Imaging of Ultrahigh Molecular Weight Cyclic Brush Polymers. Angew. Chem. Int. Ed. 2011, 50, 5882–5885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lahasky, S.H.; Serem, W.K.; Guo, L.; Garno, J.C.; Zhang, D.H. Synthesis and Characterization of Cyclic Brush-Like Polymers by N-Heterocyclic Carbene-Mediated Zwitterionic Polymerization of N-Propargyl N-Carboxyanhydride and the Grafting-to Approach. Macromolecules 2011, 44, 9063–9074. [Google Scholar] [CrossRef]
- Zhang, K.; Lackey, M.A.; Wu, Y.; Tew, G.N. Universal Cyclic Polymer Templates. J. Am. Chem. Soc. 2011, 133, 6906–6909. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Tew, G.N. Cyclic Brush Polymers by Combining Ring-Expansion Metathesis Polymerization and the “Grafting from” Technique. ACS Macro Lett. 2012, 1, 574–579. [Google Scholar] [CrossRef]
- Wei, H.; Chu, D.S.H.; Zhao, J.L.; Pahang, J.A.; Pun, S.H. Synthesis and Evaluation of Cyclic Cationic Polymers for Nucleic Acid Delivery. ACS Macro Lett. 2013, 2, 1047–1050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, K.; Zha, Y.P.; Peng, B.; Chen, Y.M.; Tew, G.N. Metallo-Supramolecular Cyclic Polymers. J. Am. Chem. Soc. 2013, 135, 15994–15997. [Google Scholar] [CrossRef] [PubMed]
- Danial, M.; Tran, C.M.N.; Jolliffe, K.A.; Perrier, S. Thermal Gating in Lipid Membranes Using Thermoresponsive Cyclic Peptide-Polymer Conjugates. J. Am. Chem. Soc. 2014, 136, 8018–8026. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Tew, G.N. Cyclic polymers as a building block for cyclic brush polymers and gels. React. Funct. Polym. 2014, 80, 40–47. [Google Scholar] [CrossRef]
- Cortez, M.A.; Godbey, W.T.; Fang, Y.L.; Payne, M.E.; Cafferty, B.J.; Kosakowska, K.A.; Grayson, S.M. The Synthesis of Cyclic Poly(ethylene imine) and Exact Linear Analogues: An Evaluation of Gene Delivery Comparing Polymer Architectures. J. Am. Chem. Soc. 2015, 137, 6541–6549. [Google Scholar] [CrossRef] [PubMed]
- Kimura, A.; Hasegawa, T.; Yamamoto, T.; Matsumoto, H.; Tezuka, Y. ESA-CF Synthesis of Linear and Cyclic Polymers Having Densely Appended Perylene Units and Topology Effects on Their Thin-Film Electron Mobility. Macromolecules 2016, 49, 5831–5840. [Google Scholar] [CrossRef]
- Roland, C.D.; Li, H.; Abboud, K.A.; Wagener, K.B.; Veige, A.S. Cyclic polymers from alkynes. Nat. Chem. 2016, 8, 791–796. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.S.; Yin, L.; Zhang, W.; Zhang, Z.B.; Zhu, X.L. Synthesis of diverse cyclic-brush polymers with cyclic polystyrene as a universal template via a grafting-from approach. Polym. Chem. 2016, 7, 2112–2120. [Google Scholar] [CrossRef]
- Zhang, H.C.; Wu, W.T.; Zhao, X.Q.; Zhao, Y.L. Synthesis and Thermoresponsive Behaviors of Thermo-, pH-, CO2- and Oxidation-Responsive Linear and Cyclic Graft Copolymers. Macromolecules 2017, 50, 3411–3423. [Google Scholar] [CrossRef]
