Biocompatibility of Poly(acrylonitrile-butadiene-styrene) Nanocomposites Modified with Silver Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
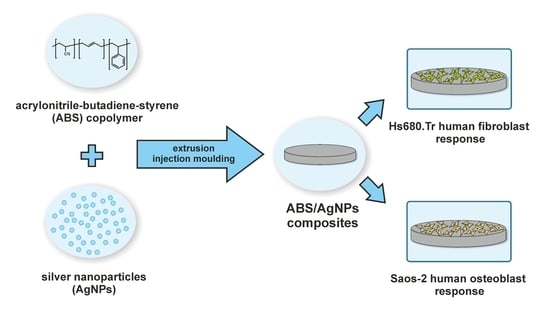
2.1. Material Manufacturing
2.2. Material Evaluation
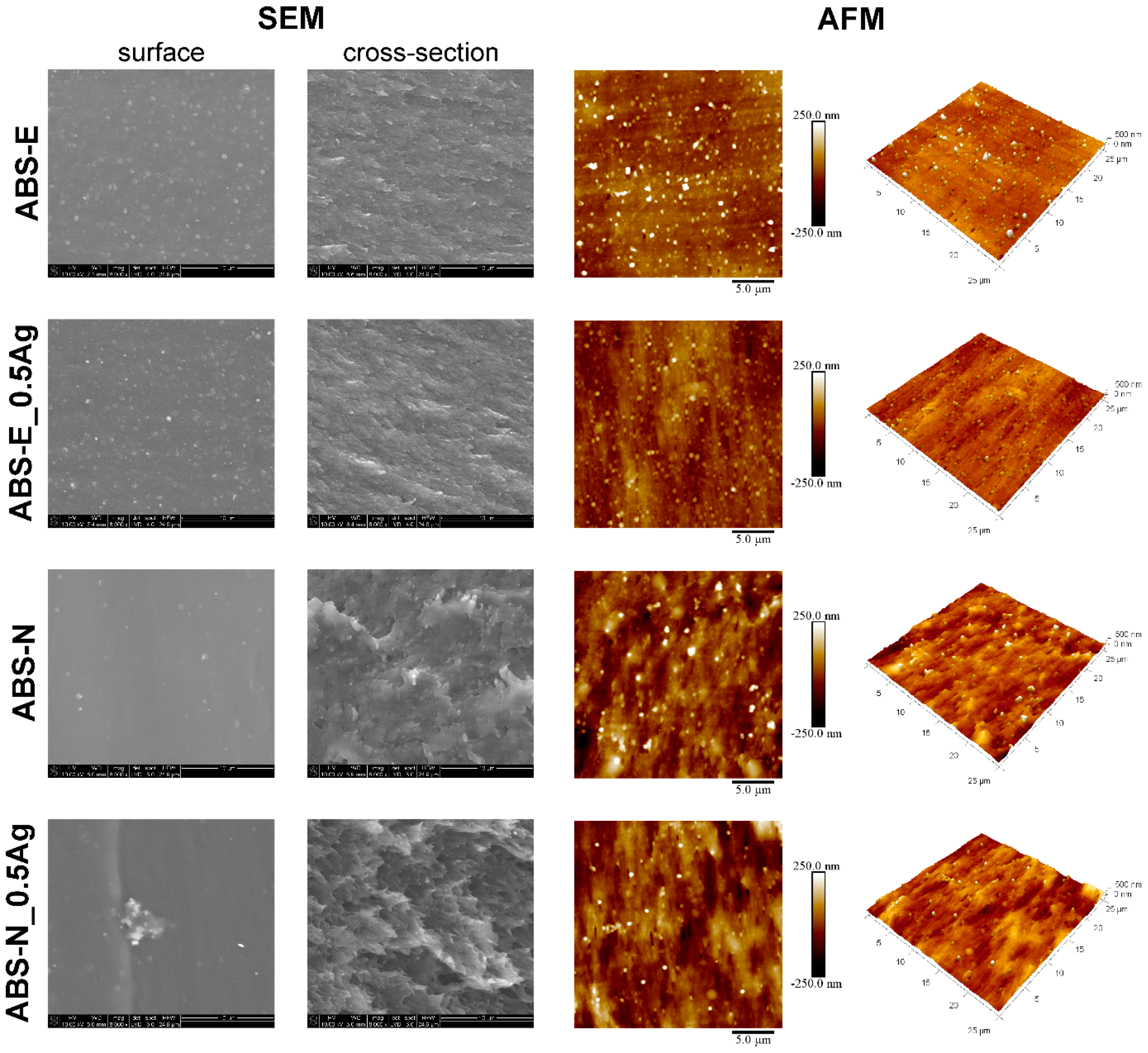
2.2.1. Scanning Electron Microscopy
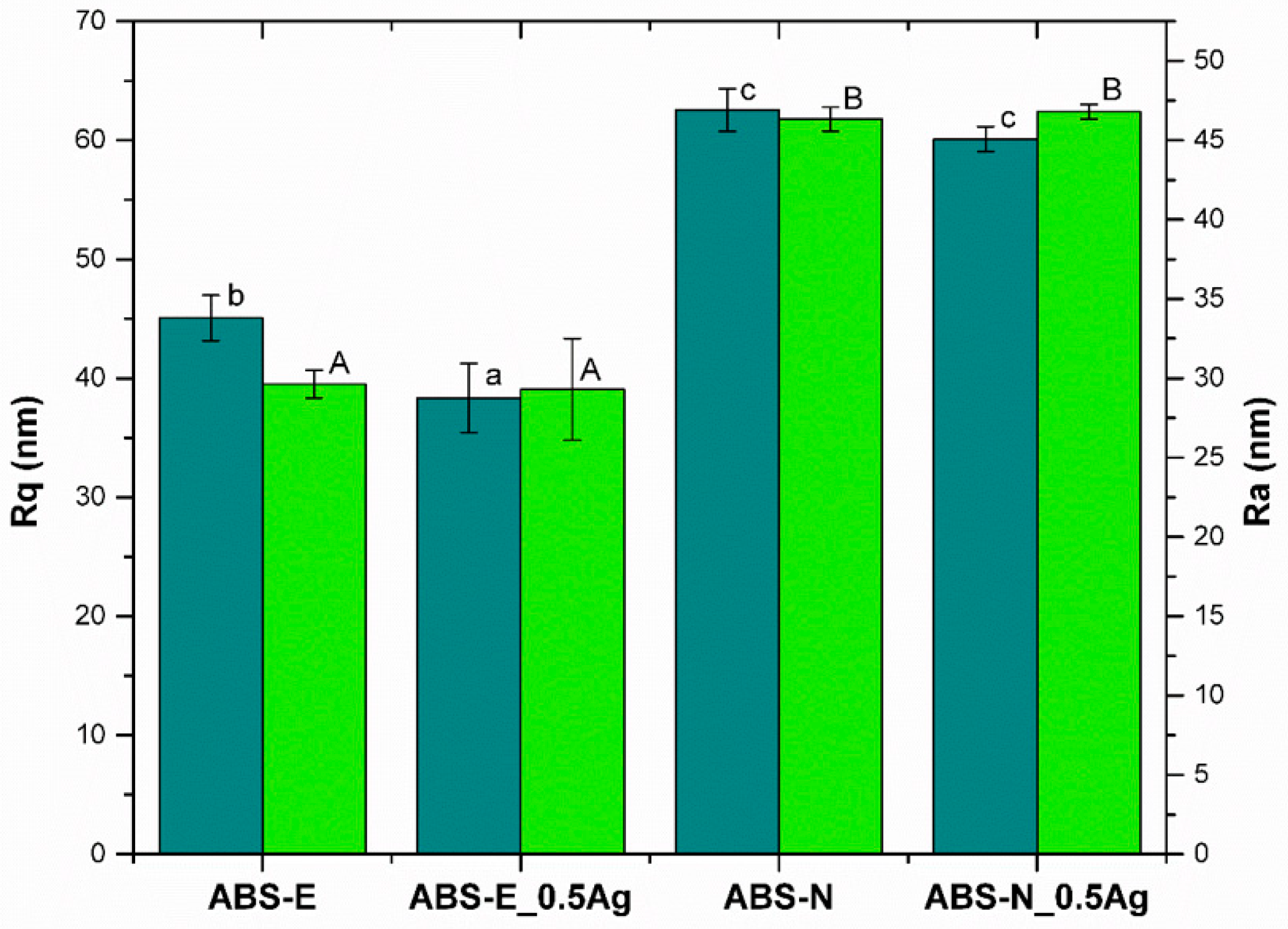
2.2.2. Atomic Force Microscopy
2.2.3. Surface Wettability
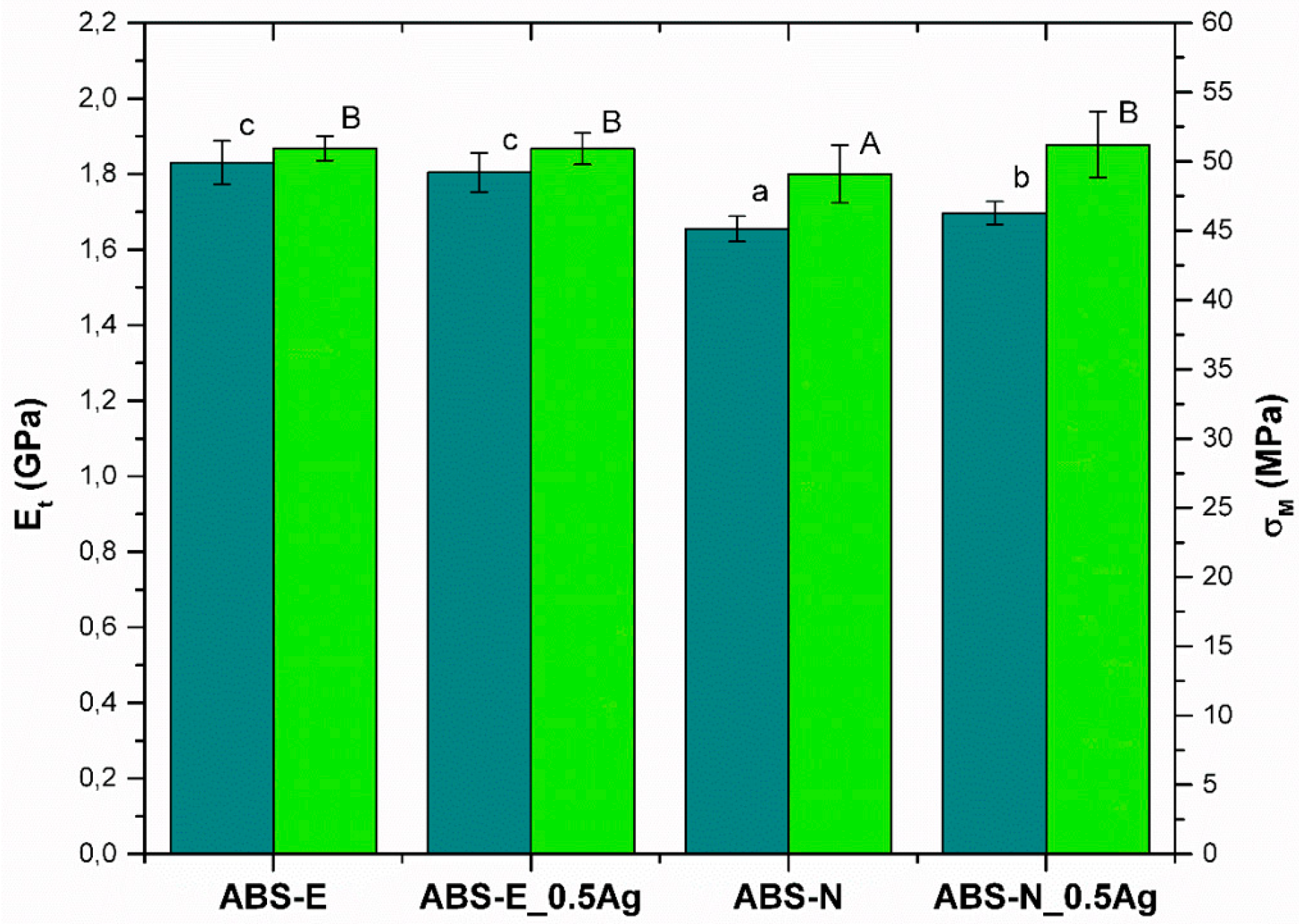
2.2.4. Tensile Test
2.2.5. In Vitro Tests
2.2.6. Statistical analysis
3. Results
3.1. SEM and AFM Measurements
3.2. Surface Wettability
3.3. Mechanical Properties
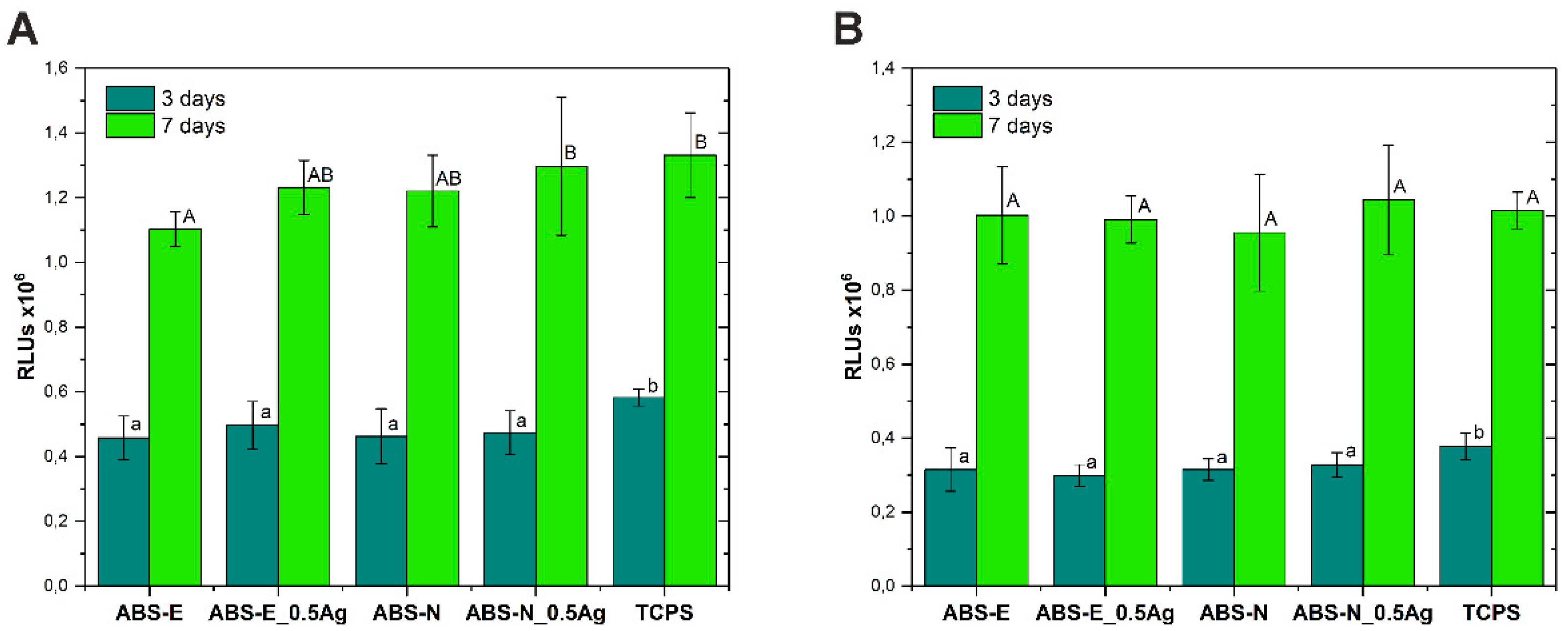
3.4. Cell Viability/Proliferation Test
