Thermal Stability and Water Content Study of Void-Free Electrospun SPEEK/Cloisite Membrane for Direct Methanol Fuel Cell Application
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of Sulfonated Poly (Ether Ether Ketone) (SPEEK)
2.3. Preparation of Electrospinning Dope Solution
2.4. Electrospinning of Nanofibers
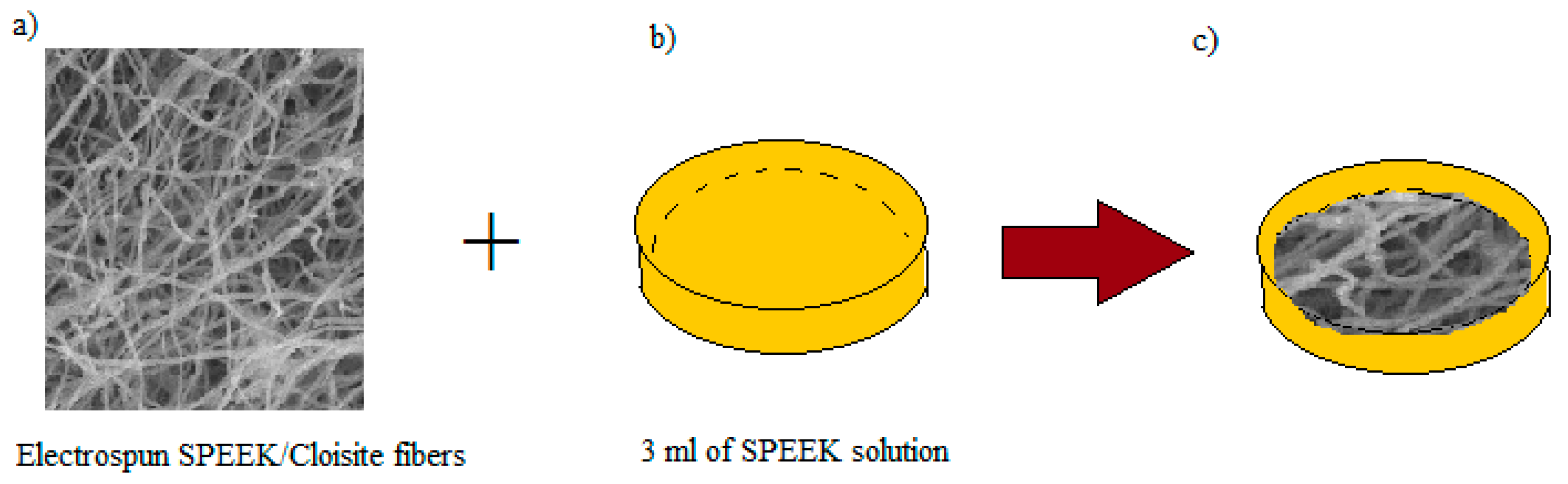
2.5. Preparation of Void-Free SP/e-spun Cloisite Membrane
2.6. Thermogravimetric Analysis (TGA)
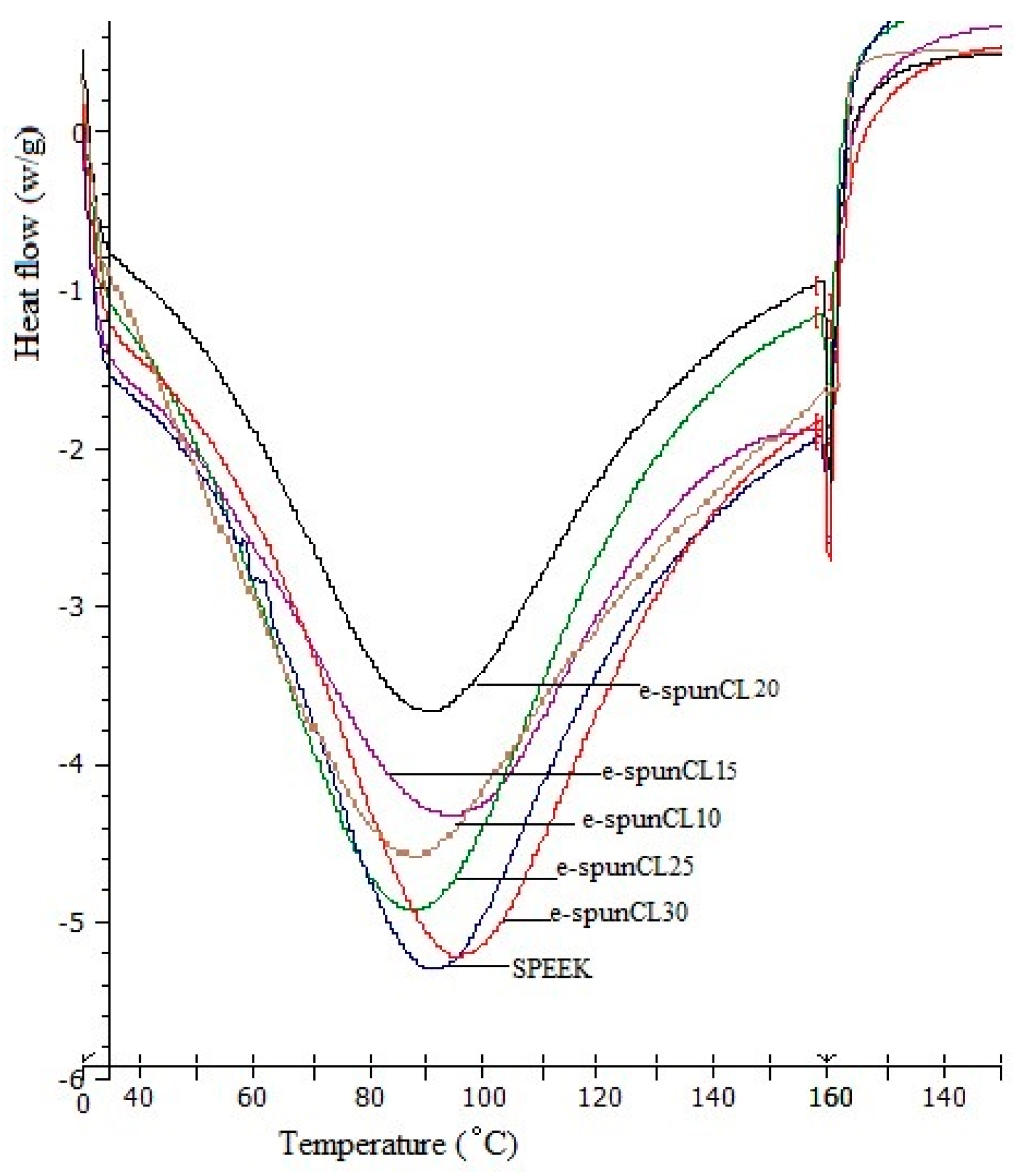
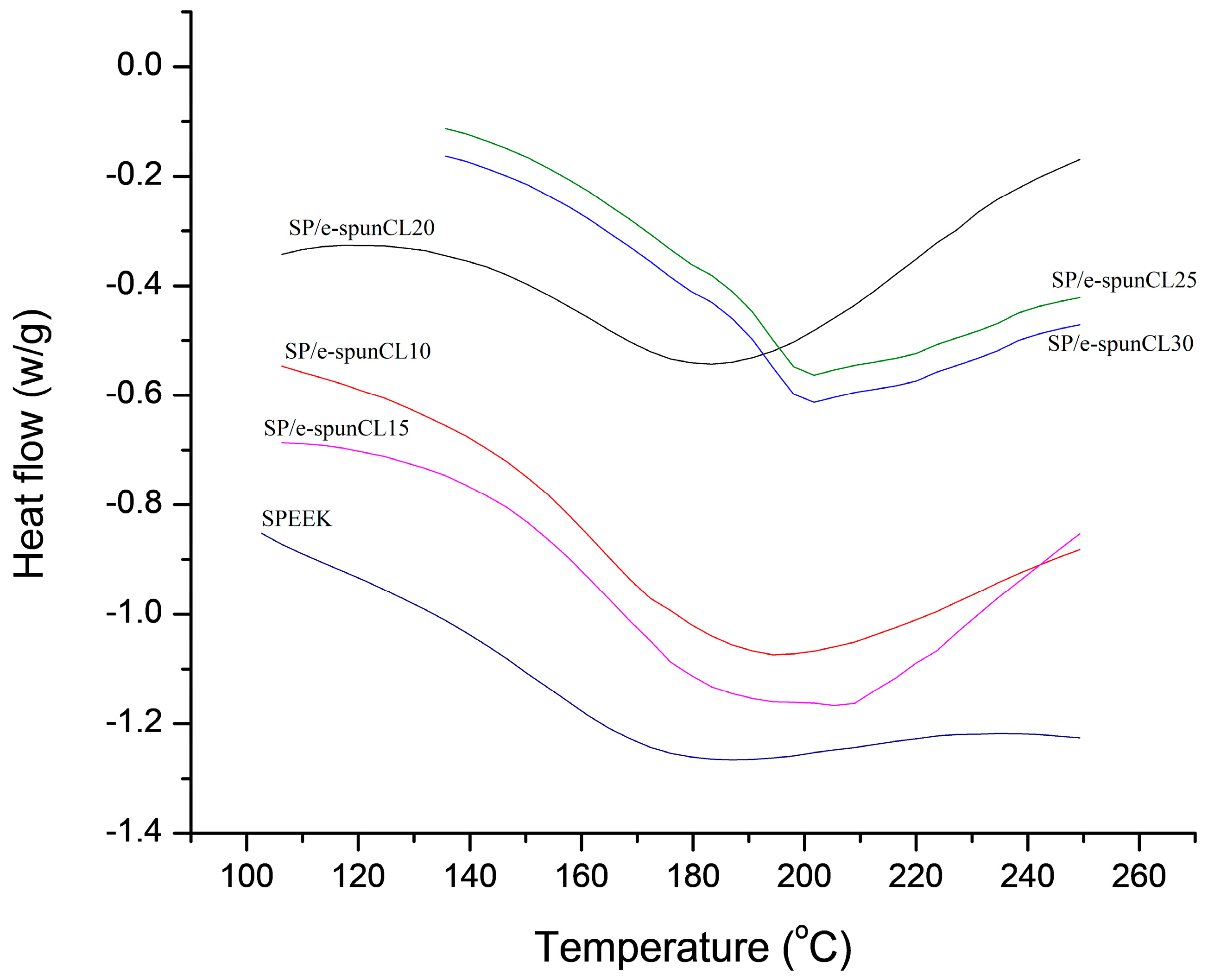
2.7. Differential Scanning Calorimetry (DSC) Analysis
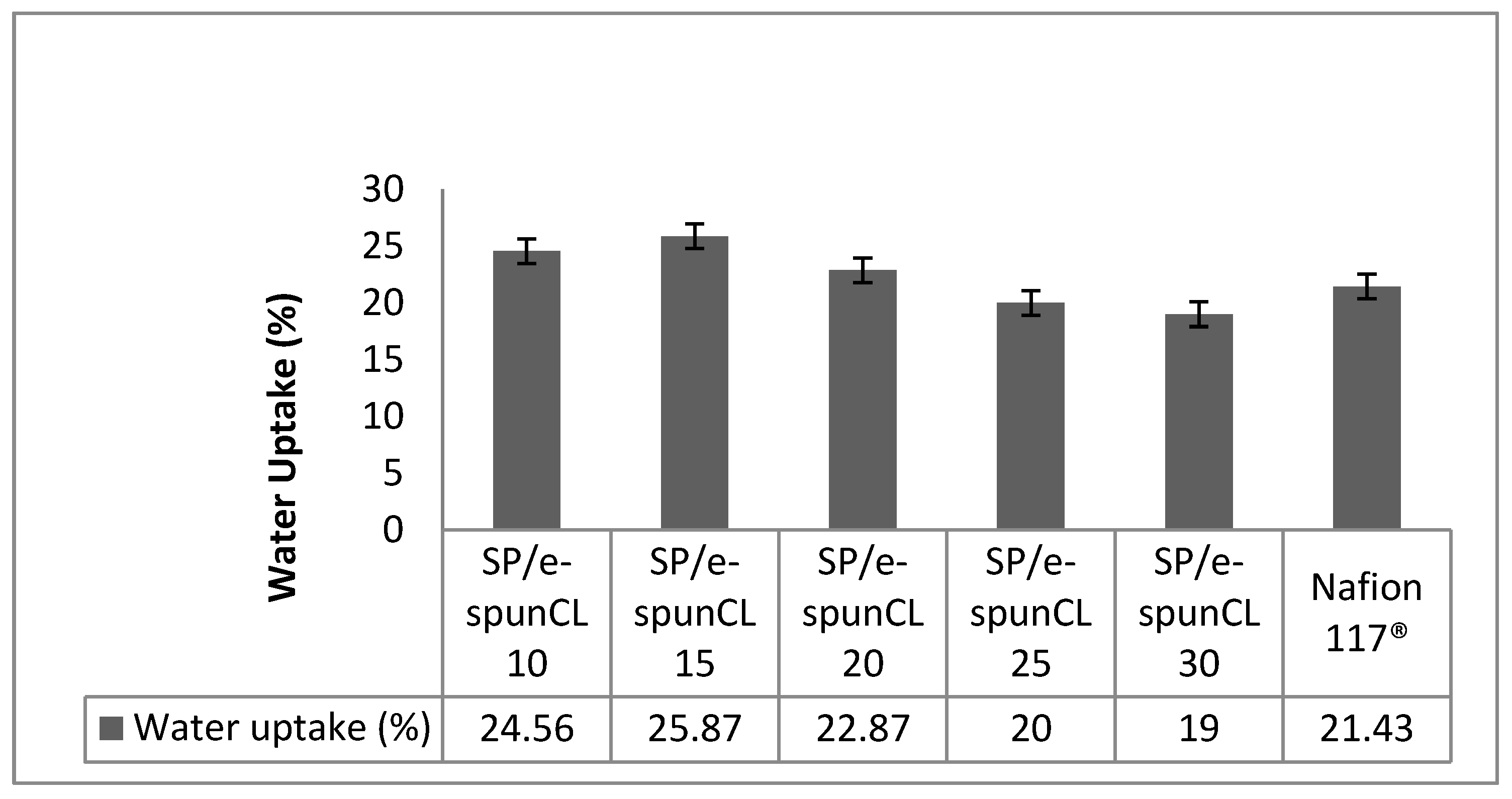
2.8. Water Uptake Measurement
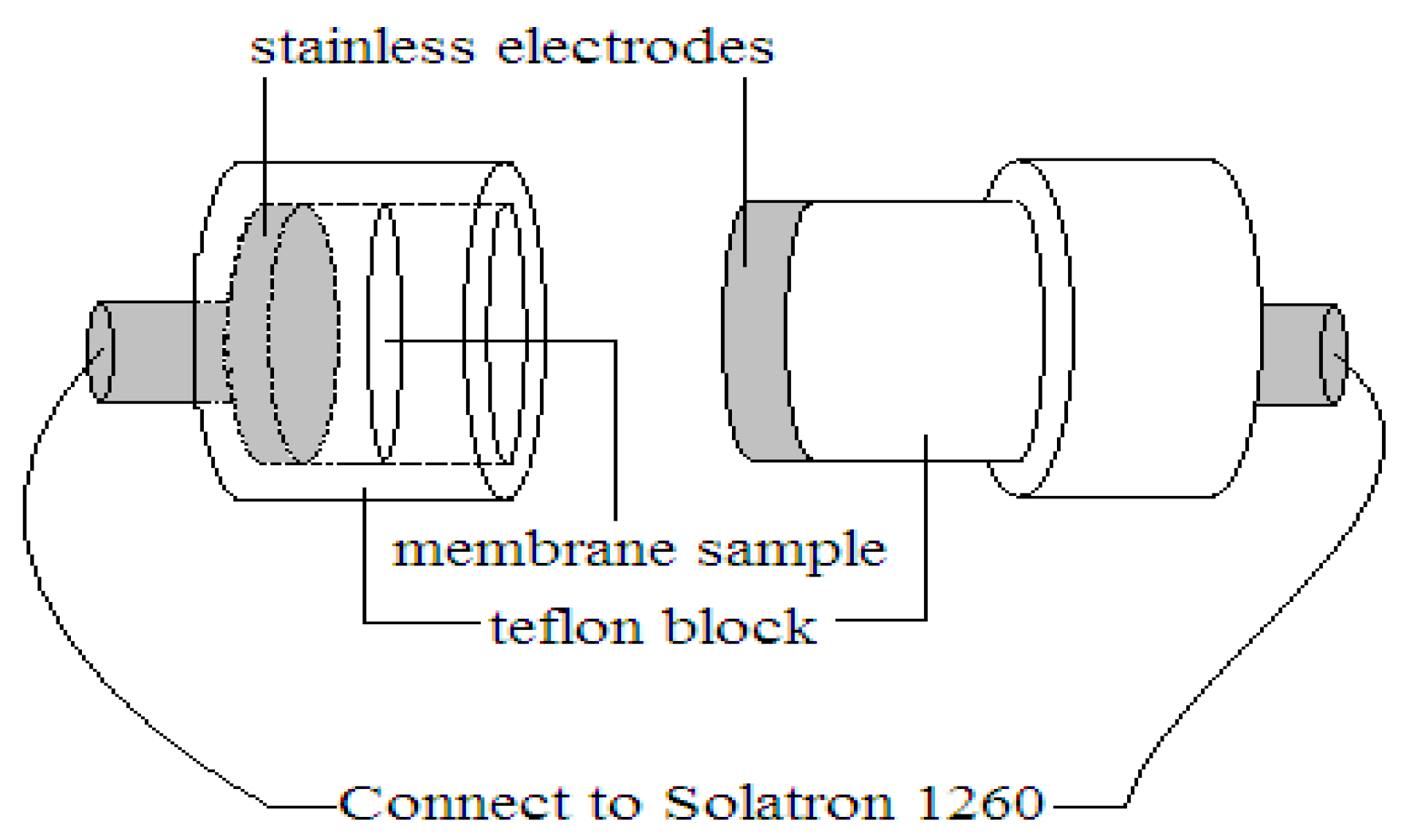
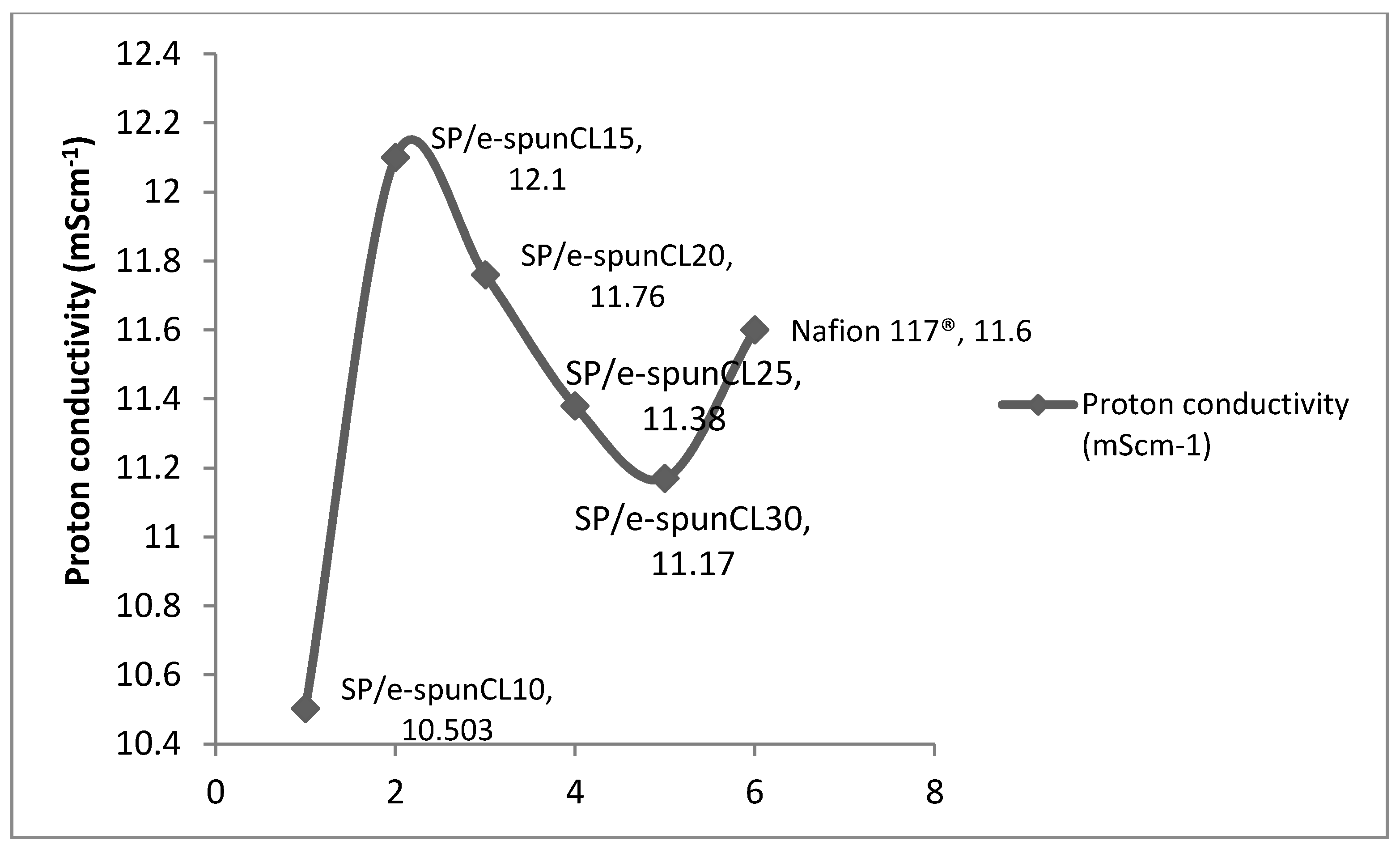
2.9. Proton Conductivity Measurement
- ∂ = proton conductivity (S·cm−1)
- d = membrane thickness (cm)
- R = resistance (ohm) (the value was derived from the low intersection of the high frequency semi-circle on a complex impedance plane with the Re (Z) axis)
- S = membrane cross section area (cm2)
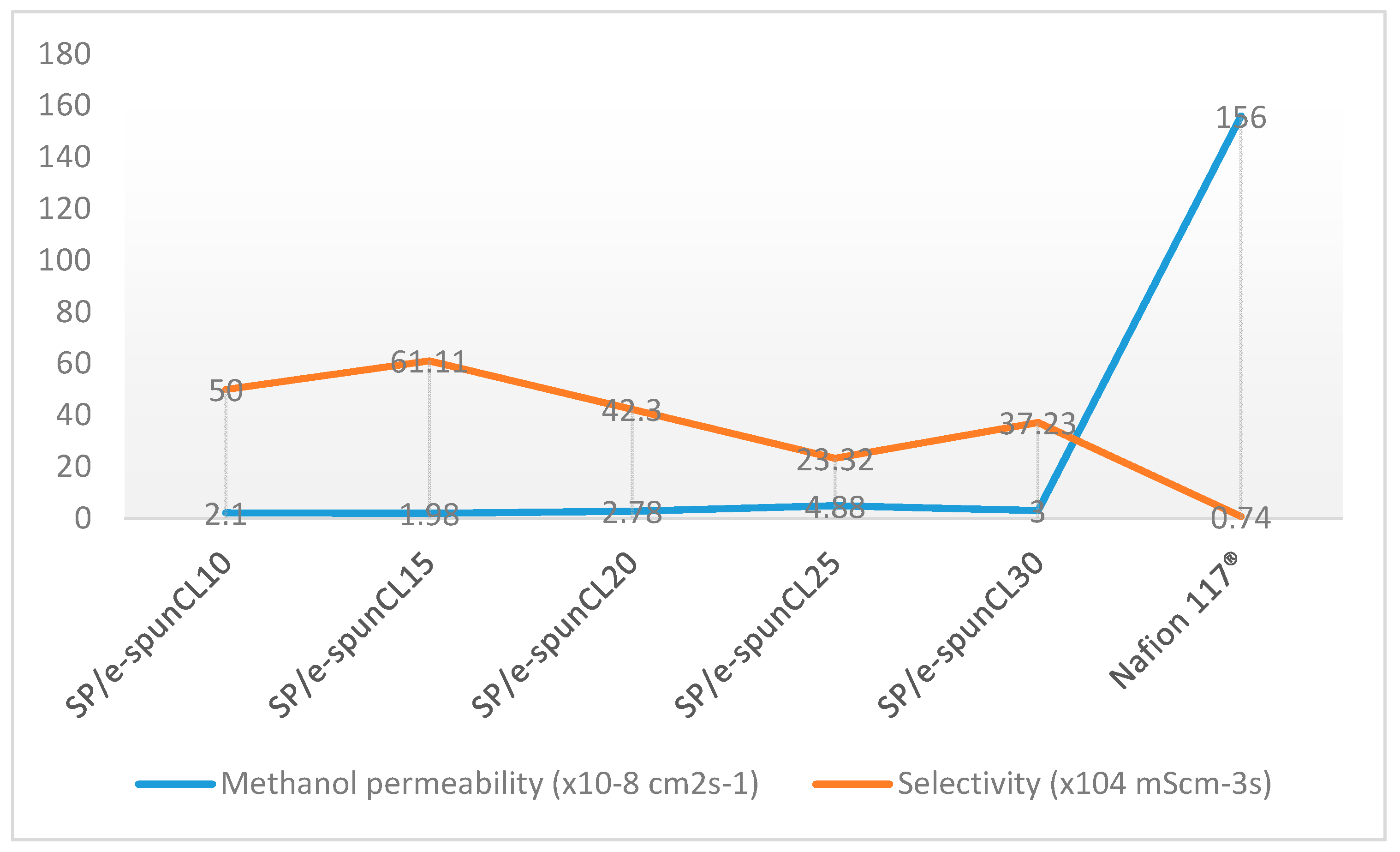
2.10. Methanol Permeability Measurement
- CB (t) = concentration of methanol in compartment B at time, t (M)
- to = time lag, related to the diffusivity (s)
- VB = volume of water in compartment B (cm3) = 200 cm3
- A = membrane cross-section area (cm2)
- L = membrane thickness (cm)
- CA = concentration of methanol in compartment A at time, t (M) = 1 M
2.11. Tensile Test
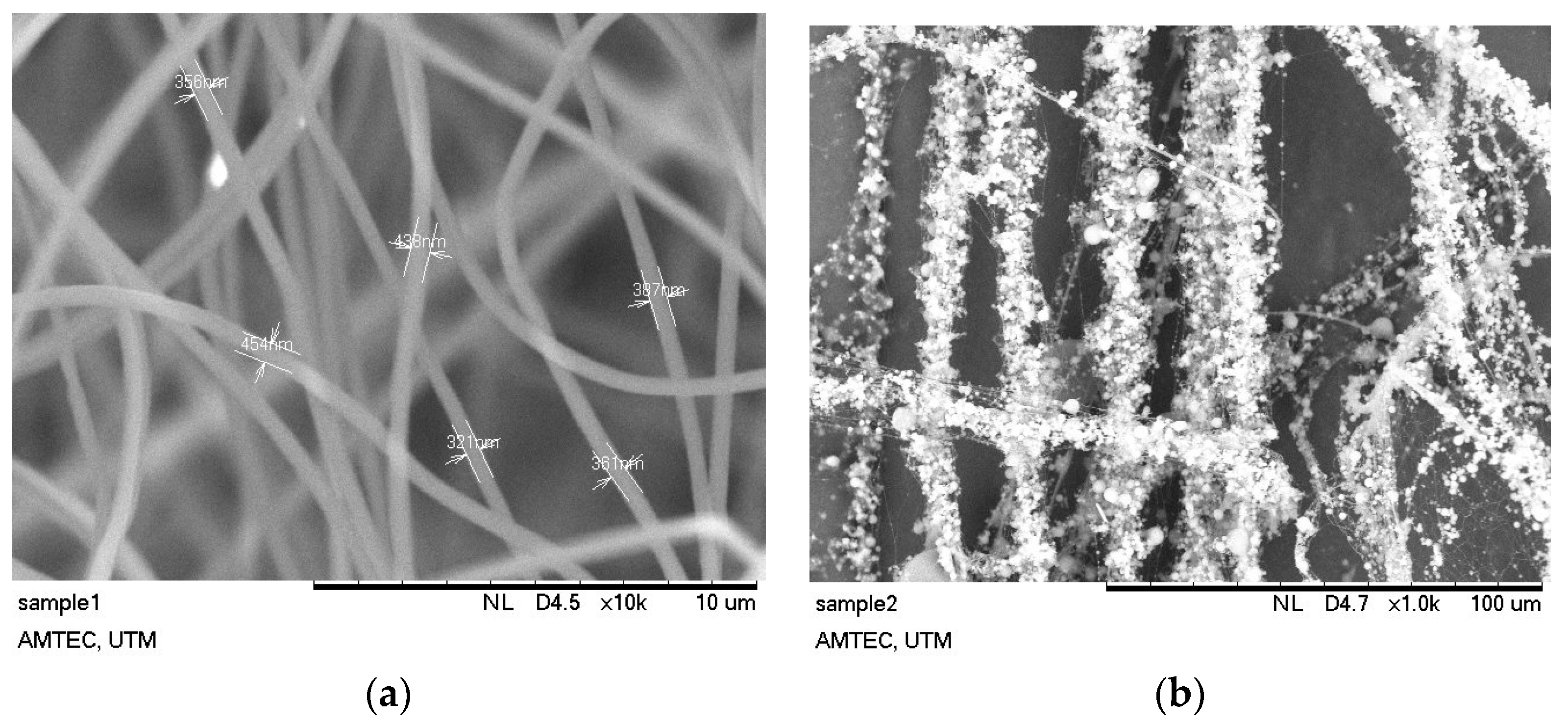
2.12. Scanning Electron Microscopy Analysis (SEM)
3. Results and Discussion
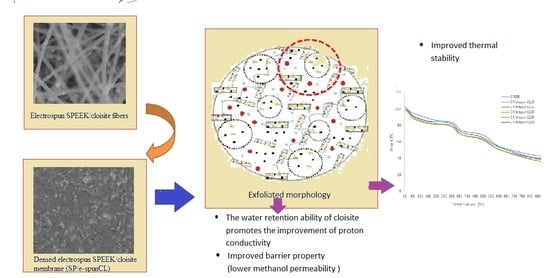
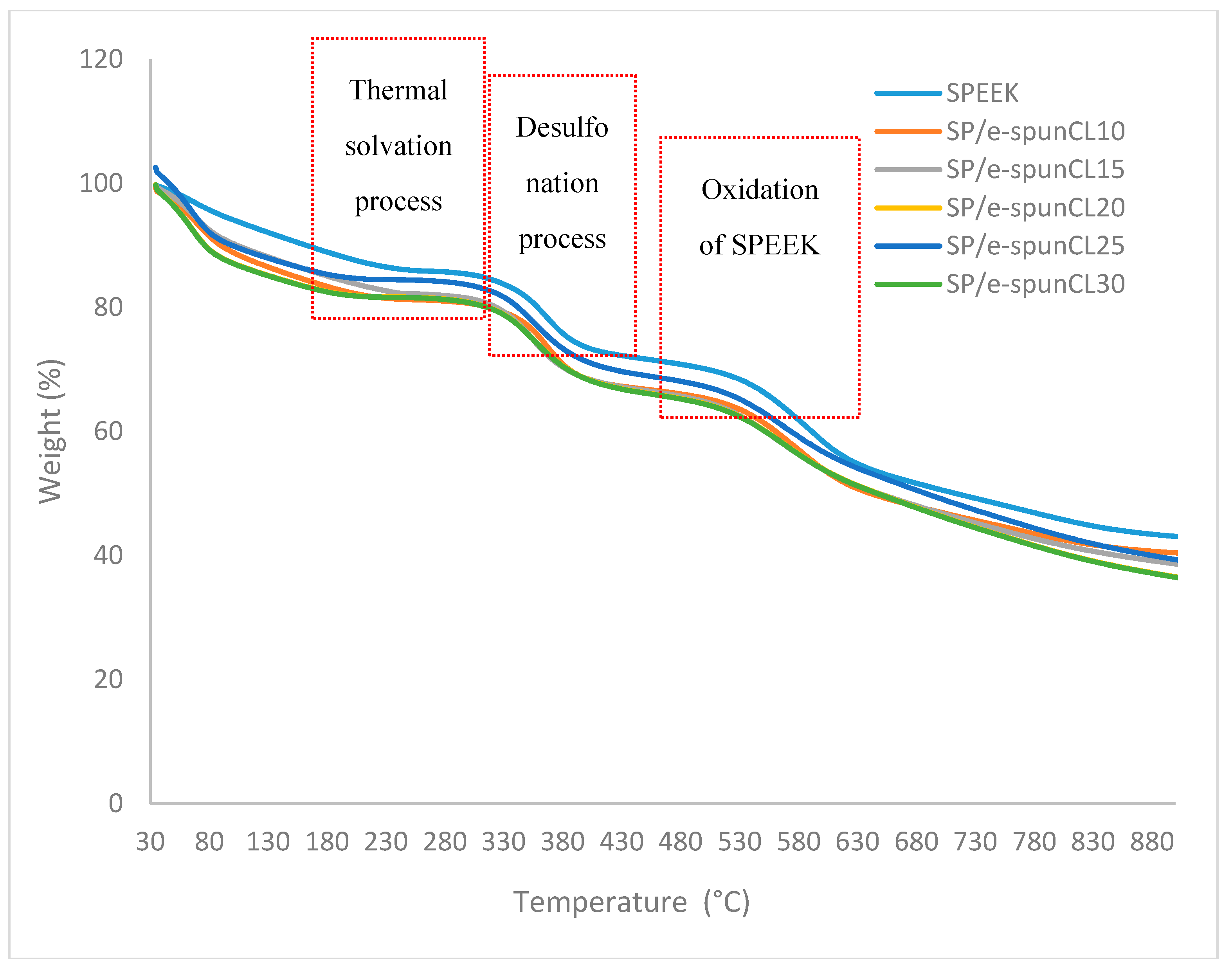
3.1. Thermal Stability Study of Void-Free SP/e-spunCL Membranes
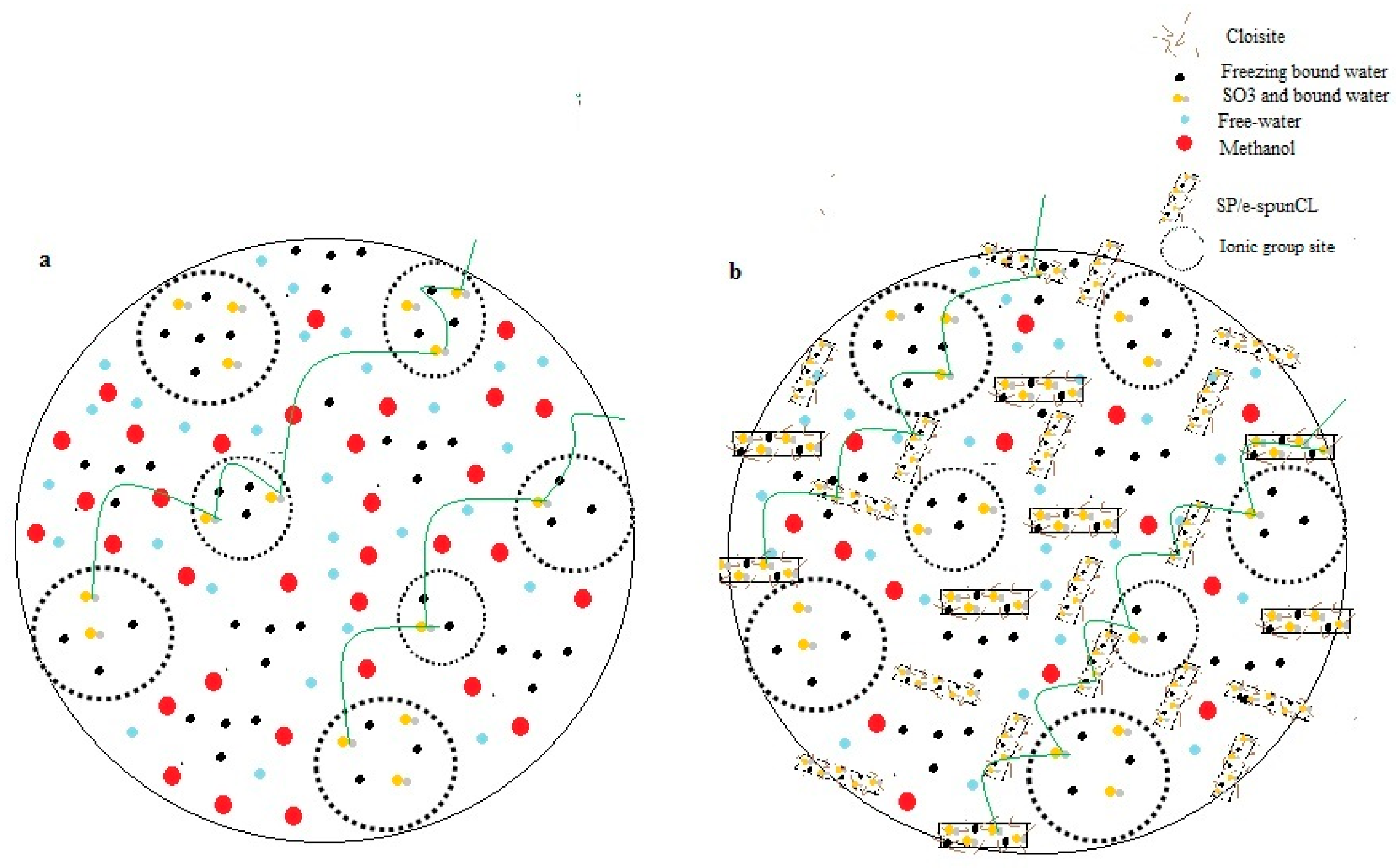
3.2. Wettability Analysis of the Void-Free SP/e-spunCL Membranes
