Fructose-Based Acrylic Copolymers by Emulsion Polymerization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
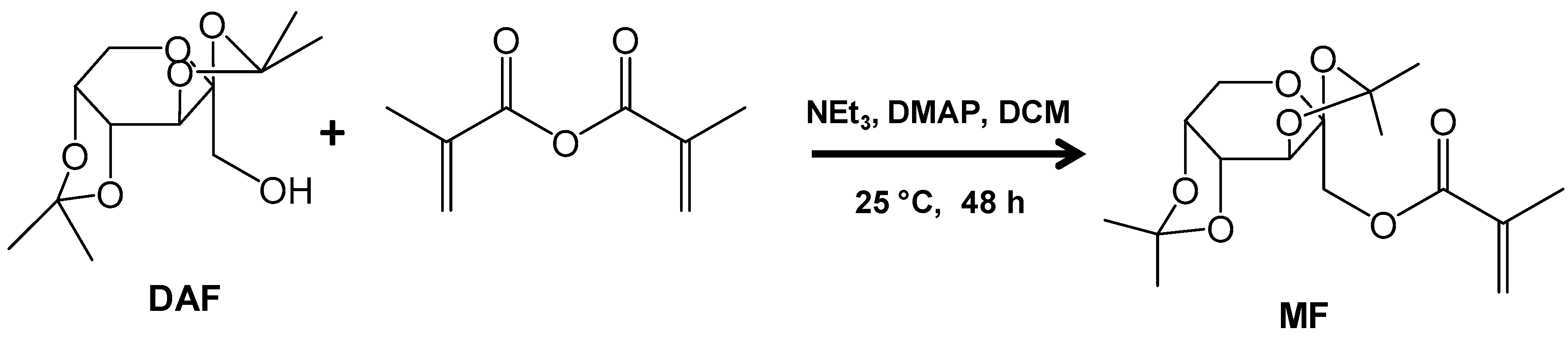
2.2. Synthesis of the Diacetone-d-Fructose Monomer
2.3. Emulsion Polymerization
2.4. Characterization
3. Results
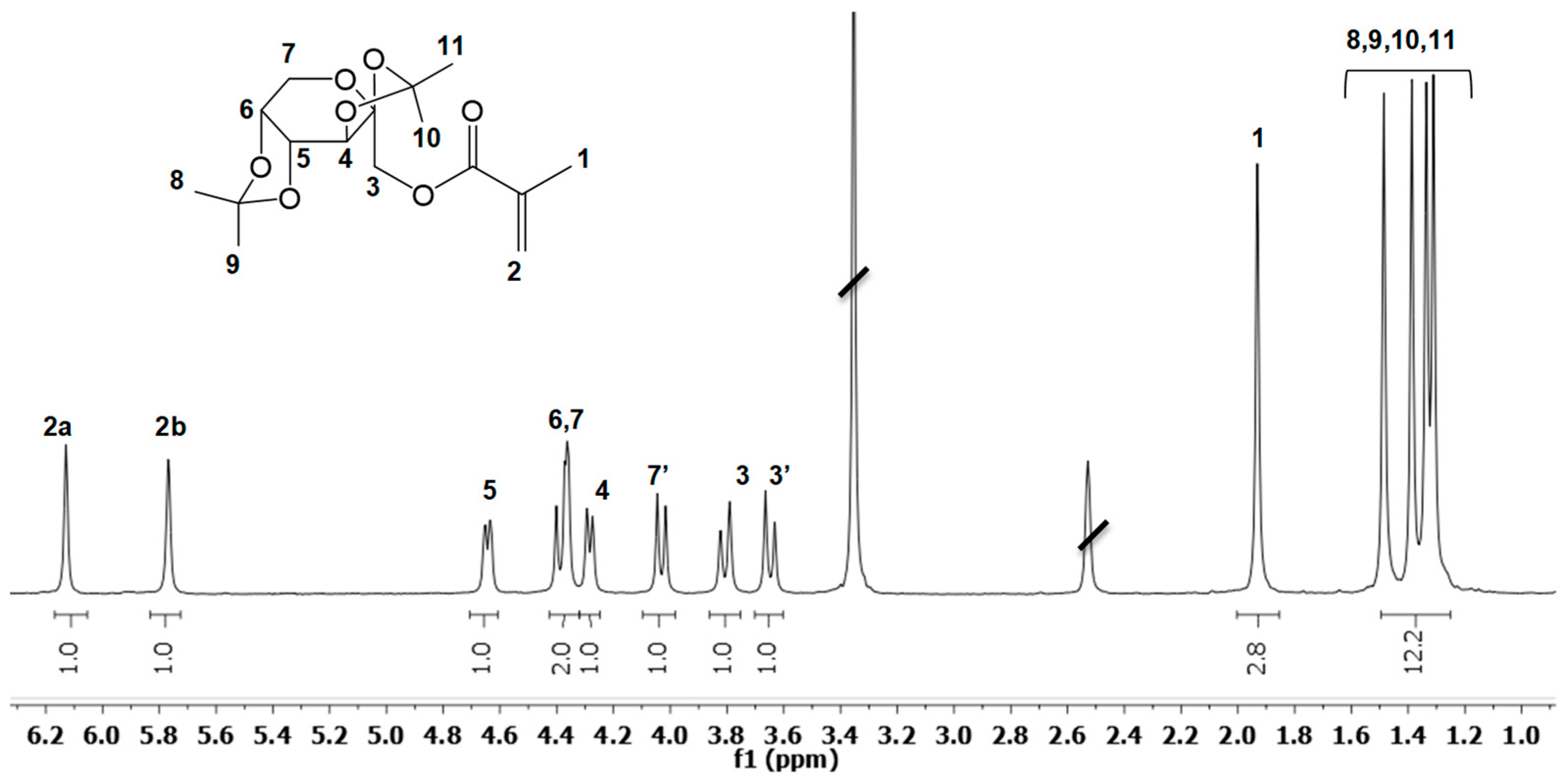
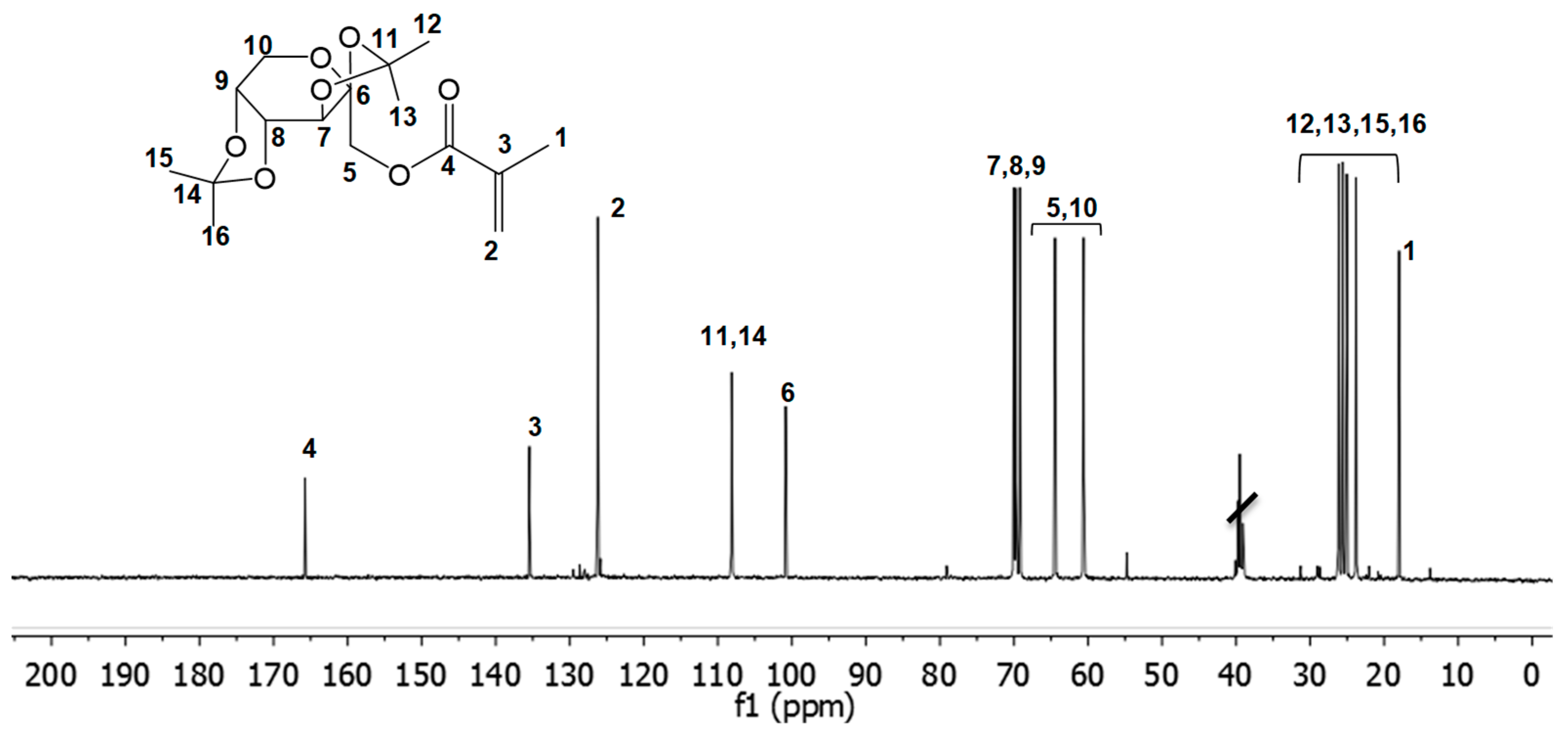
3.1. Fructose-Based Monomers
3.2. Homopolymer and Its Thermal Characterization
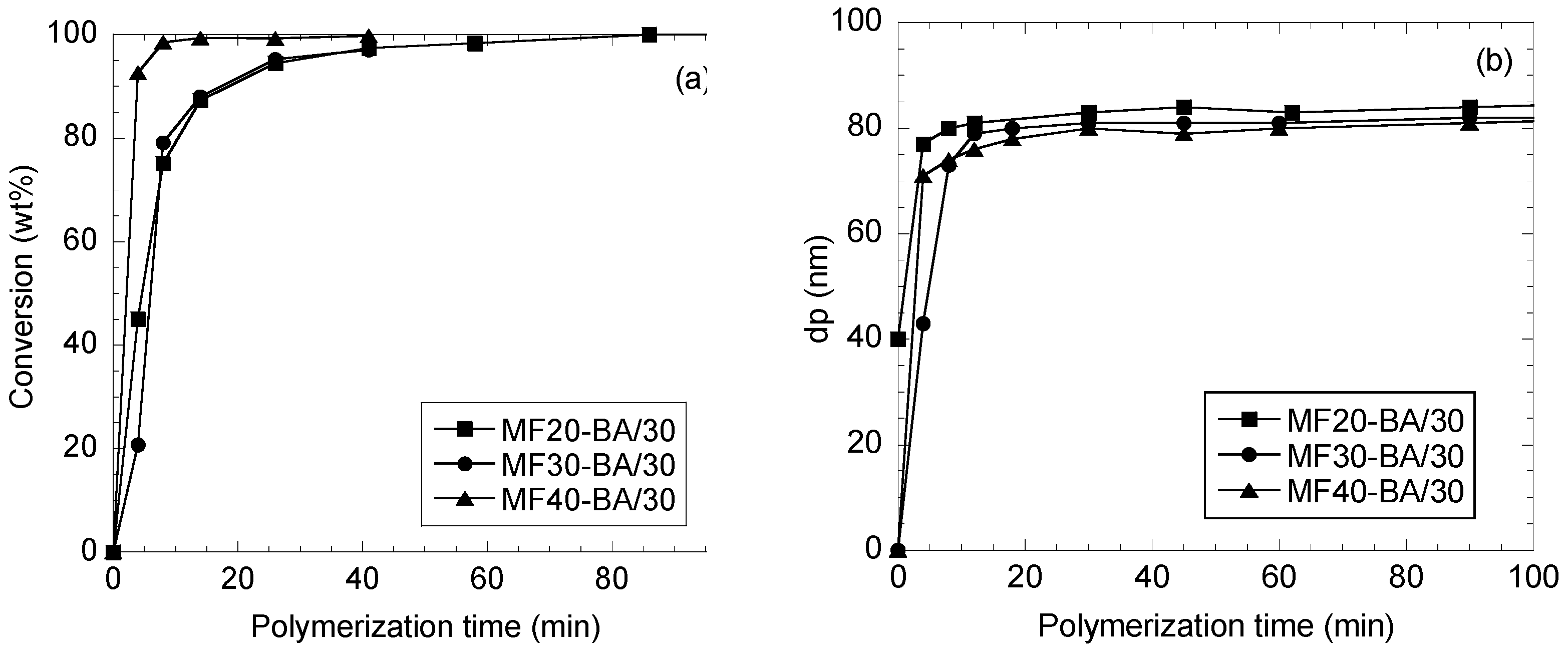
3.3. Fructose-Based Emulsion Copolymerization
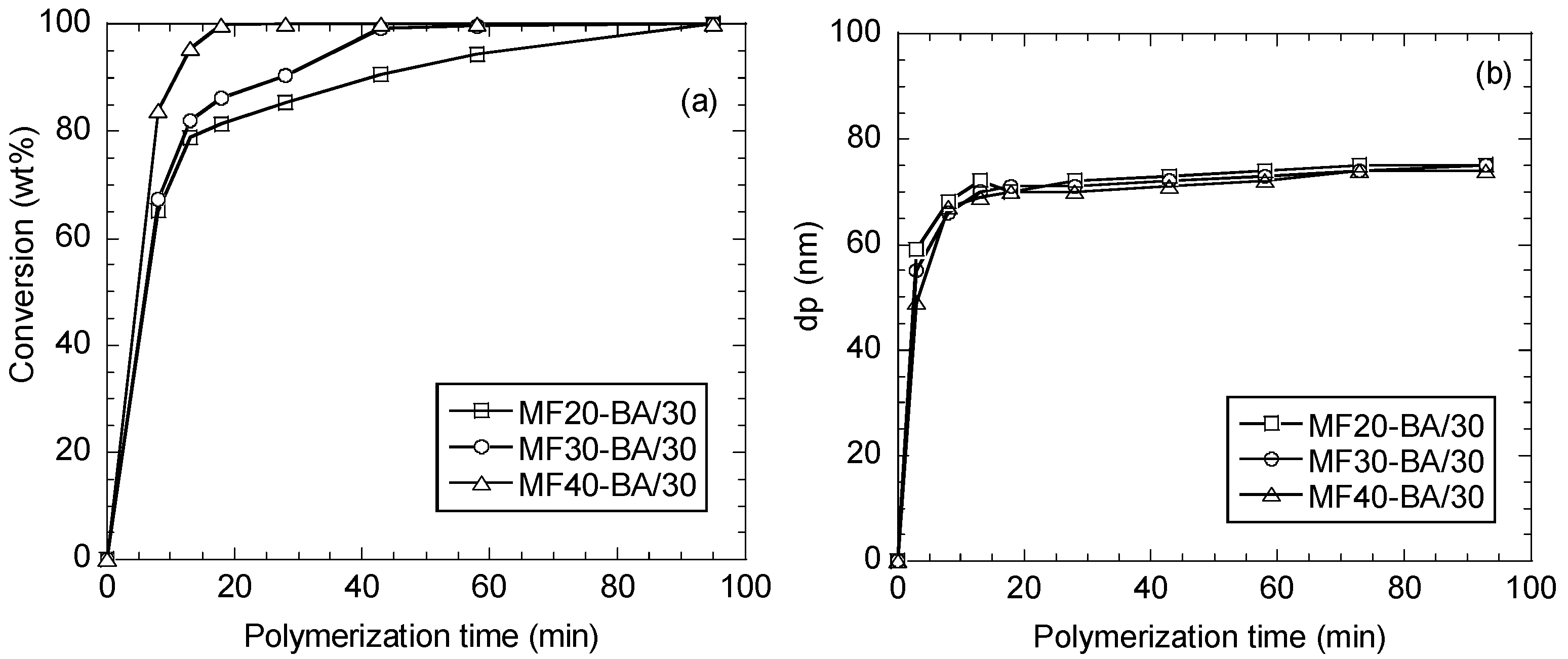
3.3.1. Polymer Characterization
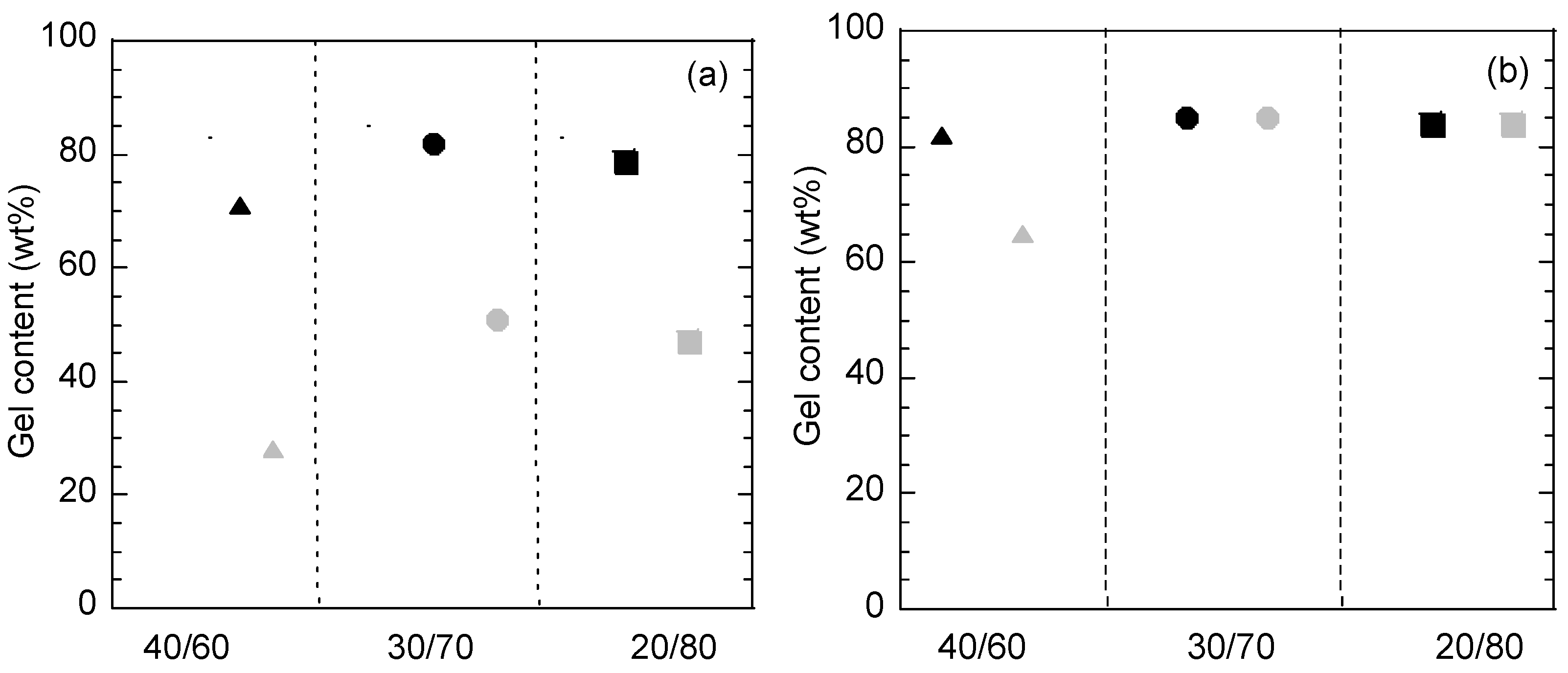
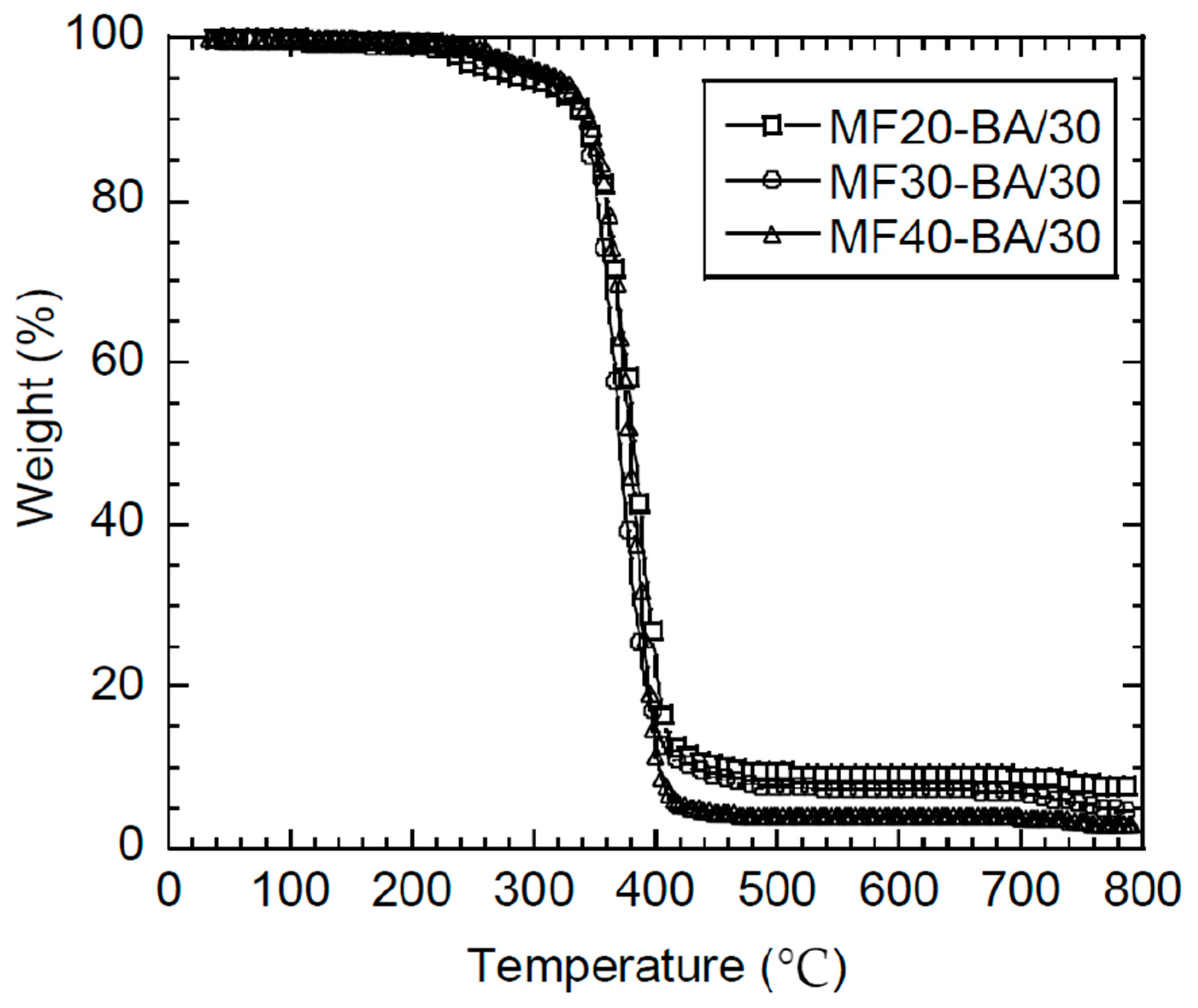
3.3.2. Thermal Characterization and Film Properties
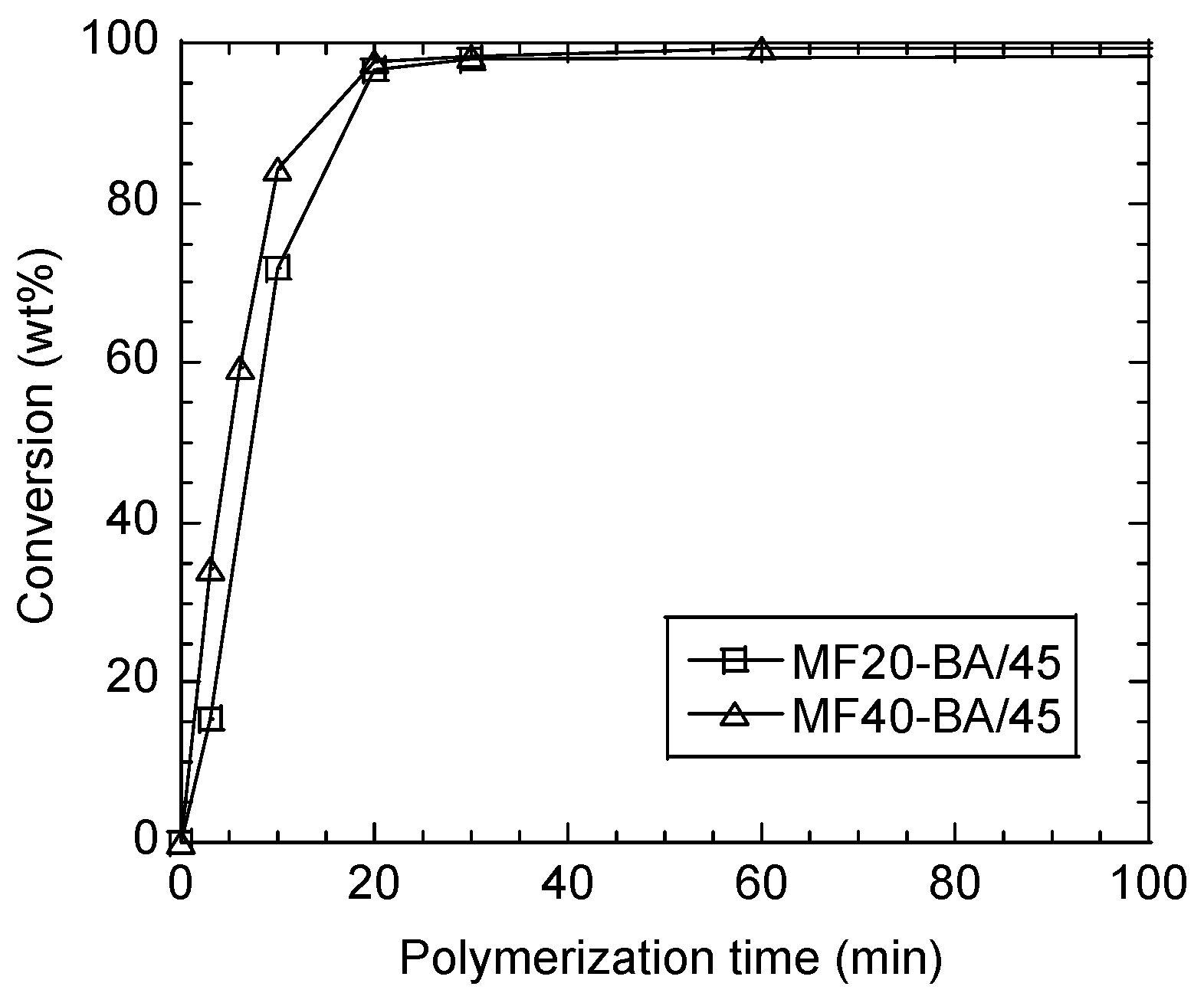
3.4. 45 wt % Solids Content Sugar-Based Copolymer Latexes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Nakajima, H.; Dijkstra, P.; Loos, K. The Recent Developments