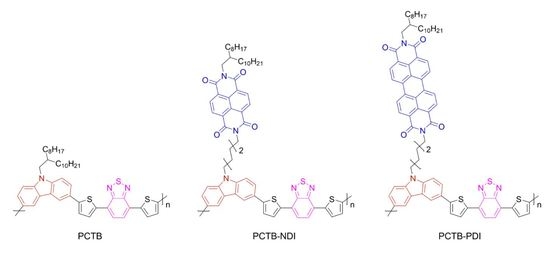
The Effect of Aromatic Diimide Side Groups on the π-Conjugated Polymer Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis
2.1.1. 3,6-Dibromo-9-(2-octyldodecanyl)-9H-carbazole (3)
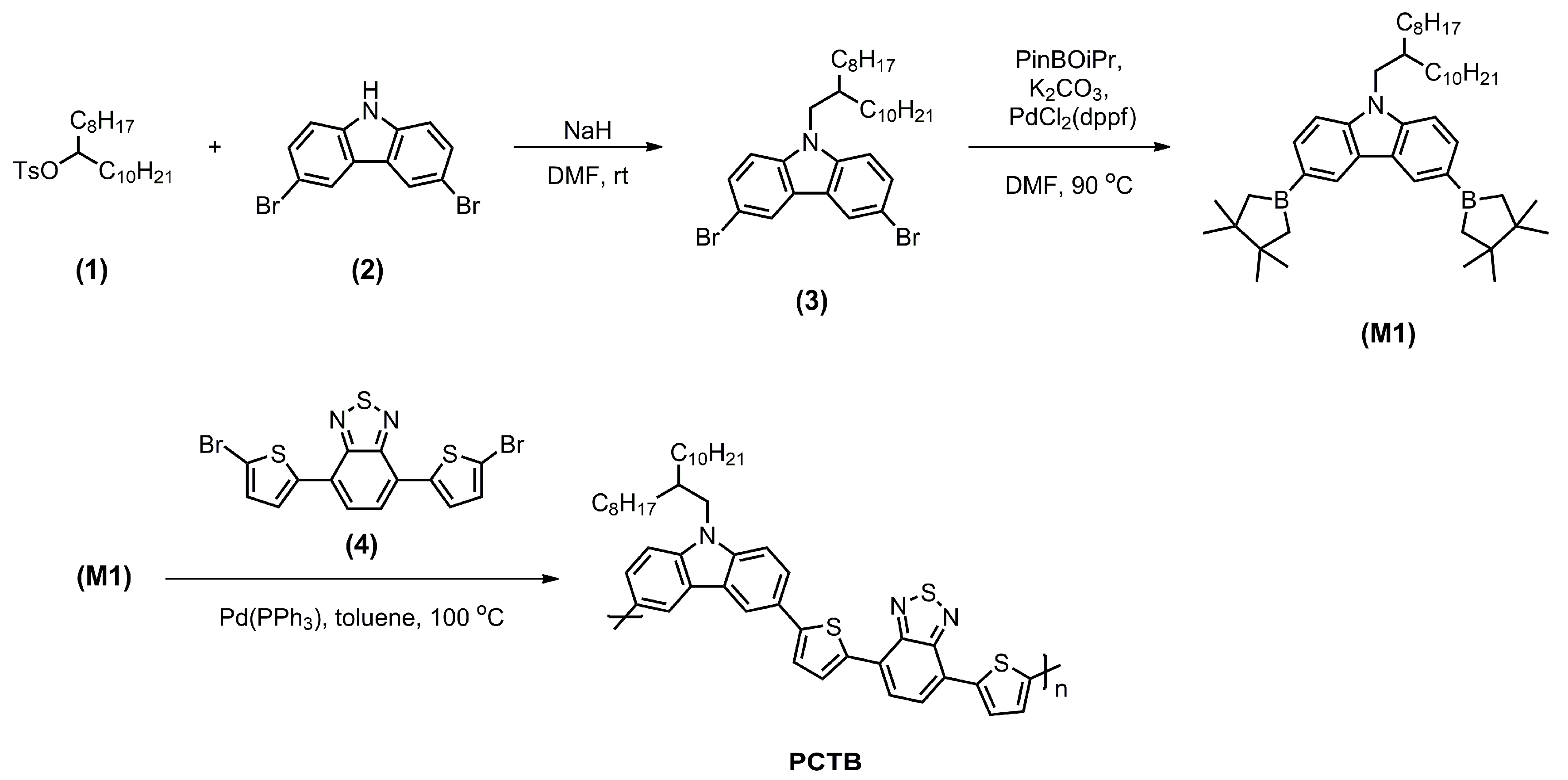
2.1.2. Monomer M1
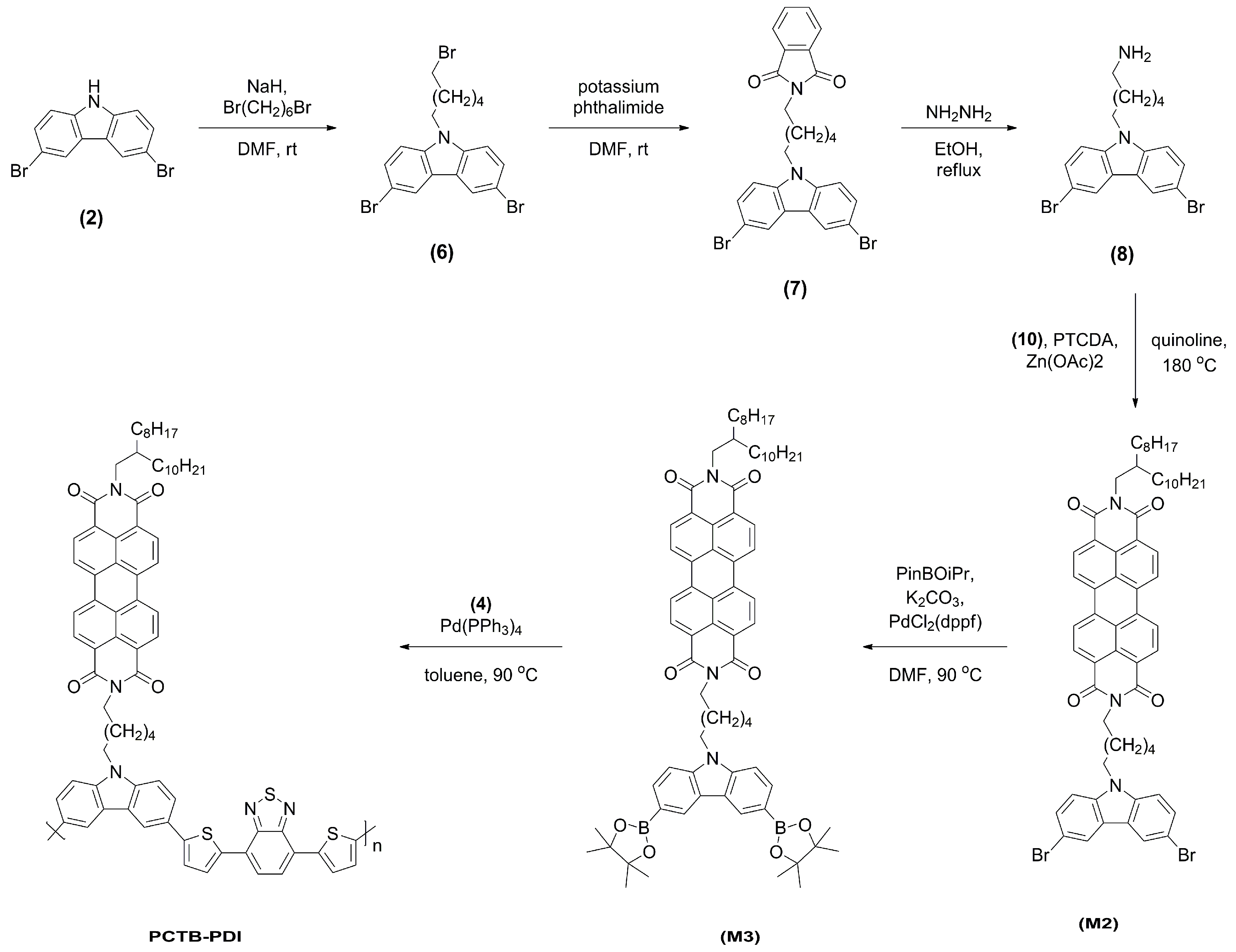
2.1.3. 3,6-Dibromo-9-(6-bromohexyl)-9H-carbazole (6)
2.1.4. 2-(6-(3,6-Dibromo-9H-carbazol-9-yl)hexyl)isoindoline-1,3-dione (7)
2.1.5. 6-(3,6-Dibromo-9H-carbazol-9-yl)hexyl-1-amine (8)
2.1.6. Monomer M2
2.1.7. Monomer M3
2.1.8. N-(2-Octyldodecyl)naphthalene-1,8-dicarboxyanhydride-4,5-dicarboxyimide (12)
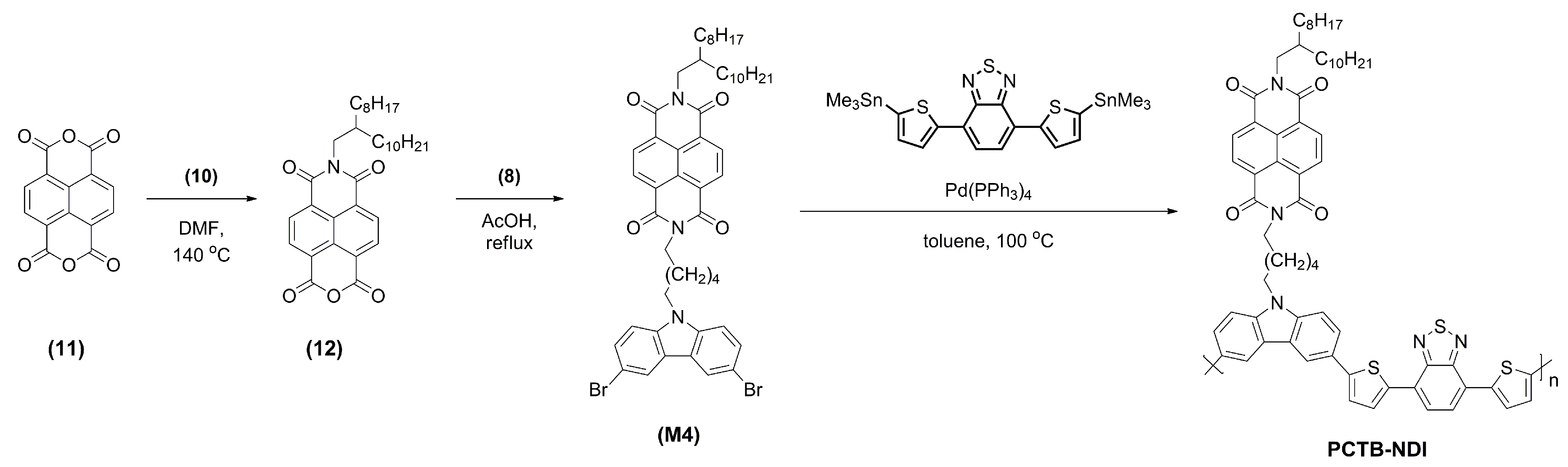
2.1.9. Monomer M4
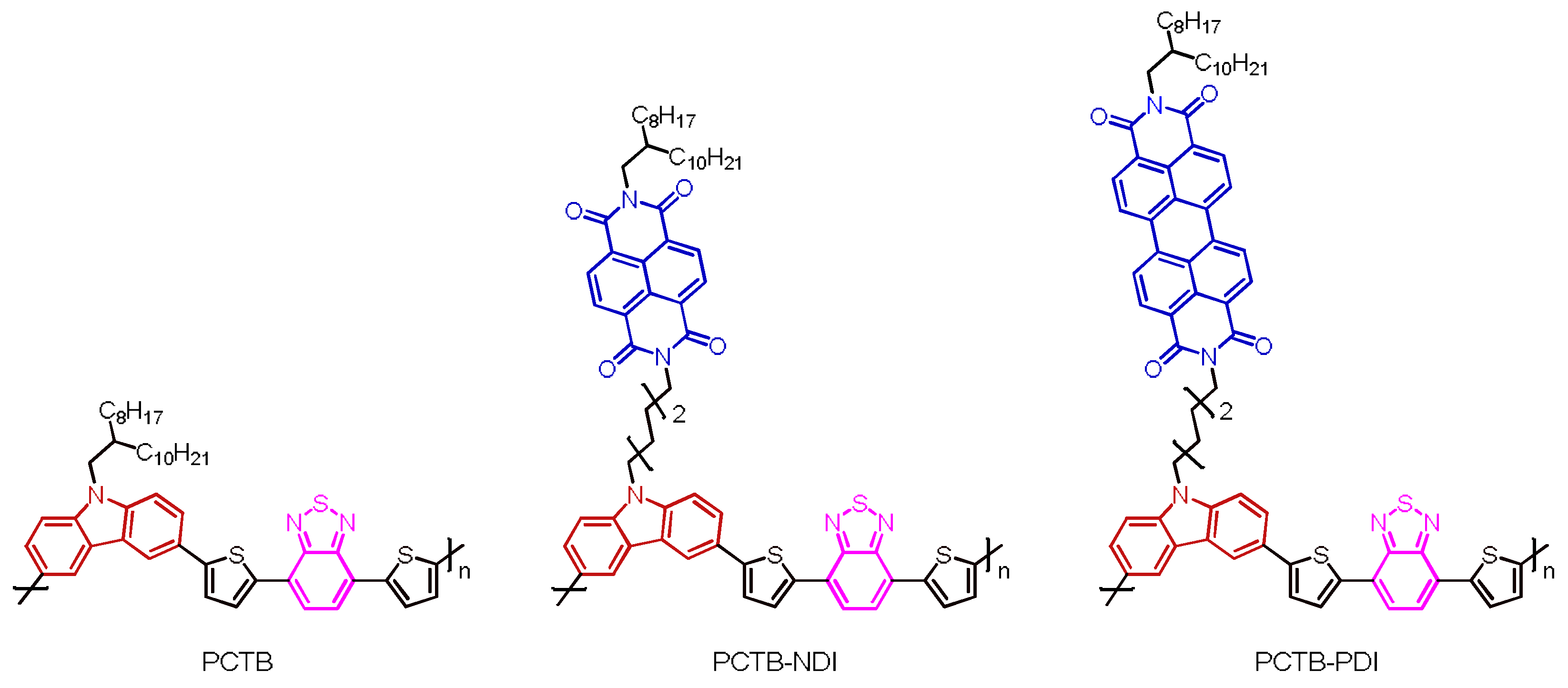
2.1.10. Polymer PCTB
2.1.11. Polymer PCTB-PDI
2.1.12. Polymer PCTB-NDI
2.2. Characterization Techniques
2.3. Device Preparation and Characterization
3. Results and Discussion
3.1. Synthesis
3.2. Physicochemical Properties
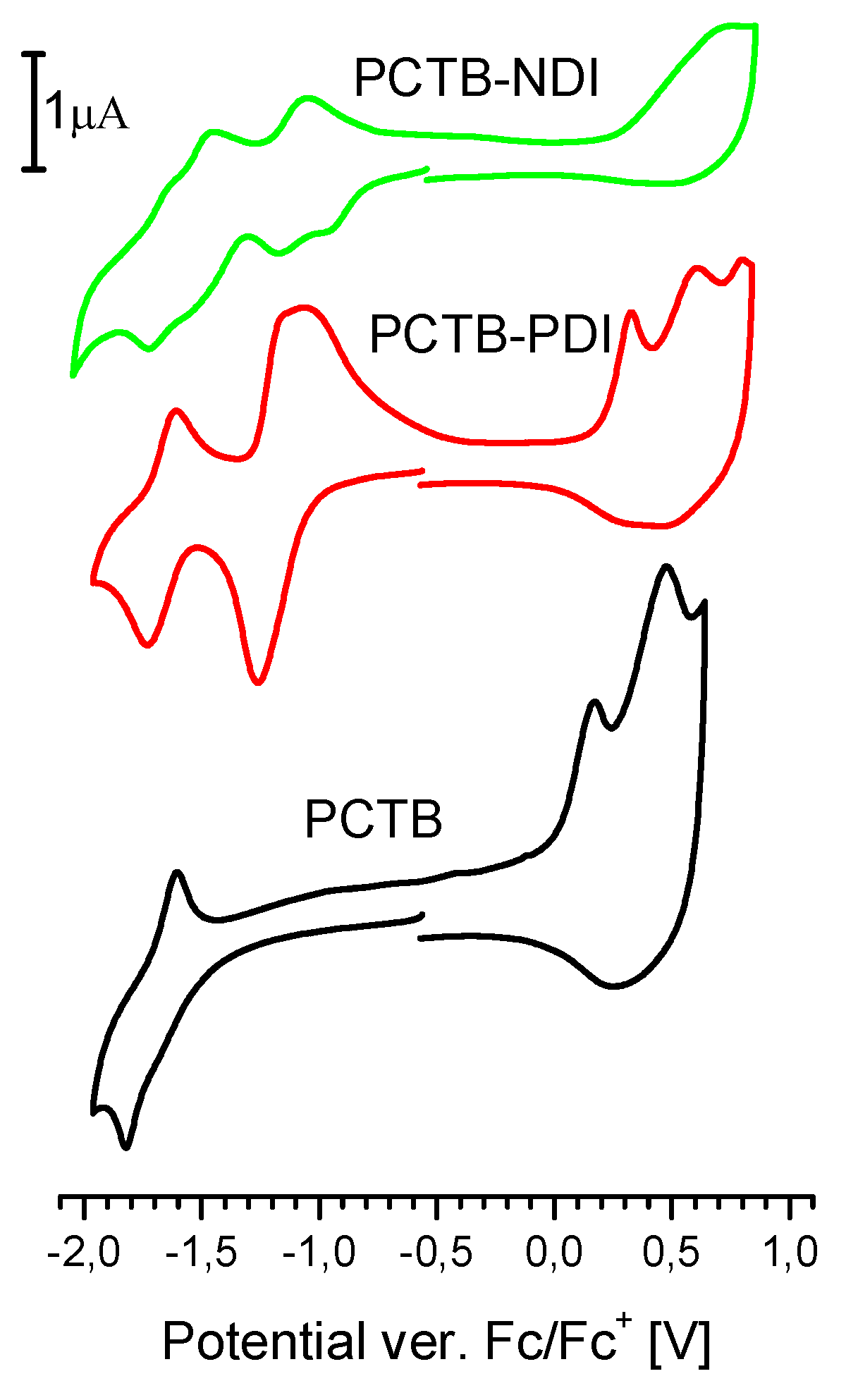
3.3. Electrochemical Properties
3.4. Electron Paramegnetic Resonance Spectroelectrochemistry of PCTB-PDI
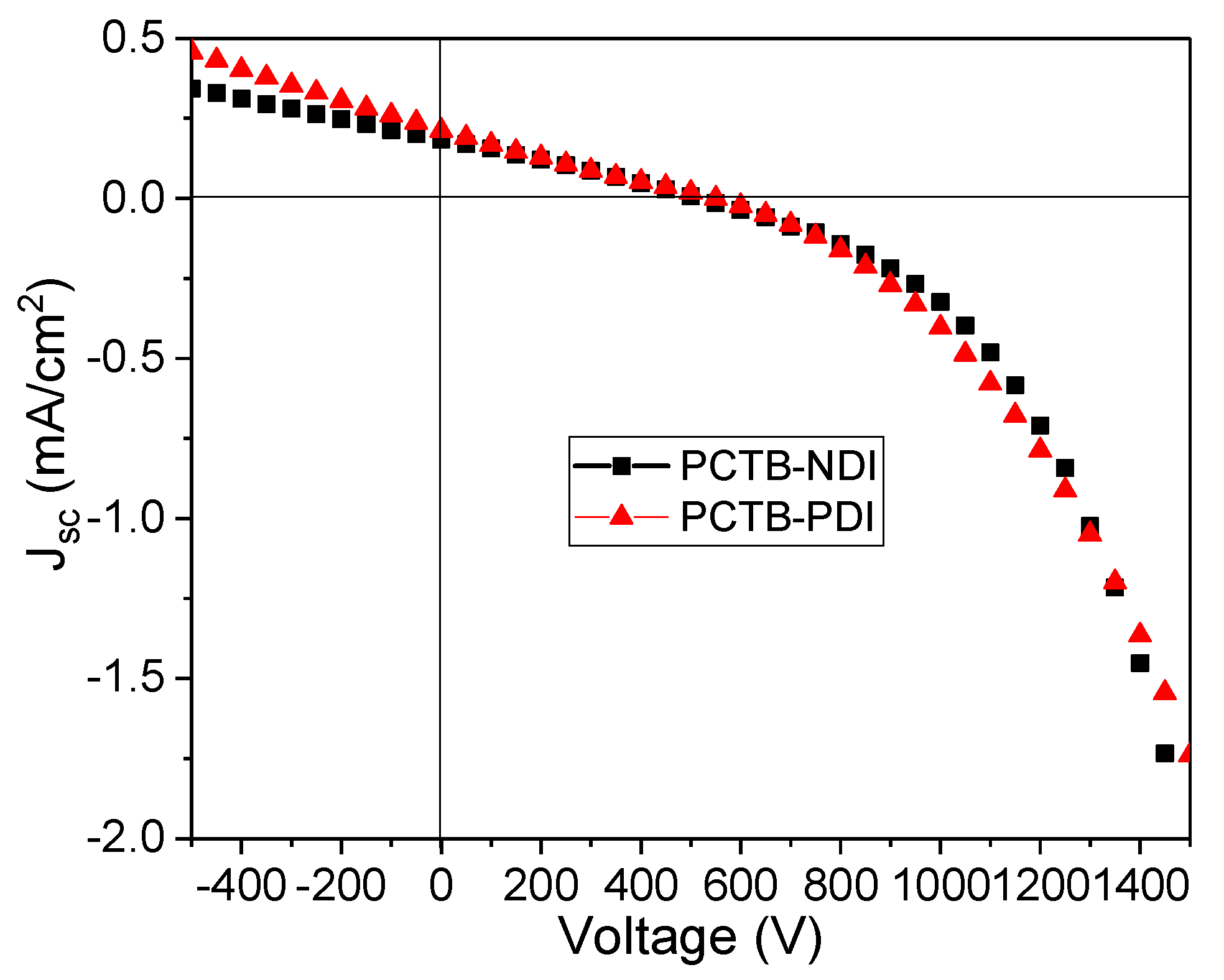
3.5. Photovoltaic Properties
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Jarosz, T.; Lapkowski, M.; Ledwon, P. Advances in star-shaped π-conjugated systems: Properties and applications. Macromol. Rapid Commun. 2014, 35, 1006–1032. [Google Scholar] [CrossRef] [PubMed]