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wan, N. High performance direct methanol fuel cell with thin electrolyte membrane. J. Power Sources 2017, 354, 167–171. [Google Scholar] [CrossRef]
- Masdar, M.S.; Zainoodin, A.M.; Rosli, M.I.; Kamarudin, S.K.; Daud, W.R.W. Performance and stability of single and 6-cell stack passive direct methanol fuel cell (DMFC) for long-term operation. Int. J. Hydrog. Energy 2017, 42, 9230–9242. [Google Scholar] [CrossRef]
- Das, V.; Padmanaban, S.; Venkitusamy, K.; Selvamuthukumaran, R.; Blaabjerg, F.; Siano, P. Recent advances and challenges of fuel cell based power system architectures and control—A review. Renew. Sustain. Energy Rev. 2017, 73, 10–18. [Google Scholar] [CrossRef]
- Branco, C.M.; Sharma, S.; de Camargo Forte, M.M.; Steinberger-Wilckens, R. New approaches towards novel composite and multilayer membranes for intermediate temperature-polymer electrolyte fuel cells and direct methanol fuel cells. J. Power Sources 2016, 316, 139–159. [Google Scholar] [CrossRef]
- Dutta, K.; Das, S.; Kumar, P.; Kundu, P.P. Polymer electrolyte membrane with high selectivity ratio for direct methanol fuel cells: A preliminary study based on blends of partially sulfonated polymers polyaniline and PVdF-co-HFP. Appl. Energy 2014, 118, 183–191. [Google Scholar] [CrossRef]
- Costamagna, P.; Yang, C.; Bocarsly, A.B.; Srinivasan, S. Nafion® 115/zirconium phosphate composite membranes for operation of PEMFCs above 100 C. Electrochim. Acta 2002, 47, 1023–1033. [Google Scholar] [CrossRef]
- Radenahmad, N.; Afif, A.; Petra, P.I.; Rahman, S.M.; Eriksson, S.G.; Azad, A.K. Proton-conducting electrolytes for direct methanol and direct urea fuel cells–A state-of-the-art review. Renew. Sustain. Energy Rev. 2016, 57, 1347–1358. [Google Scholar] [CrossRef]
