High Performance Clean Fracturing Fluid Using a New Tri-Cationic Surfactant
Abstract
:1. Introduction
2. Materials and Experiments
2.1. Materials
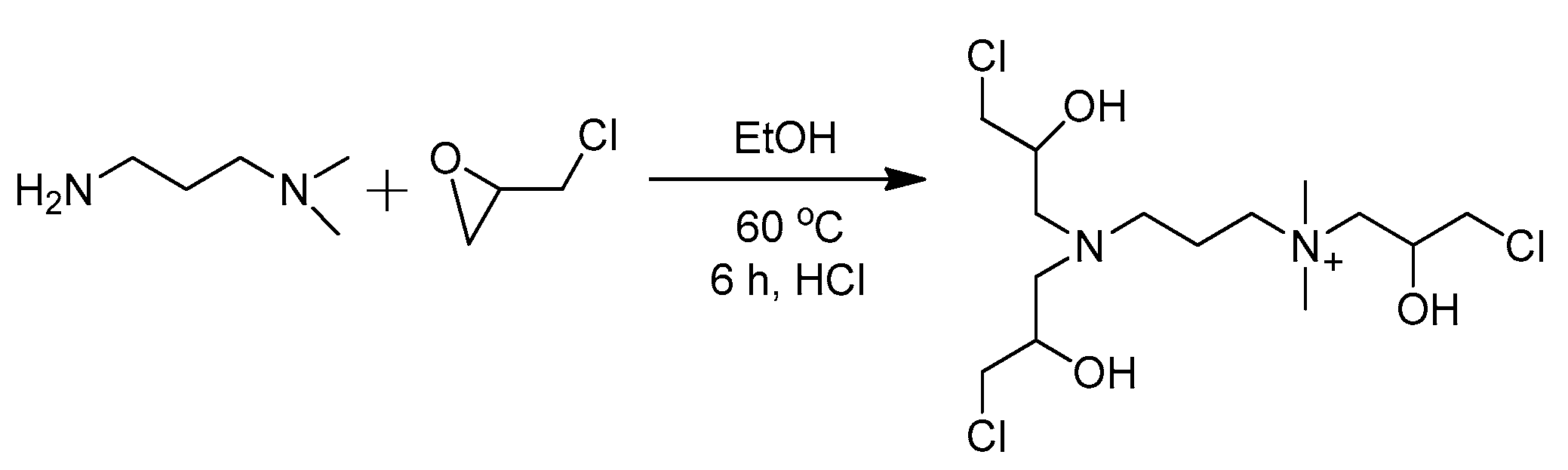
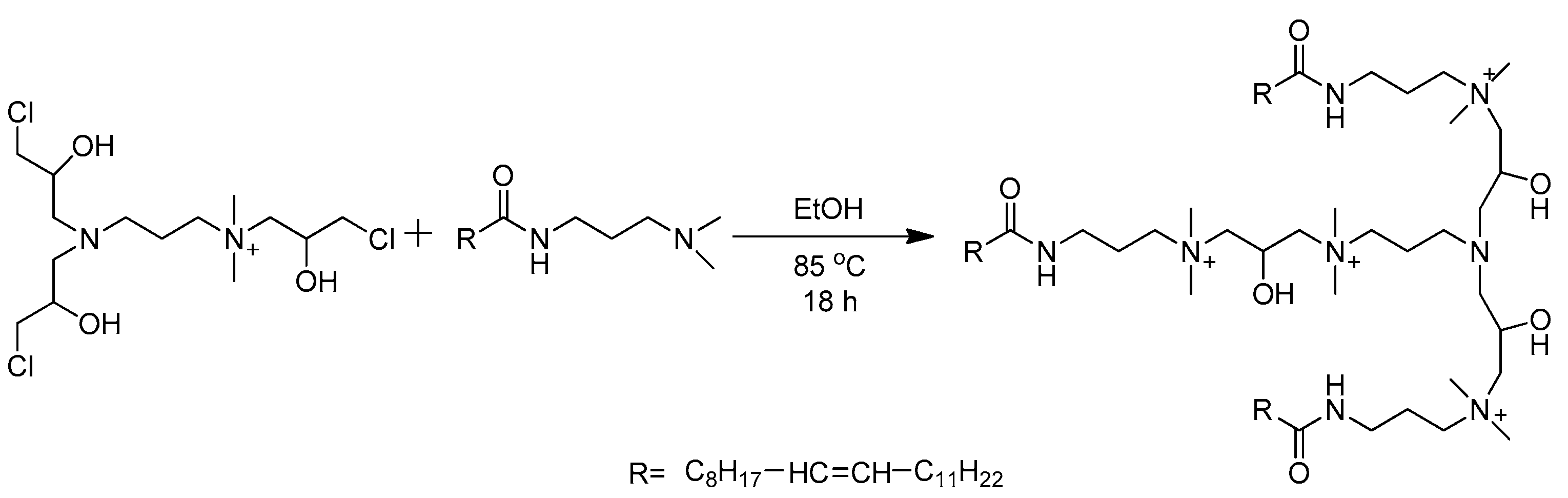
2.2. Synthesis of VES-T
2.2.1. Synthesis of Intermediate
2.2.2. Synthesis of VES-T
2.3. Measurement
Fracturing Fluid Preparation
3. Results and Discussion
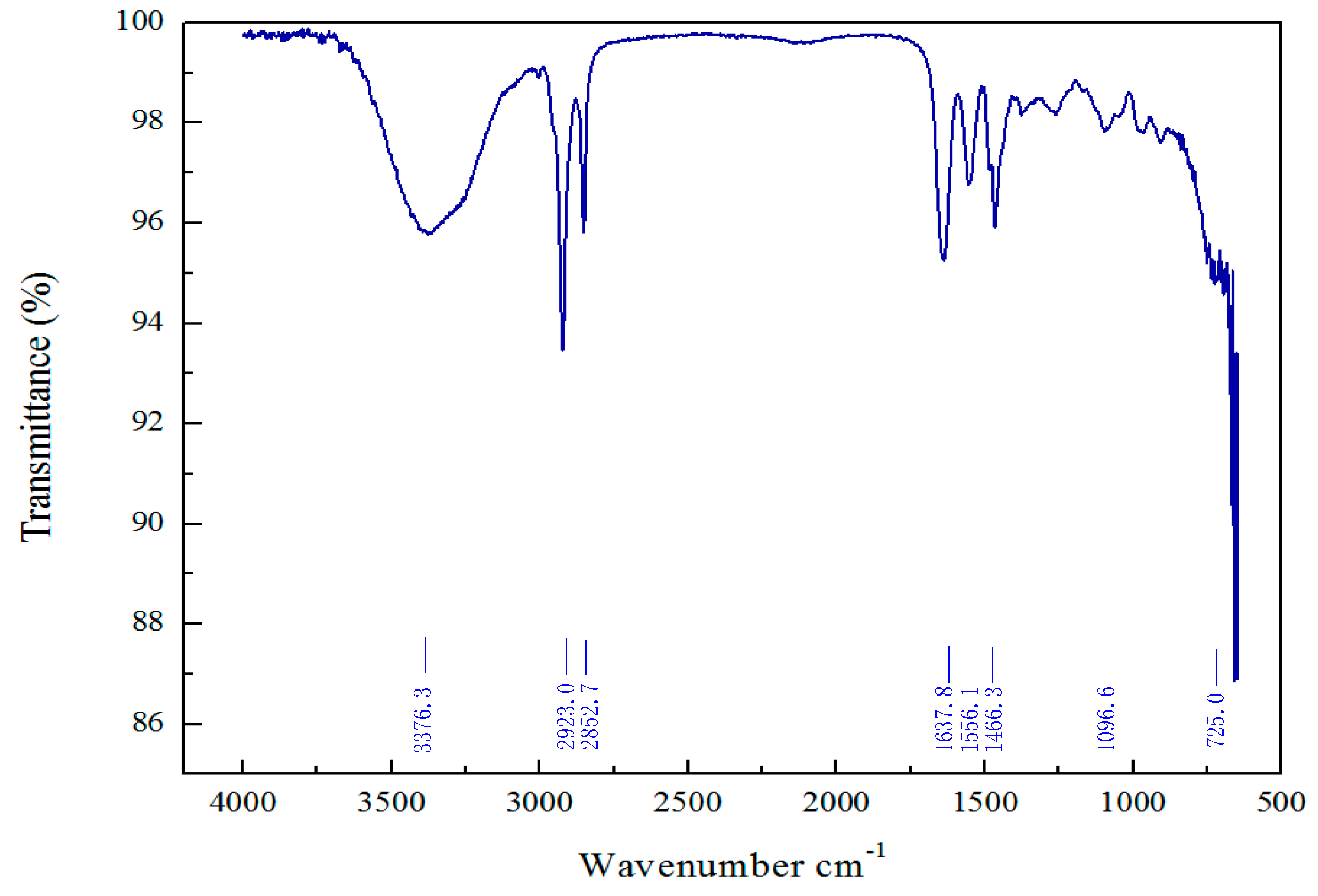