- Hemavathi, B.; Ahipa, T.N.; Pai, R.K. Polymer design for solar cell—Current trend and future scenario. Eur. Polym. J. 2015, 72, 309–340. [Google Scholar] [CrossRef]
- Jung, J.W.; Jo, J.W.; Jung, E.H.; Jo, W.H. Recent progress in high efficiency polymer solar cells by rational design and energy level tuning of low bandgap copolymers with various electron-withdrawing units. Org. Electron. 2016, 31, 149–170. [Google Scholar] [CrossRef]
- Günes, S.; Neugebauer, H.; Sariciftci, N.S. Conjugated polymer-based organic solar cells. Chem. Rev. 2007, 107, 1324–1338. [Google Scholar] [CrossRef] [PubMed]
- Facchetti, A. Polymer donor–polymer acceptor (all-polymer) solar cells. Mater. Today 2013, 16, 123–132. [Google Scholar] [CrossRef]
- Al Kobaisi, M.; Bhosale, S.V.; Latham, K.; Raynor, A.M.; Bhosale, S.V. Functional naphthalene diimides: Synthesis, properties, and applications. Chem. Rev. 2016, 116, 11685–11796. [Google Scholar] [CrossRef] [PubMed]
- Bhosale, S.V.; Jani, C.H.; Langford, S.J. Chemistry of naphthalene diimides. Chem. Soc. Rev. 2008, 37, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Würthner, F.; Saha-Möller, C.R.; Fimmel, B.; Ogi, S.; Leowanawat, P.; Schmidt, D. Perylene bisimide dye assemblies as archetype functional supramolecular materials. Chem. Rev. 2016, 116, 962–1052. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-J.; Xia, S.-B.; Duan, Y.-L.; Liu, T.; Cheng, F.-X.; Sun, C.-K. Anion-controlled architecture and photochromism of naphthalene diimide-based coordination polymers. Polymers 2018, 10, 165. [Google Scholar] [CrossRef]