- Mahajan, C.V.; Ganesan, V. Atomistic simulations of structure of solvated sulfonated poly (ether ether ketone) membranes and their comparisons to Nafion: I. Nanophase segregation and hydrophilic domains. J. Phys. Chem. B 2010, 114, 8357–8366. [Google Scholar] [CrossRef] [PubMed]
- Yang, T. Preliminary study of SPEEK/PVA blend membranes for DMFC applications. Int. J. Hydrog. Energy 2008, 33, 6772–6779. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, Z.; Bi, D.; Lin, H.; Shao, K.; Fu, T.; Na, H. Blend membranes based on disulfonated poly (aryl ether ether ketone) s (SPEEK) and poly (amide imide)(PAI) for direct methanol fuel cell usages. Polymer 2007, 48, 3090–3097. [Google Scholar] [CrossRef]
- Tsai, J.C.; Cheng, H.P.; Kuo, J.F.; Huang, Y.H.; Chen, C.Y. Blended Nafion®/SPEEK direct methanol fuel cell membranes for reduced methanol permeability. J. Power Sources 2009, 189, 958–965. [Google Scholar] [CrossRef]
- Di Vona, M.L.; Sgreccia, E.; Licoccia, S.; Alberti, G.; Tortet, L.; Knauth, P. Analysis of temperature-promoted and solvent-assisted cross-linking in sulfonated poly (ether ether ketone)(SPEEK) proton-conducting membranes. J. Phys. Chem. B 2009, 113, 7505–7512. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.; Cui, X.; Cai, H.; Fu, T.; Shao, K.; Na, H. Crosslinked SPEEK/AMPS blend membranes with high proton conductivity and low methanol diffusion coefficient for DMFC applications. J. Power Sources 2007, 168, 154–161. [Google Scholar] [CrossRef]
- Zhong, S.; Cui, X.; Cai, H.; Fu, T.; Zhao, C.; Na, H. Crosslinked sulfonated poly (ether ether ketone) proton exchange membranes for direct methanol fuel cell applications. J. Power Sources 2007, 164, 65–72. [Google Scholar] [CrossRef]
