A Facile Fabrication of High Toughness Poly(lactic Acid) via Reactive Extrusion with Poly(butylene Succinate) and Ethylene-Methyl Acrylate-Glycidyl Methacrylate
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of Samples
2.3. Mechanical Testing
2.4. Scanning Electron Microscopy (SEM)
2.5. Fourier Transform Infrared (FT-IR) Spectroscopy
2.6. Contact Angle Testing
3. Results and Discussion
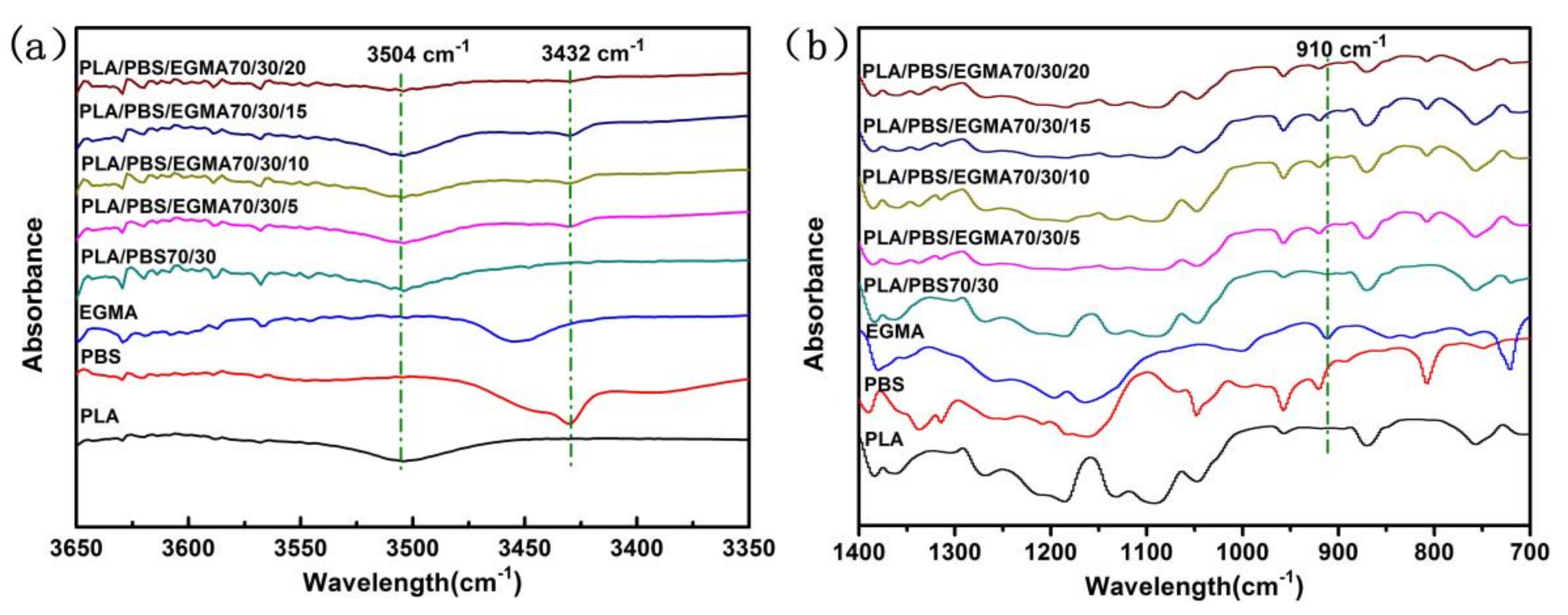
3.1. The FT-IR Analysis
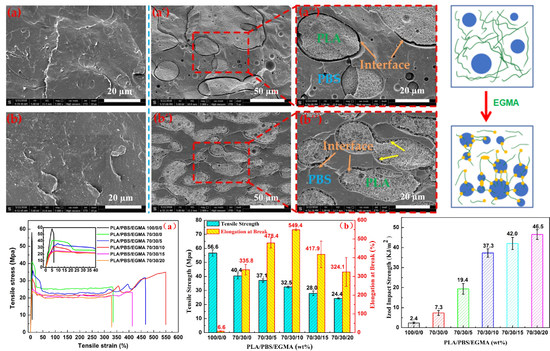
3.2. Mechanical Properties Analysis
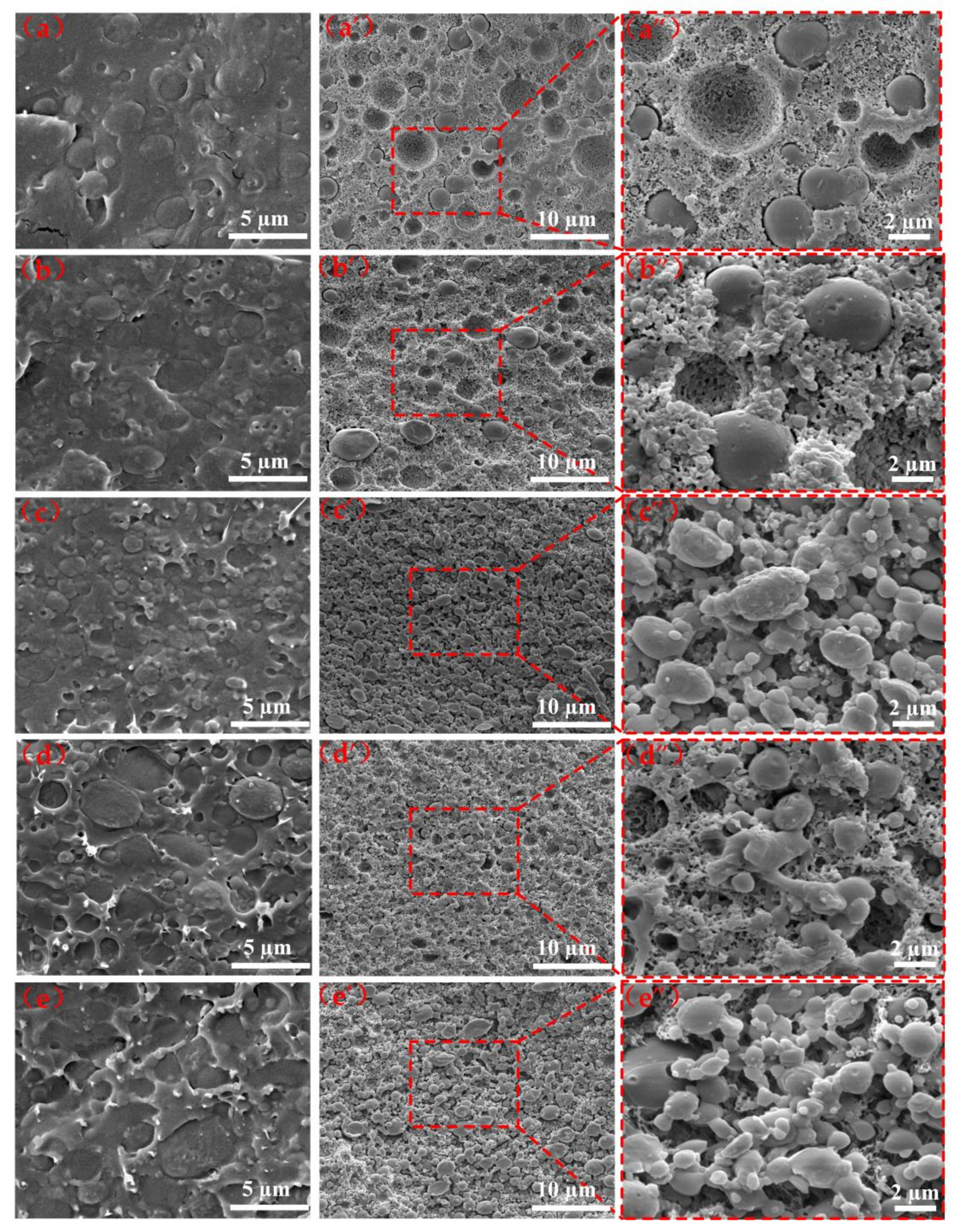
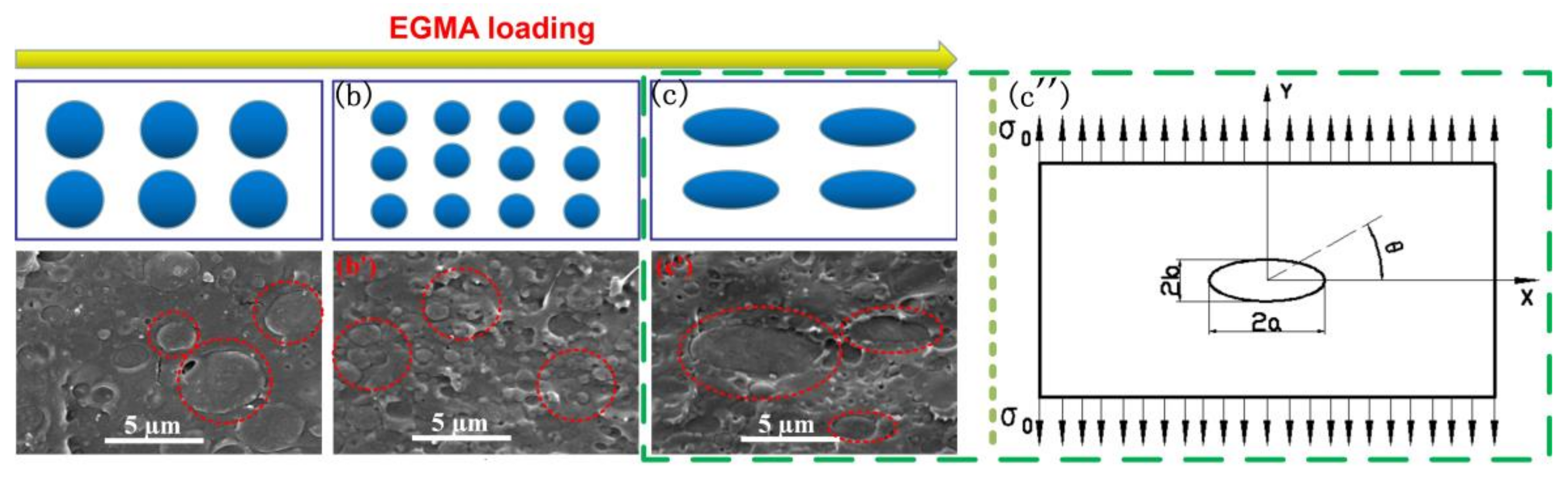
3.3. Morphological Analysis
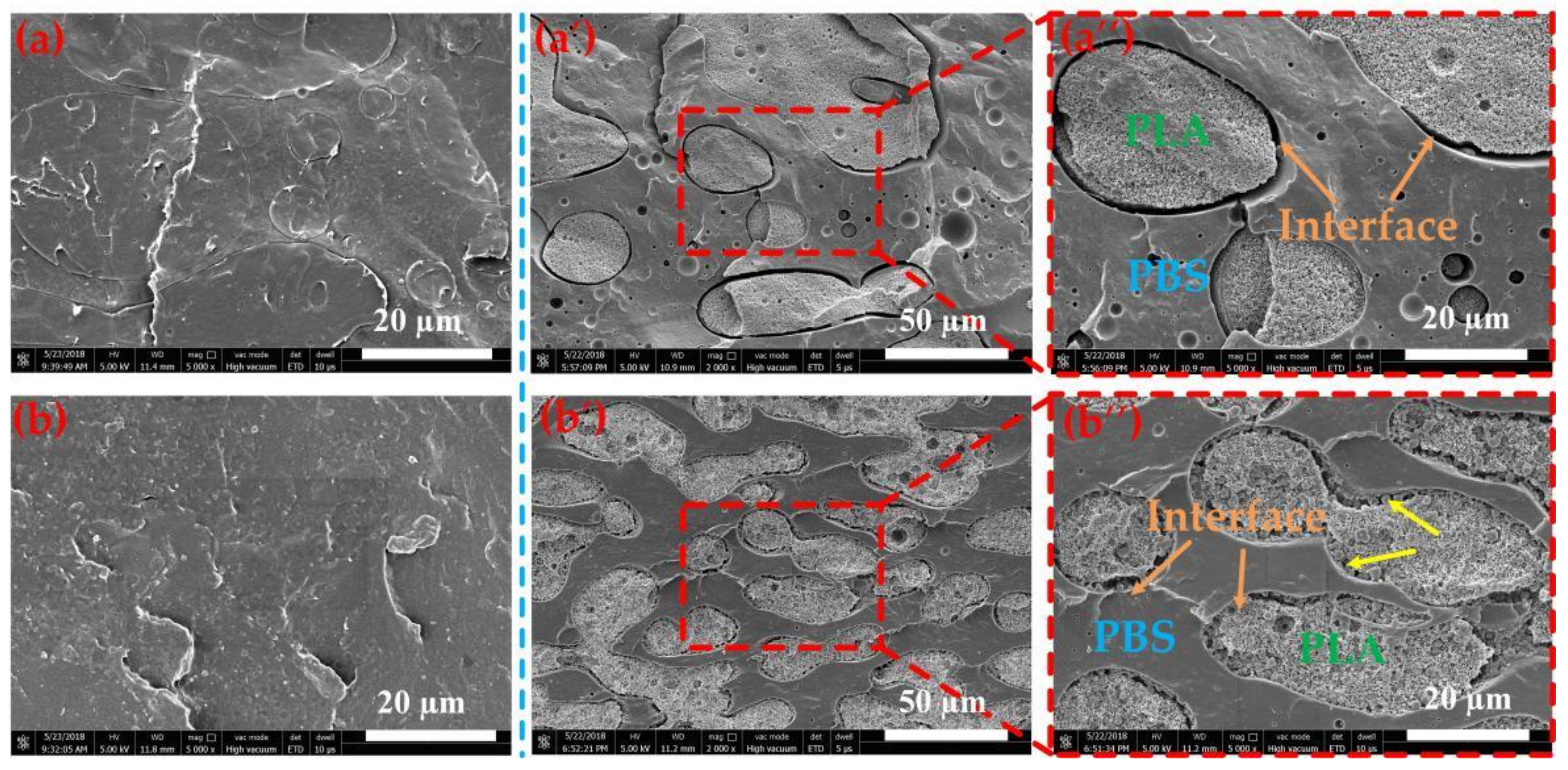
3.4. Compatibilizer EGMA Localization in the PLA/PBS Blend Analysis
3.5. Toughening Mechanism
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Álvarez-Chávez, C.R.; Edwards, S.; Moure-Eraso, R.; Geisera, K. Sustainability of bio -based plastics: General comparative analysis and recommendations for improvement. J. Clean. Prod. 2012, 23, 47–56. [Google Scholar] [CrossRef]
- Guo, Y.; Zuo, X.; Xue, Y.; Zhou, Y.; Yang, Z.; Chuang, Y.; Chang, C.; Yuan, G.; Satija, S.K.; Gersappe, D.; et al. Enhancing Impact Resistance of Polymer Blends via Self-Assembled Nanoscale Interfacial Structures. Macromolecules 2018, 51, 3897–3910. [Google Scholar] [CrossRef]
- Li, T.; Zhang, J.; Schneiderman, D.K.; Francis, L.F.; Bates, F.S. Toughening Glassy Poly(lactide) with Block Copolymer Micelles. ACS Macro Lett. 2016, 5, 359–364. [Google Scholar] [CrossRef]
- Lambert, S.; Wagner, M. Environmental performance of bio-based and biodegradable plastics: The road ahead. Chem. Soc. Rev. 2017, 46, 6855–6871. [Google Scholar] [CrossRef]
