Synthesis and Photovoltaic Properties of 2D-Conjugated Polymers Based on Alkylthiothienyl-Substituted Benzodithiophene and Different Accepting Units
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization
2.3. Device Fabrication
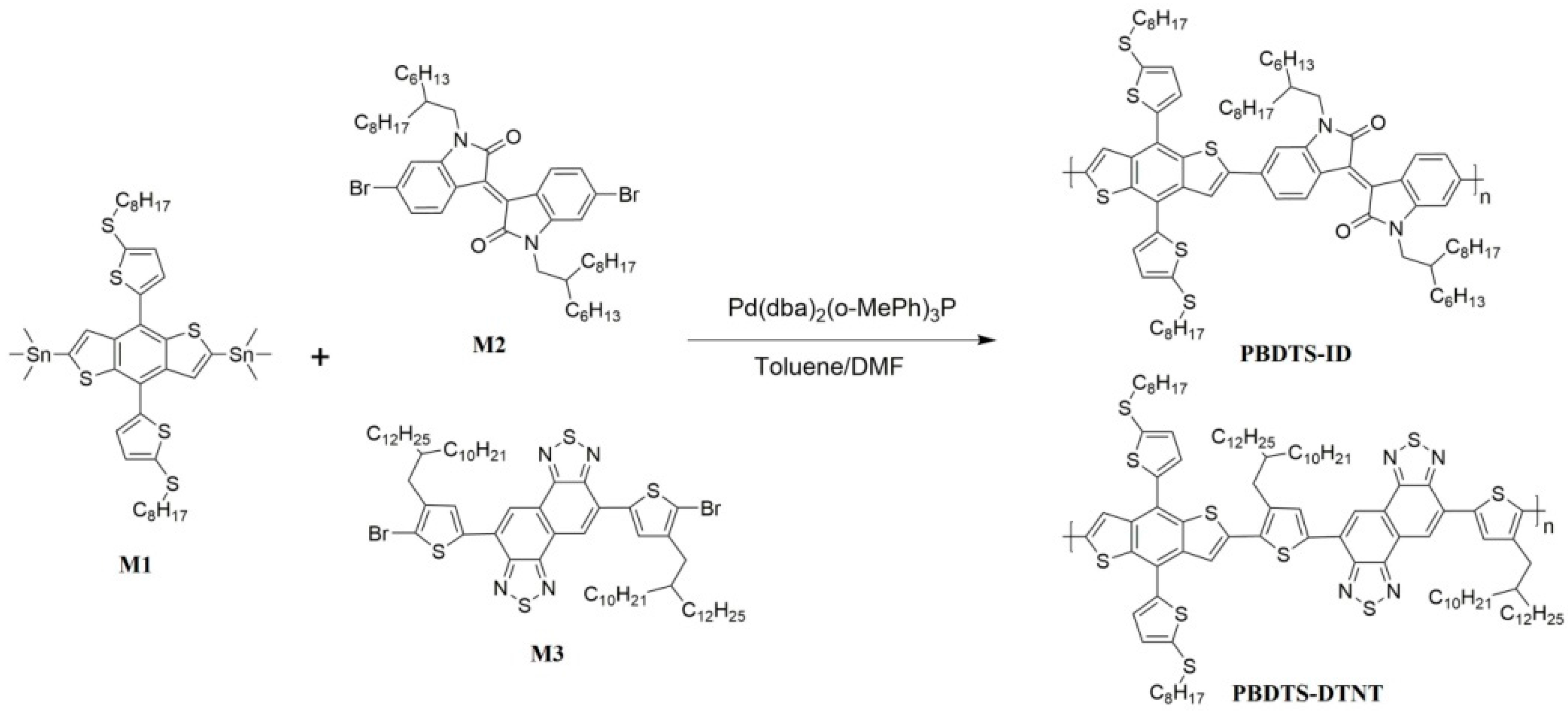
2.4. Polymer Synthesis
2.4.1. Synthesis of PBDTS-ID
2.4.2. Synthesis of PBDTS-DTNT
3. Results and Discussion
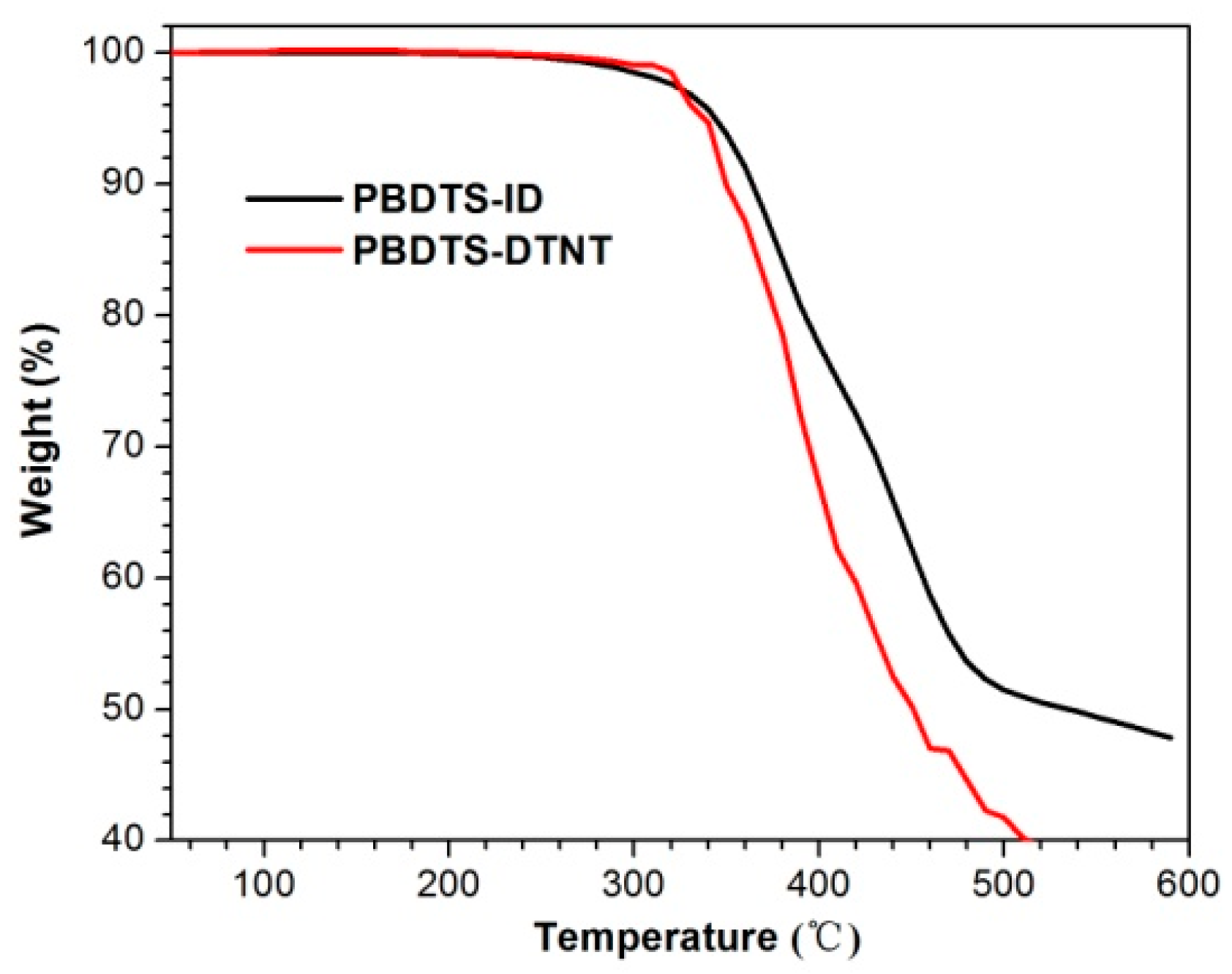
3.1. Synthesis and Characterization
3.2. Optical and Electrochemical Properties
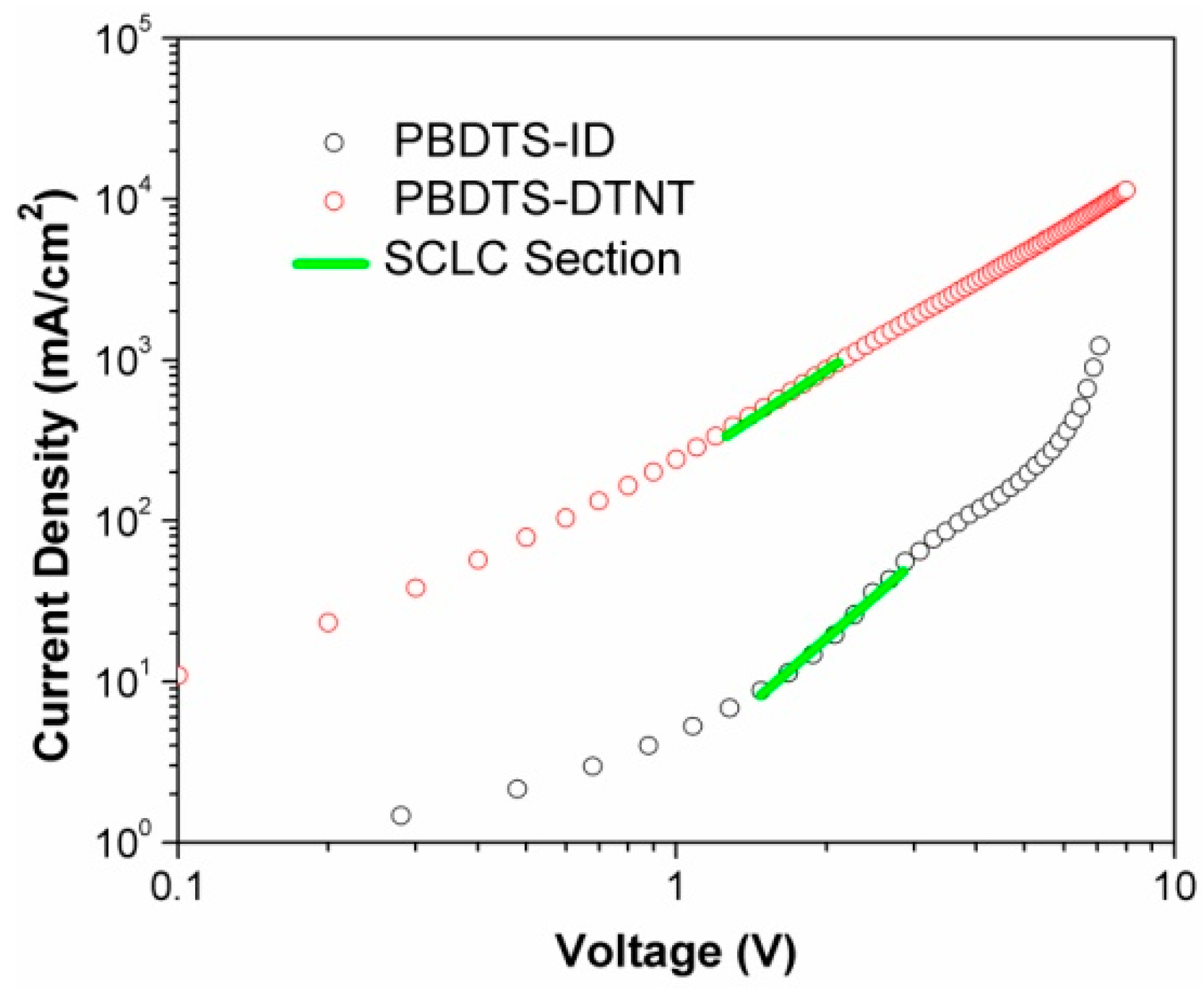
3.3. Hole Mobility
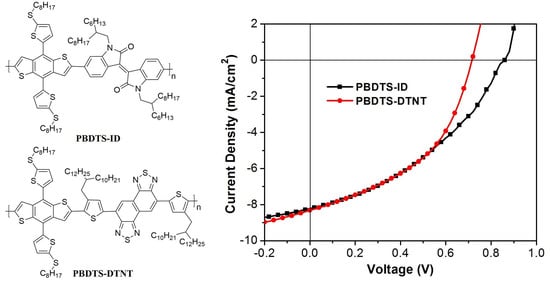
3.4. Photovoltaic Performance
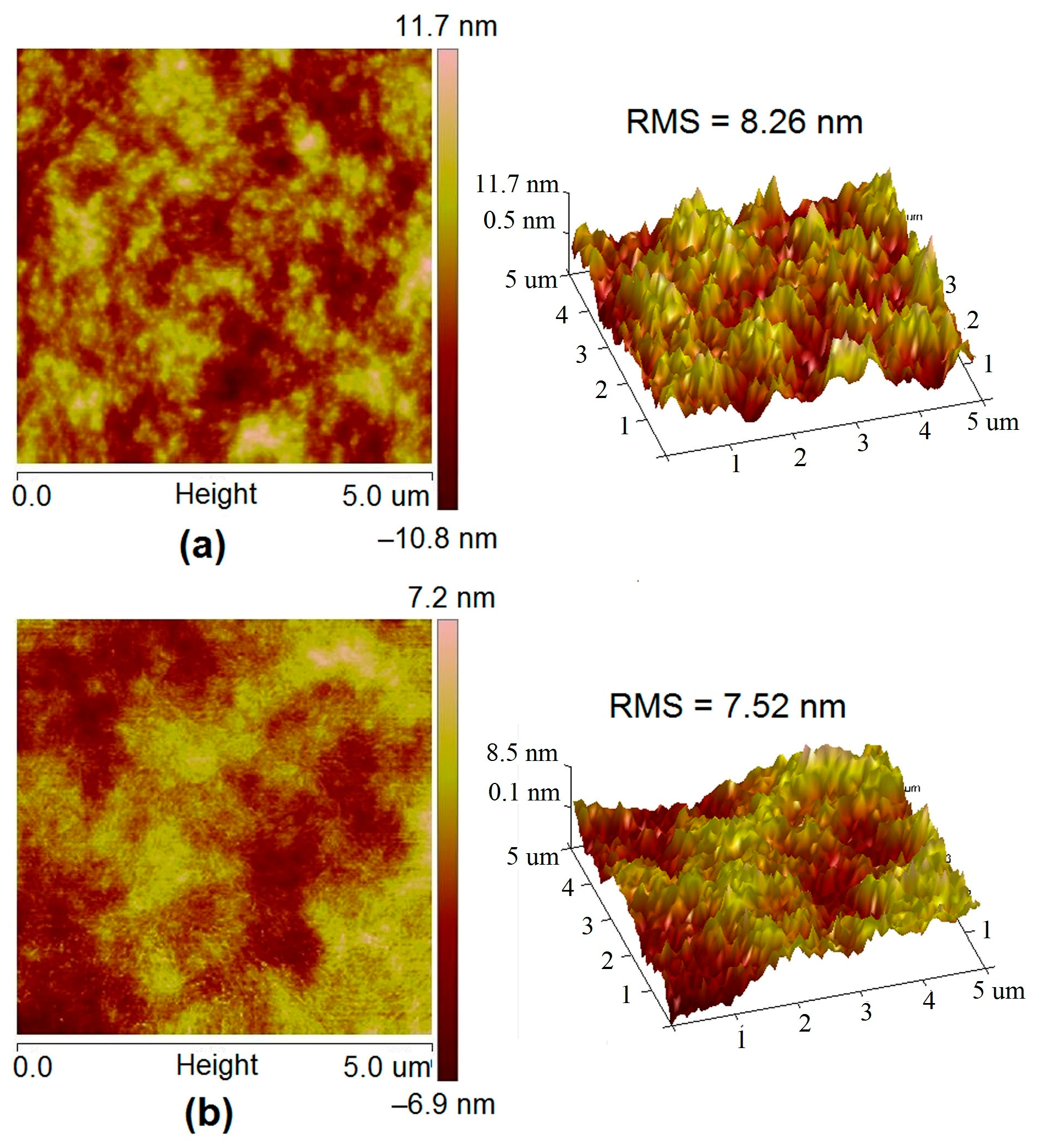
3.5. Morphology Study
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lu, L.Y.; Zheng, T.Y.; Wu, Q.H.; Schneider, A.M.; Zhao, D.L.; Yu, L.P. Recent advances in bulk heterojunction polymer solar cells. Chem. Rev. 2015, 115, 12666–12731. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.J.; Yang, S.H.; Hsu, C.S. Synthesis of conjugated polymers for organic solar cell applications. Chem. Rev. 2009, 109, 5868–5923. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.F.; Ye, L.; Zhang, H.; Li, S.S.; Zhang, S.Q.; Hou, J.H. Molecular design of benzodithiophene-based organic photovoltaic materials. Chem. Rev. 2016, 116, 7397–7457. [Google Scholar] [CrossRef] [PubMed]
- Wright, M.; Lin, R.; Tayebjee, M.J.Y.; Conibeer, G. Effect of blend composition on bulk heterojunction organic solar cells: A review. Sol. RRL 2017, 1, 31. [Google Scholar] [CrossRef]
- Cheng, P.; Zhan, X.W. Stability of organic solar cells: Challenges and strategies. Chem. Soc. Rev. 2016, 45, 2544–2582. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, C.B.; Holliday, S.; Chen, H.Y.; Cryer, S.J.; McCulloch, I. Non-fullerene electron acceptors for use in organic solar cells. Acc. Chem. Res. 2015, 48, 2803–2812. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.S.; Cheng, S.W.; Cheng, Y.J.; Hsu, C.S. Donor-acceptor conjugated polymers based on multifused ladder-type arenes for organic solar cells. Chem. Soc. Rev. 2015, 44, 1113–1154. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.C.; Li, S.S.; Yao, H.F.; Zhang, S.Q.; Zhang, Y.; Yang, B.; Hou, J.H. Molecular optimization enables over 13% efficiency in organic solar cells. J. Am. Chem. Soc. 2017, 139, 7148–7151. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.M.; Raghunath, P.; Lin, Y.C.; Lin, H.K.; Ko, C.L.; Su, Y.W.; Lin, M.C.; Wei, K.H. Linear solubilizing side chain substituents enhance the photovoltaic properties of two-dimensional conjugated benzodithiophene-based polymers. Polymer 2015, 79, 262–270. [Google Scholar] [CrossRef]
- Kim, J.H.; Park, J.B.; Yoon, S.C.; Jung, I.H.; Hwang, D.H. Enhanced and controllable open-circuit voltage using 2D-conjugated benzodithiophene (BDT) homopolymers by alkylthio substitution. J. Mater. Chem. C 2016, 4, 2170–2177. [Google Scholar] [CrossRef]
- Su, Q.; Tong, J.F.; Li, J.F.; Zhang, P.; Yang, C.Y.; Zhang, C.Z.; Wang, F.; Chen, D.J.; Xia, Y.J. Synthesis and characterization of alternating and random conjugated polymers derived from dithieno[2,3-d:2′,3′-d′]benzo[1,2-b:4,5-b']dithiophene and 2,1,3-benzothiadiazole derivatives. Polym. J. 2015, 47, 803–809. [Google Scholar] [CrossRef]
- Tong, J.F.; Li, M.J.; An, L.L.; Li, J.F.; Xu, X.H.; Zhang, P.; Yang, C.Y.; Wang, F.; Xia, Y.J. Synthesis of π–extended dithienobenzodithiophene-containing medium bandgap copolymers and their photovoltaic application. J. Macromol. Sci. Part A-Pure Appl. Chem. 2015, 52, 934–941. [Google Scholar] [CrossRef]
- Wang, X.C.; Su, Q.; Li, Y.D.; Cheng, C.; Xia, Y.J.; He, L.Y.; Li, H.; Shu, G.; Wang, F. Synthesis and photovoltaic properties of donor-acceptor conjugated polymers based on 4,7-dithienyl-2,1,3-benzothiadiazole functionalized silole. Synth. Met. 2016, 220, 433–439. [Google Scholar] [CrossRef]
- Wang, X.C.; Tong, J.H.; Guo, P.Z.; Li, Y.D.; Li, H.; Xia, Y.J.; Wang, F. Low-bandgap conjugated polymers based on alkylthiothienyl-substituted benzodithiophene for efficient bulk heterojunction polymer solar cells. Polymer 2017, 122, 96–104. [Google Scholar] [CrossRef]