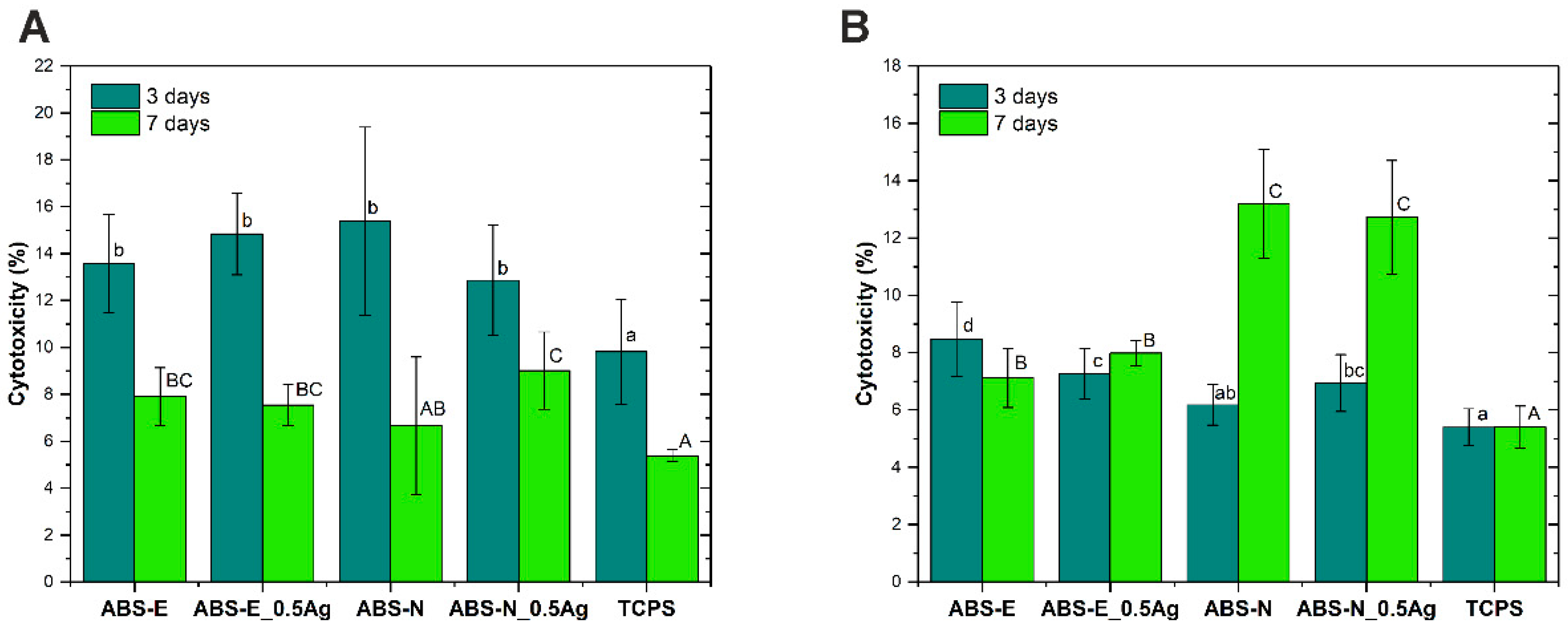
3.5. Cytotoxicity Test
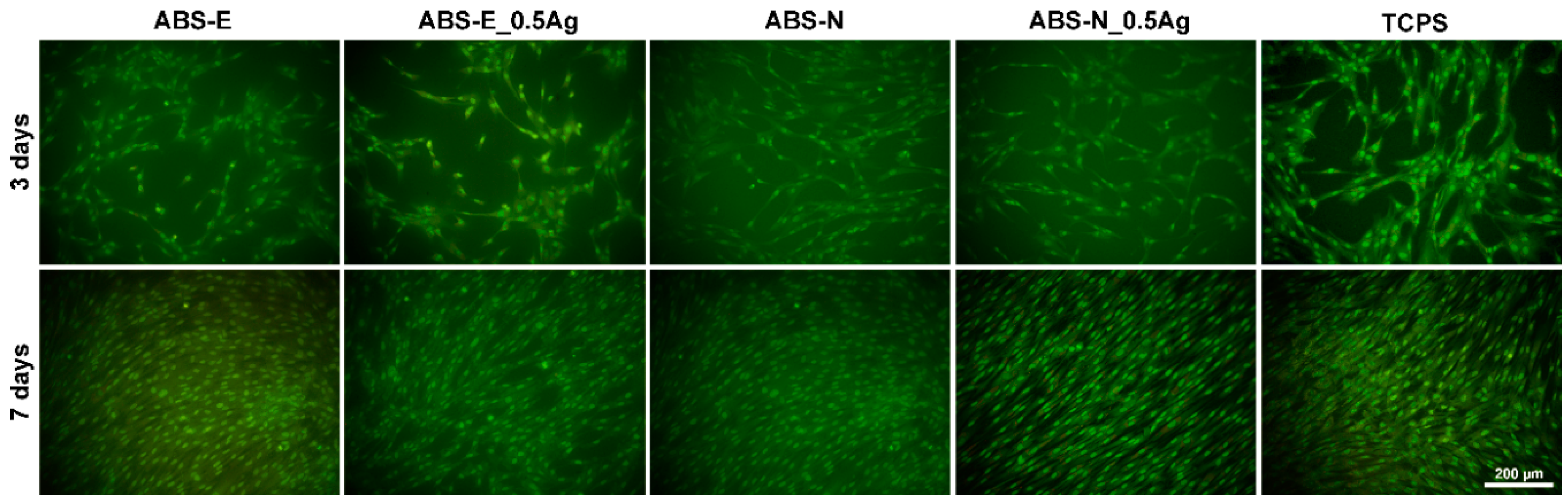
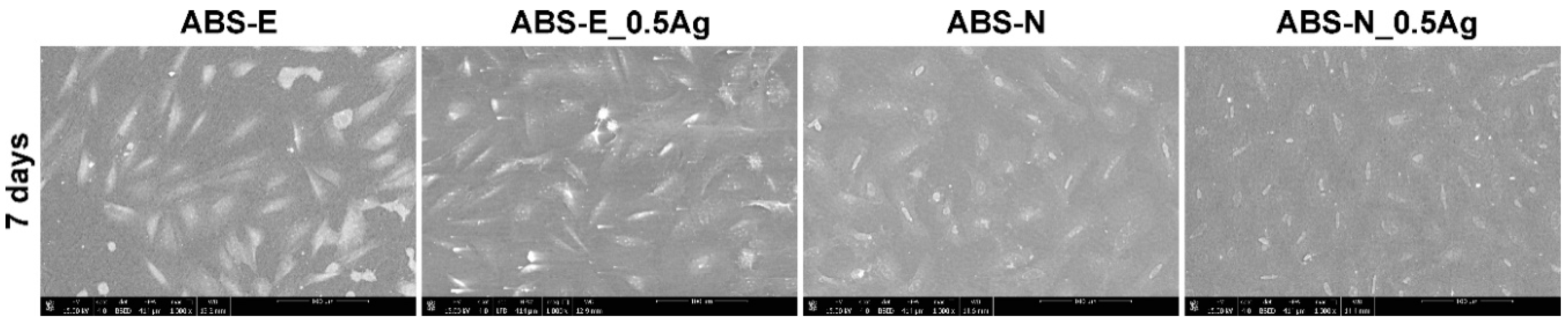
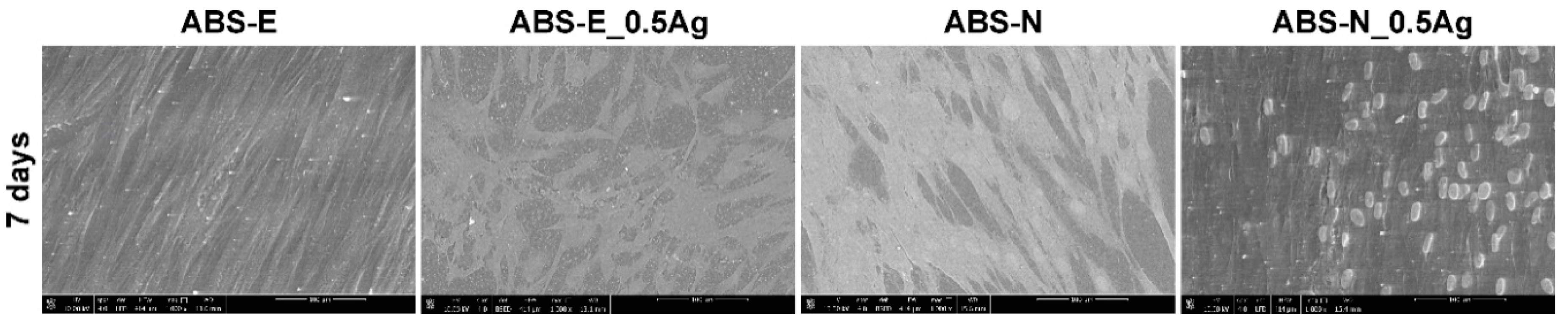
3.6. Cell Cultures Observation
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Feldman, D. Polymer nanocomposites in medicine. J. Macromol. Sci. 2016, 53, 55–62. [Google Scholar] [CrossRef]
- Luo, Q.; Gao, H.; Peng, L.; Liu, G.; Zhang, Z. Synthesis of PEGylated chitosan copolymers as efficiently antimicrobial coatings for leather. J. Appl. Polym. Sci. 2016, 133, 43465. [Google Scholar] [CrossRef]
- Bigdeli, A.; Lyer, S.; Detsch, R.; Boccaccini, A.R.; Beier, J.P.; Kneser, U.; Horch, R.E.; Arkudas, A. Nanotechnologies in tissue engineering. Nanotechnol. Rev. 2013, 2, 411–425. [Google Scholar] [CrossRef]
