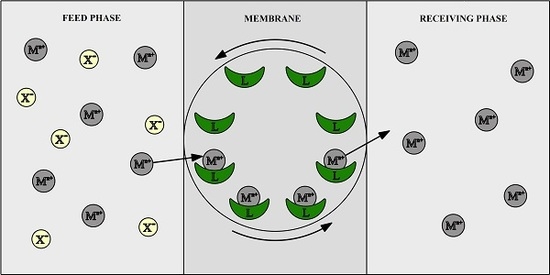
The Influence of the Morphology and Mechanical Properties of Polymer Inclusion Membranes (PIMs) on Zinc Ion Separation from Aqueous Solutions
Abstract
:1. Introduction
2. Experimental
2.1. Reagents
2.2. Membrane Preparation and Transport Experiments
2.3. AFM Analysis
2.4. Tests of Mechanical Properties of PIMs
3. Results and Discussion
3.1. Transport across PIMs Doped with Acac
- RF–recovery factor of zinc ions;
- c0–initial concentration of metal ions in the feed phase, mol/L;
- c–concentration of metal ions in the receiving phase after time t, mol/L.
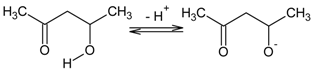
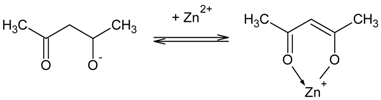
3.2. Transport across PIMs Doped with D2EHPA
- at interface: feed phase/membrane:Zn(II) + 3/2(HR)2 = ZnR2·HR + 2H+
- at interface: membrane/receiving phase:ZnR2·HR + 2H+ = Zn(II) + 3/2(HR)2
3.3. Comparision of Acac and D2EHPA in the Transport across PIMs
- formation in the feed phase of a complex composed of the carried substance and the carrier;
- diffusion of the complex across the membrane into the receiving phase;
- decomposition of the complex compound and release of the transported substance into the receiving phase [9].
3.4. Membrane Morphology
3.4.1. PIMs Characterization by AFM
3.4.2. Membrane Pore Size
3.4.3. Membrane Roughness
3.5. Characterization of Mechanical Properties of PIMs
3.5.1. Membrane Thickness
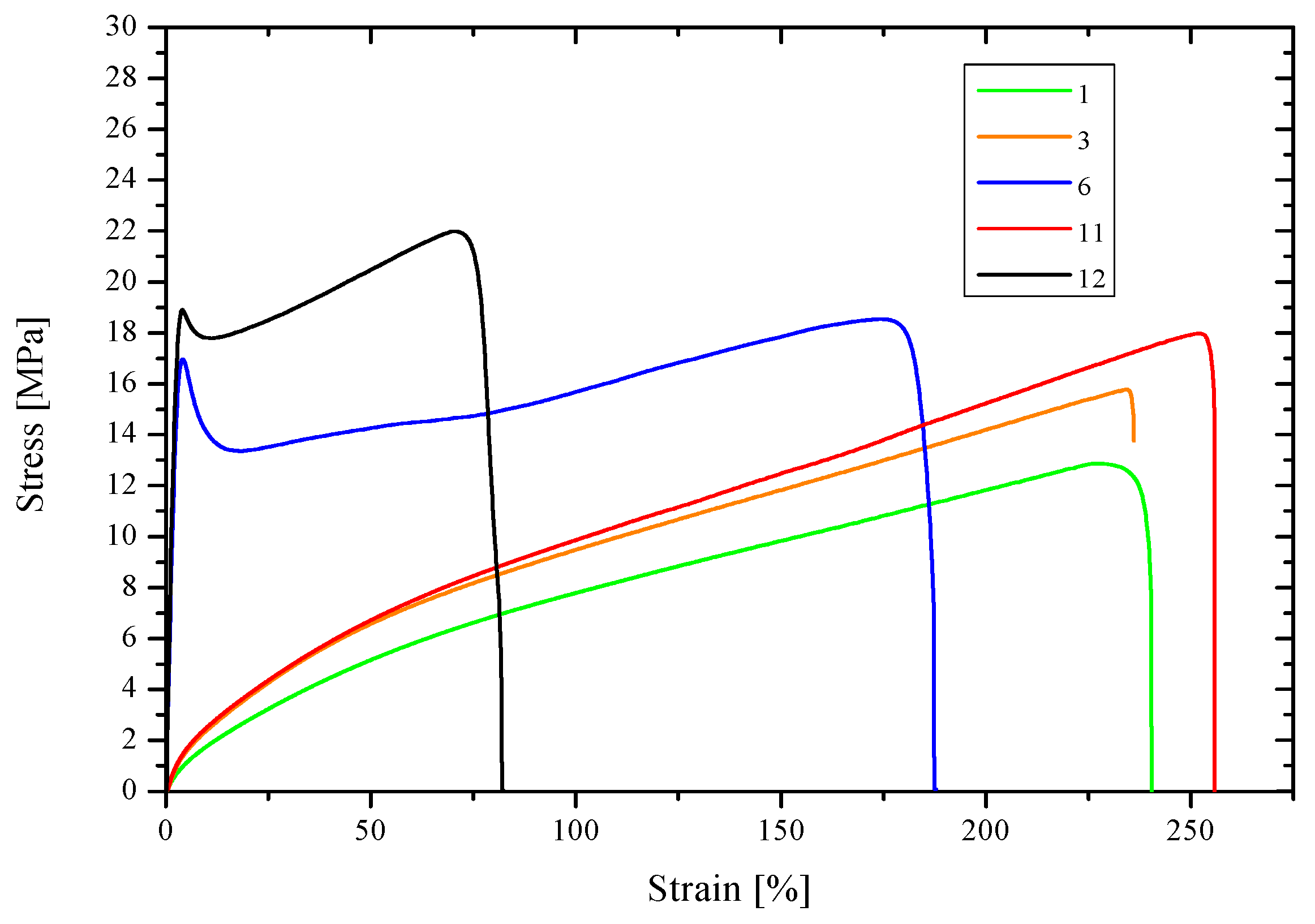
3.5.2. Resistance to Static Extension
3.5.3. Membrane Glass-Transition Temperature
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bloch, R.; Kedem, O.; Vofsi, D. Ion specific polymer membrane. Nature 1963, 199, 802–803. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, M.; Kikkawa, M.; Urita, S. Carrier-mediated transport of rare earth ions through cellulose triacetate membranes. J. Membr. Sci. 1989, 42, 47–55. [Google Scholar] [CrossRef]