- Awang, N.; Jaafar, J.; Ismail, A.F.; Othman, M.H.D.; Rahman, M.A.; Yusof, N.; Azman, W.W.M.N. Development of dense void-free electrospun SPEEK-Cloisite15A membrane for direct methanol fuel cell application: Optimization using response surface methodology. Int. J. Hydrog. Energy 2017, 42, 26496–26510. [Google Scholar] [CrossRef]
- Ren, S.; Li, C.; Zhao, X.; Wu, Z.; Wang, S.; Sun, G.; Yang, X. Surface modification of sulfonated poly (ether ether ketone) membranes using Nafion solution for direct methanol fuel cells. J. Membr. Sci. 2005, 247, 59–63. [Google Scholar] [CrossRef]
- Hudiono, Y.; Choi, S.; Shu, S.; Koros, W.J.; Tsapatsis, M.; Nair, S. Porous layered oxide/Nafion® nanocomposite membranes for direct methanol fuel cell applications. Microporous Mesoporous Mater. 2009, 118, 427–434. [Google Scholar] [CrossRef]
- Kawasumi, M. The discovery of polymer-clay hybrids. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 819–824. [Google Scholar] [CrossRef]
- Zhang, S.; He, G.; Gong, X.; Zhu, X.; Wu, X.; Sun, X.; Li, H. Electrospun nanofiber enhanced sulfonated poly (phthalazinone ether sulfone ketone) composite proton exchange membranes. J. Membr. Sci. 2015, 493, 58–65. [Google Scholar] [CrossRef]
- Frenot, A.; Chronakis, I.S. Polymer nanofibers assembled by electrospinning. Curr. Opin. Colloid Interface Sci. 2003, 8, 64–75. [Google Scholar] [CrossRef]
- Sanchez, C.; Julian, B.; Belleville, P.; Popall, M. Applications of hybrid organic–inorganic nanocomposites. J. Mater. Chem. 2005, 15, 3559–3592. [Google Scholar] [CrossRef]
- Jaafar, J.; Ismail, A.F.; Matsuura, T. Preparation and barrier properties of SPEEK/Cloisite 15A®/TAP nanocomposite membrane for DMFC application. J. Membr. Sci. 2009, 345, 119–127. [Google Scholar] [CrossRef]
- Balbaşı, M.; Gözütok, B. Poly (vinyl alcohol)-colloidal silica composite membranes for fuel cells. Synth. Metals 2010, 160, 150–155. [Google Scholar] [CrossRef]
