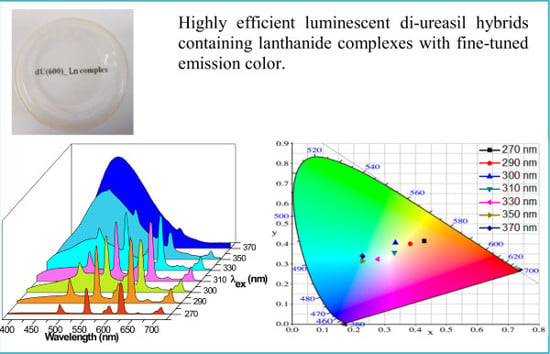
Highly Efficient Luminescent Polycarboxylate Lanthanide Complexes Incorporated into Di-Ureasils by an In-Situ Sol—Gel Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Synthesis
2.2. Characterization
2.3. Theoretical and Computational Details
3. Results and Discussion
3.1. Powder XRD Patterns
3.2. FT-IR Spectroscopy
3.3. 29Si MAS and 13C CP NMR Spectra
3.4. TG and Thermal Stability Analyses
3.5. Electronic Properties
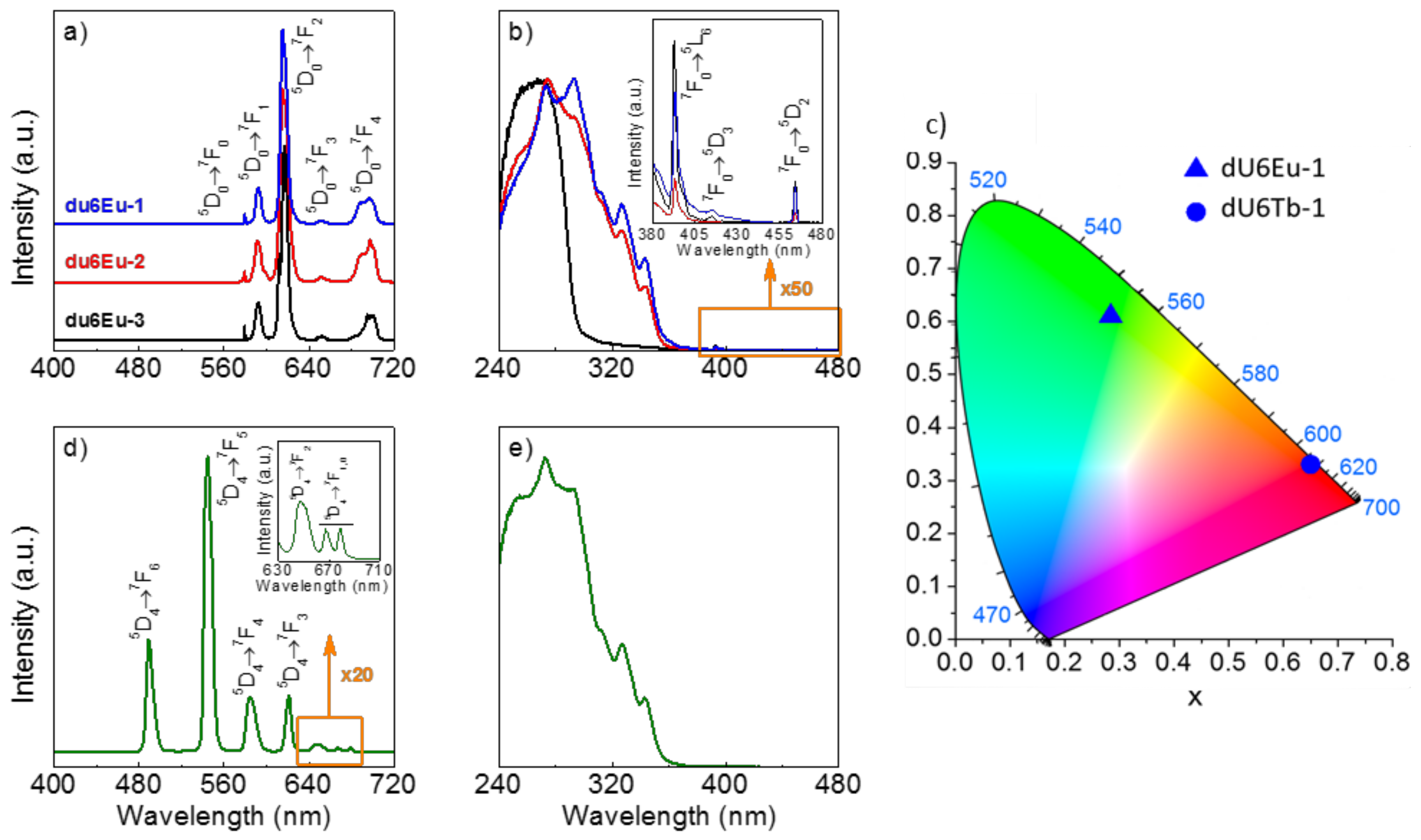
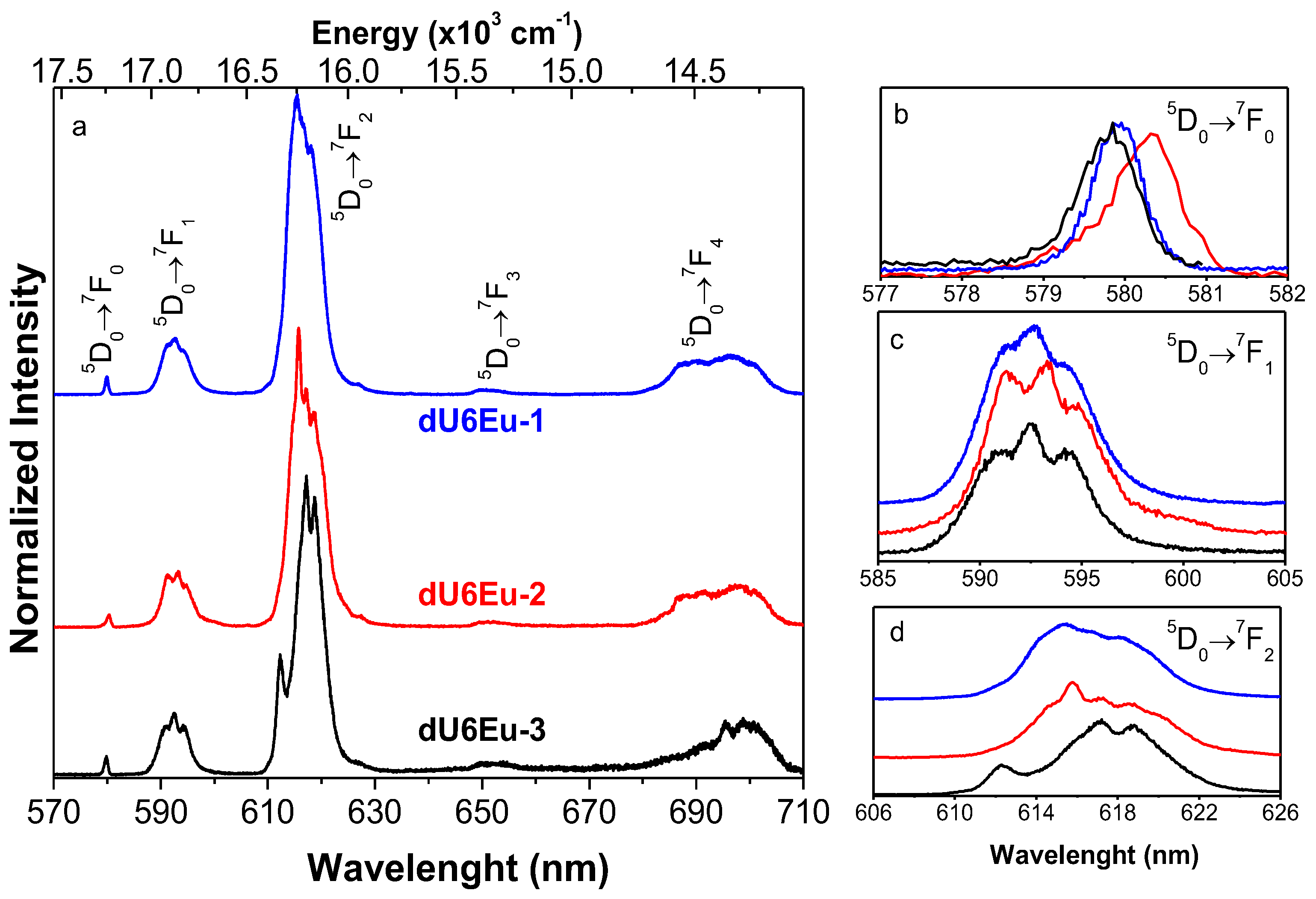
3.6. Photoluminescence
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Reisfeld, R.; Jørgensen, C.K. Lasers and Excited States of Rare Earths, 1st ed.; Springer-Verlag: Berlin/Heidelberg, Germany; New York, NY, USA, 2012; pp. 1–63. [Google Scholar]
- Bünzli, J.C.G.; Piguet, C. Taking advantage of luminescent lanthanide ions. Chem. Soc. Rev. 2005, 34, 1048–1077. [Google Scholar] [CrossRef] [PubMed]
- Carnall, W.T. The absorption and fluorescence spectra of rare earth ions in solution. In Handbook on the Physics and Chemistry of Rare Earths, 1st ed.; Blasse, G., Gschneidner, K.A., Eyring, L., Eds.; North-Holland Publishing Company: Amsterdam, The Netherlands, 1979; pp. 171–208. [Google Scholar]
- Armelao, L.; Quici, S.; Barigelletti, F.; Accorsi, G.; Bottaro, G.; Cavazzini, M.; Tondello, E. Design of luminescent lanthanide complexes: From molecules to highly efficient photo-emitting materials. Coord. Chem. Rev. 2010, 254, 487–505. [Google Scholar] [CrossRef]
- Bünzli, J.C.G. On the design of highly luminescent lanthanide complexes. Coord. Chem. Rev. 2015, 293, 19–47. [Google Scholar] [CrossRef]
- Janicki, R.; Mondry, A.; Starynowicz, P. Carboxylates of rare earth elements. Coord. Chem. Rev. 2017, 340, 98–133. [Google Scholar] [CrossRef]
- Andraud, C.; Maury, O. Lanthanide complexes for nonlinear optics: From fundamental aspects to applications. Eur. J. Inorg. Chem. 2009, 2009, 4357–4371. [Google Scholar] [CrossRef]
- Law, G.L.; Wong, K.L.; Lau, K.K.; Lap, S.T.; Tanner, P.A.; Kuo, F.; Wong, W.T. Nonlinear optical activity in dipolar organic-lanthanide complexes. J. Mater. Chem. 2010, 20, 4074–4079. [Google Scholar] [CrossRef]