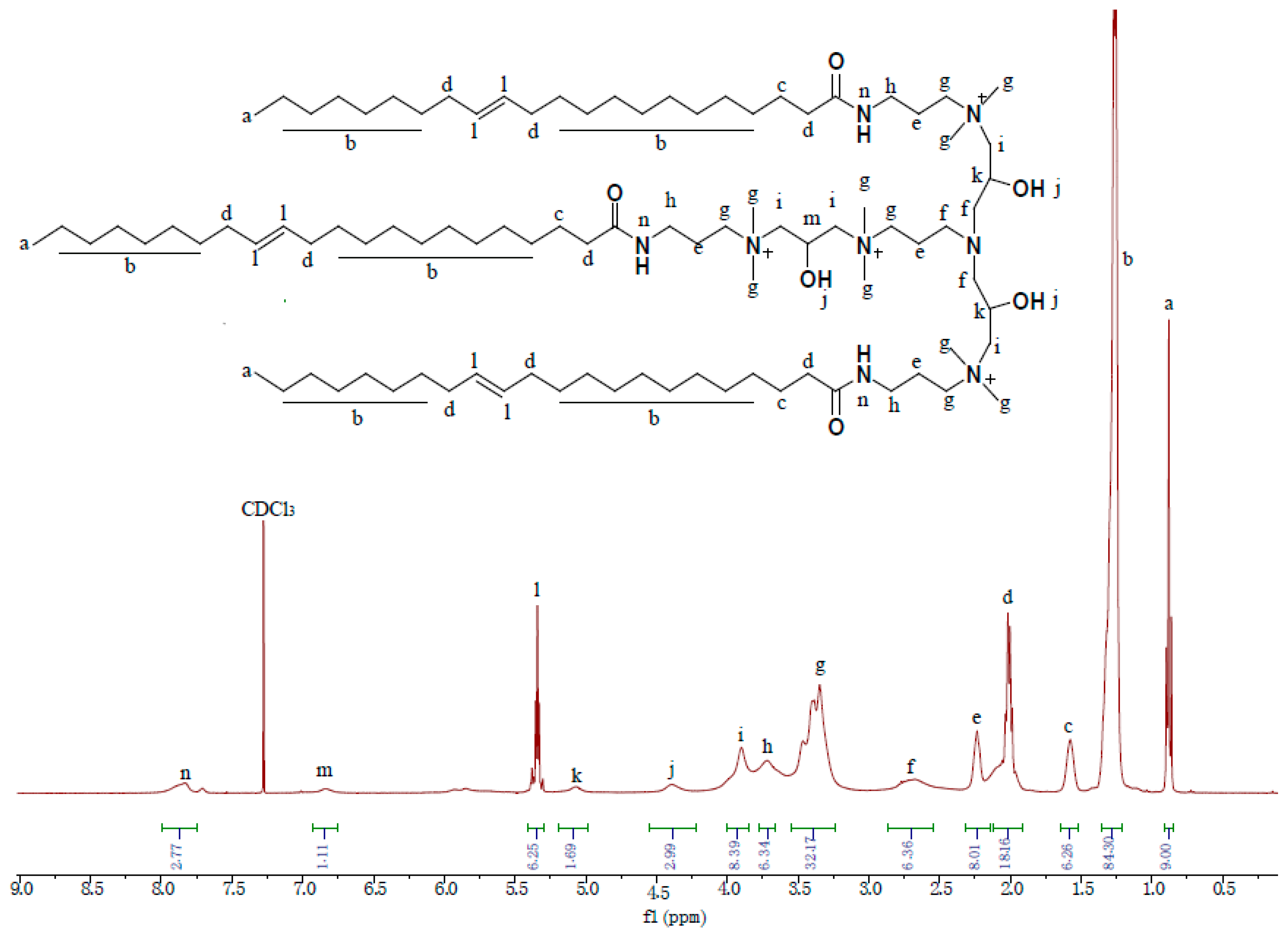
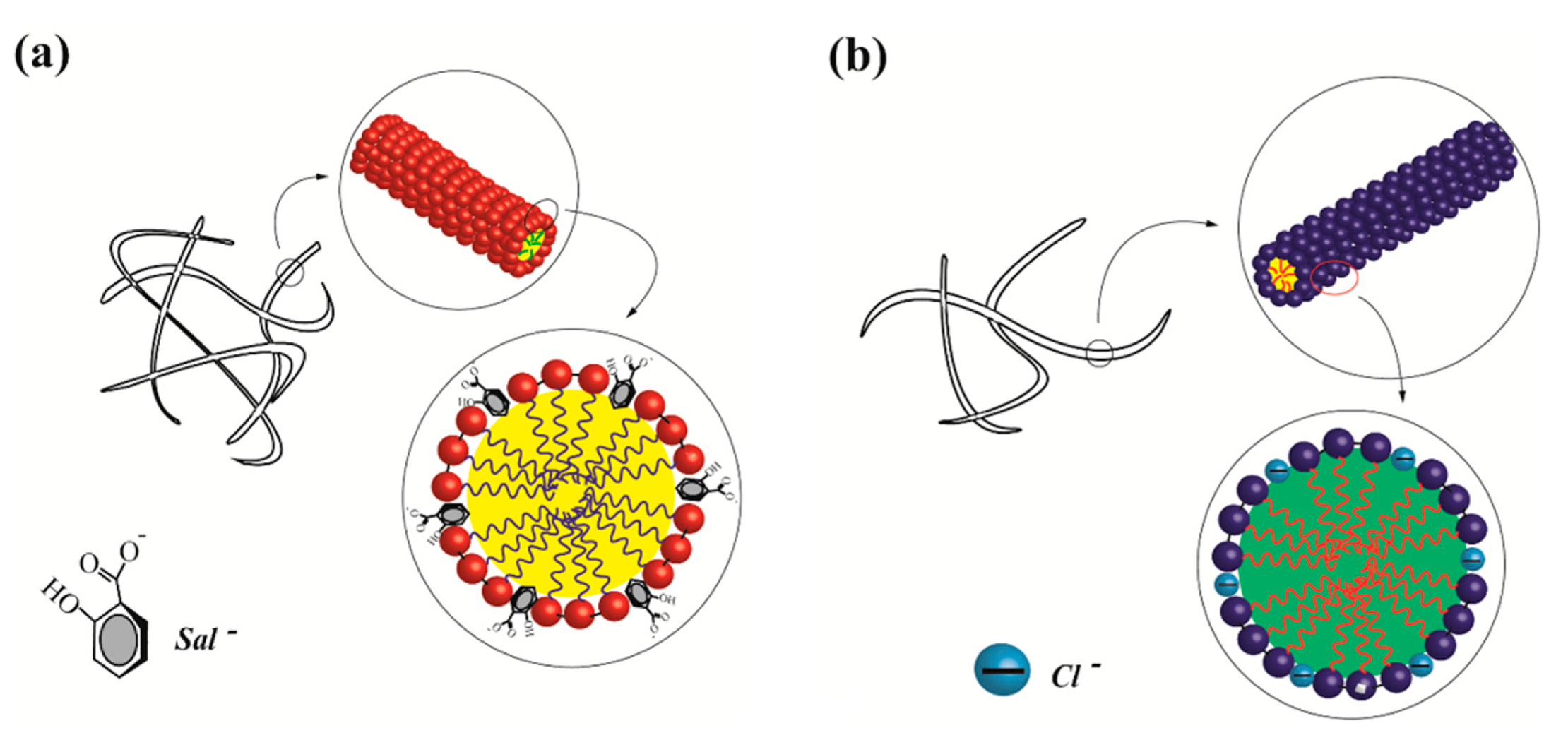
3.1. Structural Characterization of VES-T
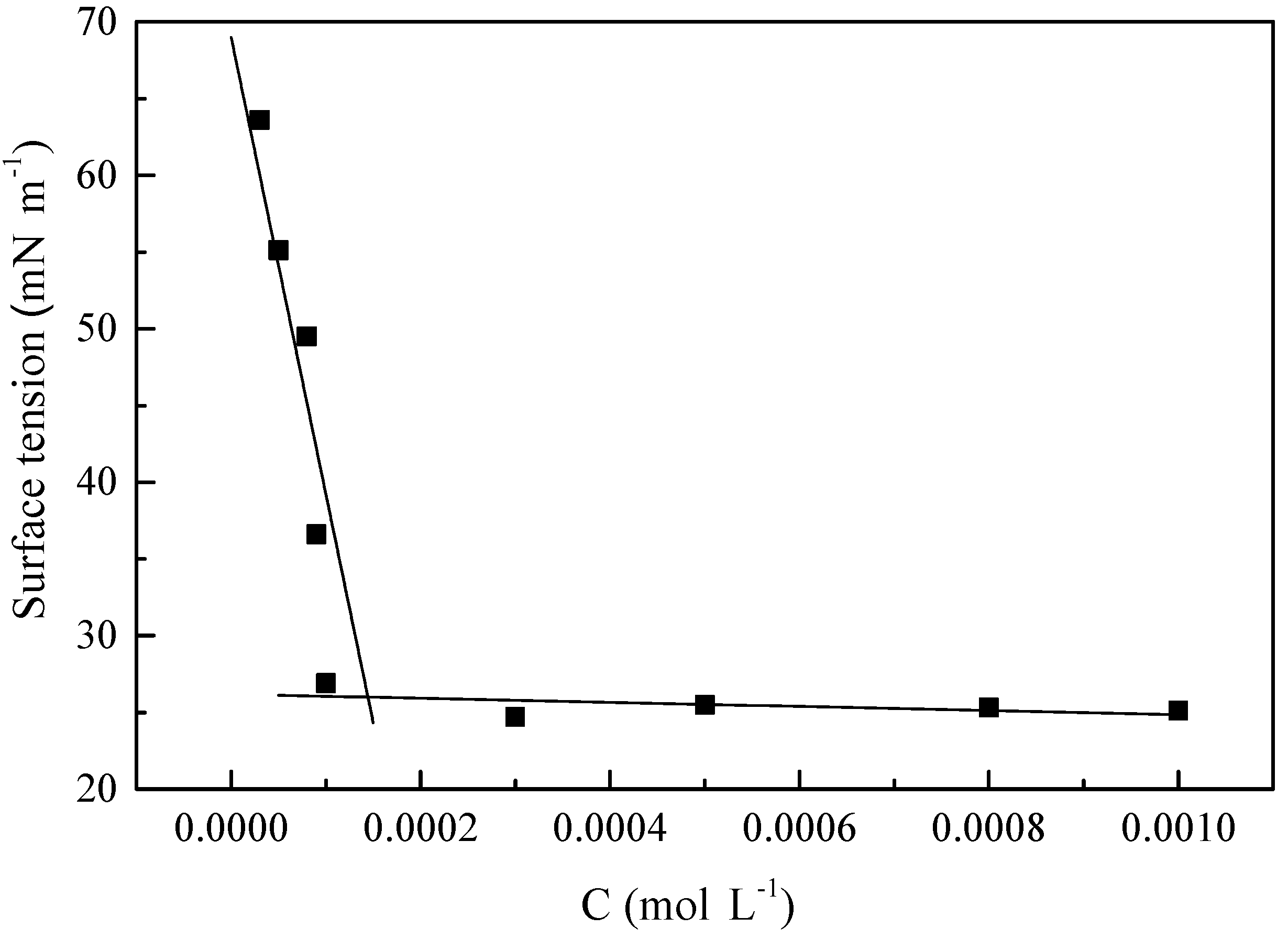
3.2. Surface Tension Measurement
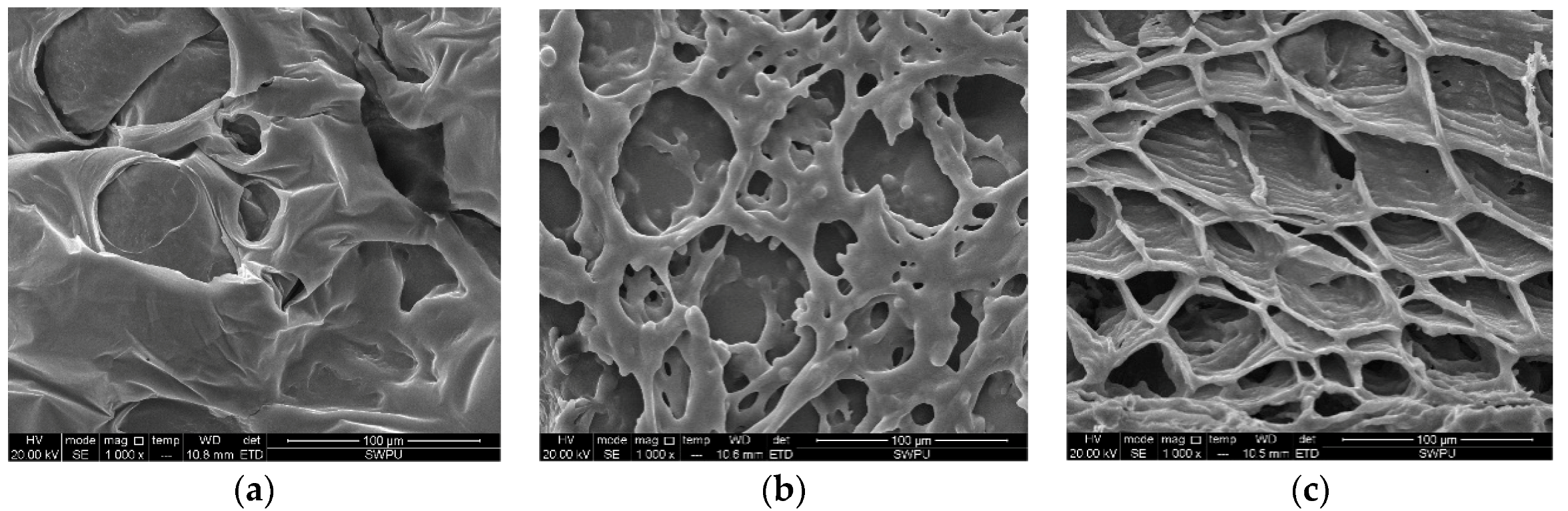
3.3. Scanning Electron Microscopy (SEM)
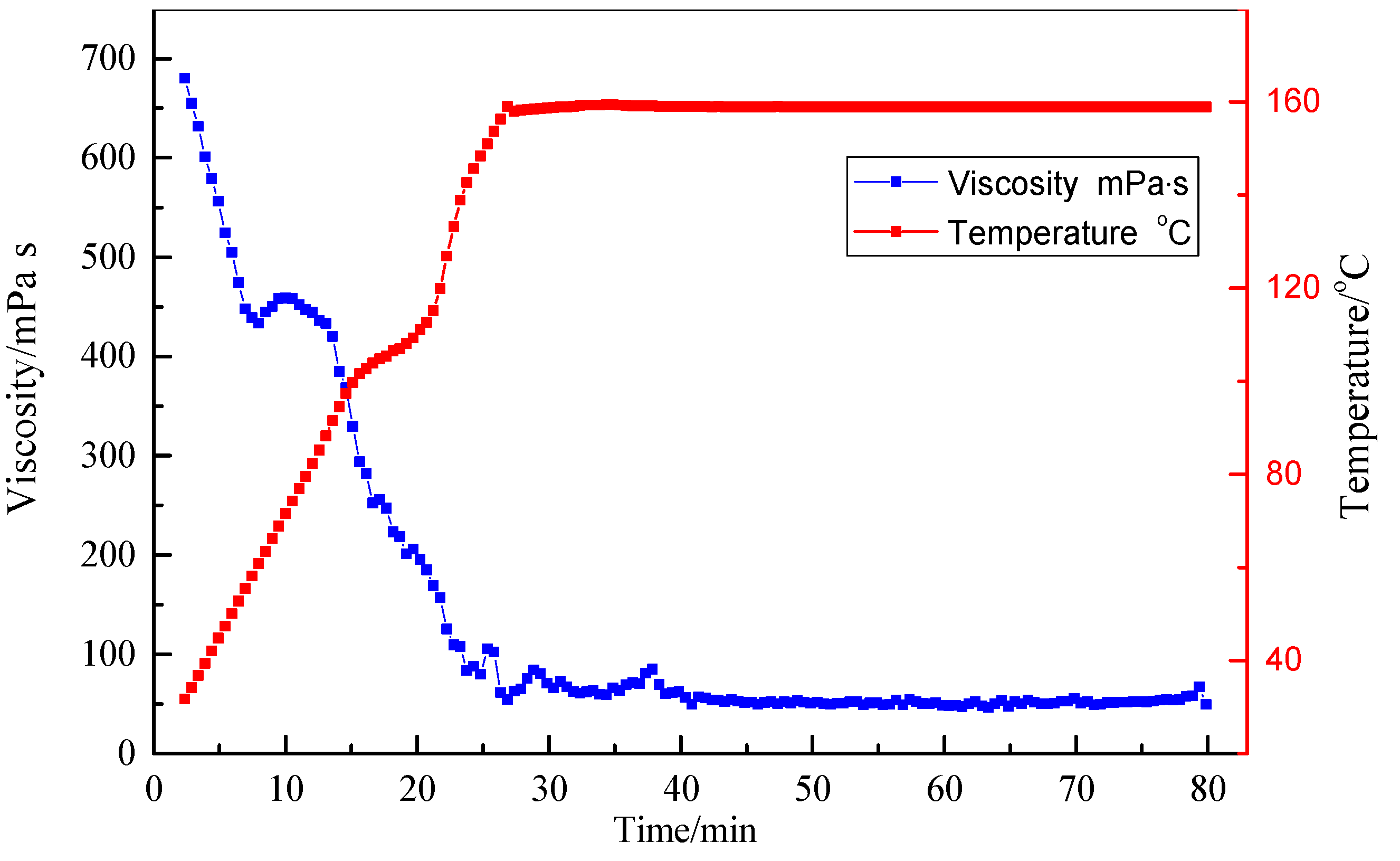
3.4. Resistance to High Temperature and High Shear
3.5. Viscoelasticity Evaluation
3.6. Proppant Suspension Measurement