- Mohtar, S.S.; Ismail, A.F.; Matsuura, T. Preparation and characterization of SPEEK/MMT-STA composite membrane for DMFC application. J. Membr. Sci. 2011, 371, 10–19. [Google Scholar] [CrossRef]
- Chou, B. Nano-Scale Modified Inorganic/Organic Hybrid Materials as Proton Conductors. Ph.D. Thesis, Case Western Reserve University, Cleveland, OH, USA, 2006. [Google Scholar]
- Jaafar, J.; Ismail, A.F.; Mustafa, A. Physicochemical study of poly (ether ether ketone) electrolyte membranes sulfonated with mixtures of fuming sulfuric acid and sulfuric acid for direct methanol fuel cell application. Mater. Sci. Eng. A 2007, 460, 475–484. [Google Scholar] [CrossRef]
- Xue, Y.; Fu, R.; Wu, C.; Lee, J.Y.; Xu, T. Acid–base hybrid polymer electrolyte membranes based on SPEEK. J. Membr. Sci. 2010, 350, 148–153. [Google Scholar] [CrossRef]
- Grot, W.G.; Rajendran, G. Membranes Containing Inorganic Fillers and Membrane and Electrode Assemblies and Electrochemical Cells Employing Same. U.S. Patent No. 5,919,583, 1999. [Google Scholar]
- Yamaguchi, T.; Miyata, F.; Nakao, S.I. Pore-filling type polymer electrolyte membranes for a direct methanol fuel cell. J. Membr. Sci. 2003, 214, 283–292. [Google Scholar] [CrossRef]
- Prasad, M.; Mohanty, S.; Nayak, S.K. Study of polymeric nanocomposite membrane made from sulfonated polysulfone and nanoclay for fuel cell applications. High Perform. Polym. 2014, 26, 578–586. [Google Scholar] [CrossRef]
- Dong, B.; Gwee, L.; Salas-de La Cruz, D.; Winey, K.I.; Elabd, Y.A. Super proton conductive high-purity Nafion nanofibers. Nano Lett. 2010, 10, 3785–3790. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.J.; Lee, H.J.; Nam, S.Y. Sulfonated poly (arylene ether sulfone) membranes blended with hydrophobic polymers for direct methanol fuel cell applications. Int. J. Hydrog. Energy 2014, 39, 17524–17532. [Google Scholar] [CrossRef]