- Xiao, L.F.; Qu, L.; Zhu, W.; Wu, Y.; Liu, Z.P.; Zhang, K. Donut-Shaped Nanoparticles Templated by Cyclic Bottlebrush Polymers. Macromolecules 2017, 50, 6762–6770. [Google Scholar] [CrossRef]
- Honda, S.; Yamamoto, T.; Tezuka, Y. Tuneable enhancement of the salt and thermal stability of polymeric micelles by cyclized amphiphiles. Nat. Commun. 2013, 4, 1574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eugene, D.M.; Grayson, S.M. Efficient Preparation of Cyclic Poly(methyl acrylate)-block-poly(styrene) by Combination of Atom Transfer Radical Polymerization and Click Cyclization. Macromolecules 2008, 41, 5082–5084. [Google Scholar] [CrossRef]
- Laurent, B.A.; Grayson, S.M. Synthetic approaches for the preparation of cyclic polymers. Chem. Soc. Rev. 2009, 38, 2202–2213. [Google Scholar] [CrossRef] [PubMed]
- Laurent, B.A.; Grayson, S.M. Synthesis of Cyclic Dendronized Polymers via Divergent “Graft-from” and Convergent Click “Graft-to” Routes: Preparation of Modular Toroidal Macromolecules. J. Am. Chem. Soc. 2011, 133, 13421–13429. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Tezuka, Y. Topological polymer chemistry: A cyclic approach toward novel polymer properties and functions. Polym. Chem. 2011, 2, 1930–1941. [Google Scholar] [CrossRef]
- Brown, H.A.; Waymouth, R.M. Zwitterionic Ring-Opening Polymerization for the Synthesis of High Molecular Weight Cyclic Polymers. Acc. Chem. Res. 2013, 46, 2585–2596. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T. Synthesis of cyclic polymers and topology effects on their diffusion and thermal properties. Polym. J. 2013, 45, 711–717. [Google Scholar] [CrossRef]
- Josse, T.; De Winter, J.; Gerbaux, P.; Coulembier, O. Cyclic Polymers by Ring-Closure Strategies. Angew. Chem. Int. Ed. 2016, 55, 13944–13958. [Google Scholar] [CrossRef] [PubMed]
- Tu, X.Y.; Liu, M.Z.; Wei, H. Recent Progress on Cyclic Polymers: Synthesis, Bioproperties, and Biomedical Applications. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 1447–1458. [Google Scholar] [CrossRef]
- Chang, Y.A.; Waymouth, R.M. Recent Progress on the Synthesis of Cyclic Polymers via Ring-Expansion Strategies. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 2892–2902. [Google Scholar] [CrossRef]
- Ruehl, J.; Ningnuek, N.; Thongpaisanwong, T.; Braslau, R. Cyclic Alkoxyamines for Nitroxide-Mediated Radical Polymerization. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 8049–8069. [Google Scholar] [CrossRef]
- Nicolaÿ, R.; Matyjaszewski, K. Synthesis of Cyclic (Co)polymers by Atom Transfer Radical Cross-Coupling and Ring Expansion by Nitroxide-Mediated Polymerization. Macromolecules 2011, 44, 240–247. [Google Scholar] [CrossRef]