- Kozma, E.; Catellani, M. Perylene diimides based materials for organic solar cells. Dye. Pigment. 2013, 98, 160–179. [Google Scholar] [CrossRef]
- Li, C.; Wonneberger, H. Perylene imides for organic photovoltaics: Yesterday, today, and tomorrow. Adv. Mater. 2012, 24, 613–636. [Google Scholar] [CrossRef] [PubMed]
- Rybakiewicz, R.; Gawrys, P.; Tsikritzis, D.; Emmanouil, K.; Kennou, S.; Zagorska, M.; Pron, A. Electronic properties of semiconducting naphthalene bisimide derivatives—Ultraviolet photoelectron spectroscopy versus electrochemistry. Electrochim. Acta 2013, 96, 13–17. [Google Scholar] [CrossRef]
- Pron, A.; Gawrys, P.; Zagorska, M.; Djurado, D.; Demadrille, R. Electroactive materials for organic electronics: Preparation strategies, structural aspects and characterization techniques. Chem. Soc. Rev. 2010, 39, 2577–2632. [Google Scholar] [CrossRef] [PubMed]
- Hartnett, P.E.; Timalsina, A.; Matte, H.S.S.R.; Zhou, N.; Guo, X.; Zhao, W.; Facchetti, A.; Chang, R.P.H.; Hersam, M.C.; Wasielewski, M.R.; et al. Slip-stacked perylenediimides as an alternative strategy for high efficiency nonfullerene acceptors in organic photovoltaics. J. Am. Chem. Soc. 2014, 136, 16345–16356. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Jiang, W.; Zhao, W.; Zhang, S.; Qian, D.; Wang, Z.; Hou, J. Selecting a donor polymer for realizing favorable morphology in efficient non-fullerene acceptor-based solar cells. Small 2014, 10, 4658–4663. [Google Scholar] [CrossRef] [PubMed]
