A Novel Ziegler–Natta-Type Catalytic System—TiCl4/2,2′-Dimethoxy-1,1′-Binaphthalene/Et3Al2Cl3/Bu2Mg for Production of Ultrahigh Molecular Weight Polyethylene Nascent Reactor Powders, Suitable for Solvent-Free Processing
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Pre-Catalyst
2.2. Polymerization of Ethylene
2.3. Polymer Evaluation Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sauter, D.W.; Taoufik, M.; Boisson, C. Polyolefins, a Success Story. Polymers 2017, 9, 185. [Google Scholar] [CrossRef]
- Baier, M.C.; Zuideveld, M.A.; Mecking, S. Post-metallocenes in the industrial production of polyolefins. Angew. Chem. Int. Ed. 2014, 53, 9722–9744. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, M.S. The UHMWPE Handbook: Ultra High Molecular Weight Polyethylene in Total Joint Replacement, 1st ed.; Elsevier Academic Press: New York, NY, USA, 2004; ISBN 9780080481463. [Google Scholar]
- Smith, P.; Chanzy, H.D.; Rotzinger, B.P. Drawing of virgin ultrahigh molecular weight polyethylene: An alternative route to high strength fibres. Polym. Commun. 1985, 26, 258–260. [Google Scholar]
- Smith, P.; Chanzy, H.D.; Rotzinger, B.P. Drawing of virgin ultrahigh molecular weight polyethylene: An alternative route to high strength/high modulus materials. J. Mater. Sci. 1987, 22, 523–531. [Google Scholar] [CrossRef]
- Rotzinger, B.P.; Chanzy, H.D.; Smith, P. High strength/high modulus polyethylene: synthesis and processing of ultra-high molecular weight virgin powders. Polymer 1989, 30, 1814–1819. [Google Scholar] [CrossRef]
- Rastogi, S.; Yao, Y.; Ronca, S.; Bos, Y.; van der Eem, J. Unprecedented High-Modulus High-Strength Tapes and Films of Ultrahigh Molecular Weight Polyethylene via Solvent-Free Route. Macromolecules 2011, 44, 5558–5568. [Google Scholar] [CrossRef]
- Ozerin, A.N.; Ivanchev, S.S.; Chvalun, S.N.; Aulov, V.A.; Ivancheva, N.I.; Bakeev, N.F. Properties of oriented film tapes prepared via solid-state processing of a nascent ultrahigh-molecular-weight polyethylene reactor powder synthesized with a postmetallocene catalyst. Polym. Sci. 2012, 54, 950–954. [Google Scholar] [CrossRef]
- Pandey, A.; Champouret, Y.; Rastogi, S. Heterogeneity in the Distribution of Entanglement Density during Polymerization in Disentangled Ultrahigh Molecular Weight Polyethylene. Macromolecules 2011, 44, 4952–4960. [Google Scholar] [CrossRef]