- Yao, H.F.; Zhao, W.C.; Zheng, Z.; Cui, Y.; Zhang, J.Q.; Wei, Z.X.; Hou, J.H. PBDT-TSR: A highly efficient conjugated polymer for polymer solar cells with a regioregular structure. J. Mater. Chem. A 2016, 4, 1708–1713. [Google Scholar] [CrossRef]
- Ye, L.; Zhang, S.Q.; Huo, L.J.; Zhang, M.J.; Hou, J.H. Molecular design toward highly efficient photovoltaic polymers based on two-dimensional conjugated benzodithiophene. Acc. Chem. Res. 2014, 47, 1595–1603. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Zhang, S.Q.; Zhao, W.C.; Yao, H.F.; Hou, J.H. Highly efficient 2D-conjugated benzodithiophene-based photovoltaic polymer with linear alkylthio side chain. Chem. Mater. 2014, 26, 3603–3605. [Google Scholar] [CrossRef]
- Yu, T.; Xu, X.P.; Li, Y.; Li, Z.J.; Peng, Q. Side-chain influence of wide-bandgap copolymers based on naphtho[1,2-b:5,6-b]bispyrazine and benzo[1,2-b:4,5-b′]dithiophene for efficient photovoltaic applications. ACS Appl. Mater. Interfaces 2017, 9, 18142–18150. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.W.; Chen, L.X.; Wang, G.; Zhang, Z.G.; Li, Y.F.; Shen, P. Synthesis and photovoltaic properties of alkylthiothienyl-substituted benzo[1,2-b:4,5-b′]dithiophene D-A copolymers with different accepting units. Synth. Met. 2016, 211, 121–131. [Google Scholar] [CrossRef]
- Zhu, E.W.; Ge, G.D.; Shu, J.K.; Yi, M.D.; Bian, L.Y.; Hai, J.F.; Yu, J.S.; Liu, Y.; Zhou, J.; Tang, W.H. Direct access to 4,8-functionalized benzo[1,2-b:4,5-b′]dithiophenes with deep low-lying HOMO levels and high mobilities. J. Mater. Chem. A 2014, 2, 13580–13586. [Google Scholar] [CrossRef]
- Cao, K.C.; Wu, Z.W.; Li, S.G.; Sun, B.Q.; Zhang, G.B.; Zhang, Q. A low bandgap polymer based on isoindigo and bis(dialkylthienyl)benzodithiophene for organic photovoltaic applications. J. Polym. Sci. Pol. Chem. 2013, 51, 94–100. [Google Scholar] [CrossRef]
- Zhang, M.J.; Guo, X.; Zhang, S.Q.; Hou, J.H. Synergistic effect of fluorination on molecular energy level modulation in highly efficient photovoltaic polymers. Adv. Mater. 2014, 26, 1118–1123. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.C.; Xiao, M.J.; Han, L.L.; Zhang, J.D.; Jiang, H.X.; Gu, C.T.; Shen, W.F.; Yang, R.Q. Unsubstituted benzodithiophene-based conjugated polymers for high-performance organic field-effect transistors and organic solar cells. ACS Appl. Mater. Interfaces 2016, 8, 19665–19671. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.Y.; Zhu, Q.Q.; Gu, C.Y.; Wang, J.Y.; Qiu, M.; Chen, W.C.; Bao, X.C.; Sun, M.L.; Yang, R.Q. High-performance photovoltaic polymers employing symmetry-breaking building blocks. Adv. Mater. 2016, 28, 8490–8498. [Google Scholar] [CrossRef] [PubMed]
- Kan, B.; Zhang, Q.; Li, M.M.; Wan, X.J.; Ni, W.; Long, G.K.; Wang, Y.C.; Yang, X.; Feng, H.R.; Chen, Y.S. Solution-processed organic solar cells based on dialkylthiol-substituted benzodithiophene unit with efficiency near 10%. J. Am. Chem. Soc. 2014, 136, 15529–15532. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.F.; Li, Z.J.; Zhang, W.; Meng, X.Y.; Zou, X.S.; Rasi, D.D.; Ma, W.; Yartsev, A.; Andersson, M.R.; Janssen, R.A.J.; et al. 8.0% efficient all-polymer solar cells with high photovoltage of 1.1 v and internal quantum efficiency near unity. Adv. Energy Mater. 2018, 8, 11. [Google Scholar] [CrossRef]
- Lu, L.Y.; Yu, L.P. Understanding low bandgap polymer PTB7 and optimizing polymer solar cells based on it. Adv. Mater. 2014, 26, 4413–4430. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Stone, S.W.; Ferraris, J.P. A novel dialkylthiobenzo[1,2-b:4,5-b′]dithiophene derivative for high open-circuit voltage in polymer solar cells. Chem. Commun. 2011, 47, 10987–10989. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Guo, X.; Liu, F.; Huo, L.J.; Chen, Y.N.; Russell, T.P.; Han, C.C.; Li, Y.F.; Hou, J.H. Improving the ordering and photovoltaic properties by extending π–conjugated area of electron-donating units in polymers with D-A structure. Adv. Mater. 2012, 24, 3383–3389. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.H.; Wong, W.Y.; Li, Y.F. Improvement of open-circuit voltage and photovoltaic properties of 2D-conjugated polymers by alkylthio substitution. Energy Environ. Sci. 2014, 7, 2276–2284. [Google Scholar] [CrossRef]
