3D Printing of Thermoresponsive Polyisocyanide (PIC) Hydrogels as Bioink and Fugitive Material for Tissue Engineering
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis and Characterisation of PIC
2.2. Rheological Analysis of PIC Hydrogel
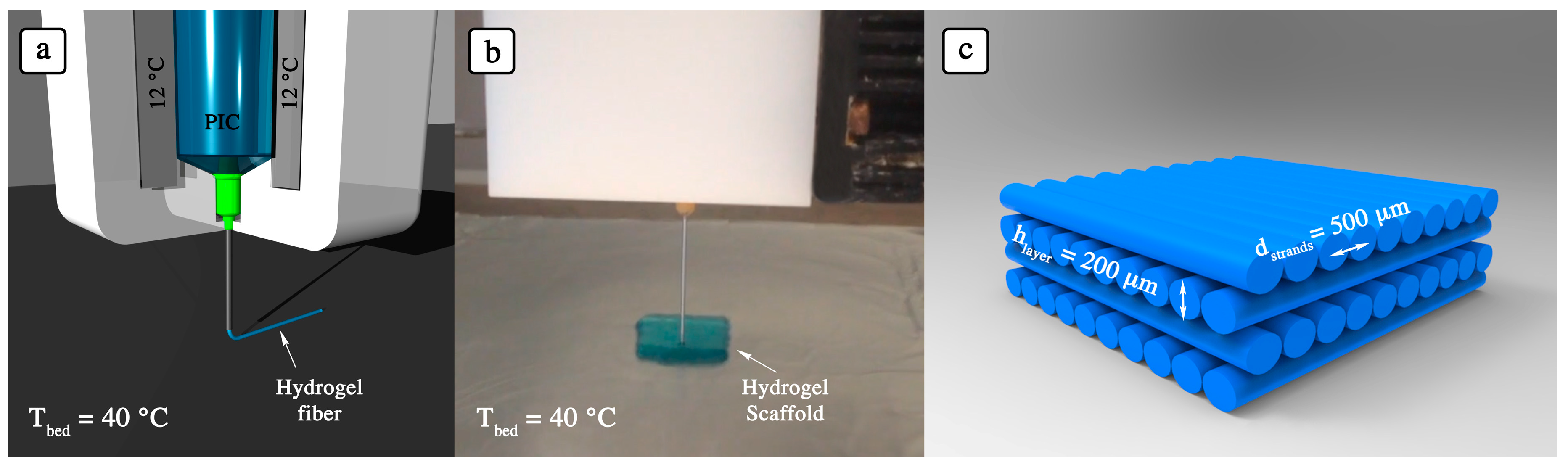
2.3. 3D Printing of PIC
2.4. Synthesis of Gelatin Methacrylate
2.5. Creating Dual Hydrogel System: PIC as Fugitive Material
3. Results and Discussion
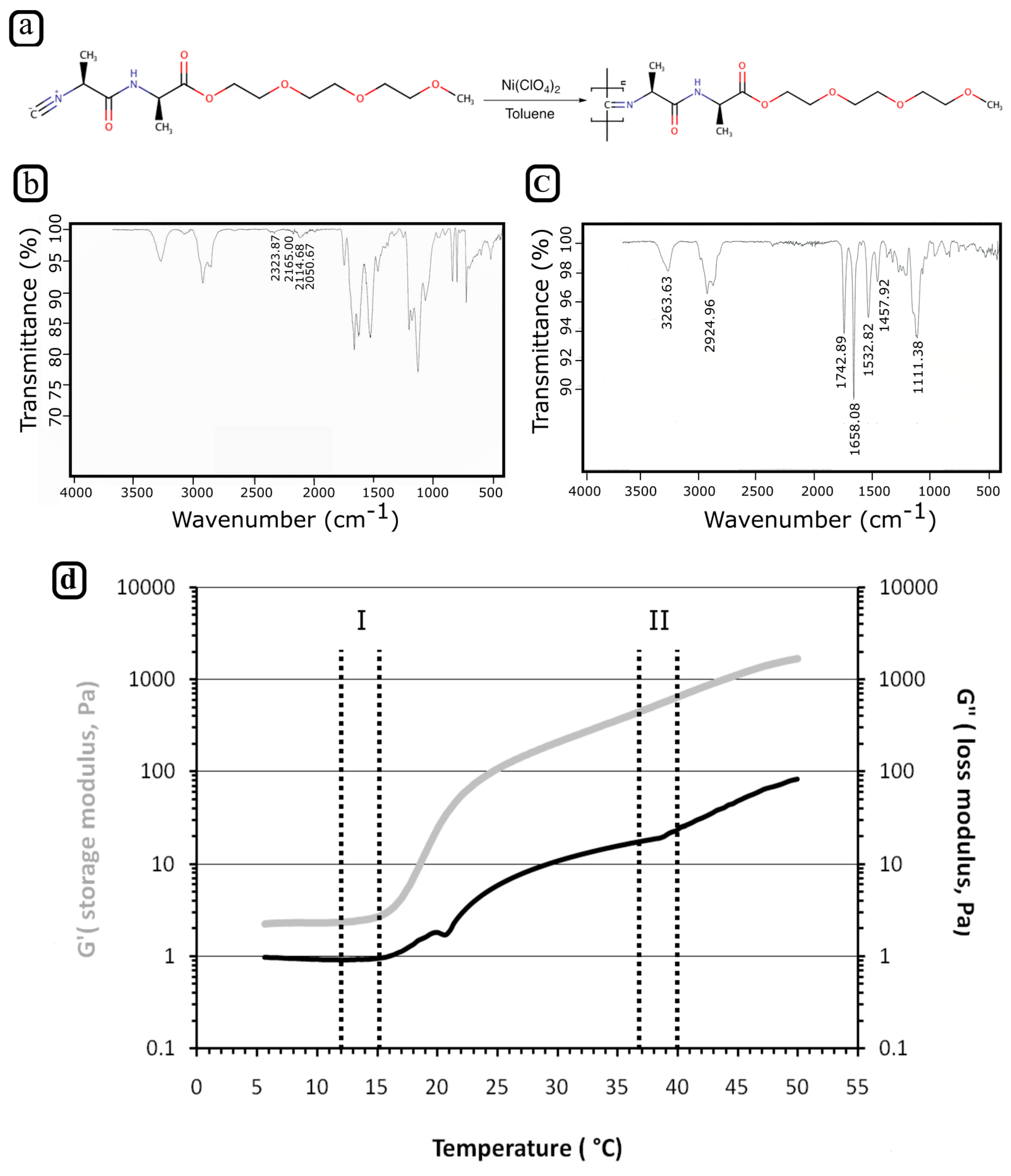
3.1. Synthesis of PIC Polymer
3.2. Rheology of PIC Solution
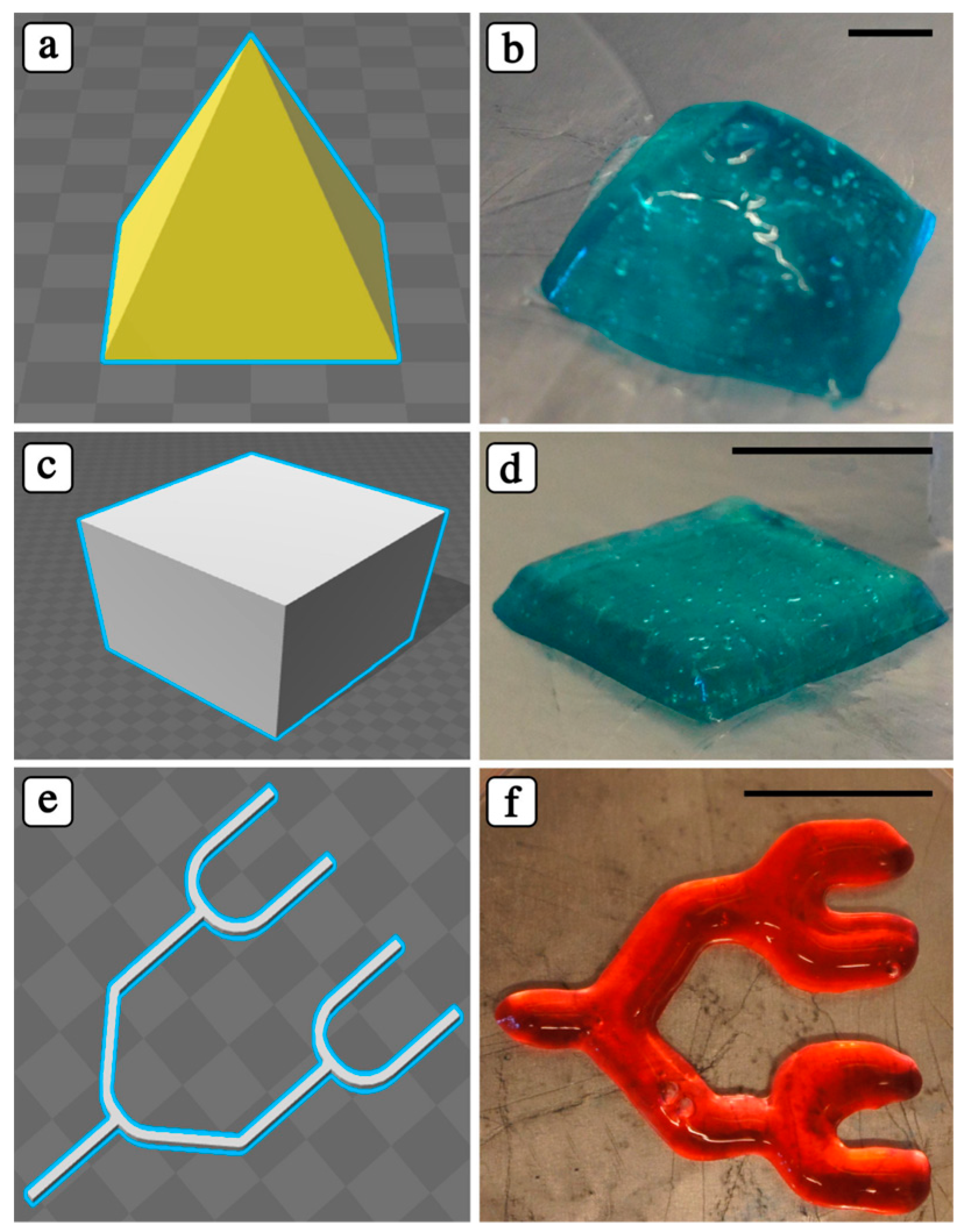
3.3. Adjusting 3D Printing Parameters for PIC Hydrogels
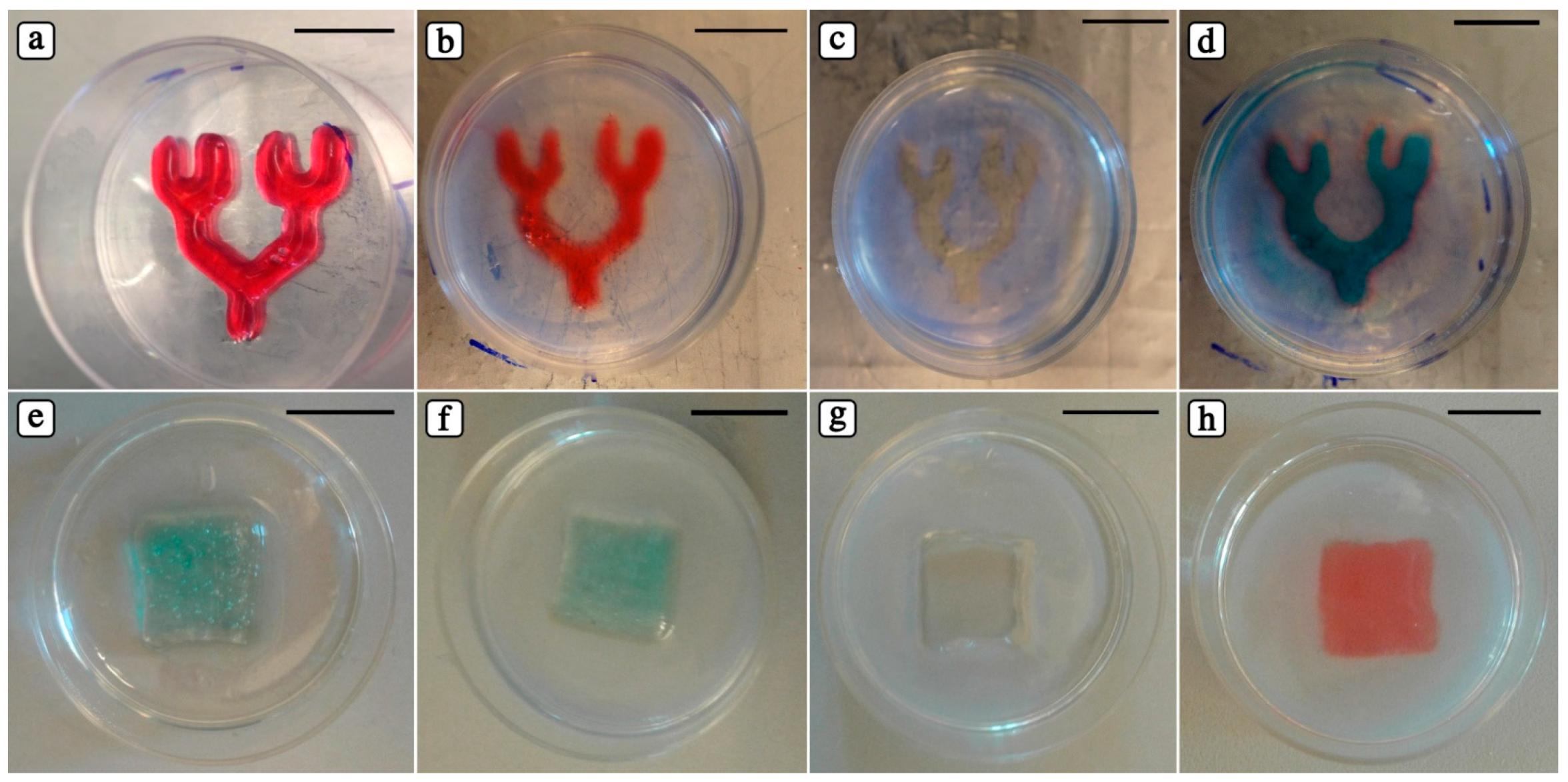
3.4. Creating Dual Hydrogel System: PIC as Fugitive Material
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Langer, R.; Vacanti, J.P. Tissue Engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Groll, J.; Boland, T.; Blunk, T.; Burdick, J.A.; Cho, D.-W.; Dalton, P.D.; Derby, B.; Forgacs, G.; Li, Q.; Mironov, V.A.; et al. Biofabrication: Reappraising the definition of an evolving field. Biofabrication 2016, 8, 13001. [Google Scholar] [CrossRef] [PubMed]
- Mironov, V.; Reis, N.; Phil, D.; Derby, B. Review: Bioprinting: A Beginning. Tissue Eng. 2006, 12, 631–634. [Google Scholar] [CrossRef] [PubMed]
- Pfister, A.; Landers, R.; Laib, A.; Hübner, U.; Schmelzeisen, R.; Mülhaupt, R. Biofunctional Rapid Prototyping for Tissue-Engineering Applications: 3D Bioplotting versus 3D Printing. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 624–638. [Google Scholar] [CrossRef]
- Landers, R.; Pfister, A.; John, H. Fabrication of soft tissue engineering scaffolds by means of rapid prototyping techniques. J. Mater. Sci. 2002, 7, 3107–3116. [Google Scholar] [CrossRef]