- Romano, D.; Andablo-Reyes, E.A.; Ronca, S.; Rastogi, S. Effect of a Cocatalyst Modifier in the Synthesis of Ultrahigh Molecular Weight Polyethylene having Reduced Number of Entanglements. J. Polym. Sci. A 2013, 51, 1630–1635. [Google Scholar] [CrossRef]
- Romano, D.; Ronca, S.; Rastogi, S. A Hemi-metallocene Chromium Catalyst with Trimethylaluminum-Free Methylaluminoxane for the Synthesis of Disentangled Ultra-High Molecular Weight Polyethylene. Macromol. Rapid Commun. 2015, 36, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Gagieva, S.C.; Tuskaev, V.A.; Fedyanin, I.V.; Sizov, A.I.; Mikhaylik, E.S.; Golubev, E.K.; Bulychev, B.M. Chloride- and alkoxo-titanium(IV) complexes stabilized by 2-hydroxymethylphenol derivative as catalysts for the formation of ultra-high molecular weight polyethylene nascent reactor powders. Polyhedron 2017, 122, 179–183. [Google Scholar] [CrossRef]
- Gagieva, S.C.; Tuskaev, V.A.; Fedyanin, I.V.; Buzin, M.I.; Vasil’ev, V.G.; Nikiforova, G.G.; Afanas’ev, E.S.; Zubkevich, S.V.; Kurmaev, D.A.; Kolosov, N.A.; et al. Novel titanium (IV) diolate complexes: Synthesis, structure and catalytic activities in ultra-high molecular weight polyethylene production. J. Organomet. Chem. 2017, 828, 89–95. [Google Scholar] [CrossRef]
- Böhm, L.L. The Ethylene Polymerization with Ziegler Catalysts: Fifty Years after the Discovery. Angew. Chem. Int. Ed. 2003, 42, 5010–5030. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.X.; Shin, Y.J.; Lee, D.H.; Yoon, K.B. Preparation of ultra high molecular weight polyethylene with MgCl2/TiCl4 catalyst: Effect of internal and external donor on molecular weight and molecular weight distribution. Polym. Bull. 2011, 66, 627–635. [Google Scholar] [CrossRef]
- Qiao, J.; Guo, M.; Wang, L.; Liu, D.; Zhang, X.; Yu, L.; Song, W.; Liu, Y. Recent advances in polyolefin technology. Polym. Chem. 2011, 2, 1611–1623. [Google Scholar] [CrossRef]
- Joo, Y.K.; Zhou, H.; Lee, S.G.; Lee, H.K.; Song, J.K. Solid-State Compaction and Drawing of Nascent Reactor Powders of Ultra-High-Molecular-Weight Polyethylene. J. Appl. Polym. Sci. 2005, 98, 718–730. [Google Scholar] [CrossRef]
- Joo, Y.L.; Han, O.H.; Lee, H.K.; Song, J.K. Characterization of ultra high molecular weight polyethyelene nascent reactor powders by X-ray diffraction and solid state NMR. Polymer 2000, 41, 1355–1368. [Google Scholar] [CrossRef]