- Kaya, A.; Alpoguz, H.K.; Yilmaz, A. Application of Cr(VI) Transport through the Polymer Inclusion Membrane with a New Synthesized Calix[4]arene Derivative. Ind. Eng. Chem. Res. 2013, 52, 5428–5436. [Google Scholar] [CrossRef]
- Nutchapurida Bonggotgetsakul, Y.Y.; Cattrall, R.W.; Kolev, S.D. Extraction of Gold(III) from Hydrochloric Acid Solutions with a PVC-based Polymer Inclusion Membrane (PIM) Containing Cyphos® IL 104. Membranes 2015, 5, 903–914. [Google Scholar] [CrossRef] [PubMed]
- Casadellà, A.; Schaetzle, O.; Nijmeijer, K.; Loos, K. Polymer Inclusion Membranes (PIM) for the Recovery of Potassium in the Presence of Competitive Cations. Polymers 2016, 8, 76. [Google Scholar] [CrossRef]
- Drioli, E.; Strathmann, H.; Giorno, L. Basic aspects in polymeric membrane preparation. In Comprehensive Membrane Science and Engineering, 1st ed.; Drioli, E., Giorno, L., Eds.; Elsevier: Milan, Italy, 2010; ISBN 978-0-444-53204-6. [Google Scholar]
- Religa, P.; Rajewski, J.; Gierycz, P. Advantages and disadvantages of SLM and PIM systems used for chromium(III) separation from aqueous solutions. Pol. J. Environ. Stud. 2015, 24, 1283–1290. [Google Scholar]
- Inês, M.; Almeida, G.S.; Cattrall, R.W.; Kolev, S.D. Recent trends in extraction and transport of metal ions using polymer inclusion membranes (PIMs). J. Membr. Sci. 2012, 415–416, 9–23. [Google Scholar] [CrossRef]
- Nghiem, L.D.; Mornane, P.; Potter, I.D.; Perera, J.M.; Cattrall, R.W.; Kolev, S.D. Extraction and transport of metal ions and small organic compounds using polymer inclusion membranes (PIMs). J. Membr. Sci. 2006, 281, 7–41. [Google Scholar] [CrossRef]
- Annane, K.; Sahmoune, A.; Montels, P.; Tingry, S. Polymer inclusion membrane extraction of cadmium(II) with Aliquat 336 in micro-channel cell. Chem. Eng. Res. Des. 2015, 94, 605–610. [Google Scholar] [CrossRef]
- Baczyńska, M.; Regel-Rosocka, M.; Nowicki, M.; Wiśniewski, M. Effect of the structure of polymer inclusion membranes on Zn(II) transport from chloride aqueous solutions. J. Appl. Polym. Sci. 2015, 132, 42319–42329. [Google Scholar] [CrossRef]