- Liu, T.; Pan, X.X.; Meng, X.Y.; Liu, Y.; Wei, D.H.; Ma, W.; Huo, L.J.; Sun, X.B.; Lee, T.H.; Huang, M.; et al. Alkyl side-chain engineering in wide-bandgap copolymers leading to power conversion efficiencies over 10%. Adv. Mater. 2017, 29, 7. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.C.; Chen, C.A.; Chang, C.Y.; Darling, S.B.; Su, W.F. Isoindigo-based copolymers for polymer solar cells with efficiency over 7%. J. Mater. Chem. A 2014, 2, 8026–8032. [Google Scholar] [CrossRef]
- Jung, I.H.; Kim, J.H.; Nam, S.Y.; Lee, C.; Hwang, D.H.; Yoon, S.C. A di(1-benzothieno)[3,2-b:2′,3′-d]pyrrole and isoindigo-based electron donating conjugated polymer for efficient organic photovoltaics. J. Mater. Chem. C 2016, 4, 663–667. [Google Scholar] [CrossRef]
- Lei, T.; Wang, J.Y.; Pei, J. Design, synthesis, and structure-property relationships of isoindigo-based conjugated polymers. Acc. Chem. Res. 2014, 47, 1117–1126. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.C.; Dong, S.; Cai, P.; Liu, P.; Liu, S.J.; Chen, J.W.; Liu, F.; Ying, L.; Russell, T.P.; Huang, F.; et al. Donor-acceptor copolymers based on thermally cleavable indigo, isoindigo, and DPP units: Synthesis, field effect transistors, and polymer solar cells. ACS Appl. Mater. Interfaces 2015, 7, 9038–9051. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.F.; Dang, D.F.; Tang, Z.; Gedefaw, D.; Bergqvist, J.; Zhu, W.G.; Mammo, W.; Andersson, M.R.; Inganas, O.; Zhang, F.L.; et al. A facile method to enhance photovoltaic performance of benzodithiophene-isoindigo polymers by inserting bithiophene spacer. Adv. Energy Mater. 2014, 4. [Google Scholar] [CrossRef]
- Stalder, R.; Grand, C.; Subbiah, J.; So, F.; Reynolds, J.R. An isoindigo and dithieno[3,2-b:2′,3′-d]silole copolymer for polymer solar cells. Polym. Chem. 2012, 3, 89–92. [Google Scholar] [CrossRef]
- Tong, J.F.; An, L.L.; Li, J.F.; Zhang, P.; Guo, P.Z.; Yang, C.Y.; Su, Q.; Wang, X.C.; Xia, Y.J. Large branched alkylthienyl bridged naphtho[1,2-c:5,6-c′]bis[1,2,5]thiadiazole-containing low bandgap copolymers: Synthesis and photovoltaic application. J. Macromol. Sci. Part A Pure Appl. Chem. 2017, 54, 176–185. [Google Scholar] [CrossRef]