- Tabone, M.D.; Cregg, J.J.; Beckman, E.J.; Landis, A.E. Sustainability Metrics: Life Cycle Assessment and Green Design in Polymers. Environ. Sci. Technol. 2010, 44, 8264–8269. [Google Scholar] [CrossRef] [PubMed]
- Gan, Z.; Abe, H.; Kurokawa, H.; Doi, Y. Solid-State Microstructures, Thermal Properties, and Crystallization of Biodegradable Poly(butylene succinate)(PBS) and Its Copolyesters. Biomacromolecules 2001, 2, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Javadi, A.; Kramschuster, A.J.; Pilla, S.; Lee, J.; Gong, S.; Turng, L. Processing and Characterization of Microcellular PHBV/PBAT Blends. Polym. Eng. Sci. 2010, 50, 1440–1448. [Google Scholar] [CrossRef]
- Huang, L.; Chen, Z.; Wen, Q.; Lee, D. Enhanced polyhydroxyalkanoate production by mixed microbial culture with extended cultivation strategy. Bioresour. Technol 2017, 241, 802–811. [Google Scholar] [CrossRef]
- Tee, Y.B.; Rosnita, A.T.; Khalina, A.; Chin, N.L.; Roseliza, K.B.; Faezah, M.Y.K. Reinforcing mechanical, water absorption and barrier properties of Poly(Lactic acid) composites with kenaf-Derived cellulose of thermally-Grafted aminosilane Pertanika. J. Trop. Agric. Sci. 2015, 38, 563–573, ISSN 1511-3701. [Google Scholar]
- Laaziz, S.A.; Raji, M.; Hilali, E.; Essabir, H.; Rodrigue, D.; Bouhfid, R.; Qaiss, A. Bio-composites based on polylactic acid and argan nut shell: Production and properties. Int. J. Biol. Macromol. 2017, 104, 30–42. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, J.; Huang, J. Poly(lactic acid)/polyoxymethylene blends: Morphology, rheology, and thermal mechanical properties. Polymer 2015, 69, 103–109. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, W.; Yuan, D.; Xu, C.; Cao, L.; Liang, X. Bio-Based PLA/NR-PMMA/NR Ternary Thermoplastic Vulcanizates with Balanced Stiffness and Toughness: “Soft−Hard” Core−Shell Continuous Rubber Phase, In Situ Compatibilization, and Properties. ACS Sustain. Chem. Eng. 2018, 6, 6488–6496. [Google Scholar] [CrossRef]
- Marra, A.; Silvestre, C.; Duraccio, D.; Cimmino, S. Polylactic acid/zinc oxide biocomposite films for food packaging application. Int. J. Biol. Macromol. 2016, 88, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Wei, X.; Huang, J.; Yang, L.; Zhang, G.; He, G.; Wang, M.; Qu, J. Supertoughened Poly(lactic acid)/Polyurethane Blend Material by in Situ Reactive Interfacial Compatibilization via Dynamic Vulcanization. Ind. Eng. Chem. Res. 2014, 53, 17386–17393. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, J. Research progress in toughening modification of poly(lactic acid). J. Polym. Sci. Polym. Phys. 2011, 49, 1051–1083. [Google Scholar] [CrossRef]
- Choe, I.; Lee, J.H.; Yu, J.H.; Yoon, J. Mechanical Properties of Acrylonitrile-Butadiene-Styrene Copolymer/Poly(L-lactic acid) Blends and Their Composites. J. Appl. Polym. Sci. 2014, 131, 40329. [Google Scholar] [CrossRef]
- Gu, L.; Nessim, E.E.; Macosko, C.W. Reactive compatibilization of poly(lactic acid)/polystyrene blends and its application to preparation of hierarchically porous poly(lactic acid). Polymer 2018, 134, 104–116. [Google Scholar] [CrossRef]