- Rumiński, S.; Ostrowska, B.; Jaroszewicz, J.; Skirecki, T.; Włodarski, K.; Święszkowski, W.; Lewandowska-Szumieł, M. Three-dimensional printed polycaprolactone-based scaffolds provide an advantageous environment for osteogenic differentiation of human adipose-derived stem cells. J. Tissue Eng. Regen. Med. 2018, 12, e473–e485. [Google Scholar] [CrossRef] [PubMed]
- Moroni, L.; de Wijn, J.R.; van Blitterswijk, C. A 3D fiber-deposited scaffolds for tissue engineering: Influence of pores geometry and architecture on dynamic mechanical properties. Biomaterials 2006, 27, 974–985. [Google Scholar] [CrossRef] [PubMed]
- Vandenbroucke, B.; Kruth, J. Selective laser melting of biocompatible metals for rapid manufacturing of medical parts. Rapid Prototyp. J. 2007, 13, 196–203. [Google Scholar] [CrossRef]
- Wysocki, B.; Maj, P.; Krawczyńska, A.; Rożniatowski, K.; Zdunek, J.; Kurzydłowski, K.J.; Święszkowski, W. Microstructure and mechanical properties investigation of CP titanium processed by selective laser melting (SLM). J. Mater. Process. Technol. 2017, 241, 13–23. [Google Scholar] [CrossRef]
- Scheithauer, U.; Weingarten, S.; Johne, R.; Schwarzer, E.; Abel, J.; Richter, H.-J.; Moritz, T.; Michaelis, A. Ceramic-Based 4D Components: Additive Manufacturing (AM) of Ceramic-Based Functionally Graded Materials (FGM) by Thermoplastic 3D Printing (T3DP). Materials 2017, 10, 1368. [Google Scholar] [CrossRef] [PubMed]
- Gbureck, U.; Hoelzel, T.; Doillon, C.J.; Mueller, F.A.; Barralet, J.E. Direct printing of bioceramic implants with spatially localized angiogenic factors. Adv. Mater. 2007, 19, 795–800. [Google Scholar] [CrossRef]