- Tong, J.F.; Li, J.F.; Zhang, P.; Ma, X.Y.; Wang, M.; An, L.L.; Sun, J.B.; Guo, P.Z.; Yang, C.Y.; Xia, Y.J. Naphtho[1,2-c:5,6-c′]bis[1,2,5]thiadiazole-based conjugated polymers consisting of oligothiophenes for efficient polymer solar cells. Polymer 2017, 121, 183–195. [Google Scholar] [CrossRef]
- Wang, E.G.; Mammo, W.; Andersson, M.R. 25th anniversary article: Isoindigo-based polymers and small molecules for bulk heterojunction solar cells and field effect transistors. Adv. Mater. 2014, 26, 1801–1826. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.G.; Zhao, J.; Li, Y.; Peng, Q. Low band-gap copolymers derived from fluorinated isoindigo and dithienosilole: Synthesis, properties and photovoltaic applications. Polym. Chem. 2014, 5, 4984–4992. [Google Scholar] [CrossRef]
- Zheng, Y.Q.; Wang, Z.; Dou, J.H.; Zhang, S.D.; Luo, X.Y.; Yao, Z.F.; Wang, J.Y.; Pei, J. Effect of halogenation in isoindigo-based polymers on the phase separation and molecular orientation of bulk heterojunction solar cells. Macromolecules 2015, 48, 5570–5577. [Google Scholar] [CrossRef]
- Chatterjee, S.; Ie, Y.; Karakawa, M.; Aso, Y. Naphtho[1,2-c:5,6-c′]bis[1,2,5]thiadiazole-containing π–conjugated compound: Nonfullerene electron acceptor for organic photovoltaics. Adv. Funct. Mater. 2016, 26, 1161–1168. [Google Scholar] [CrossRef]
- Jin, Y.C.; Chen, Z.M.; Xiao, M.J.; Peng, J.J.; Fan, B.B.; Ying, L.; Zhang, G.C.; Jiang, X.F.; Yin, Q.W.; Liang, Z.Q.; et al. Thick film polymer solar cells based on naphtho[1,2-c:5,6-c]bis[1,2,5]thiadiazole conjugated polymers with efficiency over 11%. Adv. Energy Mater. 2017, 7. [Google Scholar] [CrossRef]
- Li, W.; Li, Q.D.; Liu, S.J.; Duan, C.H.; Ying, L.; Huang, F.; Cao, Y. Synthesis of two-dimensional π–conjugated polymers pendent with benzothiadiazole and naphtho[1,2-c:5,6-c]bis[1,2,5]thiadiazole moieties for polymer solar cells. Sci. China Chem. 2015, 58, 257–266. [Google Scholar] [CrossRef]
- Liu, L.Q.; Zhang, G.C.; He, B.T.; Huang, F. Polymer solar cells based on the copolymers of naphtho[1,2-c:5,6-c]bis(1,2,5-thiadiazole) and alkoxylphenyl substituted benzodithiophene with high open-circuit voltages. Chin. J. Chem. 2015, 33, 902–908. [Google Scholar] [CrossRef]