- Tuskaev, V.A.; Gagieva, S.C.; Kurmaev, D.A.; Kolosov, N.A.; Mikhaylik, E.S.; Golubev, E.K.; Sizov, A.I.; Zubkevich, S.V.; Vasil’ev, V.G.; Nikiforova, G.G.; et al. Titanium(III, IV)-Containing Catalytic Systems for Production of Ultrahigh Molecular Weight Polyethylene Nascent Reactor Powders, Suitable for Solventless Processing—Impact of Oxidation States of Transition Metal. Polymers 2018, 10, 2. [Google Scholar] [CrossRef]
- Zahedi, R.; Taromi, F.A.; Mirjahanmardi, S.H.; Haghighi, M.N.; Jadidie, K.; Jamjah, R. New Penta-ether as the Internal Donor in the MgCl2-supported Ziegler-Natta Catalysts for Propylene Polymerization. Chin. J. Polym. Sci. 2016, 34, 268–279. [Google Scholar] [CrossRef]
- Mirjahanmardi, S.H.; Taromi, F.A.; Zahedi, R.; Haghighi, M.N. Effects of Various Amounts of New Hepta-Ether as the Internal Donor on the Polymerization of Propylene with and without the External Donor. Polym. Sci. Ser. B 2017, 59, 639–649. [Google Scholar] [CrossRef]
- Zahedi, R.; Taromi, F.A.; Mirjahanmardi, S.H.; Haghighi, M.N.; Jadidi, K.; Jamjah, R. Propylene Polymerization over MgCl2-Supported Ziegler–Natta Catalysts Containing Tri-Ether as the Internal Donor. Adv. Powder Technol. 2018, 37, 144–153. [Google Scholar] [CrossRef]
- Zhang, L.T.; Fan, Z.Q.; Fu, Z. Effect of internal electron donor on copolymerization of ethylene and 1-hexene catalyzed by supported Ziegler- Natta catalysts. E-Polymers 2008, 8, 143. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, L.; Fu, Z.; Fan, Z. Effect of internal electron donor on the active center distribution in MgCl2-supported Ziegler-Natta catalyst. Catal. Commun. 2015, 69, 147–149. [Google Scholar] [CrossRef]
- Raj, K.V.; Vanka, K. Understanding Ziegler-Natta Catalysis through Your Laptop. Resonance 2017, 22, 1025–1037. [Google Scholar] [CrossRef]
- Ishiwari, F.; Fukasawa, K.; Sato, T.; Nakazono, K.; Koyama, Y.; Takata, T. A Rational Design for the Directed Helicity Change of Polyacetylene Using Dynamic Rotaxane Mobility by Means of Through-Space Chirality Transfer. Chem. Eur. J. 2011, 17, 12067–12075. [Google Scholar] [CrossRef] [PubMed]