- Kosik-Kozioł, A.; Costantini, M.; Bolek, T.; Szöke, K.; Barbetta, A.; Brinchmann, J.; Święszkowski, W. PLA short sub-micron fiber reinforcement of 3D bioprinted alginate constructs for cartilage regeneration. Biofabrication 2017, 9, 44105. [Google Scholar] [CrossRef] [PubMed]
- Costantini, M.; Testa, S.; Mozetic, P.; Barbetta, A.; Fuoco, C.; Fornetti, E.; Tamiro, F.; Bernardini, S.; Jaroszewicz, J.; Swieszkowski, W.; et al. Microfluidic-enhanced 3D bioprinting of aligned myoblast-laden hydrogels leads to functionally organized myofibers in vitro and in vivo. Biomaterials 2017, 131, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Guvendiren, M. Recent Advances in Bioink Design for 3D Bioprinting of Tissues and Organs. Front. Bioeng. Biotechnol. 2017, 5, 23. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Yeong, W.Y. Design and Printing Strategies in 3D Bioprinting of Cell-Hydrogels: A Review. Adv. Healthc. Mater. 2016, 5, 2856–2865. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Hydrogels for Tissue Engineering. Chem. Rev. 2001, 101, 1869–1880. [Google Scholar] [CrossRef] [PubMed]
- Guvendiren, M.; Molde, J.; Soares, R.M.D.; Kohn, J. Designing biomaterials for 3D printing. ACS Biomater. Sci. Eng. 2016, 2, 1679–1693. [Google Scholar] [CrossRef] [PubMed]
- Khattak, S.F.; Bhatia, S.R.; Roberts, S.C. Pluronic F127 as a cell encapsulation material: Utilization of membrane-stabilizing agents. Tissue Eng. 2005, 11, 974–983. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Deconinck, A.; Lewis, J.A. Omnidirectional printing of 3D microvascular networks. Adv. Mater. 2011, 23, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.