- Hosseini, S.S.; Bringas, E.; Tan, N.R.; Ortiz, I.; Ghahramani, M.; Shahmirzadi, M.A.A. Recent progress in development of high performance polymeric membranes and materials for metal plating wastewater treatment: A review. J. Water Process Eng. 2016, 9, 78–110. [Google Scholar] [CrossRef]
- Narębska, A. Wprowadzenie do technik membranowych. Podstawowe prawa transportu. In Membrany I Membranowe Techniki Rozdziału, 1st ed.; Narębska, A., Ed.; Wydawnictwo UMK: Torun, Poland, 1997; ISBN 83-231-0937-0. [Google Scholar]
- Senhadji-Kebiche, O.; Belaid, T.; Benamor, M. Polymer Inclusion Membrane (PIM) as Competitive Material for Applications in SPE for Water Treatment Process. Open Access Libr. J. 2014, 1, 1–7. [Google Scholar] [CrossRef]
- Ulewicz, M.; Radzyminska-Lenarcik, E. Supported Liquid (SLM) and Polymer Inclusion (PIM) Membranes Pertraction of Copper(II) from Aqueous Nitrate Solutions by 1-Hexyl-2-Methylimidazole. Sep. Sci. Technol. 2012, 47, 1383–1389. [Google Scholar] [CrossRef]
- Kumari, A.; Sinha, M.K.; Sahu, S.K.; Pandey, B.D. Solvent extraction and separation of tri-valent lanthanides using Cyphos IL 104, A novel phosphonium ionic liquid as extractant. Solvent Extr. Ion Exch. 2016, 34, 469–484. [Google Scholar] [CrossRef]
- Cieszynska, A.; Wisniewski, M. Extraction of palladium(II) from chloride solutions with Cyphos® IL 101/toluene mixtures as novel extractant. Sep. Purif. Technol. 2010, 73, 202–207. [Google Scholar] [CrossRef]
- Araki, T.; Tsukube, H. Liquid Membranes: Chemical Applications; CRC Press: Boca Raton, FL, USA, 1990. [Google Scholar]
- Saf, A.Ö.; Alpaydin, S.; Coskun, A.; Ersoz, M. Selective transport and removal of Cr(VI) through polymer inclusion membrane containing 5-(4-phenoxyphenyl)-6H-1,3,4-thiadiazin-2-amine as a carrier. J. Membr. Sci. 2011, 377, 241–248. [Google Scholar] [CrossRef]
- Sgarlata, C.; Arena, G.; Longo, E.; Zhang, D.; Yang, Y.; Bartsch, R.A. Heavy metal separation with polymer inclusion membranes. J. Membr. Sci. 2008, 323, 444–451. [Google Scholar] [CrossRef]