- Zang, Y.; Li, C.-Z.; Chueh, C.-C.; Williams, S.T.; Jiang, W.; Wang, Z.-H.; Yu, J.-S.; Jen, A.K.-Y. Integrated molecular, interfacial, and device engineering towards high-performance non-fullerene based organic solar cells. Adv. Mater. 2014, 26, 5708–5714. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Sun, K.; Jiang, W.; Zhang, S.; Zhao, W.; Yao, H.; Wang, Z.; Hou, J. Enhanced efficiency in fullerene-free polymer solar cell by incorporating fine-designed donor and acceptor materials. ACS Appl. Mater. Interfaces 2015, 17, 9274–9280. [Google Scholar] [CrossRef] [PubMed]
- Meng, D.; Sun, D.; Zhong, C.; Liu, T.; Fan, B.; Huo, L.; Li, Y.; Jiang, W.; Choi, H.; Kim, T.; et al. High-performance solution-processed non-fullerene organic solar cells based on selenophene-containing perylene bisimide acceptor. J. Am. Chem. Soc. 2016, 138, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Meng, D.; Cai, Y.; Fan, B.; Li, Y.; Jiang, W.; Huo, L.; Sun, Y.; Wang, Z. Non-fullerene-acceptor-based bulk-heterojunction organic solar cells with efficiency over 7%. J. Am. Chem. Soc. 2015, 137, 11156–11162. [Google Scholar] [CrossRef] [PubMed]
- Benten, H.; Mori, D.; Ohkita, H.; Ito, S. Recent research progress of polymer donor/polymer acceptor blend solar cells. J. Mater. Chem. A 2016, 4, 5340–5365. [Google Scholar] [CrossRef]