-W.; Lee, S.J.; Ko, I.K.; Kengla, C.; Yoo, J.J.; Atala, A. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat. Biotechnol. 2016, 34, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.; Becher, J.; Schnabelrauch, M.; Zenobi-Wong, M. Nanostructured Pluronic hydrogels as bioinks for 3D bioprinting. Biofabrication 2015, 7, 35006. [Google Scholar] [CrossRef] [PubMed]
- Das, R.K.; Gocheva, V.; Hammink, R.; Zouani, O.F.; Rowan, A.E. Stress-stiffening-mediated stem-cell commitment switch in soft responsive hydrogels. Nat. Mater. 2016, 15, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Rowan, A.E.; Eksteen, A.Z.H.; Wilson, C.; Geutjes, P.J.; FEITZ, W.F.; Oosterwijk, E. Polymer Suitable for Use in Cell Culture. U.S. Patent 14/905,579, 19 May 2016. [Google Scholar]
- Kouwer, P.H.J.; Koepf, M.; Le Sage, V.A.A.; Jaspers, M.; van Buul, A.M.; Eksteen-Akeroyd, Z.H.; Woltinge, T.; Schwartz, E.; Kitto, H.J.; Hoogenboom, R.; et al. Responsive biomimetic networks from polyisocyanopeptide hydrogels. Nature 2013, 493, 651–655. [Google Scholar] [CrossRef] [PubMed]
- Jaspers, M.; Dennison, M.; Mabesoone, M.F.J.; MacKintosh, F.C.; Rowan, A.E.; Kouwer, P.H.J. Ultra-responsive soft matter from strain-stiffening hydrogels. Nat. Commun. 2014, 5, 5808. [Google Scholar] [CrossRef] [PubMed]
- Costantini, M.; Idaszek, J.; Szöke, K.; Jaroszewicz, J.; Dentini, M.; Barbetta, A.; Brinchmann, J.E.; Święszkowski, W. 3D bioprinting of BM-MSCs-loaded ECM biomimetic hydrogels for in vitro neocartilage formation. Biofabrication 2016, 8, 35002. [Google Scholar] [CrossRef] [PubMed]