- Dong, D.W.; Jiang, S.C.; Men, Y.F.; Ji, X.L.; Jiang, B.Z. Nanostructured hybrid organic–inorganic lanthanide complex films produced in situ via a sol-gel approach. Adv. Mater. 2000, 12, 646–649. [Google Scholar] [CrossRef]
- Carlos, L.D.; Ferreira, R.A.S.; de Zea Bermudez, V.; Ribeiro, S.J. Lanthanide-containing light-emitting organic-inorganic hybrids: A bet on the future. Adv. Mater. 2009, 21, 509–534. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.Q.; Wang, Y.G.; Wang, Y.; Li, Z.Q.; Li, H.R. Luminescence enhancement after adding organic salts to nanohybrid under aqueous condition. ACS Appl. Mater. Interfaces 2015, 7, 2097–2103. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, H.R. Luminescent materials of zeolite functionalized with lanthanides. CrystEngComm 2014, 16, 9764–9778. [Google Scholar] [CrossRef]
- Esquivel, D.; Kaczmarek, A.M.; Jimenez-Sanchidrian, C.; Van Deun, R.; Romero-Salguero, F.J.; Van der Voort, P. Eu3+@PMO: Synthesis, characterization and luminescence properties. J. Mater. Chem. C 2015, 3, 2909–2917. [Google Scholar] [CrossRef]
- Xu, Q.H.; Fu, L.S.; Li, L.S.; Zhang, H.J.; Xu, R.R. Preparation, characterization and photophysical properties of layered zirconium bis(monohydrogenphosphate) intercalated with rare earth complexes. J. Mater. Chem. 2000, 10, 2532–2536. [Google Scholar] [CrossRef]
- Bhowmik, S.; Banerjee, S.; Maitra, U. A self-assembled, luminescent europium cholate hydrogel: A novel approach towards lanthanide sensitization. Chem. Commun. 2010, 46, 8642–8644. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.G.; Wang, Q.; Yan, X.H.; Xu, J.; Liu, W.S.; Wang, Q.; Tang, Y. Encapsulating a ternary europium complex in a silica/polymer hybrid matrix for high performance luminescence application. J. Phys. Chem. C 2011, 115, 2332–2340. [Google Scholar] [CrossRef]
- Singh, K.; Boddula, R.; Vaidyanathan, S. Versatile luminescent europium(III)-beta-diketonate-imidazo-bipyridyl complexes intended for white LEDs: A detailed photophysical and theoretical study. Inorg. Chem. 2017, 56, 9376–9390. [Google Scholar] [CrossRef] [PubMed]