in Biobased Polymers toward General and Engineering Applications: Polymers that are Upgraded from Biodegradable Polymers, Analogous to Petroleum-Derived Polymers, and Newly Developed. Polymers 2017, 9, 523. [Google Scholar] [CrossRef]
- Picchio, M.L.; Passeggi, M.C.G., Jr.; Barandiaran, M.J.; Gugliotta, L.M.; Minari, R.J. Waterborne Acrylic-Casein Latexes as Eco-friendly Binders for Coatings. Prog. Org. Coat. 2015, 88, 8–16. [Google Scholar] [CrossRef]
- Moreno, M.; Goikoetxea, M.; Barandiaran, M.J. Biobased-Waterborne Homopolymers from Oleic Acid Derivatives. J. Polym. Sci. Part Polym. Chem. 2012, 50, 4628–4637. [Google Scholar] [CrossRef]
- Moreno, M.; Miranda, J.I.; Goikoetxea, M.; Barandiaran, M.J. Sustainable Polymer Latexes Based on Linoleic Acid for Coatings Applications. Prog. Org. Coat. 2014, 77, 1709–1714. [Google Scholar] [CrossRef]
- Lichtenthaler, F.W.; Peters, S. Carbohydrates as Green Raw Materials for the Chemical Industry. Comptes Rendus Chim. 2004, 7, 65–90. [Google Scholar] [CrossRef]
- Klein, J.; Herzog, D.; Hajibegli, A. Poly (Vinyl Saccharide) S, 1. Emulsion Polymerization of Poly (Methacryloylglucose). Makromol. Chem. Rapid Commun. 1985, 6, 675–678. [Google Scholar] [CrossRef]
- Imaz, A.; Forcada, J. N-Vinylcaprolactam-Based Microgels for Biomedical Applications. J. Polym. Sci. Part Polym. Chem. 2010, 48, 1173–1181. [Google Scholar] [CrossRef]
- Imaz, A.; Forcada, J. N-Vinylcaprolactam-Based Microgels: Synthesis and Characterization. J. Polym. Sci. Part Polym. Chem. 2008, 46, 2510–2524. [Google Scholar] [CrossRef]
- Takasu, A.; Baba, M.; Hirabayashi, T. Preparation and biodegradation of sugar-containing poly(vinyl acetate) emulsions. Macromol. Biosci. 2008, 8, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Koch, U.; Yaacoub, E.-J. Emulsion Polymerization of a Sugar Derivative–Kinetic Studies and Properties of the Sugar Latexes. Macromol. Chem. Phys. 2003, 204, 803–812. [Google Scholar] [CrossRef]