- Colosi, C.; Shin, S.R.; Manoharan, V.; Massa, S.; Costantini, M.; Barbetta, A.; Dokmeci, M.R.; Dentini, M.; Khademhosseini, A. Microfluidic Bioprinting of Heterogeneous 3D Tissue Constructs Using Low-Viscosity Bioink. Adv. Mater. 2016, 28, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Schilling, A.F.; Hubbell, K.; Yonezawa, T.; Truong, D.; Hong, Y.; Dai, G.; Cui, X. Improved properties of bone and cartilage tissue from 3D inkjet-bioprinted human mesenchymal stem cells by simultaneous deposition and photocrosslinking in PEG-GelMA. Biotechnol. Lett. 2015, 37, 2349–2355. [Google Scholar] [CrossRef] [PubMed]
- Yue, K.; Santiago, G.T.; Tamayol, A.; Annabi, N.; Khademhosseini, A.; Hospital, W.; Arabia, S.; Trujillo-De Santiago, G.; Alvarez, M.M.; Tamayol, A.; et al. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials 2015, 73, 254–271. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Eksteen-Akeroyd, Z.H.; Jacobs, M.J.; Hammink, R.; Koepf, M.; Lambeck, A.J.A.; van Hest, J.C.M.; Wilson, C.J.; Blank, K.; Figdor, C.G. Therapeutic nanoworms: Towards novel synthetic dendritic cells for immunotherapy. Chem. Sci. 2013, 4, 4168–4174. [Google Scholar] [CrossRef]
- Van Den Bulcke, A.I.; Bogdanov, B.; De Rooze, N.; Schacht, E.H.; Cornelissen, M.; Berghmans, H. Structural and rheological properties of methacrylamide modified gelatin hydrogels. Biomacromolecules 2000, 1, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Zimoch, J.; Padial, J.S.; Klar, A.S.; Vallmajo-Martin, Q.; Meuli, M.; Biedermann, T.; Wilson, C.J.; Rowan, A.; Reichmann, E. Polyisocyanopeptide hydrogels: A novel thermo-responsive hydrogel supporting pre-vascularization and the development of organotypic structures. Acta Biomater. 