3.7. Gel Breaking Test
3.8. General Description
4. Conclusions
- (1).
- The surface tension measurement data confirm that the combination of three single-chain surfactants leads to a lower CMC and higher surface activity than the conventional Gemini surfactants.
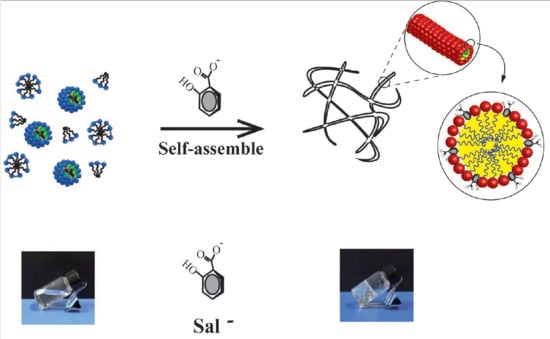
- (2).
- Microstructure study suggests that the organic (NaSal) and inorganic (KCl) salts both have significant effects on the micellar transition from spherical micelles to wormlike micelles.
- (3).
- The fracturing fluids with the two concentrations (3 and 5 wt %) of VES-T both exhibit excellent rheological properties under high temperature. Especially, the viscosity of fluid sample containing 5 wt % VES-T and 1.2 wt % NaSal maintains at about 42 mPa·s at 180 °C, which is the highest temperature recorded till now.
- (4).