- Directive 93/42/EEC of the European Parliament and of the Council of 14 June 1993 Concerning Medical Devices. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CONSLEG:1993L0042:20071011:en:PDF (accessed on 1 February 2003).
- PN-EN ISO 10993-5:2009. Biological Evaluation of Medical Devices Part 5: Tests for In Vitro Cytotoxicity; International Organization for Standardization: Geneva, Switzerland, 2009. [Google Scholar]
- Kononenko, V.; Narat, M.; Drobne, D. Nanoparticle interaction with the immune system. Arch. Ind. Hyg. Toxicol. 2015, 66, 97–108. [Google Scholar]
- Hirai, T.; Yoshioka, Y.; Ichihashi, K.; Mori, T.; Nishijima, N.; Handa, T.; Takahashi, H.; Tsunoda, S.; Higashisaka, K.; Tsutsumi, Y. Silver nanoparticles induce silver nanoparticle-specific allergic responses. J. Immunol. 2014, 192, 118–119. [Google Scholar]
- Capasso, L.; Camatini, M.; Gualtieri, M. Nickel oxide nanoparticles induce inflammation and genotoxic effect in lung epithelial cells. Toxicol. Lett. 2014, 226, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Radomski, A.; Jurasz, P.; Alonso-Escolano, D.; Drews, M.; Morandi, M.; Malinski, T.; Radomski, M.W. Nanoparticle-induced platelet aggregation and vascular thrombosis. Br. J. Pharmacol. 2005, 146, 882–893. [Google Scholar] [CrossRef] [PubMed]