2018, 70, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Blaeser, A.; Duarte Campos, D.F.; Puster, U.; Richtering, W.; Stevens, M.M.; Fischer, H. Controlling Shear Stress in 3D Bioprinting is a Key Factor to Balance Printing Resolution and Stem Cell Integrity. Adv. Healthc. Mater. 2016, 5, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Melchels, F.P.; Blokzijl, M.M.; Levato, R.; Peiffer, Q.C.; De Ruijter, M.; Hennink, W.E.; Vermonden, T.; Malda, J. Hydrogel-based reinforcement of 3D bioprinted constructs. Biofabrication 2016, 8, 35004. [Google Scholar] [CrossRef] [PubMed]
- Billiet, T.; Gevaert, E.; De Schryver, T.; Cornelissen, M.; Dubruel, P. The 3D printing of gelatin methacrylamide cell-laden tissue-engineered constructs with high cell viability. Biomaterials 2014, 35, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Hansen, C.J.; Wu, W.; Toohey, K.S.; Sottos, N.R.; White, S.R.; Lewis, J.A. Self-healing materials with interpenetrating microvascular networks. Adv. Mater. 2009, 21, 4143–4147. [Google Scholar] [CrossRef]
- Kolesky, D.B.; Truby, R.L.; Gladman, A.S.; Busbee, T.A.; Homan, K.A.; Lewis, J.A. 3D bioprinting of vascularized, heterogeneous cell-laden tissue constructs. Adv. Mater. 2014, 26, 3124–3130. [Google Scholar] [CrossRef] [PubMed]
- Bae, H.; Nichol, J.; Foudeh, A.; Zamanian, B.; Kwon, C.H.; Khadenhosseini, A. Microengineering Approach for Directing Embryonic Stem Cell Differentiation. Biomater. Stem Cell Niche 2010, 2, 153–171. [Google Scholar] [CrossRef]
- Ko, D.Y.; Shinde, U.P.; Yeon, B.; Jeong, B. Recent progress of in situ formed gels for biomedical applications. Prog. Polym. Sci. 2013, 38, 672–701. [Google Scholar] [CrossRef]