- Talebi, S.; Duchateau, R.; Rastogi, S.; Kaschta, J.; Peters, G.W.M.; Lemstra, P.J. Molar Mass and Molecular Weight Distribution Determination of UHMWPE Synthesized Using a Living Homogeneous Catalyst. Macromolecules 2010, 43, 2780–2788. [Google Scholar] [CrossRef]
- Ivancheva, N.I.; Sanieva, D.V.; Fedorov, S.P.; Oleinik, I.V.; Oleinik, I.I.; Tolstikov, G.A.; Ivancheva, S.S. Self-immobilized catalysts for ethylene polymerization based on various phenoxyimine titanium halide complexes. Russ. Chem. Bull. 2012, 61, 836–842. [Google Scholar] [CrossRef]
- Uehara, H.; Nakae, M.; Kanamoto, T.; Ohtsu, O.; Sano, A.; Matsuura, K. Structural characterization of ultrahigh molecular-weight polyethylene reactor powders based on fuming nitric acid etching. Polymer 1998, 39, 6127–6135. [Google Scholar] [CrossRef]
- Michler, G.H.; Seydewitz, V.; Buschnakowski, M.; Myasnikowa, L.P.; Ivan’kova, E.M.; Marikhin, V.A.; Boiko, Y.M.; Goerlitz, S. Correlation among powder morphology, compactability, and mechanical properties of consolidated nascent UHMWPE. J. Appl. Polymer Sci. 2010, 118, 866–875. [Google Scholar] [CrossRef]
- Graff, R.J.L.; Kortleve, G.; Vonk, C.G. On the size of the primary particles in ziegler catalysts. J. Polym. Sci. Polym. Lett. 1970, 8, 735–739. [Google Scholar] [CrossRef]
- Chanzy, H.D.; Revol, J.F.; Marchessault, R.H.; Lamandé, A. Nascent structures during the polymerization of ethylene. Kolloid-Z. Z. Polym. 1973, 251, 563–576. [Google Scholar] [CrossRef]
- Aulov, V.A.; Shcherbina, M.A.; Chvalun, S.N.; Makarov, S.V.; Kuchkina, I.O.; Pantyukhin, A.A.; Bakeev, N.F.; Pavlov Yu, S. Monoclinic phase in reactor powders of ultra-high-molecular-weight polyethylene and its changes during compaction and monolithization. Polym. Sci. Ser. A 2004, 46, 620–626. [Google Scholar]
- Litvinov, V.M.; Xu, J.; Melian, C.; Demco, D.E.; Möller, M.; Simmelink, J. Morphology, Chain Dynamics, and Domain Sizes in Highly Drawn Gel-Spun Ultrahigh Molecular Weight Polyethylene Fibers at the Final Stages of Drawing by SAXS, WAXS, and 1H Solid-State NMR. Macromolecules 2011, 44, 9254–9266. [Google Scholar] [CrossRef]
- Kikuchi, Y.; Krimm, S.J. Infrared studies of the role of monoclinic structure in the deformation of polyethylene. J. Macromol. Sci. Phys. 1970, 4, 461–472. [Google Scholar] [CrossRef]
- Jarrett, W.L.; Mathias, L.J.; Porter, R.S. Solid-state carbon-13 NMR study of polyethylene reactor powders. Macromolecules 1990, 23, 5164–5166. [Google Scholar] [CrossRef]