- Carlos, L.D.; Ferreira, R.A.S.; de Zea Bermudez, V.; Julián-López, B.; Escribano, P. Progress on lanthanide-based organic-inorganic hybrid phosphors. Chem. Soc. Rev. 2011, 40, 536–549. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Zhang, H.J. Hybrid materials based on lanthanide organic complexes: A review. Chem. Soc. Rev. 2013, 42, 387–410. [Google Scholar] [CrossRef] [PubMed]
- Eliseeva, S.V.; Bünzli, J.G. Lanthanide luminescence for functional materials and bio-sciences. Chem. Soc. Rev. 2010, 39, 189–227. [Google Scholar] [CrossRef] [PubMed]
- De Zea Bermudez, V.; Carlos, L.D.; Alcácer, L. Sol-gel derived urea cross-linked organically modified silicates. 1. Room temperature mid-infrared spectra. Chem. Mater. 1999, 11, 569–580. [Google Scholar] [CrossRef]
- Carlos, L.D.; de Zea Bermudez, V.; Ferreira, R.A.S.; Marques, L.; Assunção, M. Sol-gel derived urea cross-linked organically modified silicates. 2. Blue-light emission. Chem. Mater. 1999, 11, 581–588. [Google Scholar] [CrossRef]
- Carlos, L.D.; Ferreira, R.A.S.; De Zea Bermudez, V.; Ribeiro, S.J.L. Full-color phosphors from amine-functionalized crosslinked hybrids lacking metal activator ions. Adv. Funct. Mater. 2001, 11, 111–115. [Google Scholar] [CrossRef]
- Willis-Fox, N.; Kraft, M.; Arlt, J.; Scherf, U.; Evans, R.C. Tunable white-light emission from conjugated polymer-di-ureasil materials. Adv. Opt. Mater. 2016, 26, 532–542. [Google Scholar] [CrossRef]
- Kaniyoor, A.; McKenna, B.; Comby, S.; Evans, R.C. Design and response of high-efficiency, planar, doped luminescent solar concentrators using organic–inorganic di-ureasil waveguides. Adv. Opt. Mater. 2016, 4, 444–456. [Google Scholar] [CrossRef]
- Correia, S.F.; Lima, P.P.; Pecoraro, E.; Ribeiro, S.J.; André, P.S.; Ferreira, R.A.; Carlos, L.D. Scale up the collection area of luminescent solar concentrators towards metre-length flexible waveguiding photovoltaics. Prog. Photovolt. Res. Appl. 2016, 24, 1178–1193. [Google Scholar] [CrossRef]
- Fu, L.S.; Ferreira, R.A.S.; Silva, N.J.O.; Fernandes, A.J.; Ribeiro-Claro, P.; Gonçalves, I.S.; de Zea Bermudez, V.; Carlos, L.D. Structure-photoluminescence relationship in Eu(III) beta-diketonate-based organic-inorganic hybrids. Influence of the synthesis method: Carboxylic acid solvolysis versus conventional hydrolysis. J. Mater. Chem. 2005, 15, 3117–3125. [Google Scholar] [CrossRef]