- Wang, S.; Pang, S.; Pan, L.; Xu, N.; Huang, H.; Li, T. Compatibilization of poly(lactic acid)/ethylene-propylene-diene rubber blends by using organic montmorillonite as a compatibilizer. J. Appl. Polym. Sci. 2016, 133, 44192. [Google Scholar] [CrossRef]
- Luyt, A.S.; Gasmi, S. Influence of blending and blend morphology on the thermal properties and crystallization behaviour of PLA and PCL in PLA/ PCL blends. J. Mater. Sci. 2016, 51, 4670–4681. [Google Scholar] [CrossRef]
- Ojijo, V.; Ray, S.S.; Sadiku, R. Toughening of Biodegradable Polylactide/Poly(butylene succinateco-adipate) Blends via in Situ Reactive Compatibilization. ACS Appl. Mater. Interfaces 2013, 5, 4266–4276. [Google Scholar] [CrossRef]
- Fang, H.; Jiang, F.; Wu, Q.; Ding, Y.; Wang, Z. Supertough Polylactide Materials Prepared through In Situ Reactive Blending with PEG-Based Diacrylate Monomer. ACS Appl. Mater. Interfaces 2014, 6, 13552–13563. [Google Scholar] [CrossRef] [PubMed]
- Supthanyakul, R.; Kaabbuathong, N.; Chirachanchaia, S. Random poly(butylene succinate-co-lactic acid) as a multi-functional additive for miscibility, toughness, and clarity of PLA/PBS blends. Polymer 2016, 105, 1–9. [Google Scholar] [CrossRef]
- Yokohara, T.; Yamaguchi, M. Structure and properties for biomass-based polyester blends of PLA and PBS. Eur. Polym. J. 2008, 44, 677–685. [Google Scholar] [CrossRef]
- Liu, Z.; Luo, Y.; Bai, H.; Zhang, Q.; Fu, Q. Remarkably Enhanced Impact Toughness and Heat Resistance of poly(L-Lactide)/Thermoplastic Polyurethane Blends by Constructing Stereocomplex Crystallites in the Matrix. ACS Sustain. Chem. Eng. 2016, 4, 111–120. [Google Scholar] [CrossRef]