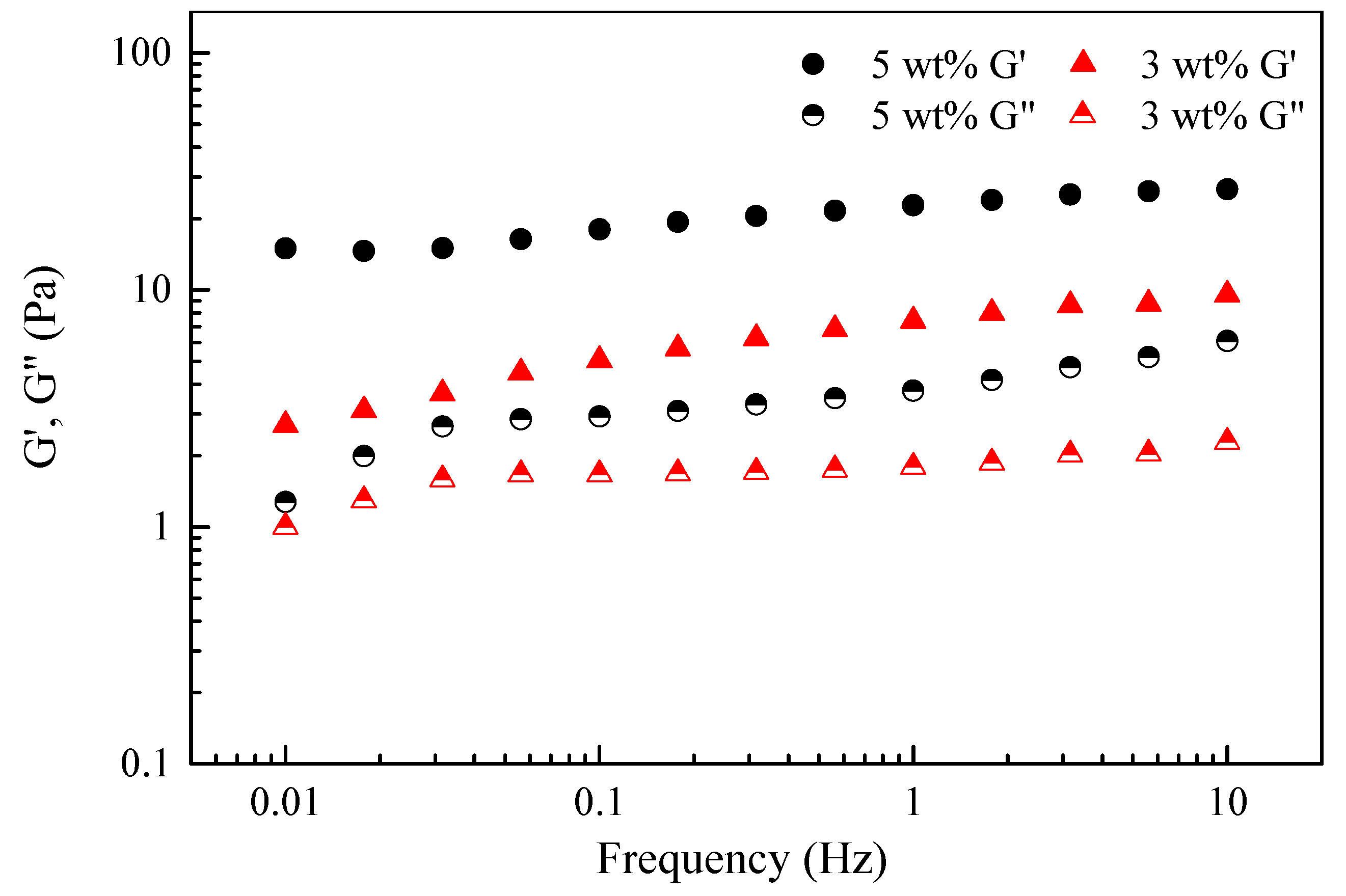
- Viscoelasticity evaluation shows typical elastic properties of the two fracturing fluids. Additionally, the storage modulus G′ and loss modulus G″ of the VES solution both increased with the increasing concentration of VES-T, indicating a more obvious viscoelasticity of the VES fracturing fluid. In other words, a higher VES concentration leads to a stronger network structure.
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Montgomery, C.T.; Smith, M.B. Hydraulic fracturing: History of an enduring technology. J. Petrol. Technol. 2010, 62, 26–32. [Google Scholar] [CrossRef]
- Waters, G.A.; Dean, B.K.; Downie, R.C.; Kerrihard, K.; Austbo, L.; Mcpherson, B. Simultaneous Hydraulic Fracturing of Adjacent Horizontal Wells in the Woodford Shale. In Proceedings of the SPE Hydraulic Fracturing Technology Conference, The Woodlands, TX, USA, 19–21 January 2009. [Google Scholar]
- Yang, C.; Hu, Z.; Song, Z.; Bai, J.; Zhang, Y.; Luo, J.; Du, Y.; Jiang, Q. Self-assembly properties of ultra-long-chain Gemini surfactant with high performance in a fracturing fluid application. J. Appl. Polym. Sci. 2017, 134, 44602. [Google Scholar] [CrossRef]
- Marec, A.; Thomas, J.H.; Guerjouma, R.E. Damage characterization of polymer-based composite materials: Multivariable analysis and wavelet transform for clustering acoustic emission data. Mech. Syst. Signal Process. 2008, 22, 1441–1464. [Google Scholar] [CrossRef]
- Yang, J.; Guan, B.; Lu, Y.; Cui, W.; Qiu, X.; Yang, Z.; Qin, W. Viscoelastic Evaluation of Gemini Surfactant Gel for Hydraulic Fracturing. In Proceedings of the SPE European Formation Damage Conference & Exhibition, Noordwijk, The Netherlands, 5–7 June 2013. [Google Scholar]
- Samuel, M.; Dan, P.; Graham, D.; Kordziel, W.; Waters, G.; Waite, T.; Vinod, P.S.; Fu, D.; Downey, R. Viscoelastic Surfactant Fracturing Fluids: Applications in Low Permeability Reservoirs. In Proceedings of the SPE Rocky Mountain Regional/Low-Permeability Reservoirs Symposium and Exhibition, Denver, CO, USA, 12–15 March 2000. [Google Scholar]
- Barbati, A.C.; Desroches, J.; Robisson, A.; Mckinley, G.H. Complex Fluids and Hydraulic Fracturing. Annu. Rev. Chem. Biomol. 2016, 7, 415–453. [Google Scholar] [CrossRef] [PubMed]
- Samuel, M.M.; Card, R.J.; Nelson, E.B.; Brown, J.E.; Vinod, P.S.; Temple, H.L.; Qu, Q.; Fu, D.K. Polymer-Free Fluid for Fracturing Applications. SPE Drill. Complet. 1999, 14, 240–246. [Google Scholar] [CrossRef]
- Nasr-El-Din, H.A.; Li, L.; Crews, J.B.; Cawiezel, K.E. Impact of Organic Acids/Chelating Agents on the Rheological Properties of an Amidoamine-Oxide Surfactant. SPE Prod. Oper. 2010, 26, 30–40. [Google Scholar] [CrossRef]