- Lima, P.P.; Ferreira, R.A.S.; Freire, R.O.; Paz, F.A.A.; Fu, L.S.; Alves, S.; Carlos, L.D.; Malta, O.L. Spectroscopic study of a UV-photostable organic-inorganic hybrids incorporating an Eu3+ β-diketonate complex. ChemPhysChem 2006, 7, 735–746. [Google Scholar] [CrossRef] [PubMed]
- Lima, P.P.; Junior, S.A.; Malta, O.L.; Carlos, L.D.; Ferreira, R.A.S.; Pavithran, R.; Reddy, M.L.P. Synthesis, characterization, and luminescence properties of Eu3+ 3-phenyl-4-(4-toluoyl)-5-isoxazolonate based organic–inorganic hybrids. Eur. J. Inorg. Chem. 2006, 2006, 3923–3929. [Google Scholar] [CrossRef]
- Fernandes, M.; de Zea Bermudez, V.; Ferreira, R.A.S.; Carlos, L.D.; Martins, N.V. Incorporation of the Eu(tta)3(H2O)2 complex into a co-condensed d-U(600)/d-U(900) matrix. J. Lumin. 2008, 128, 205–212. [Google Scholar] [CrossRef]
- Lima, P.P.; Paz, F.A.A.; Ferreira, R.A.S.; de Zea Bermudez, V.; Carlos, L.D. Ligand-assisted rational design and supramolecular tectonics toward highly luminescent Eu3+-containing organic–inorganic hybrids. Chem. Mater. 2009, 21, 5099–5111. [Google Scholar] [CrossRef]
- Lima, P.P.; Ferreira, R.A.S.; Júnior, S.A.; Malta, O.L.; Carlos, L.D. Terbium(III)-containing organic–inorganic hybrids synthesized through hydrochloric acid catalysis. J. Photochem. Photobiol. A Chem. 2009, 201, 214–221. [Google Scholar] [CrossRef]
- Brinker, C.J.; Scherer, G.W. Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing, 1st ed.; Academic: New York, NY, USA, 1990. [Google Scholar]
- Yan, B. Recent progress in photofunctional lanthanide hybrid materials. RSC Adv. 2012, 2, 9304–9324. [Google Scholar] [CrossRef]
- Binnemans, K. Lanthanide-based luminescent hybrid materials. Chem. Rev. 2009, 109, 4283–4374. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, C.; Belleville, P.; Popall, M.; Nicole, L. Applications of advanced hybrid organic–inorganic nanomaterials: From laboratory to market. Chem. Soc. Rev. 2011, 40, 696–753. [Google Scholar] [CrossRef] [PubMed]
- Escribano, P.; Julián-López, B.; Planelles-Aragó, J.; Cordoncillo, E.; Viana, B.; Sanchez, C. Photonic and nanobiophotonic properties of luminescent lanthanide-doped hybrid organic–inorganic materials. J. Mater. Chem. 2008, 18, 23–40. [Google Scholar] [CrossRef]