- Peng, S.; Lin, J.-Y.; Cheng, M.-H.; Wu, C.-W.; Chu, I.-M. A cell-compatible PEO–PPO–PEO (Pluronic®)-based hydrogel stabilized through secondary structures. Mater. Sci. Eng. C 2016, 69, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.C.; Pak, P.J.; Joo, Y.H.; Lee, H.-S.; Chung, N. In vitro and in vivo comparison of the immunotoxicity of single- and multi-layered graphene oxides with or without pluronic F.-127. Sci. Rep. 2016, 6, 38884. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Celikkin, N.; Simó Padial, J.; Costantini, M.; Hendrikse, H.; Cohn, R.; Wilson, C.J.; Rowan, A.E.; Święszkowski, W. 3D Printing of Thermoresponsive Polyisocyanide (PIC) Hydrogels as Bioink and Fugitive Material for Tissue Engineering. Polymers 2018, 10, 555. https://doi.org/10.3390/polym10050555
Celikkin N, Simó Padial J, Costantini M, Hendrikse H, Cohn R, Wilson CJ, Rowan AE, Święszkowski W. 3D Printing of Thermoresponsive Polyisocyanide (PIC) Hydrogels as Bioink and Fugitive Material for Tissue Engineering. Polymers. 2018; 10(5):555. https://doi.org/10.3390/polym10050555
Chicago/Turabian StyleCelikkin, Nehar, Joan Simó Padial, Marco Costantini, Hans Hendrikse, Rebecca Cohn, Christopher J. Wilson, Alan Edward Rowan, and Wojciech Święszkowski. 2018. "3D Printing of Thermoresponsive Polyisocyanide (PIC) Hydrogels as Bioink and Fugitive Material for Tissue Engineering" Polymers 10, no. 5: 555. https://doi.org/10.3390/polym10050555
APA StyleCelikkin, N., Simó Padial, J., Costantini, M., Hendrikse, H., Cohn, R., Wilson, C. J., Rowan, A. E., & Święszkowski, W. (2018). 3D Printing of Thermoresponsive Polyisocyanide (PIC) Hydrogels as Bioink and Fugitive Material for Tissue Engineering. Polymers, 10(5), 555. https://doi.org/10.3390/polym10050555