- Malhotra, S.; Sharma, M.M. A General Correlation for Proppant Settling in VES Fluids. In Proceedings of the SPE Hydraulic Fracturing Technology Conference, The Woodlands, TX, USA, 24–26 January 2011. [Google Scholar]
- Wang, Z.; Wang, S.; Jing, Z.; Luo, X. Viscoelastic Drag of Particles Settling in Wormlike Micellar Solutions of Varying Surfactant Concentration. J. Dispers. Sci. Technol. 2016, 37, 442–449. [Google Scholar] [CrossRef]
- Brown, J.E.; Lee, R.; Nelson, E.B.; Ali, S.A. Use of a Viscoelastic Carrier Fluid in Frac-Pack Applications. In Proceedings of the SPE Formation Damage Control Symposium, Lafayette, LA, USA, 14–15 February 1996. [Google Scholar]
- Samuel, M.; Card, R.; Nelson, E.; Brown, J.E.; Vinod, P.S.; Temple, H.L.; Qu, Q.; Fu, D.K. Polymer-free fluid for hydraulic fracturing. In Proceedings of the SPE Annual Technical Conference and Exhibition, San Antonio, TX, USA, 5–8 October 1997. [Google Scholar]
- Crews, J.B.; Huang, T.; Wood, W.R. New Fluid Technology Improves Performance and Provides a Method To Treat High-Pressure and Deepwater Wells. In Proceedings of the SPE Annual Technical Conference and Exhibition, San Antonio, TX, USA, 24–27 September 2006. [Google Scholar]
- Fontana, C.; Muruaga, E.; Perez, D.R.; Cavazzoli, G.D.; Krenz, A. Successful Application of a High Temperature Viscoelastic Surfactant (VES) Fracturing Fluids under Extreme Conditions in Patagonian Wells, San Jorge Basin. In Proceedings of the EUROPEC/EAGE Conference and Exhibition, London, UK, 11–14 June 2007. [Google Scholar]
- Gu, M.; Mohanty, K.K. Rheology of polymer-free foam fracturing fluids. J. Pet. Sci. Eng. 2015, 134, 87–96. [Google Scholar] [CrossRef]
- Barati, R.; Liang, J.T. A review of fracturing fluid systems used for hydraulic fracturing of oil and gas wells. J. Appl. Polym. Sci. 2014, 131, 318–323. [Google Scholar] [CrossRef]
- Cawiezel, K.E.; Gupta, D.V.S. Successful Optimization of Viscoelastic Foamed Fracturing Fluids with Ultralightweight Proppants for Ultralow-Permeability Reservoirs. SPE Prod. Oper. 2010, 25, 80–88. [Google Scholar] [CrossRef]
- Akram, M.; Bhat, I.A.; KabirudDin. Self-aggregation of surfactant ethane-1,2-diyl bis(N,N-dimethyl-N-hexadecylammoniumacetoxy) dichloride: Tensiometric, microscopic, and spectroscopic studies. J. Phys. Chem. B 2015, 119, 3499–3509. [Google Scholar] [CrossRef] [PubMed]
- Kern, F.; Lequeux, F.; Zana, R.; Candau, S.J. Dynamic Properties of Salt-Free Viscoelastic Micellar Solutions. Langmuir 1994, 10, 1714–1723. [Google Scholar] [CrossRef]
- Li, Q.; Yue, X.; Shang, P.; Quan, Y.; Ren, M.; Ma, Y.; Chen, X. Environmental stimuli induced phase transition in the aqueous mixture solution of Gemini surfactants and sodium deoxycholate. Colloids Surf. A 2016, 489, 67–74. [Google Scholar] [CrossRef]