- Jung, D.H.; Cho, S.Y.; Peck, D.H.; Shin, D.R.; Kim, J.S. Preparation and performance of a Nafion®/montmorillonite nanocomposite membrane for direct methanol fuel cell. J. Power Sources 2003, 118, 205–211. [Google Scholar] [CrossRef]
- Lin, Y.F.; Yen, C.Y.; Hung, C.H.; Hsiao, Y.H.; Ma, C.C.M. A novel composite membranes based on sulfonated montmorillonite modified Nafion® for DMFCs. J. Power Sources 2007, 168, 162–166. [Google Scholar] [CrossRef]
- Nakamura, K.; Hatakeyama, T.; Hatakeyama, H. Relationship between hydrogen bonding and bound water in polyhydroxystyrene derivatives. Polymer 1983, 24, 871–876. [Google Scholar] [CrossRef]
- Morishige, K.; Kawano, K. Freezing and melting of water in a single cylindrical pore: The pore-size dependence of freezing and melting behavior. J. Chem. Phys. 1999, 110, 4867–4872. [Google Scholar] [CrossRef]
- Kim, D.S.; Liu, B.; Guiver, M.D. Influence of silica content in sulfonated poly (arylene ether ether ketone ketone)(SPAEEKK) hybrid membranes on properties for fuel cell application. Polymer 2006, 47, 7871–7880. [Google Scholar] [CrossRef]
- Awang, N.; Jaafar, J.; Ismail, A.F.; Othman, M.H.D.; Rahman, M.A. Effects of SPEEK/Cloisite Concentration as Electrospinning Parameter on Proton Exchange Membrane for Direct Methanol Fuel Cell Application. Mater. Sci. Forum 2017, 890, 278–284. [Google Scholar] [CrossRef]
- Awang, N.; Ismail, A.F.; Jaafar, J.; Matsuura, T.; Junoh, H.; Othman, M.H.D.; Rahman, M.A. Functionalization of polymeric materials as a high performance membrane for direct methanol fuel cell: A review. React. Funct. Polym. 2015, 86, 248–258. [Google Scholar] [CrossRef]
- Kreuer, K.D. Proton conductivity: Materials and applications. Chem. Mater. 1996, 8, 610–641. [Google Scholar] [CrossRef]
| Samples | Voltage (kV) | Distance (cm) | Designation |
|---|---|---|---|