- Narumi, A.; Zeidler, S.; Barqawi, H.; Enders, C.; Binder, W.H. Cyclic Alkoxyamine-Initiator Tethered by Azide/Alkyne-“Click”-Chemistry Enabling Ring-Expansion Vinyl Polymerization Providing Macrocyclic Polymers. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 3402–3416. [Google Scholar] [CrossRef]
- Narumi, A.; Hasegawa, S.; Yanagisawa, R.; Tomiyama, M.; Yamada, M.; Binder, W.H.; Kikuchi, M.; Kawaguchi, S. Ring expansion-controlled radical polymerization: Synthesis of cyclic polymers and ring component quantification based on SEC-MALS analysis. React. Funct. Polym. 2016, 104, 1–8. [Google Scholar] [CrossRef]
- Bunha, A.; Cao, P.F.; Mangadlao, J.D.; Advincula, R.C. Cyclic poly(vinylcarbazole) via ring-expansion polymerization-RAFT (REP-RAFT). React. Funct. Polym. 2014, 80, 33–39. [Google Scholar] [CrossRef]
- Kammiyada, H.; Konishi, A.; Ouchi, M.; Sawamoto, M. Ring-Expansion Living Cationic Polymerization via Reversible Activation of a Hemiacetal Ester Bond. ACS Macro Lett. 2013, 2, 531–534. [Google Scholar] [CrossRef]
- Ouchi, M.; Kammiyada, H.; Sawamoto, M. Ring-expansion cationic polymerization of vinyl ethers. Polym. Chem. 2017, 8, 4970–4977. [Google Scholar] [CrossRef]
- Kammiyada, H.; Ouchi, M.; Sawamoto, M. A Study on Physical Properties of Cyclic Poly(vinyl ether)s Synthesized via Ring-Expansion Cationic Polymerization. Macromolecules 2017, 50, 841–848. [Google Scholar] [CrossRef]
- Kammiyada, H.; Ouchi, M.; Sawamoto, M. Expanding Vinyl Ether Monomer Repertoire for Ring-Expansion Cationic Polymerization: Various Cyclic Polymers with Tailored Pendant Groups. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 3082–3089. [Google Scholar] [CrossRef]
- He, T.; Zheng, G.H.; Pan, C.Y. Synthesis of cyclic polymers and block copolymers by monomer insertion into cyclic initiator by a radical mechanism. Macromolecules 2003, 36, 5960–5966. [Google Scholar] [CrossRef]
- Yamaguchi, G.; Higaki, Y.; Otsuka, H.; Takahara, A. Reversible Radical Ring-Crossover Polymerization of an Alkoxyamine-Containing Dynamic Covalent Macrocycle. Macromolecules 2005, 38, 6316–6320. [Google Scholar] [CrossRef]
- Harth, E.; Hawker, C.J.; Fan, W.; Waymouth, R.M. Chain end functionalization in nitroxide-mediated “Living” free radical polymerizations. Macromolecules 2001, 34, 3856–3862. [Google Scholar] [CrossRef]
- Otsuka, H.; Aotani, K.; Higaki, Y.; Takahara, A. A dynamic (reversible) covalent polymer: Radical crossover behaviour of TEMPO-containing poly(alkoxyamine ester)s. Chem. Commun. 2002, 23, 2838–2839. [Google Scholar] [CrossRef]
- Otsuka, H.; Aotani, K.; Higaki, Y.; Takahara, A. Polymer scrambling: Macromolecular radical crossover reaction between the main chains of alkoxyamine-based dynamic covalent polymers. J. Am. Chem. Soc. 2003, 125, 4064–4065. [Google Scholar] [CrossRef] [PubMed]