- Ivan’kova, E.M.; Myasnikova, L.P.; Marikhin, V.A.; Baulin, A.A.; Volchek, B.Z. On the memory effect in Uhmwpe nascent powders. J. Macromol. Sci. Phys. 2001, 40, 813–832. [Google Scholar] [CrossRef]
- Tashiro, K.; Sasaki, S.; Kobayashi, M. Structural Investigation of Orthorhombic-to-Hexagonal Phase Transition in Polyethylene Crystal: The Experimental Confirmation of the Conformationally Disordered Structure by X-ray Diffraction and Infrared/Raman Spectroscopic Measurements. Macromolecules 1996, 29, 7460–7469. [Google Scholar] [CrossRef]
- Tsubakihara, S.; Nakamura, A.; Yasuniwa, M. Hexagonal Phase of Polyethylene Fibers under High Pressure. Polym. J. 1991, 23, 1317–1324. [Google Scholar] [CrossRef]
- Fu, Y.; Chen, W.; Pyda, M.; Londono, D.; Annis, B.; Boller, A.; Habenschuss, A.; Cheng, J.; Wunderlich, B. Structure-property analysis for gel-spun, ultrahigh molecular mass polyethylene fibers. J. Macromol. Sci. Part B Phys. 1996, 35, 37–87. [Google Scholar] [CrossRef]


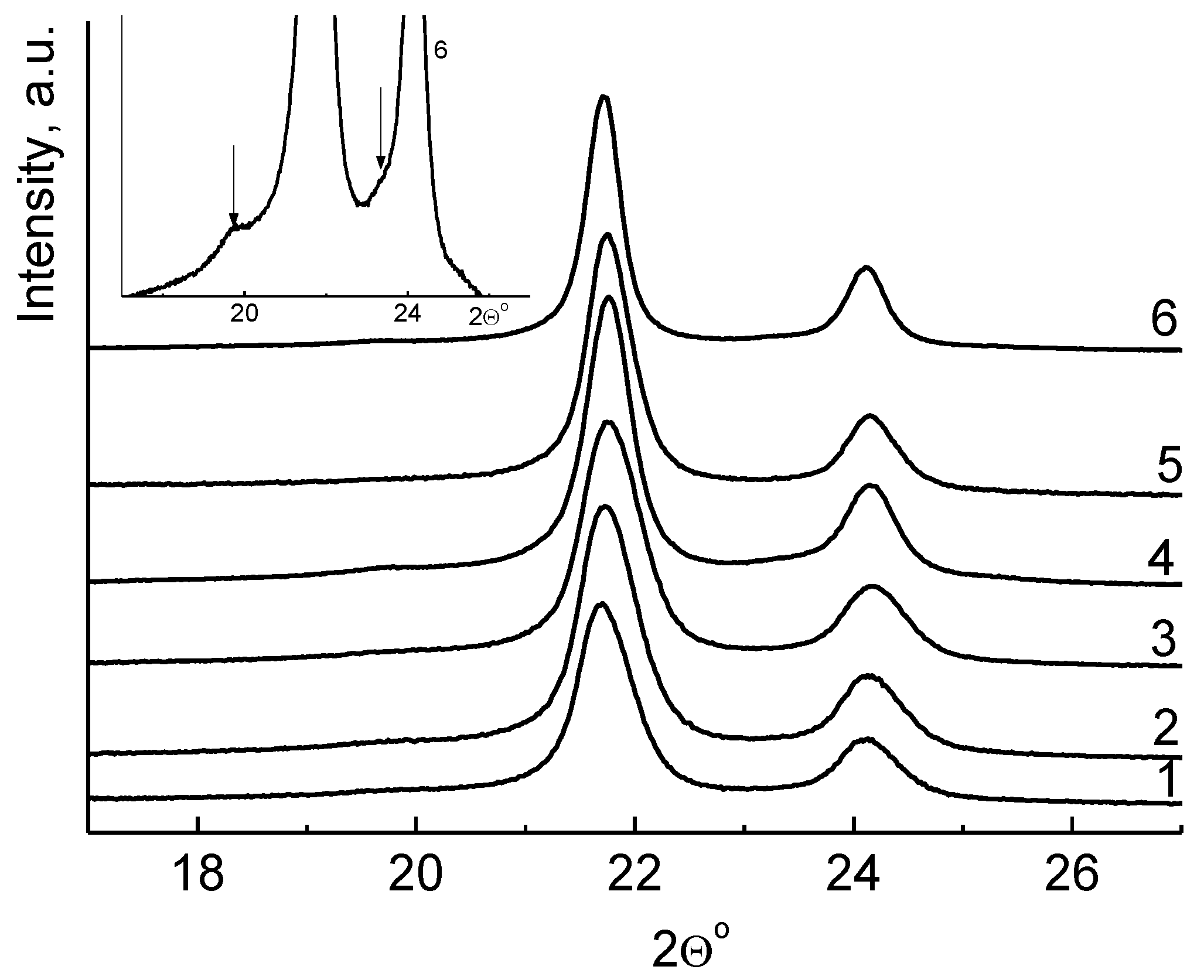
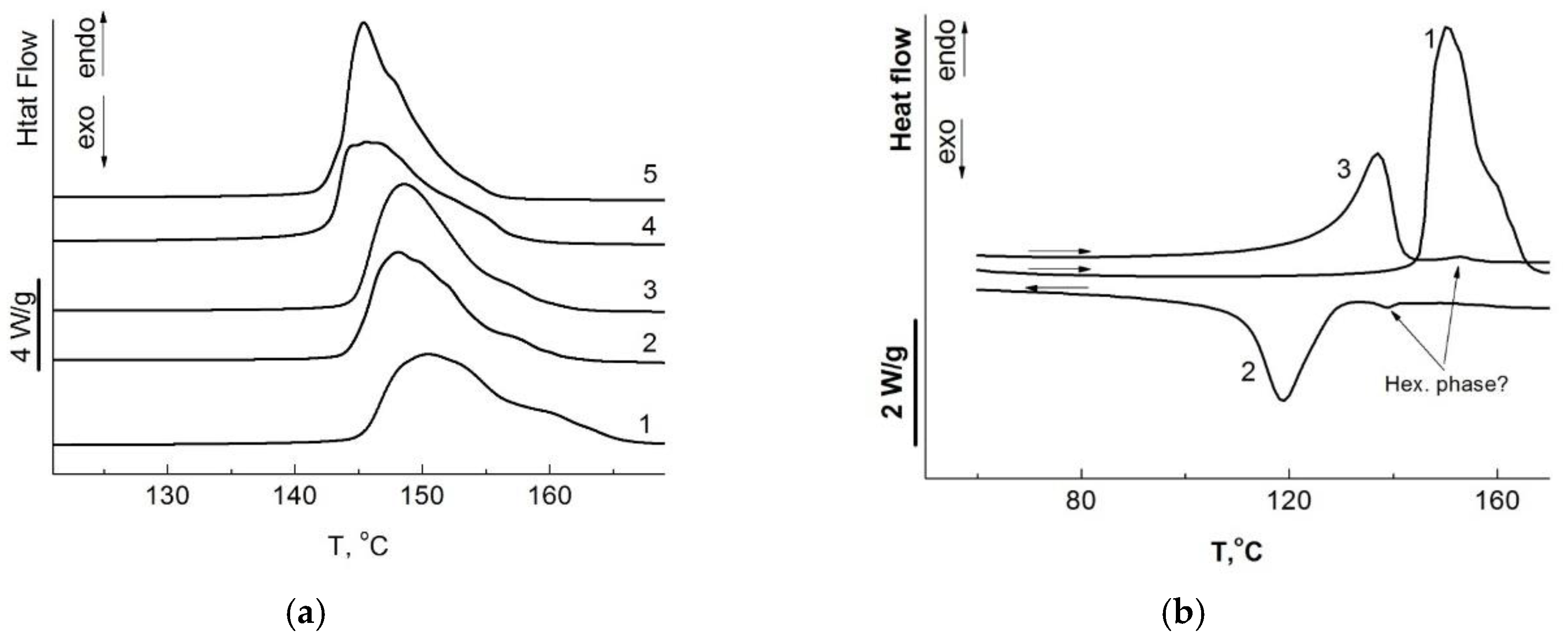
| Entry | Tpoly, °C | Activity, kg polyethylene /(mol Ti h atm) | Activity, kg polyethylene /(mol Ti h [C2H4]) | Mv, b 106 Da |
|---|---|---|---|---|
| 1 | 10 | 1200 | 4970 | 5.6 |
| 2 | 22 | 3085 | 15,200 | 4.8 |
| 3 | 30 | 3097 | 15,260 | 4.6 |
| 4 | 40 | 2630 | 16,283 | 2.0 |
| 5 | 50 | 2485 | 20,470 | 1.6 |
| 6 | 70 | 2000 | 18,700 | 1.7 |
| Tpoly, °C | First Heating | Cooling | Reheating | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Tm1, °C | ΔHm1, J/g | αm1, % | Tc, °C | ΔHc, J/g | αc, % | Tm2, °C | ΔHm2, J/g | αm2, % | |
| 10 | 142 | 224 | 75 | 118 | 123 | 41 | 136 | 120 | 40 |
| 22 | 141 | 216 | 72 | 116 | 130 | 43 | 136 | 130 | 44 |
| 30 | 142 | 220 | 74 | 115 | 131 | 44 | 137 | 132 | 44 |
| 40 | 138 | 207 | 69 | 117 | 149 | 50 | 137 | 149 | 50 |
| 50 | 138 | 206 | 69 | 116 | 156 | 52 | 136 | 154 | 52 |
| 70 | 137 | 207 | 69 | 116 | 165 | 55 | 137 | 165 | 55 |
| Tpoly, °C | Mw, 106 Da | Drawing Ratio | E, GPa | σ, GPa | ε, % |
|---|---|---|---|---|---|
| 10 | 5.6 | 32 | 136 | 2.3 | 2.0 |
| 22 | 4.8 | 32 | 136 | 2.4 | 2.2 |
| 30 | 4.6 | 28 | 125 | 2.5 | 2.5 |
| 40 | 2.0 | 20 | 100 | 1.8 | 2.5 |
| 70 | 1.7 | 20 | 96 | 1.8 | 3.3 |
| Tpoly, °C | First Heating | Cooling | Reheating | |||