- Parola, S.; Julián-López, B.; Carlos, L.D.; Sanchez, C. Optical properties of hybrid organic–inorganic materials and their applications. Adv. Funct. Mater. 2016, 26, 6506–6544. [Google Scholar] [CrossRef]
- Guo, X.M.; Guo, H.D.; Fu, L.S.; Deng, R.P.; Chen, W.; Feng, J.; Dang, S.; Zhang, H.J. Synthesis, spectroscopic properties, and stabilities of ternary europium complex in SBA-15 and periodic mesoporous organosilica: A comparative study. J. Phys. Chem. C 2009, 113, 2603–2610. [Google Scholar] [CrossRef]
- Qian, G.D.; Wang, M.Q.; Wang, M.; Fan, X.P.; Hong, Z.L. Synthesis in situ of 2,2’-dipyridyl-Tb(III) complexes in silica gel. J. Mater. Sci. Lett. 1997, 16, 322–323. [Google Scholar]
- Zhang, H.J.; Fu, L.S.; Wang, S.B.; Meng, Q.G.; Yang, K.Y.; Ni, J.Z. Luminescence characteristics of europium and terbium complexes with 1,10-phenanthroline in-situ synthesized in a silica matrix by a two-step sol-gel process. Mater. Lett. 1999, 38, 260–264. [Google Scholar] [CrossRef]
- Li, B.; Wen, H.M.; Cui, Y.J.; Qian, G.D.; Chen, B.L. Multifunctional lanthanide coordination polymers. Prog. Polym. Sci. 2015, 48, 40–84. [Google Scholar] [CrossRef]
- Ma, D.; Li, X.; Huo, R. A high-efficiency white light-emitting lanthanide–organic framework assembled from 4,4’-oxybis(benzoic acid), 1,10-phenanthroline and oxalate. J. Mater. Chem. C 2014, 2, 9073–9076. [Google Scholar] [CrossRef]
- Wang, Y.B.; Wang, Z.M.; Yan, C.H.; Jin, L.P. Hydrothermal synthesis and structure of new lanthanide coordination polymers with dicarboxylic acid and 1,10-phenanthroline. J. Mol. Struct. 2004, 692, 177–186. [Google Scholar] [CrossRef]
- Li, X.X.; Wei, Z.Q.; Yue, S.T.; Wang, N.; Mo, H.H.; Liu, Y.L. A new lanthanide coordination polymer with 4’4-oxybis(benzoic acid) ligand: Hydrothermal synthesis, crystal structure and photoluminescence. J. Chem. Crystallogr. 2011, 41, 757–761. [Google Scholar] [CrossRef]
- McCamy, C.S. Correlated color temperature as an explicit function of chromaticity coordinates. Color Res. Appl. 1992, 17, 142–144. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colic-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F.W. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD-Visual molecular dynamics. J. Mol. Gr. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Rev. B.01; Gaussian Inc: Wallingford, CT, USA, 2009. [Google Scholar]