- Ceynowa, J. Membrany selektywne i procesy membranowe. In Membrany Teoria i Praktyka; Zeszyt, I., Wodzki, R., Eds.; Wydział Chemii, Uniwersytet Mikołaja Kopernika: Torun, Poland, 2003; ISBN 83-231-1604-0. [Google Scholar]
- Godlewski, M. Zinc oxide for electronic, photovoltaic and optoelectronic applications. J. Low Temp. Phys. 2011, 37, 235–240. [Google Scholar] [CrossRef]
- Councell, T.B.; Duckenfield, K.U.; Landa, E.R.; Callender, E. Tire-Wear Particles as a Source of Zinc to the Environment. Environ. Sci. Technol. 2004, 38, 4206–4214. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.F.O.; Oliveira, M.L.S.; Serra, C.; Hower, J.C. Zinc Speciation in Power Burning Mixturea of Coal and Tires. Coal Combust. Gasif. Prod. 2011, 3, 41–50. [Google Scholar] [CrossRef]
- Radzymińska-Lenarcik, E.; Sulewski, M.; Urbaniak, W. Recovery of Zinc from Metallurgic Waste Sludges. Pol. J. Environ. Stud. 2015, 24, 1277–1282. [Google Scholar] [CrossRef]
- Biswas, R.K.; Karmakar, A.K.; Kumar, S.L.; Hossain, M.N. Recovery of manganese and zinc from waste Zn-C cell powder: Characterization and leaching. Waste Manag. 2015, 46, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Varghese, V.; Revanna, M.; Kiran, R.; Shivakumar, C.; Kumar, D. Hydrometallurgical Recovery of Zinc from Zinc Ash, Silver from Waste X-ray and Photographic Films. Int. J. Adv. Res. Technol. 2015, 4, 878–885. [Google Scholar]
- European Council. Dyrektywa 2000/60/WE Parlamentu Europejskiego i Rady z dnia 23 Października 2000 r; European Council: Bruxelles, Belgium, 2000. [Google Scholar]
- Cabała, J. Cynk w technosferze. Górnictwo Geol. 2010, 5, 63–76. [Google Scholar]
- Gherasim, C.-V.; Cristea, M.; Grigoras, C.-V.; Bourceanu, G. New polymer inclusion membrane. Preparation and characterization. Dig. J. Nanomater. Biostruct. 2011, 6, 1507–1516. [Google Scholar]