|---|---|---|---|---|---|---|
| Tm1, °C | ΔHm1, J/g | Tc, °C | ΔHc, J/g | Tm2, °C | ΔHm2, J/g | |
| 10 (32) * | 150 | 267 | 119 | 119 | 136 | 124 |
| 139 ** | 0.05 ** | 153 | 2,0 | |||
| 22 (32) | 148 | 265 | 117 | 140 | 136 | 142 |
| 30 (28) | 148 | 271 | 119 | 129 | 136 | 131 |
| 40 (20) | 145 | 259 | - | - | 136 | 155 |
| 70 (20) | 145 | 253 | 114 | 156 | 137 | 164 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serenko, O.A.; Buzin, M.I.; Tuskaev, V.A.; Gagieva, S.C.; Kolosov, N.A.; Kurmaev, D.A.; Savel’eva, T.F.; Golubev, E.K.; Zubkevich, S.V.; Vasil’ev, V.G.; et al. A Novel Ziegler–Natta-Type Catalytic System—TiCl4/2,2′-Dimethoxy-1,1′-Binaphthalene/Et3Al2Cl3/Bu2Mg for Production of Ultrahigh Molecular Weight Polyethylene Nascent Reactor Powders, Suitable for Solvent-Free Processing. Polymers 2018, 10, 1281. https://doi.org/10.3390/polym10111281
Serenko OA, Buzin MI, Tuskaev VA, Gagieva SC, Kolosov NA, Kurmaev DA, Savel’eva TF, Golubev EK, Zubkevich SV, Vasil’ev VG, et al. A Novel Ziegler–Natta-Type Catalytic System—TiCl4/2,2′-Dimethoxy-1,1′-Binaphthalene/Et3Al2Cl3/Bu2Mg for Production of Ultrahigh Molecular Weight Polyethylene Nascent Reactor Powders, Suitable for Solvent-Free Processing. Polymers. 2018; 10(11):1281. https://doi.org/10.3390/polym10111281
Chicago/Turabian StyleSerenko, Olga A., Mikhail I. Buzin, Vladislav A. Tuskaev, Svetlana C. Gagieva, Nikolay A. Kolosov, Dmitrii A. Kurmaev, Tatyana F. Savel’eva, Evgenii K. Golubev, Sergey V. Zubkevich, Viktor G. Vasil’ev, and et al. 2018. "A Novel Ziegler–Natta-Type Catalytic System—TiCl4/2,2′-Dimethoxy-1,1′-Binaphthalene/Et3Al2Cl3/Bu2Mg for Production of Ultrahigh Molecular Weight Polyethylene Nascent Reactor Powders, Suitable for Solvent-Free Processing" Polymers 10, no. 11: 1281. https://doi.org/10.3390/polym10111281
APA StyleSerenko, O. A., Buzin, M. I., Tuskaev, V. A., Gagieva, S. C., Kolosov, N. A., Kurmaev, D. A., Savel’eva, T. F., Golubev, E. K., Zubkevich, S. V., Vasil’ev, V. G., Nikiforova, G. G., Korlyukov, A. A., & Bulychev, B. M. (2018). A Novel Ziegler–Natta-Type Catalytic System—TiCl4/2,2′-Dimethoxy-1,1′-Binaphthalene/Et3Al2Cl3/Bu2Mg for Production of Ultrahigh Molecular Weight Polyethylene Nascent Reactor Powders, Suitable for Solvent-Free Processing. Polymers, 10(11), 1281. https://doi.org/10.3390/polym10111281