- Jørgensen, M.; Norrman, K.; Gevorgyan, S.A.; Tromholt, T.; Andreasen, B.; Krebs, F.C. Stability of polymer solar cells. Adv. Mater. 2012, 24, 580–612. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhan, C.; Yao, J. Non-fullerene organic solar cells with 6.1% efficiency through fine-tuning parameters of the film-forming process. Chem. Mater. 2015, 27, 166–173. [Google Scholar] [CrossRef]
- Hua, J.; Meng, F.; Li, J.; Ding, F.; Fan, X.; Tian, H. Synthesis and characterization of new highly soluble and thermal-stable perylene-PPV copolymers containing triphenylamine moiety. Eur. Polym. J. 2006, 42, 2686–2694. [Google Scholar] [CrossRef]
- Tao, Y.; McCulloch, B.; Kim, S.; Segalman, R.A. The relationship between morphology and performance of donor-acceptor rod-coil block copolymer solar cells. Soft Matter 2009, 5, 4219–4230. [Google Scholar] [CrossRef]
- Zhang, Q.; Cirpan, A.; Russell, T.P.; Emrick, T. Donor-acceptor poly(thiophene-block-perylene diimide) copolymers: Synthesis and solar cell fabrication. Macromolecules 2009, 42, 1079–1082. [Google Scholar] [CrossRef]
- Sommer, M.; Hüttner, S.; Steiner, U.; Thelakkat, M. Influence of molecular weight on the solar cell performance of double-crystalline donor-acceptor block copolymers. Appl. Phys. Lett. 2009, 95. [Google Scholar] [CrossRef]
- Yuan, M.-C.; Su, M.-H.; Chiu, M.-Y.; Wei, K.-H. Synthesis and characterization of donor-bridge-acceptor alternating copolymers containing perylene diimide units and their application to photovoltaic cells. J. Polym. Sci. Part A 2010, 48, 1298–1309. [Google Scholar] [CrossRef]