- Mao, J.; Yang, X.; Wang, D.; Li, Y.; Zhao, J. A Novel Gemini Viscoelastic Surfactant (VES) for Fracturing Fluid with Good Temperature Stability. RSC Adv. 2016, 6, 88426–88432. [Google Scholar] [CrossRef]
- Yan, Z.; Dai, C.; Zhao, M.; Sun, Y.; Zhao, G. Development, formation mechanism and performance evaluation of a reusable viscoelastic surfactant fracturing fluid. J. Ind. Eng. Chem. 2016, 37, 115–122. [Google Scholar] [CrossRef]
- Khatory, A.; Lequeux, F.; Kern, F.; Candau, S.J. Linear and nonlinear viscoelasticity of semidilute solutions of wormlike micelles at high salt content. Langmuir 1993, 9, 1456–1464. [Google Scholar] [CrossRef]
- Mu, J.; Li, G.; Jia, X.; Wang, H.; Zhang, G. Rheological Properties and Microstructures of Anionic Micellar Solutions in the Presence of Different Inorganic Salts. J. Phys. Chem. B 2002, 106, 622–643. [Google Scholar] [CrossRef]
- Hartmann, V.; Cressely, R. Simple salts effects on the characteristics of the shear thickening exhibited by an aqueous micellar solution of CTAB/NaSal. EPL 2007, 40, 691. [Google Scholar] [CrossRef]
- Imae, T.; Ikeda, S. Characteristics of rodlike micelles of cetyltrimethylammonium chloride in aqueous NaCl solutions: Their flexibility and the scaling laws in dilute and semi dilute regimes. Colloid Polym. Sci. 1987, 265, 1090–1098. [Google Scholar] [CrossRef]
- And, L.J.M.; Han, Z.; Warr, G.G.; Cassidy, M.A.; Butler, P.D.; Hamilton, W.A. Effect of Counterion Competition on Micellar Growth Horizons for Cetyltrimethylammonium Micellar Surfaces: Electrostatics and Specific Binding. J. Phys. Chem. B 1997, 101, 7919–7927. [Google Scholar]
- Maillet, J.B.; Lachet, V.; Coveney, P.V. Large scale molecular dynamics simulation of self-assembly processes in short and long chain cationic surfactants. Phys. Chem. Chem. Phys. 1999, 1, 5277–5290. [Google Scholar] [CrossRef]
- And, S.R.R.; Kaler, E.W. Highly Viscoelastic Wormlike Micellar Solutions Formed by Cationic Surfactants with Long Unsaturated Tails. Langmuir 2007, 17, 300–306. [Google Scholar]
- Mohanty, S.; Davis, H.T.; Mccormick, A.V. Complementary Use of Simulations and Free Energy Models for CTAB/NaSal Systems. Langmuir 2015, 17, 7160–7171. [Google Scholar] [CrossRef]
- Clausen, T.M.; Vinson, P.K.; Minter, J.R.; Davis, H.T.; Talmon, Y.; Miller, W.G. Viscoelastic micellar solutions: Microscopy and rheology. J. Phys. Chem. 1992, 1, 474–484. [Google Scholar] [CrossRef]
- And, W.J.K.; Yang, S.M. Flow-Induced Silica Structure during in Situ Gelation of Wormy Micellar Solutions. Langmuir 2000, 16, 4761–4765. [Google Scholar]