- Yilmaza, A.; Arslana, G.; Tor, A.; Akin, I. Selectively facilitated transport of Zn(II) through a novel polymer inclusion membrane containing Cyanex 272 as a carrier reagent. Desalination 2011, 277, 301–307. [Google Scholar] [CrossRef]
- Resina, M.; Macanas, J.; de Gyves, J.; Munoz, M. Zn(II), Cd(II) and Cu(II) separation through organic-inorganic Hybrid Membranes containing di-(2-ethylhexyl) phosphoric acid or di-(2-ethylhexyl) dithiophosphoric acid as a carrier. J. Membr. Sci. 2006, 268, 57–64. [Google Scholar] [CrossRef]
- Kozlowski, C.A. Facilitated transport of metal ions through composite and polymer inclusion membranes. Desalination 2006, 198, 132–140. [Google Scholar] [CrossRef]
- Pranolo, Y.; Zhu, Z.; Cheng, C.Y. Separation of lithium from sodium in chloride solutions using SSX systems with LIX 54 and Cyanex 923. Hydrometallurgy 2015, 154, 33–39. [Google Scholar] [CrossRef]
- Wejman-Gibas, K.; Pilsniak-Rabiega, M.; Ochromowicz, K. solvent extraction of zinc(II) from ammonia leaching solution by LIX 54-100, LIX 84 I and TOA. Physicochem. Probl. Miner. Process. 2017, 53, 202–211. [Google Scholar] [CrossRef]
- Kebiche-Senhadji, O.; Mansouri, L.; Tingry, S.; Seta, P.; Benamora, M. Facilitated Cd(II) transport across CTA polymer inclusion membrane using anion (Aliquat 336) and cation (D2EHPA) metal carriers. J. Membr. Sci. 2008, 310, 438–445. [Google Scholar] [CrossRef]
- Kolev, S.D.; Baba, Y.; Cattrall, R.W.; Tasaki, T.; Pereira, N.; Perera, J.M.; Stevens, G.W. Solid phase extraction of zinc(II) using a PVC-based polymer inclusion membrane with di(2-ethylhexyl)phosphoric acid (D2EHPA) as the carrier. Talanta 2009, 78, 795–799. [Google Scholar] [CrossRef] [PubMed]
- Baczyńska, M.; Regel-Rosocka, M.; Coll, M.T.; Fortuny, A.; Sastre, A.M.; Wiśniewski, M. Transport of Zn(II), Fe(II), Fe(III) across polymer inclusion membranes (PIM) and flat sheet supported liquid membranes (SLM) containing phosphonium ionic liquids as metal ion carriers. Sep. Sci. Technol. 2016, 51, 2639–2648. [Google Scholar] [CrossRef]