- Jhaveri, S.B.; Beinhoff, M.; Hawker, C.J.; Carter, K.R.; Sogah, D.Y. Chain-end functionalized nanopatterned polymer brushes grown via in situ nitroxide free radical exchange. ACS Nano 2008, 2, 719–727. [Google Scholar] [CrossRef] [PubMed]
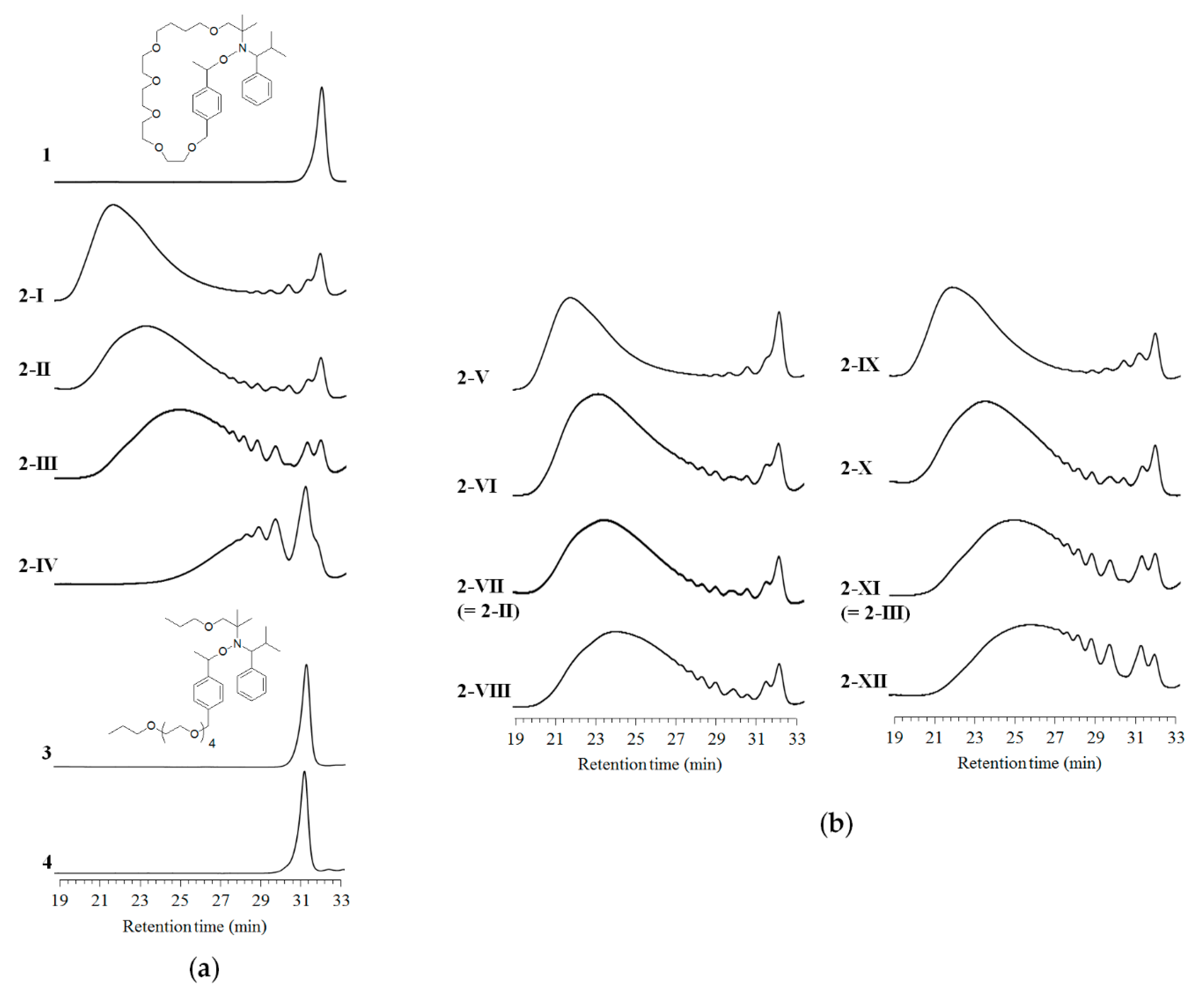

| Entry | Starting Material | Temp. (°C) | Time (h) | Product Code | Mw1 | Mw/Mn1 | Mp1 | φ 2 (%) |
|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 105 | 12 | 2-I | 42,800 | 18.1 | 57,100 | 93 |
| 2 | 1 | 115 | 12 | 2-II | 29,600 | 11.0 | 24,900 | 90 |
| 3 | 1 | 125 | 12 | 2-III | 13,600 | 7.04 | 12,400 | 74 |
| 4 | 1 | 135 | 12 | 2-IV | 2930 | 3.33 | 446 | - |
| 5 | 3 (control) | 115 | 12 | 4 | 506 | 1.06 | 426 | 90 |
| 6 | 1 | 115 | 3 | 2-V | 38,000 | 24.8 | 58,400 | 98 |
| 7 | 1 | 115 | 6 | 2-VI | 26,600 | 11.3 | 28,600 | 92 |
| 8 (=2) | 1 | 115 | 12 | 2-VII (=2-II) | 29,600 | 11.0 | 24,900 | 90 |
| 9 | 1 | 115 | 24 | 2-VIII | 20,300 | 8.5 | 19,300 | 82 |
| 10 | 1 | 125 | 3 | 2-IX | 38,600 | 15.9 | 50,600 | 92 |
| 11 | 1 | 125 | 6 | 2-X | 23,500 | 9.27 | 24,500 | 84 |
| 12 (=3) | 1 | 125 | 12 | 2-XI (=2-III) | 13,600 | 7.04 | 12,400 | 74 |
| 13 | 1 | 125 | 24 | 2-XII | 10,300 | 6.11 | 9940 | 64 |
| Entry | Starting Material | Temp. (°C) | Time (h) | Product Code | Mw1 | Mw/Mn1 | Mp1 | φ 2 (%) |
|---|---|---|---|---|---|---|---|---|
| 14 | 2-V | 115 | 9 | 5-V | 690 | 1.87 | 231 | 90 |
| 15 | 5-V | 115 | 3 | 6-V | 13,500 | 6.59 | 14,700 | 84 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Narumi, A.; Kobayashi, T.; Yamada, M.; Binder, W.H.; Matsuda, K.; Shaykoon, M.S.A.; Enomoto, K.; Kikuchi, M.; Kawaguchi, S. Ring-Expansion/Contraction Radical Crossover Reactions of Cyclic Alkoxyamines: A Mechanism for Ring Expansion-Controlled Radical Polymerization. Polymers 2018, 10, 638. https://doi.org/10.3390/polym10060638
Narumi A, Kobayashi T, Yamada M, Binder WH, Matsuda K, Shaykoon MSA, Enomoto K, Kikuchi M, Kawaguchi S. Ring-Expansion/Contraction Radical Crossover Reactions of Cyclic Alkoxyamines: A Mechanism for Ring Expansion-Controlled Radical Polymerization. Polymers. 2018; 10(6):638. https://doi.org/10.3390/polym10060638
Chicago/Turabian StyleNarumi, Atsushi, Tetsuya Kobayashi, Masatsugu Yamada, Wolfgang H. Binder, Keigo Matsuda, Montaser Shaykoon Ahmed Shaykoon, Kazushi Enomoto, Moriya Kikuchi, and Seigou Kawaguchi. 2018. "Ring-Expansion/Contraction Radical Crossover Reactions of Cyclic Alkoxyamines: A Mechanism for Ring Expansion-Controlled Radical Polymerization" Polymers 10, no. 6: 638. https://doi.org/10.3390/polym10060638
APA StyleNarumi, A., Kobayashi, T., Yamada, M., Binder, W. H., Matsuda, K., Shaykoon, M. S. A., Enomoto, K., Kikuchi, M., & Kawaguchi, S. (2018). Ring-Expansion/Contraction Radical Crossover Reactions of Cyclic Alkoxyamines: A Mechanism for Ring Expansion-Controlled Radical Polymerization. Polymers, 10(6), 638. https://doi.org/10.3390/polym10060638