- Mohamad, D.K.; Fischereder, A.; Yi, H.; Cadby, A.J.; Lidzey, D.G.; Iraqi, A. A novel 2,7-linked carbazole based “double cable” polymer with pendant perylene diimide functional groups: Preparation, spectroscopy and photovoltaic properties. J. Mater. Chem. 2011, 21, 851–862. [Google Scholar] [CrossRef]
- Huettner, S.; Sommer, M.; Hodgkiss, J.; Kohn, P.; Thurn-Albrecht, T.; Friend, R.H.; Steiner, U.; Thelakkat, M. Tunable charge transport using supramolecular self-assembly of nanostructured crystalline block copolymers. ACS Nano 2011, 5, 3506–3515. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.; Li, J.; Colberts, F.J.M.; Li, M.; Zhang, J.; Yang, F.; Jin, Y.; Zhang, F.; Janssen, R.A.J.; Li, C.; et al. “Double-Cable” Conjugated polymers with linear backbone toward high quantum efficiencies in single-component polymer solar cells. J. Am. Chem. Soc. 2017, 139, 18647–18656. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.L.; Lee, T.H.; Gautam, B.; Park, S.Y.; Gundogdu, K.; Kim, J.Y.; Woo, H.Y. Single component organic solar cells based on oligothiophene-fullerene conjugate. Adv. Funct. Mater. 2017, 27, 1702474. [Google Scholar] [CrossRef]
- Huang, C.; Sartin, M.M.; Siegel, N.; Cozzuol, M.; Zhang, Y.; Hales, J.M.; Barlow, S.; Perry, J.W.; Marder, S.R. Photo-induced charge transfer and nonlinear absorption in dyads composed of a two-photon-absorbing donor and a perylene diimide acceptor. J. Mater. Chem. 2011, 21, 16119–16128. [Google Scholar] [CrossRef]