- Koch, U.; Yaacoub, E.-J. Batch Emulsion Copolymerization of 3-O-Methacryloyl-1,2:5,6-Di-O-Isopropylidene-α-d-Glucofuranose and Butyl Acrylate: Synthesis and Properties of the Sugar Latices. J. Polym. Sci. Part Polym. Chem. 2003, 41, 788–803. [Google Scholar] [CrossRef]
- Al-Bagoury, M.; Yaacoub, E.-J. Semicontinuous Emulsion Copolymerization of 3-O-Methacryloyl-1, 2: 5, 6-Di-O-Isopropylidene-α-d-Glucofuranose (3-MDG) and Butyl Acrylate (BA). Monomer Feed Addition. J. Appl. Polym. Sci. 2003, 90, 2091–2102. [Google Scholar] [CrossRef]
- Al-Bagoury, M.; Yaacoub, E.-J. Semicontinuous Emulsion Copolymerization of 3-O-Methacryloyl-1,2:5,6-Di-O-Isopropylidene-α-d-Glucofuranose (3-MDG) and Butyl Acrylate (BA) by Pre-Emulsion Addition Technique. Eur. Polym. J. 2004, 40, 2617–2627. [Google Scholar] [CrossRef]
- Koch, U.; Yaacoub, E.-J. Carbohydrat Latex, Process for Its Prepation and Its Use. Eur. Patent EP1086965 B1, 22 October 2003. [Google Scholar]
- Al-Bagoury, M.; Yaacoub, E.-J. Synthesis of Saccharidic Polymer Colloids via Free Radical Mini-Emulsion Polymerization. Polym. Adv. Technol. 2004, 15, 499–507. [Google Scholar] [CrossRef]
- Al-Bagoury, M.; Buchholz, K.; Yaacoub, E.-J. Synthesis of Well-Designed Polymers Carrying Saccharide Moieties via RAFT Miniemulsion Polymerization. Polym. Adv. Technol. 2007, 18, 313–322. [Google Scholar] [CrossRef]
- Albertin, L.; Stenzel, M.; Barner-Kowollik, C.; Foster, L.J.R.; Davis, T.P. Well-Defined Glycopolymers from RAFT Polymerization: Poly(methyl 6-O-Methacryloyl-α-d-Glucoside) and Its Block Copolymer with 2-Hydroxyethyl Methacrylate. Macromolecules 2004, 37, 7530–7537. [Google Scholar] [CrossRef]