- Heshmati, V.; Favis, B.D. High performance poly (lactic acid)/bio-polyamide11 through controlled chain mobility. Polymer 2017, 123, 184–193. [Google Scholar] [CrossRef]
- Rigoussena, A.; Vergea, P.; Raquez, J.; Habibi, Y.; Dubois, P. In-depth investigation on the effect and role of cardanol in the compatibilization of PLA/ABS immiscible blends by reactive extrusion. Eur. Polym. J. 2017, 93, 272–283. [Google Scholar] [CrossRef]
- Garcia-Campo, M.J.; Quiles-Carrillo, L.; Masia, J.; Reig-Pérez, M.J.; Montanes, N.; Balart, R. Environmentally Friendly Compatibilizers from Soybean Oil for Ternary Blends of Poly(lactic acid)-PLA, Poly(ε-caprolactone)-PCL and Poly(3-hydroxybutyrate)-PHB. Materials 2017, 10, 1339. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lv, S.S.I.; Sun, C.; Wan, L.; Tan, H.; Zhang, Y. Effect of MAH-g-PLA on the Properties of Wood Fiber/Polylactic Acid Composites. Polymers 2017, 9, 591. [Google Scholar] [CrossRef]
- Phetwarotai, W.; Phusunti, N.; Aht-Ong, D. Preparation and Characteristics of Poly(butylene adipate-coterephthalate)/Polylactide Blend Films via Synergistic Efficiency of Plasticization and Compatibilization. Chin. J. Polym. Sci. 2019, 37, 68–78. [Google Scholar] [CrossRef]
- He, Y.; Yang, Z.; Qu, J. Super-toughed poly(lactic acid)/thermoplastic poly(ether)urethane nanofiber composites with in-situ formation of aligned nanofibers prepared by an innovative eccentric rotor extruder. Compos. Sci. Technol. 2019, 169, 135–141. [Google Scholar] [CrossRef]
- Virgilio, N.; Marc-Aurele, C.; Basil, D. Favis, Novel Self-Assembling Close-Packed Droplet Array at the Interface inTernary Polymer Blends. Macromolecules 2009, 42, 3405–3416. [Google Scholar] [CrossRef]