- Yu, L.-F.; Ge, C.-W.; Wang, J.-T.; Xiang, X.; Li, W.-S. Modification of a donor-acceptor photovoltaic polymer by integration of optoelectronic moieties into its side chains. Polymer 2015, 59, 57–66. [Google Scholar] [CrossRef]
- Aasmundtveit, K.E.; Samuelsen, E.J.; Mammo, W.; Svensson, M.; Andersson, M.R.; Pettersson, L.A.A.; Inganäs, O. Structural ordering in phenyl-substituted polythiophenes. Macromolecules 2000, 33, 5481–5489. [Google Scholar] [CrossRef]
- Deng, Y.; Chen, Y.; Zhang, X.; Tian, H.; Bao, C.; Yan, D.; Geng, Y.; Wang, F. Donor–acceptor conjugated polymers with dithienocarbazoles as donor units: Effect of structure on semiconducting properties. Macromolecules 2012, 45, 8621–8627. [Google Scholar] [CrossRef]
- Ledwon, P.; Thomson, N.; Angioni, E.; Findlay, N.J.; Skabara, P.J.; Domagala, W. The role of structural and electronic factors in shaping the ambipolar properties of donor-acceptor polymers of thiophene and benzothiadiazole. RSC Adv. 2015, 5, 77303–77315. [Google Scholar] [CrossRef]
- Ledwon, P.; Pluczyk, S.; Idzik, K.R.; Beckert, R.; Lapkowski, M. Bipolar properties of polythiophene derivatives with 1, 3, 5-triazineunits. Electrochim. Acta 2013, 109, 395–402. [Google Scholar] [CrossRef]
- Kamm, V.; Battagliarin, G.; Howard, I.A.; Pisula, W.; Mavrinskiy, A.; Li, C.; Müllen, K.; Laquai, F. Polythiophene: Perylene Diimide Solar Cells—The impact of alkyl-substitution on the photovoltaic performance. Adv. Energy Mater. 2011, 1, 297–302. [Google Scholar] [CrossRef]