- Morones, J.; Elechiguerra, J.; Camacho, A.; Holt, K.; Kouri, J.B.; Ramírez, J.T.; Yacaman, M.J. The bactericidal effect of silver nanoparticles. Nanotechnology 2005, 16, 2346–2353. [Google Scholar] [CrossRef] [PubMed]
- Duran, N.; Marcato, P.; De Conti, R.; Alves, O.L.; Costa, F.T.M.; Brocchi, M. Potential use of silver nanoparticles on pathogenic bacteria, their toxicity and possible mechanisms of action. J. Braz. Chem. Soc. 2010, 21, 949–959. [Google Scholar] [CrossRef]
- Morones-Ramirez, J.; Winkler, J.; Spina, C.; Collins, J. Silver enhances antibiotic activity against gram-negative bacteria. Sci. Transl. Med. 2013, 5, 190ra81. [Google Scholar] [CrossRef] [PubMed]
- Visani de Luna, L.; Mazarin de Moraes, A.; Consonni, S. Comparative in vitro toxicity of a graphene oxide-silver nanocomposite and the pristine counterparts toward macrophages. J. Nanobiotechnol. 2016, 14, 12. [Google Scholar] [CrossRef] [PubMed]
- Menéndez-Colino, L.M.; Bernal-Sprekelsen, M.; Alobid, I. Preliminary functional results of tympanoplasty with titanium prostheses. Otolaryngol. Head Neck Surg. 2004, 131, 747–749. [Google Scholar]
- Ziąbka, M. Study of mechanical properties of polymer composites containing silver nanoparticles for middle ear prostheses. Compos. Theor. Pract. 2016, 16, 42–46. [Google Scholar]
- Woods, O.; El Fata, F.; Saliba, I. Ossicular reconstruction: Incus versus universal titanium prosthesis. Auris Nasus Larynx 2009, 36, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Hales, N.W.; Shakir, F.A.; Saunders, J.E. Titanium