- Vasudevan, M.; Shen, A.; Khomami, B.; Sureshkumar, R. Self-similar shear thickening behavior in CTAB/NaSal surfactant solutions. J. Rheol. 2007, 52, 527–550. [Google Scholar] [CrossRef]
- Wang, L.; Wang, D.; Shen, Y.; Lai, X.; Guo, X. Study on properties of hydrophobic associating polymer as drag reduction agent for fracturing fluid. J. Polym. Res. 2016, 23, 235. [Google Scholar] [CrossRef]
- Reinicke, A.; Rybacki, E.; Stanchits, S.; Huenges, E.; Dresen, G. Hydraulic fracturing stimulation techniques and formation damage mechanisms-Implications from laboratory testing of tight sandstone–proppant systems. Chem. Erde-Geochem. 2010, 70, 107–117. [Google Scholar] [CrossRef]
- Goel, N.; Shah, S.N.; Grady, B.P. Correlating viscoelastic measurements of fracturing fluid to particles suspension and solids transport. J. Petrol. Sci. Eng. 2002, 35, 59–81. [Google Scholar] [CrossRef]
- Zhang, Y.M.; An, P.Y.; Liu, X.F. A “worm”-containing viscoelastic fluid based on single amine oxide surfactant with an unsaturated C22-tail. RSC Adv. 2015, 5, 19135–19144. [Google Scholar] [CrossRef]
- Samuel, M.; Marcinew, R.; Al-Harbi, M.; Samuel, E.; Xiao, Z.; Ezzat, A.M.; Khamees, S.A.; Jarrett, C.; Ginest, N.H.; Bartko, K.; et al. A New Solids-Free Non-Damaging High Temperature Lost-Circulation Pill: Development and First Field Applications. In Proceedings of the SPE Middle East Oil Show, Manama, Bahrain, 9–12 June 2003. [Google Scholar]
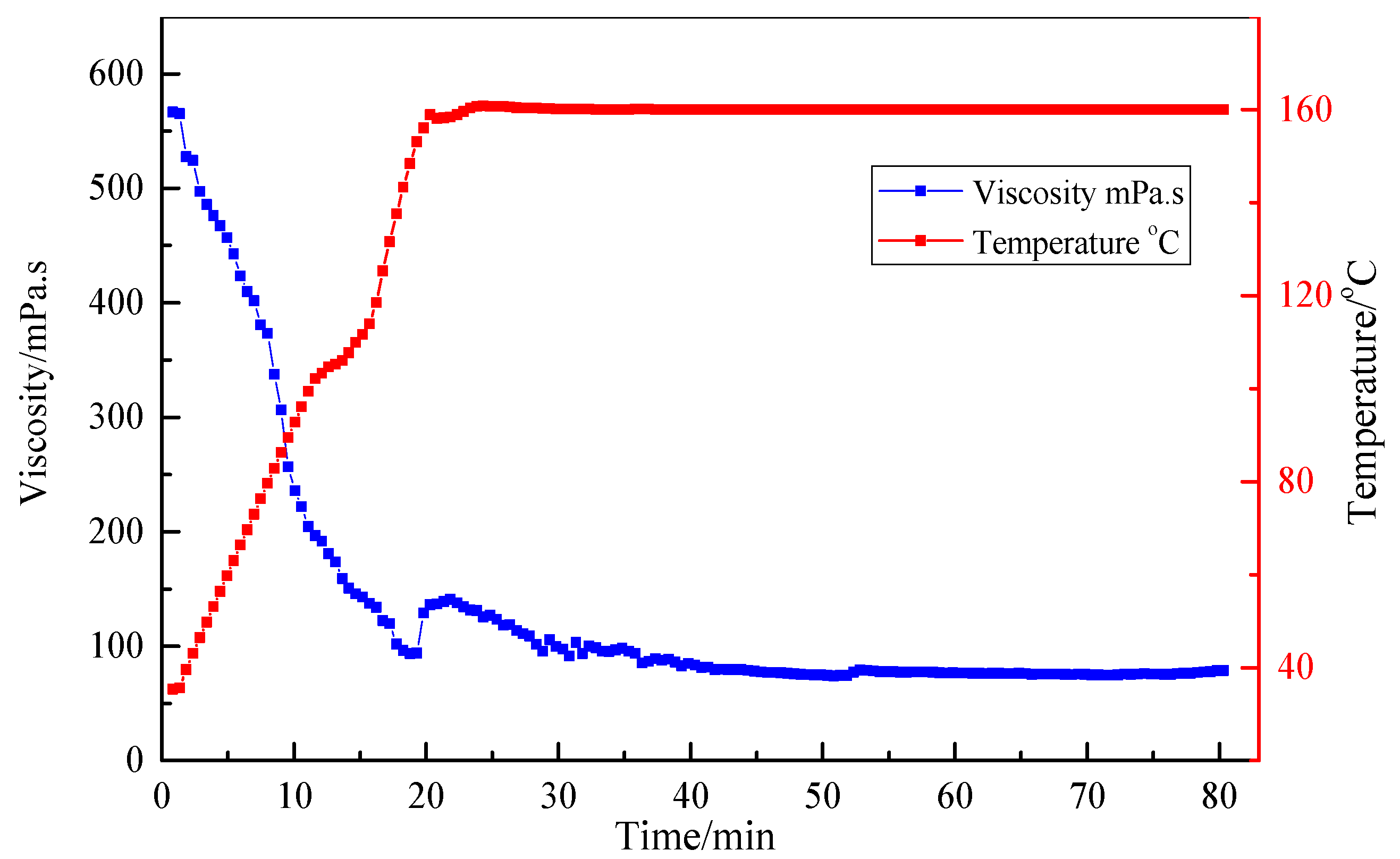
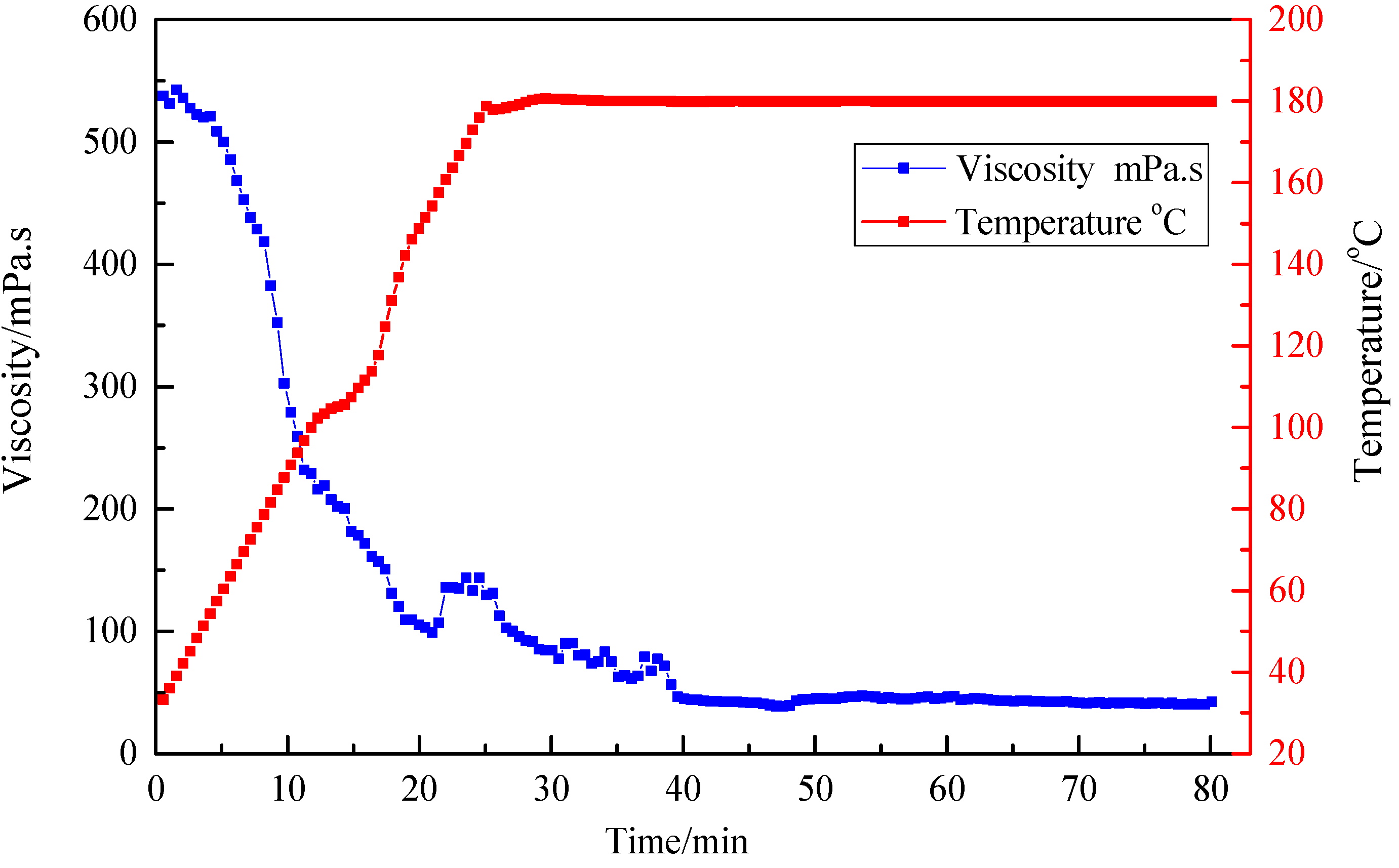
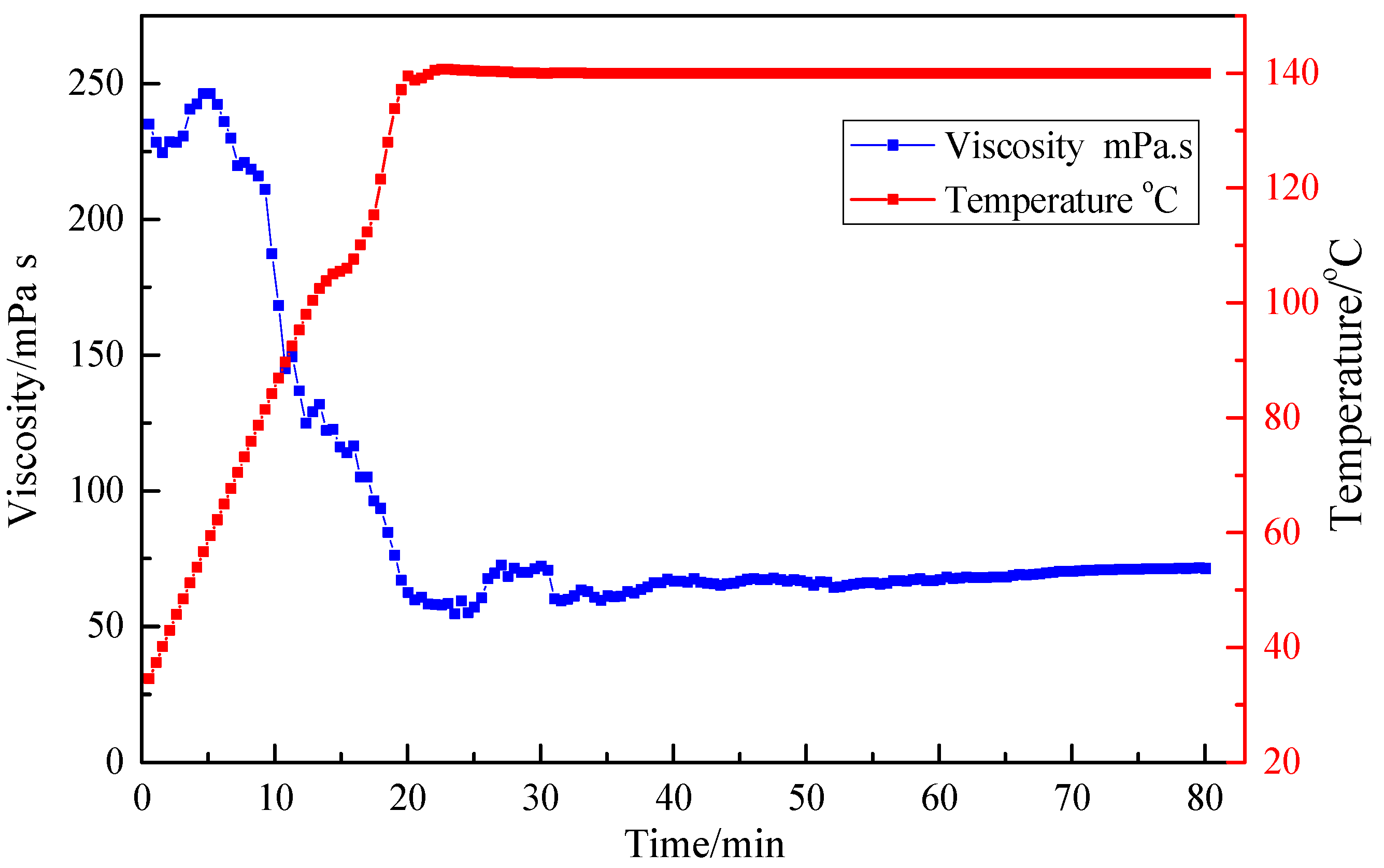
| Ratio (Standard Brine to Fluid) | Breaking Time (h) | Viscosity of Breaking Fluid (mPa·s) |
|---|---|---|
| 1:5 | 2.3 | 4.9 |
| 1:4 | 1.8 | 4.1 |
| 1:3 | 1.1 | 3.1 |
| Ratio (Standard Brine to Fluid) | Breaking Time (h) | Viscosity of Breaking Fluid (mPa·s) |
|---|---|---|
| 1:12 | 2.6 | 4.3 |
| 1:10 | 1.9 | 3.8 |
| 1:8 | 1.3 | 2.9 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Fan, J.; Mao, J.; Yang, X.; Zhang, H.; Zhang, W. High Performance Clean Fracturing Fluid Using a New Tri-Cationic Surfactant. Polymers 2018, 10, 535. https://doi.org/10.3390/polym10050535
Zhao J, Fan J, Mao J, Yang X, Zhang H, Zhang W. High Performance Clean Fracturing Fluid Using a New Tri-Cationic Surfactant. Polymers. 2018; 10(5):535. https://doi.org/10.3390/polym10050535
Chicago/Turabian StyleZhao, Jinzhou, Jinming Fan, Jincheng Mao, Xiaojiang Yang, Heng Zhang, and Wenlong Zhang. 2018. "High Performance Clean Fracturing Fluid Using a New Tri-Cationic Surfactant" Polymers 10, no. 5: 535. https://doi.org/10.3390/polym10050535
APA StyleZhao, J., Fan, J., Mao, J., Yang, X., Zhang, H., & Zhang, W. (2018). High Performance Clean Fracturing Fluid Using a New Tri-Cationic Surfactant. Polymers, 10(5), 535. https://doi.org/10.3390/polym10050535