| Compound | λ 1 [nm] | Eox 2 [V] | Ered 3 [V] | IP 4 [eV] | EA 5 [eV] | Tg 6 [°C] |
|---|---|---|---|---|---|---|
| PCTB | 352; 535 | 0 | −1.41 | 5.1 | 3.8 | 140 |
| PCTB-NDI | 361; 382; 521 | 0.26 | −0.85 | 5.4 | 4.2 | |
| PCTB-PDI | 359; 494; 527 | 0.18 | −1.04 | 5.3 | 4.1 | 155 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drewniak, A.; Tomczyk, M.D.; Hanusek, L.; Mielanczyk, A.; Walczak, K.; Nitschke, P.; Hajduk, B.; Ledwon, P. The Effect of Aromatic Diimide Side Groups on the π-Conjugated Polymer Properties. Polymers 2018, 10, 487. https://doi.org/10.3390/polym10050487
Drewniak A, Tomczyk MD, Hanusek L, Mielanczyk A, Walczak K, Nitschke P, Hajduk B, Ledwon P. The Effect of Aromatic Diimide Side Groups on the π-Conjugated Polymer Properties. Polymers. 2018; 10(5):487. https://doi.org/10.3390/polym10050487
Chicago/Turabian StyleDrewniak, Anna, Mateusz D. Tomczyk, Lukasz Hanusek, Anna Mielanczyk, Krzysztof Walczak, Pawel Nitschke, Barbara Hajduk, and Przemyslaw Ledwon. 2018. "The Effect of Aromatic Diimide Side Groups on the π-Conjugated Polymer Properties" Polymers 10, no. 5: 487. https://doi.org/10.3390/polym10050487
APA StyleDrewniak, A., Tomczyk, M. D., Hanusek, L., Mielanczyk, A., Walczak, K., Nitschke, P., Hajduk, B., & Ledwon, P. (2018). The Effect of Aromatic Diimide Side Groups on the π-Conjugated Polymer Properties. Polymers, 10(5), 487. https://doi.org/10.3390/polym10050487