- Min, E.H.; Ting, S.R.S.; Billon, L.; Stenzel, M.H. Thermo-Responsive Glycopolymer Chains Grafted onto Honeycomb Structured Porous Films via RAFT Polymerization as a Thermo-Dependent Switcher for Lectin Concanavalin a Conjugation. J. Polym. Sci. Part Polym. Chem. 2010, 48, 3440–3455. [Google Scholar] [CrossRef]
- Müller, A.H.E.; Roos, S.G.; Matyjaszewski, K. Copolymerization of N-Butyl Acrylate with Methyl Methacrylate and PMMA Macromonomers: Comparison of Reactivity Ratios in Conventional and Atom Transfer Radical Copolymerization. Am. Chem. Soc. Polym. Prepr. Div. Polym. Chem. 1999, 40, 352–353. [Google Scholar]
- Plessis, C.; Arzamendi, G.; Leiza, J.R.; Schoonbrood, H.A.S.; Charmot, D.; Asua, J.M. A Decrease in Effective Acrylate Propagation Rate Constants Caused by Intramolecular Chain Transfer. Macromolecules 2000, 33, 4–7. [Google Scholar] [CrossRef]
- González, I.; Asua, J.M.; Leiza, J.R. The Role of Methyl Methacrylate on Branching and Gel Formation in the Emulsion Copolymerization of BA/MMA. Polymer 2007, 48, 2542–2547. [Google Scholar] [CrossRef]