- Liu, L.Q.; Zhang, G.C.; Liu, P.; Zhang, J.; Dong, S.; Wang, M.; Ma, Y.G.; Yip, H.L.; Huang, F. Donor-acceptor-type copolymers based on a naphtho[1,2-c:5,6-c]bis(1,2,5-thiadiazole) scaffold for high-efficiency polymer solar cells. Chem. -Asian J. 2014, 9, 2104–2112. [Google Scholar] [CrossRef] [PubMed]
- Osaka, I.; Shimawaki, M.; Mori, H.; Doi, I.; Miyazaki, E.; Koganezawa, T.; Takimiya, K. Synthesis, characterization, and transistor and solar cell applications of a naphthobisthiadiazole-based semiconducting polymer. J. Am. Chem. Soc. 2012, 134, 3498–3507. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.M.; Seifter, J.; Wang, M.; Perez, L.A.; Luo, C.; Bazan, G.C.; Huang, F.; Cao, Y.; Heeger, A.J. Effect of molecular order on the performance of naphthobisthiadiazole-based polymer solar cells. Adv. Energy Mater. 2014, 4. [Google Scholar] [CrossRef]
- Wang, M.; Hu, X.W.; Liu, P.; Li, W.; Gong, X.; Huang, F.; Cao, Y. Donor acceptor conjugated polymer based on naphtho[1,2-c:5,6-c]bis[1,2,5]thiadiazole for high-performance polymer solar cells. J. Am. Chem. Soc. 2011, 133, 9638–9641. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, T.; Shinohara, Y.; Kusagaki, Y.; Matsuda, T.; Han, L.; Ishi-I, T. Synthesis and photovoltaic properties of naphthobisthiadiazole-triphenylamine-based donor-acceptor π–conjugated polymer. Polymer 2015, 58, 139–145. [Google Scholar] [CrossRef]
- Zhang, L.H.; Pei, K.; Yu, M.D.; Huang, Y.L.; Zhao, H.B.; Zeng, M.; Wang, Y.; Gao, J.W. Theoretical investigations on donor-acceptor conjugated copolymers based on naphtho[1,2-c:5,6-c]bis[1,2,5]thiadiazole for organic solar cell applications. J. Phys. Chem. C 2012, 116, 26154–26161. [Google Scholar] [CrossRef]
- Zhu, X.W.; Fang, J.; Lu, K.; Zhang, J.Q.; Zhu, L.Y.; Zhao, Y.F.; Shuai, Z.G.; Wei, Z.X. Naphtho[1,2-b:5,6-b′]dithiophene based two-dimensional conjugated polymers for highly efficient thick-film inverted polymer solar cells. Chem. Mater. 2014, 26, 6947–6954. [Google Scholar] [CrossRef]
- Li, Y.D.; Zhang, H.; Wang, X.C.; Wang, F.; Xia, Y.J. Synthesis and photovoltaic properties of silole-containing conjugated polymers. Acta Chim. Sin. 2015, 73, 1055–1060. [Google Scholar]