- West, A.R. Solid State Chemistry and Its Applications, 2nd ed.; Wiley: Sheffield, UK, 2014. [Google Scholar]
- Lima, P.P.; Nobre, S.S.; Freire, R.O.; Junior, S.A.; Ferreira, R.A.S.; Pischel, U.; Malta, O.L.; Carlos, L.D. Energy transfer mechanisms in organic–inorganic hybrids incorporating europium(III): A quantitative assessment by light emission spectroscopy. J. Phys. Chem. C 2007, 111, 17627–17634. [Google Scholar] [CrossRef]
- Warren, B.E. X-ray Diffraction; Adisson Wesley: New York, NY, USA, 1969; pp. 15–26. [Google Scholar]
- Freitas, V.T.; Lima, P.P.; Ferreira, R.A.S.; Pecoraro, E.; Fernandes, M.; de Zea Bermudez, V.; Carlos, L.D. Luminescent urea cross-linked tripodal siloxane-based hybrids. J. Sol-Gel Sci. Technol. 2013, 65, 83–92. [Google Scholar] [CrossRef]
- Fu, L.S.; Ferreira, R.A.S.; Silva, N.J.O.; Carlos, L.D.; de Zea Bermudez, V.; Rocha, J. Photoluminescence and quantum yields of urea and urethane cross-linked nanohybrids derived from carboxylic acid solvolysis. Chem. Mater. 2004, 16, 1507–1516. [Google Scholar] [CrossRef]
- De Zea Bermudez, V.; Ferreira, R.A.S.; Carlos, L.D.; Molina, C.; Dahmouche, K.; Ribeiro, S.J.L. Coordination of Eu3+ ions in siliceous nanohybrids containing short polyether chains and bridging urea cross-links. J. Phys. Chem. B 2001, 105, 3378–3386. [Google Scholar] [CrossRef]
- Wang, L.Y.; Zhu, Y.; Du, C.X.; Ma, H.B. Novel supramolecular compounds with three-dimensional hydrogen-bonded network [M(H2O)6][H2L] (H4L=1,2,4,5-benzenetetracarboxylic acid, M=MnII and CoII): Syntheses, characterization and crystal structures. J. Mol. Struct. 2002, 610, 191–196. [Google Scholar] [CrossRef]
- De Zea Bermudez, V.; Alcácer, L.; Acosta, J.; Morales, E. Synthesis and characterization of novel urethane cross-linked ormolytes for solid-state lithium batteries. Solid State Ion. 1999, 116, 197–209. [Google Scholar] [CrossRef]
- Carlos, L.D.; Ferreira, R.A.S.; Orion, I.; de Zea Bermudez, V.; Rocha, J. Sol-gel derived nanocomposite hybrids for full color displays. J. Lumin. 2000, 87–89, 702–705. [Google Scholar] [CrossRef]