- Mahalingam, S.M.; Aidhen, I.S. Efficient and Rapid Regioselective Deprotection of Isopropylidene Ketals. Z. Naturforschung B 2014, 60, 962–966. [Google Scholar] [CrossRef]
- Bird, T.P.; Black, W.A.P.; Colquhoun, J.A.; Dewar, E.T.; Rutherford, D. Preparation and Derivatives of Poly-(6-O-Methacryloyl-d-Galactose) and Poly-(6-O-Acryloyl-d-Galactose). J. Chem. Soc. C Org. 1966, 1913–1918. [Google Scholar] [CrossRef]
- Lindemann, M. Vinyl Ester Aqueous Adhesive Emulsions Including Acrylamide. U.S. Patent US4339552 A, 4 July 1982. [Google Scholar]

| Latex Name | MF/BA (wt %) | MF/BA (mol %) | |
|---|---|---|---|
| Low Solids | High Solids | ||
| MF20-BA/30 | MF20-BA/45 | 20/80 | 9/91 |
| MF30-BA/30 | MF30-BA/45 | 30/70 | 14/86 |
| MF40-BA/30 | MF40-BA/45 | 40/60 | 21/79 |
| Monomer Composition MF/BA | 20/80 | 30/70 | 40/60 |
|---|---|---|---|
| Tg (°C) measured | −30 | −25 | −2 |
| Tg (°C) Fox-equation prediction | −29 | −17 | −4 |
| Run | dp (nm) | Tg (°C) | Gel (wt %) |
|---|---|---|---|
| MF20-BA/45 | 107 | −24 | 76 |
| MF40-BA/45 | 106 | 3 | 75 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Desport, J.S.; Moreno, M.; Barandiaran, M.J. Fructose-Based Acrylic Copolymers by Emulsion Polymerization. Polymers 2018, 10, 488. https://doi.org/10.3390/polym10050488
Desport JS, Moreno M, Barandiaran MJ. Fructose-Based Acrylic Copolymers by Emulsion Polymerization. Polymers. 2018; 10(5):488. https://doi.org/10.3390/polym10050488
Chicago/Turabian StyleDesport, Jessica S., Mónica Moreno, and María J. Barandiaran. 2018. "Fructose-Based Acrylic Copolymers by Emulsion Polymerization" Polymers 10, no. 5: 488. https://doi.org/10.3390/polym10050488
APA StyleDesport, J. S., Moreno, M., & Barandiaran, M. J. (2018). Fructose-Based Acrylic Copolymers by Emulsion Polymerization. Polymers, 10(5), 488. https://doi.org/10.3390/polym10050488