- Arslana, G.; Yılmazb, A.; Torc, A.; Ersoz, M. Preparation of polymer inclusion membrane with sodium diethyldithiocarbamate as a carrier reagent for selective transport of zinc ions. Desalination Water Treat. 2017, 75, 348–356. [Google Scholar] [CrossRef]
- Radzyminska-Lenarcik, E.; Ulewicz, M. The use of 1-alkylimidzoles for selective separation of zinc ions in the transport process across a polymeric inclusion membrane. Physicochem. Probl. Miner. Process. 2014, 50, 131–142. [Google Scholar] [CrossRef]
- Witt, K.; Radzyminska-Lenarcik, E.; Urbaniak, W. Selective transport of zinc ions through novel polymer inclusion membranes (PIMs) containing β-diketone derivatives as carrier reagents. Sep. Sci. Technol. 2016, 51, 2620–2627. [Google Scholar] [CrossRef]
- Xu, J.; Wang, L.; Shen, W.; Paimin, R.; Wang, X. The Influence of the Interior Structure of Aliquat 336/PVC Membranes to their Extraction Behavior. Sep. Sci. Technol. 2005, 39, 3527–3539. [Google Scholar] [CrossRef]
- Gherrou, A.; Kerdjoudj, H.; Molinari, R.; Seta, P.; Drioli, E. Fixed sites plasticized cellulose triacetate membranes containing crown ethers for silver(I), copper(II) and gold(III) ions transport. J. Membr. Sci. 2004, 228, 149–157. [Google Scholar] [CrossRef]
- Gherrou, A.; Kerdjoudj, H.; Molinari, R.; Drioli, E. Modelization of the transport of silver and copper in acidic thiourea medium through a supported liquid membrane. Desalination 2001, 139, 317–325. [Google Scholar] [CrossRef]
- Arous, O.; Kerdjoudj, H.; Seta, P. Comparison of carrier-facilitated silver (I) and copper (II) ions transport mechanisms in a supported liquid membrane and in a plasticized cellulose triacetate membrane. J. Membr. Sci. 2004, 241, 177–185. [Google Scholar] [CrossRef]
- Arous, O.; Amara, M.; Kerdjoudj, H. Synthesis and characterization of cellulose triacetate and poly(ethylene imine) membranes containing a polyether macrobicyclic: Their application to the separation of copper(II) and silver(I) ions. J. Appl. Polym. Sci. 2004, 93, 1401–1410. [Google Scholar] [CrossRef]