- Fu, L.S.; Ferreira, R.A.S.; Valente, A.; Rocha, J.; Carlos, L.D. Optically functional nanocomposites with poly(oxyethylene)-based di-ureasils and mesoporous MCM-41. Microporous Mesoporous Mater. 2006, 94, 185–192. [Google Scholar] [CrossRef]
- Fu, L.S.; Ferreira, R.A.S.; Fernandes, M.; Nunes, S.; de Zea Bermudez, V.; Hungerford, G.; Rocha, J.; Carlos, L.D. Photoluminescence and quantum yields of organic/inorganic hybrids prepared through formic acid solvolysis. Opt. Mater. 2008, 30, 1058–1064. [Google Scholar] [CrossRef]
- El Nahhal, I.M.; Chehimi, M.M.; Cordier, C.; Dodin, G. XPS, NMR and FT-IR structural characterization of polysiloxane-immobilized amine ligand systems. J. Non-Cryst. Solids 2000, 275, 142–146. [Google Scholar] [CrossRef]
- Irfanullah, M.; Iftikhar, K. The correlation between f–f absorption and sensitized visible light emission of luminescent Pr (III) complexes: Role of solvents and ancillary ligands on sensitivity. J. Fluoresc. 2011, 21, 673–686. [Google Scholar] [CrossRef] [PubMed]
- Dutra, J.D.L.; Bispo, T.D.; Freire, R.O. LUMPAC lanthanide luminescence software: Efficient and user friendly. J. Comput. Chem. 2014, 35, 772–775. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Zheng, Y.; Calvez, G.; Freslon, S.; Bernot, K.; Daiguebonne, C.; Roisnel, T.; Guillou, O. Synthesis, crystal structure and luminescent properties of new lanthanide-containing coordination polymers involving 4, 4′-oxy-bis-benzoate as ligand. CrystEngComm 2013, 15, 706–720. [Google Scholar] [CrossRef]
- Yang, T.H.; Fu, L.S.; Ferreira, R.A.S.; Nolasco, M.M.; Rocha, J.; Carlos, L.D.; Shi, F.N. Influence of the crystal structure on the luminescence properties of mixed Eu,La-(1,10-phenanthroline) complexes. Eur. J. Inorg. Chem. 2015, 29, 4861–4868. [Google Scholar] [CrossRef]
- Nolasco, M.M.; Vaz, P.M.; Freitas, V.T.; Lima, P.P.; André, P.S.; Ferreira, R.A.S.; Vaz, P.D.; Ribeiro-Claro, P.; Carlos, L.D. Engineering highly efficient Eu(III)-based tri-ureasil hybrids toward luminescent solar concentrators. J. Mater. Chem. A 2013, 1, 7339–7350. [Google Scholar] [CrossRef]