- Harkins, W.D.; Feldman, A. Films. The spreading of liquids and the spreading coefficient. J. Am. Chem. Soc. 1922, 44, 2665–2685. [Google Scholar] [CrossRef]
- Margolina, A.; Wu, S. Percolation model for brittle-tough transition in nylon/rubber blends. Polymer 1988, 29, 2170–2173. [Google Scholar] [CrossRef]
- Su, J.; Zhu, F.; Meng, Y.; Han, J.; Wang, K.; Fu, Q. Significant toughness improvement in iPP/PLLA/EGMA blend by introducing dicumyl peroxide as the morphology governor. Colloid Polym. Sci. 2018, 296, 31–39. [Google Scholar] [CrossRef]
- Hristov, V.; Krumova, M.; Michler, G. The Influence of Excess Coupling Agent on the Microdeformation Processes and Mechanical Properties of Poly(propylene)/Wood-Flour Composites. Macromol. Mater. Eng. 2006, 291, 677–683. [Google Scholar] [CrossRef]
- Zhang, K.; Nagarajan, V.; Misra, M.; Mohanty, A.K. Supertoughened Renewable PLA Reactive Multiphase Blends System: Phase Morphology and Performance. ACS Appl. Mater. Interfaces 2014, 6, 12436–12448. [Google Scholar] [CrossRef] [PubMed]
- Najafi, N.; Heuzey, M.C.; Carreau, P.J. Polylactide (PLA)-clay nanocomposites prepared by melt compounding in the presence of a chain extender. Compos. Sci. Technol. 2012, 72, 608–615. [Google Scholar] [CrossRef]
- Lan, H.; Wang, H.; Cao, L.; Wang, Z. Analysis on Stress Concentration Factor for Elliptical Hole Based on FEM Theory. In Informatics and Management Science III. Lecture Notes in Electrical Engineering; Du, W., Ed.; Springer: London, UK, 2013; Volume 206, pp. 707–715. [Google Scholar]






| Sample | Surface Tension (mN/m) | Interfacial Tension (mN/m) | Spreading Coefficient (mN/m) | ||