| 0.10 wt % electrospun SPEEK/Cloisite membrane | 22.5 | 20 | SP/e-spunCL10 |
| 0.15 wt % electrospun SPEEK/Cloisite membrane | 22.5 | 20 | SP/e-spunCL15 |
| 0.20 wt % electrospun SPEEK/Cloisite membrane | 22.5 | 20 | SP/e-spunCL20 |
| 0.25 wt % electrospun SPEEK/Cloisite membrane | 22.5 | 20 | SP/e-spunCL25 |
| 0.30 wt % electrospun SPEEK/Cloisite membrane | 22.5 | 20 | SP/e-spunCL30 |
| Membrane | First Weight Loss (%) | Second Weight Loss (%) | Third Weight Loss (%) | Td1 (°C) | Td2 (°C) | Td3 (°C) |
|---|---|---|---|---|---|---|
| SP/e-spunCL10 | 17.85 ± 0.76 | 12.70 ± 0.98 | 15.77 ± 0.76 | 192.1 ± 0.77 | 389.1 ± 0.70 | 602.1 ± 0.77 |
| SP/e-spunCL15 | 16.08 ± 0.56 | 15.26 ± 0.87 | 15.74 ± 0.85 | 201.1 ± 0.87 | 398.1 ± 0.80 | 610.1 ± 0.86 |
| SP/e-spunCL20 | 17.44 ± 0.45 | 13.69 ± 0.08 | 15.72 ± 0.07 | 196.1 ± 0.06 | 395.1 ± 0.08 | 606.1 ± 0.06 |
| SP/e-spunCL25 | 12.61 ± 0.67 | 14.21 ± 0.65 | 16.62 ± 0.64 | 208.1 ± 0.56 | 406.1 ± 0.56 | 613.1 ± 0.58 |
| SP/e-spunCL30 | 9.44 ± 0.34 | 15.31 ± 0.34 | 17.95 ± 0.32 | 209.1 ± 0.34 | 410.1 ± 0.38 | 617.1 ± 0.38 |
| SPEEK | 20.56 ± 0.23 | 14.41 ± 0.12 | 11.12 ± 0.34 | 163.1 ± 0.45 | 388.1 ± 0.89 | 564.1 ± 0.67 |
| Concentration (wt %) | Fiber Diameter, nm |
|---|---|
| SP/e-spunCL10 | 67,680.0 |
| SP/e-spunCL15 | 429.2 |
| SP/e-spunCL20 | 386.17 |
| SP/e-spunCL25 | 495.4 |
| SP/e-spunCL30 | 9257.0 |
| Samples | Tg (°C) |
|---|---|
| SP/e-spunCL10 | 151.00 |
| SP/e-spunCL15 | 156.67 |
| SP/e-spunCL20 | 153.00 |
| SP/e-spunCL25 | 160.33 |
| SP/e-spunCL30 | 164.00 |
| SPEEK | 150.20 |
| Sample | Tensile Strength (MPa) | Young’s Modulus (MPa) |
|---|---|---|
| SP/e-spunCL10 | 29.97 ± 0.78 | 2743.79 ± 0.56 |
| SP/e-spunCL15 | 36.35 ± 0.97 | 3640.74 ± 0.98 |
| SP/e-spunCL20 | 33.40 ± 0.74 | 1713.62 ± 0.35 |
| SP/e-spunCL25 | 28.58 ± 0.67 | 2681.99 ± 0.37 |