- Fernandes, M.; de Zea Bermudez, V.; Ferreira, R.A.S.; Carlos, L.D.; Charas, A.; Morgado, J.; Silva, M.M.; Smith, M.J. Highly photostable luminescent poly(ε-caprolactone)siloxane biohybrids doped with europium complexes. Chem. Mater. 2007, 19, 3892–3901. [Google Scholar] [CrossRef]
- Steemers, F.J.; Verboom, W.; Reinhoudt, D.N.; van der Tol, E.B.; Verhoeven, J.W. New sensitizer-modified calix[4]arenes enabling near-UV excitation of complexed luminescent lanthanide ions. J. Am. Chem. Soc. 1995, 117, 9408–9414. [Google Scholar] [CrossRef]
- Latva, M.; Takalo, H.; Mukkala, V.M.; Matachescu, C.; Rodríguez-Ubis, J.C.; Kankare, J. Correlation between the lowest triplet state energy level of the ligand and lanthanide(III) luminescence quantum yield. J. Lumin. 1997, 75, 149–169. [Google Scholar] [CrossRef]




| q | τ | η | kr | knr | nw | |||
|---|---|---|---|---|---|---|---|---|
| λx (nm) | 275 | 295 | 325 | 295 | ||||
| dU6Eu-1 | 35 ± 4 | 0.39 ± 0.04 | 26 ± 3 | 1.00 ± 0.05 | 0.48 | 0.487 | 0.518 | 0.2 |
| dU6Eu-2 | 48 ± 5 | 0.50 ± 0.05 | 40 ± 4 | 0.92 ± 0.01 | 0.43 | 0.465 | 0.626 | 0.4 |
| dU6Eu-3 | 12 ± 1 | 0.83 ± 0.01 | 0.37 | 0.441 | 0.762 | 0.5 | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, M.; Fu, L.; Correia, S.F.H.; Ferreira, R.A.S.; Carlos, L.D. Highly Efficient Luminescent Polycarboxylate Lanthanide Complexes Incorporated into Di-Ureasils by an In-Situ Sol—Gel Process. Polymers 2018, 10, 434. https://doi.org/10.3390/polym10040434
Fang M, Fu L, Correia SFH, Ferreira RAS, Carlos LD. Highly Efficient Luminescent Polycarboxylate Lanthanide Complexes Incorporated into Di-Ureasils by an In-Situ Sol—Gel Process. Polymers. 2018; 10(4):434. https://doi.org/10.3390/polym10040434
Chicago/Turabian StyleFang, Ming, Lianshe Fu, Sandra F. H. Correia, Rute A. S. Ferreira, and Luís D. Carlos. 2018. "Highly Efficient Luminescent Polycarboxylate Lanthanide Complexes Incorporated into Di-Ureasils by an In-Situ Sol—Gel Process" Polymers 10, no. 4: 434. https://doi.org/10.3390/polym10040434
APA StyleFang, M., Fu, L., Correia, S. F. H., Ferreira, R. A. S., & Carlos, L. D. (2018). Highly Efficient Luminescent Polycarboxylate Lanthanide Complexes Incorporated into Di-Ureasils by an In-Situ Sol—Gel Process. Polymers, 10(4), 434. https://doi.org/10.3390/polym10040434