- Tor, A.; Arslan, G.; Muslu, H.; Celiktas, A.; Cengeloglu, Y.; Ersoz, M. Facilitated transport of Cr(III) through polymer inclusion membrane with di(2-ethylhexyl)phosphoric acid (DEHPA). J. Membr. Sci. 2004, 329, 169–174. [Google Scholar] [CrossRef]
- Walkowiak, W.; Bartsch, R.A.; Kozlowski, C.; Gega, J.; Charewicz, W.; Amiri-Eliasi, B. Separation and removal of metal ionic species by polymer inclusion membrane. J. Radioanal. Nucl. Chem. 2000, 246, 643–650. [Google Scholar] [CrossRef]
- Boussu, K.; Vandecasteele, B.; Van der Bruggen, B. Relation between membrane characteristics and performance in nanofiltration. J. Membr. Sci. 2008, 310, 51–65. [Google Scholar] [CrossRef]
- Boussu, K.; Belpaire, A.; Volodin, A.; Van Haesendonck, C.; Van der Meerenc, P.; Vandecasteele, C.; Van der Bruggen, B. Influence of membrane and colloid characteristics on fouling of nanofiltration membranes. J. Membr. Sci. 2007, 289, 220–230. [Google Scholar] [CrossRef]
- Kozlowski, C.A.; Walkowiak, W. Transport of Cr(VI), Zn(II), and Cd(II) Ions Across Polymer Inclusion Membranes with Tridecyl(pyridine) Oxide and Tri-n-Octylamine. Sep. Sci. Technol. 2004, 39, 3127–3141. [Google Scholar] [CrossRef]
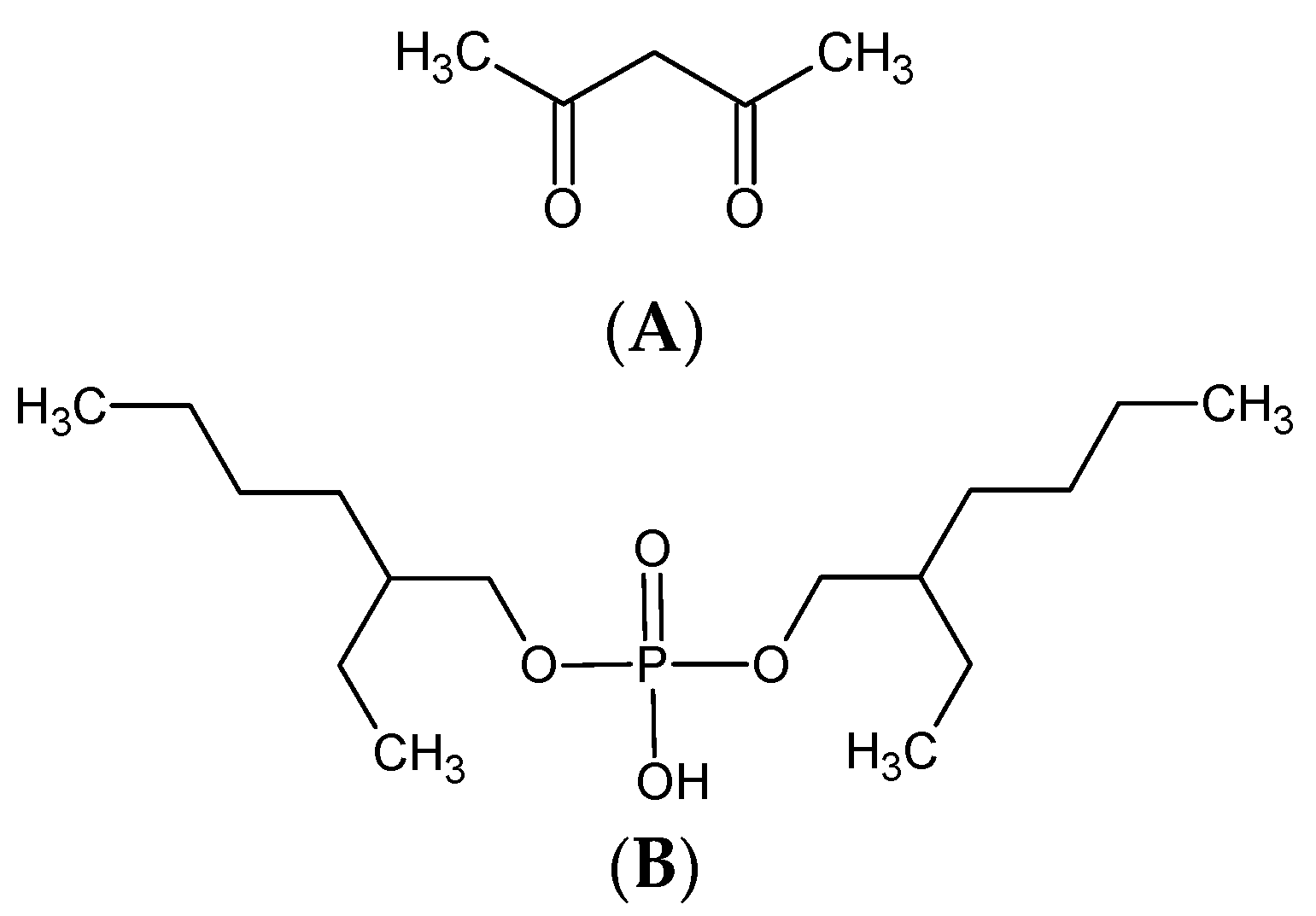
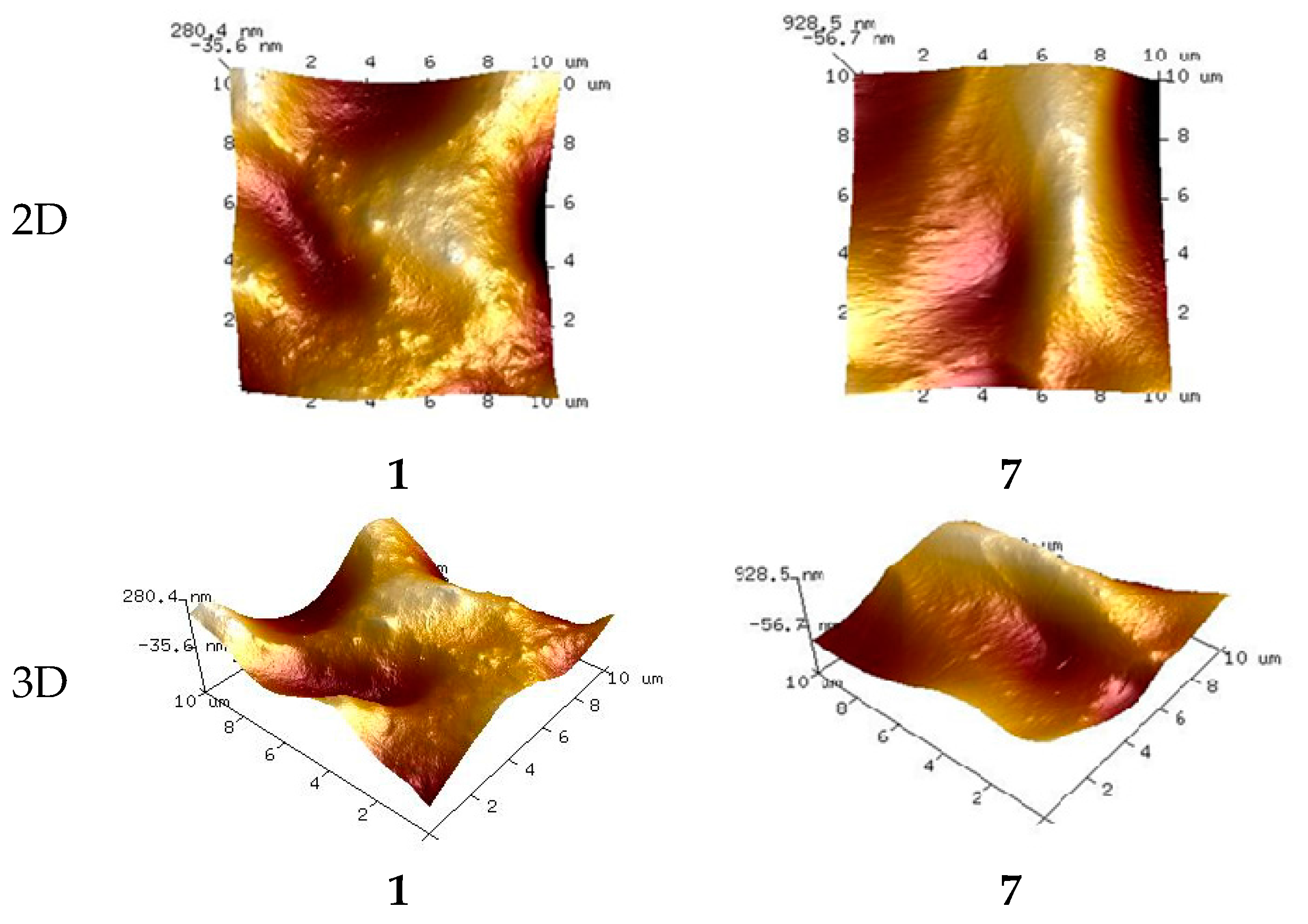
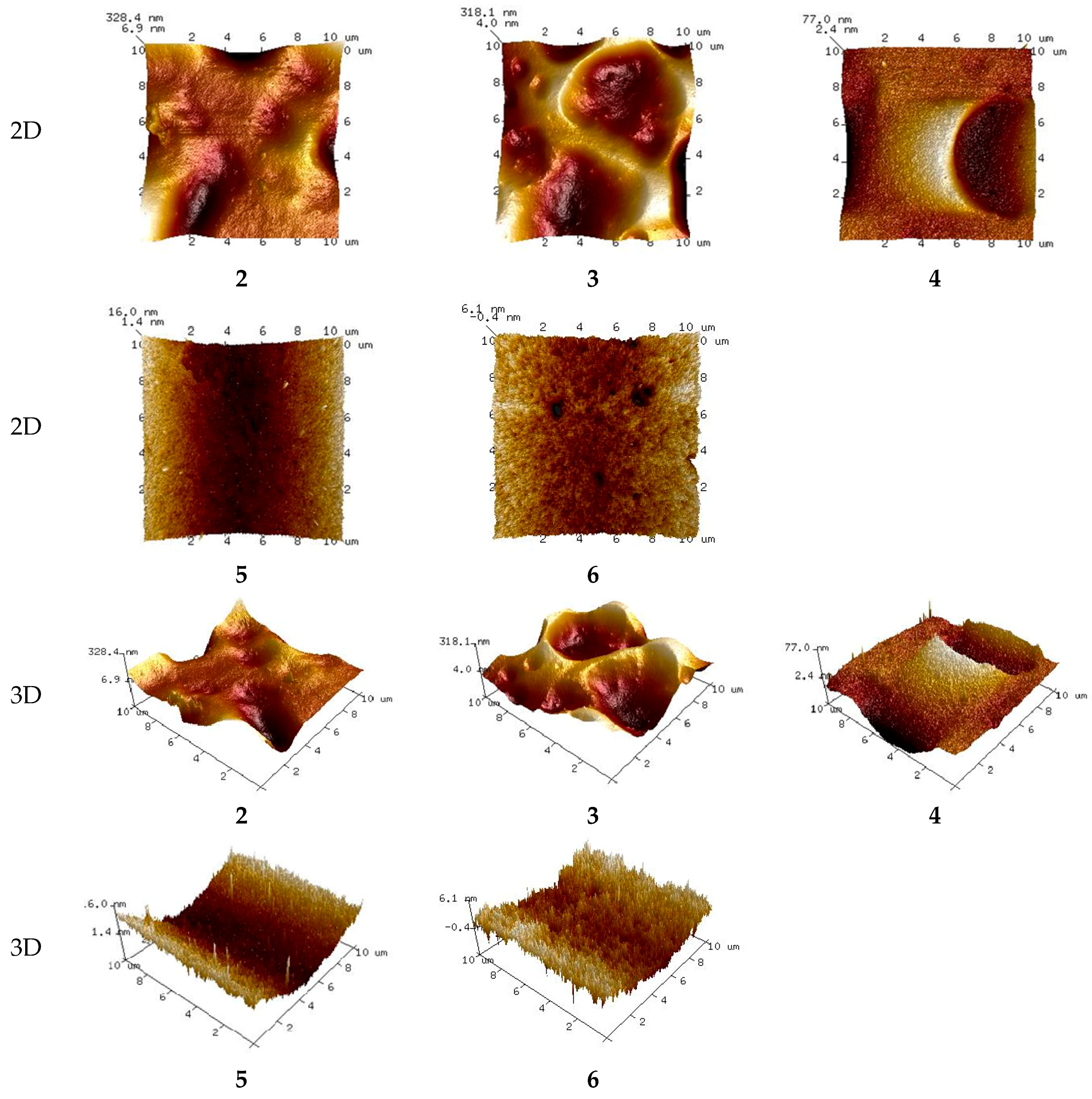
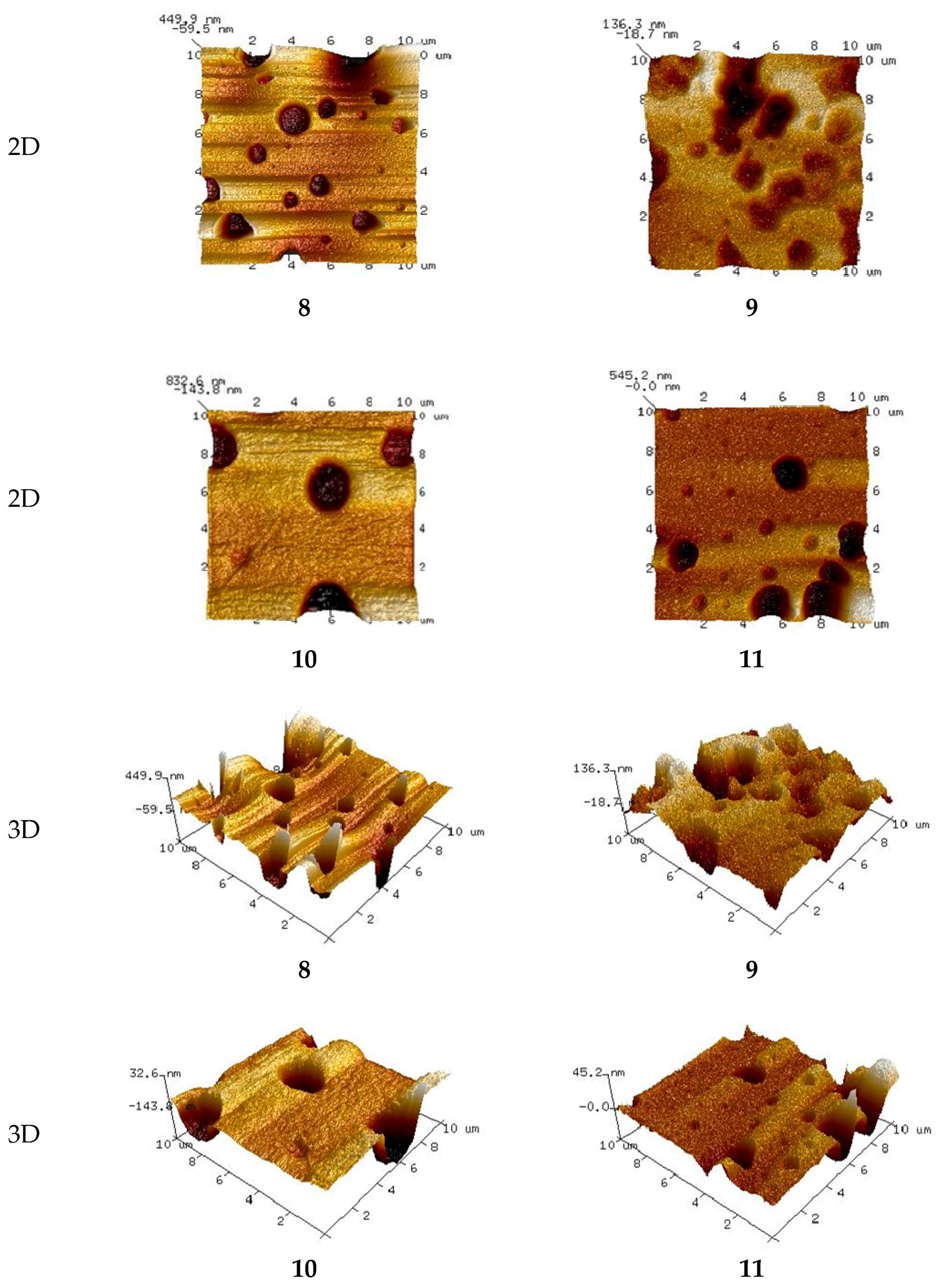
| Membrane, No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Matrix, PVC, % | 90 | 70 | 50 | 40 | 30 | 20 | 95 | 75 | 55 | 45 | 35 |
| Plasticizer, DAO, % | 10 | 10 | 10 | 10 | 10 | 10 | 5 | 5 | 5 | 5 | 5 |
| Carrier | acac | D2EHPA | |||||||||
| Carrier, % | 0 | 20 | 40 | 50 | 60 | 70 | 0 | 20 | 40 | 50 | 60 |
| Membrane No. | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| % ACAC | 0% | 20% | 40% | 50% | 60% | 80% |
| RF, % | 0.51 | 99.65 | 23.54 | 16.64 | 12.45 | 6.29 |
| Membrane No. | 7 | 8 | 9 | 10 | 11 |
|---|---|---|---|---|---|
| % D2EHPA | 0% | 20% | 40% | 50% | 60% |