- Liu, T.F.; Jiang, F.Y.; Tong, J.H.; Qin, F.; Meng, W.; Jiang, Y.Y.; Li, Z.F.; Zhou, Y.H. Reduction and oxidation of poly(3,4-ethylenedioxythiophene):Poly(styrenesulfonate) induced by methylamine (CH3NH2)–containing atmosphere for perovskite solar cells. J. Mater. Chem. A 2016, 4, 4305–4311. [Google Scholar] [CrossRef]
- Zhang, S.Q.; Uddin, M.A.; Zhao, W.C.; Ye, L.; Woo, H.Y.; Liu, D.L.; Yang, B.; Yao, H.F.; Cui, Y.; Hou, J.H. Optimization of side chains in alkylthiothiophene-substituted benzo[1,2-b:4,5-b']dithiophene-based photovoltaic polymers. Polym. Chem. 2015, 6, 2752–2760. [Google Scholar] [CrossRef]




| Polymers | λonset (nm) | Egopt (eV) 1 | EHOMO (eV) 2 | ELUMO (eV) 3 |
|---|---|---|---|---|
| PBDTS-ID | 803 | 1.54 | −5.40 | −3.86 |
| PBDTS-DTNT | 842 | 1.47 | −5.24 | −3.77 |
| Polymers | μ (×10−5, cm2·V−1·S−1) | JSC (mA·cm−2) | VOC (V) | FF (%) | PCE (%) |
|---|---|---|---|---|---|
| PBDTS-ID | 3.83 | 8.21 | 0.84 | 39.2 | 2.70 |
| PBDTS-DTNT | 8.36 | 8.31 | 0.72 | 45.8 | 2.71 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Cheng, C.; Li, Y.; Wang, F. Synthesis and Photovoltaic Properties of 2D-Conjugated Polymers Based on Alkylthiothienyl-Substituted Benzodithiophene and Different Accepting Units. Polymers 2018, 10, 331. https://doi.org/10.3390/polym10030331
Wang X, Cheng C, Li Y, Wang F. Synthesis and Photovoltaic Properties of 2D-Conjugated Polymers Based on Alkylthiothienyl-Substituted Benzodithiophene and Different Accepting Units. Polymers. 2018; 10(3):331. https://doi.org/10.3390/polym10030331
Chicago/Turabian StyleWang, Xunchang, Chang Cheng, Yuda Li, and Feng Wang. 2018. "Synthesis and Photovoltaic Properties of 2D-Conjugated Polymers Based on Alkylthiothienyl-Substituted Benzodithiophene and Different Accepting Units" Polymers 10, no. 3: 331. https://doi.org/10.3390/polym10030331
APA StyleWang, X., Cheng, C., Li, Y., & Wang, F. (2018). Synthesis and Photovoltaic Properties of 2D-Conjugated Polymers Based on Alkylthiothienyl-Substituted Benzodithiophene and Different Accepting Units. Polymers, 10(3), 331. https://doi.org/10.3390/polym10030331