|---|---|---|---|---|---|
| Total (λ) | Dispersion (λd) (cd) | Polar (λp) | |||
| PLA | 42.29 | 33.27 | 9.02 | γPLA/PBS = 0.9 | λPLA/EGMA/PBS = −9.36 |
| PBS | 45.46 | 36.44 | 9.01 | γPBS/EGMA = 5.24 | λEGMA/PLA/PBS = −1.12 |
| EGMA | 30.72 | 30.11 | 0.61 | γPLA/EGMA = 5.02 | λEGMA/PBS/PLA = −0.68 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, B.; He, H.; Zhu, Z.; Li, J.; Huang, Z.; Wang, G.; Chen, M.; Zhan, Z. A Facile Fabrication of High Toughness Poly(lactic Acid) via Reactive Extrusion with Poly(butylene Succinate) and Ethylene-Methyl Acrylate-Glycidyl Methacrylate. Polymers 2018, 10, 1401. https://doi.org/10.3390/polym10121401
Xue B, He H, Zhu Z, Li J, Huang Z, Wang G, Chen M, Zhan Z. A Facile Fabrication of High Toughness Poly(lactic Acid) via Reactive Extrusion with Poly(butylene Succinate) and Ethylene-Methyl Acrylate-Glycidyl Methacrylate. Polymers. 2018; 10(12):1401. https://doi.org/10.3390/polym10121401
Chicago/Turabian StyleXue, Bin, Hezhi He, Zhiwen Zhu, Jiqian Li, Zhaoxia Huang, Guozhen Wang, Ming Chen, and Zhiming Zhan. 2018. "A Facile Fabrication of High Toughness Poly(lactic Acid) via Reactive Extrusion with Poly(butylene Succinate) and Ethylene-Methyl Acrylate-Glycidyl Methacrylate" Polymers 10, no. 12: 1401. https://doi.org/10.3390/polym10121401
APA StyleXue, B., He, H., Zhu, Z., Li, J., Huang, Z., Wang, G., Chen, M., & Zhan, Z. (2018). A Facile Fabrication of High Toughness Poly(lactic Acid) via Reactive Extrusion with Poly(butylene Succinate) and Ethylene-Methyl Acrylate-Glycidyl Methacrylate. Polymers, 10(12), 1401. https://doi.org/10.3390/polym10121401