| RF, % | 0.95 | 1.80 | 25.71 | 44.27 | 56.33 |
| Membrane No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| RF, nm | 2.91 | 4.71 | 3.86 | 2.23 | 3.55 | 1.66 | 1.62 | 2.42 | 3.09 | 5.82 | 6.15 |
| Membrane No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean membrane thickness, mm | 0.242 | 0.292 | 0.281 | 0.273 | 0.271 | 0.269 | 0.185 | 0.194 | 0.196 | 0.187 | 0.179 |
| Standard deviation | 0.050 | 0.038 | 0.041 | 0.046 | 0.011 | 0.017 | 0.080 | 0.030 | 0.043 | 0.018 | 0.047 |
| Membrane | Young’s Modulus (Mpa) | Tensile Strength (Mpa) | Strain at Break (%) |
|---|---|---|---|
| 1 | 61 | 17.5 | 240 |
| 2 | 36 | 14.6 | 279 |
| 4 | 58 | 15.7 | 264 |
| 7 | 794 | 17.1 * | 127 |
| 8 | 747 | 18.1 * | 191 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Witt, K.; Radzyminska-Lenarcik, E.; Kosciuszko, A.; Gierszewska, M.; Ziuziakowski, K. The Influence of the Morphology and Mechanical Properties of Polymer Inclusion Membranes (PIMs) on Zinc Ion Separation from Aqueous Solutions. Polymers 2018, 10, 134. https://doi.org/10.3390/polym10020134
Witt K, Radzyminska-Lenarcik E, Kosciuszko A, Gierszewska M, Ziuziakowski K. The Influence of the Morphology and Mechanical Properties of Polymer Inclusion Membranes (PIMs) on Zinc Ion Separation from Aqueous Solutions. Polymers. 2018; 10(2):134. https://doi.org/10.3390/polym10020134
Chicago/Turabian StyleWitt, Katarzyna, Elzbieta Radzyminska-Lenarcik, Artur Kosciuszko, Magdalena Gierszewska, and Kamil Ziuziakowski. 2018. "The Influence of the Morphology and Mechanical Properties of Polymer Inclusion Membranes (PIMs) on Zinc Ion Separation from Aqueous Solutions" Polymers 10, no. 2: 134. https://doi.org/10.3390/polym10020134
APA StyleWitt, K., Radzyminska-Lenarcik, E., Kosciuszko, A., Gierszewska, M., & Ziuziakowski, K. (2018). The Influence of the Morphology and Mechanical Properties of Polymer Inclusion Membranes (PIMs) on Zinc Ion Separation from Aqueous Solutions. Polymers, 10(2), 134. https://doi.org/10.3390/polym10020134