| SP/e-spunCL30 | 28.61 ± 0.86 | 1626.70 ± 0.27 |
| Sample | Total Water (%) | ΔHf Normalized (J·g−1 Sample) a | ΔHf Per Mass Water (J·g−1 Water) b | Freezing Water/Total Water (%) c | Non-freezing Water/Total Water (%) | Freezing Water/Sample (%) | Non-freezing Water/Sample (%) |
|---|---|---|---|---|---|---|---|
| SP/e-spunCL10 | 24.56 | 67.04 | 272.96 | 81.97 | 18.03 | 20.13 | 4.43 |
| SP/e-spunCL15 | 25.87 | 50.84 | 196.52 | 59.01 | 40.99 | 15.27 | 10.60 |
| SP/e-spunCL20 | 30.00 | 70.15 | 233.83 | 70.22 | 29.78 | 21.07 | 8.93 |
| SP/e-spunCL25 | 20.00 | 57.55 | 287.75 | 86.41 | 13.59 | 17.28 | 2.72 |
| SP/e-spunCL30 | 19.00 | 59.76 | 314.52 | 94.45 | 5.55 | 17.95 | 1.05 |
| SPEEK | 26.76 | 85.46 | 319.35 | 95.90 | 4.10 | 25.66 | 1.10 |
| Nafion®112 | 21.43 | 64.19 | 299.53 | 89.95 | 10.05 | 19.28 | 2.15 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Awang, N.; Jaafar, J.; Ismail, A.F. Thermal Stability and Water Content Study of Void-Free Electrospun SPEEK/Cloisite Membrane for Direct Methanol Fuel Cell Application. Polymers 2018, 10, 194. https://doi.org/10.3390/polym10020194
Awang N, Jaafar J, Ismail AF. Thermal Stability and Water Content Study of Void-Free Electrospun SPEEK/Cloisite Membrane for Direct Methanol Fuel Cell Application. Polymers. 2018; 10(2):194. https://doi.org/10.3390/polym10020194
Chicago/Turabian StyleAwang, Nuha, Juhana Jaafar, and Ahmad Fauzi Ismail. 2018. "Thermal Stability and Water Content Study of Void-Free Electrospun SPEEK/Cloisite Membrane for Direct Methanol Fuel Cell Application" Polymers 10, no. 2: 194. https://doi.org/10.3390/polym10020194
APA StyleAwang, N., Jaafar, J., & Ismail, A. F. (2018). Thermal Stability and Water Content Study of Void-Free Electrospun SPEEK/Cloisite Membrane for Direct Methanol Fuel Cell Application. Polymers, 10(2), 194. https://doi.org/10.3390/polym10020194