middle-ear prostheses in staged ossiculoplasty: Does mass really matter? Am. J. Otolaryngol-Head Neck Med. Surg. 2007, 28, 164–167. [Google Scholar] [CrossRef] [PubMed]
- Pathan, F.; Satpathy, S.; Bhalekar, S.; Sudarshan, K. Tragal Cartilage Versus Polytetrafluoroethylene (TEFLON) Partial Ossicular Replacement Prosthesis (PORP): A Comparative Study of Outcomes of Ossiculoplasty. IJIRMS 2016, 1, 2455–8737. [Google Scholar]
- Trabandt, N.; Brandes, G.; Wintermantel, E.; Lenarz, T.; Stieve, M. Limitations of Titanium Dioxide and Aluminum Oxide as Ossicular Replacement Materials: An Evaluation of the Effects of Porosity on Ceramic Prostheses. Otol. Neurotol. 2004, 25, 682–693. [Google Scholar] [CrossRef] [PubMed]
- Truy, E.; Naiman, A.N.; Pavillon, C.; Abedipour, D.; Lina-Granade, G.; Rabilloud, M. Hydroxyapatite Versus Titanium Ossiculoplasty. Otol. Neurotol. 2007, 28, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Bojrab, D.I.; Causse, J.B.; Battista, R.A.; Vincent, R.; Gratacap, B.; Vandeventer, G. Ossiculoplasty with composite prostheses. Overview and analysis. Otolaryngol. Clin. N. Am. 1994, 27, 759–776. [Google Scholar]
- Hahn, Y.; Bojrab, D.I. Outcomes following ossicular chain reconstruction with composite prostheses: Hydroxyapatite-polyethylene vs. hydroxyapatite-titanium. Ear. Nose Throat J. 2013, 92, 250–254. [Google Scholar] [PubMed]
- Battista, R.A.; Esquivel, C. Middle Ear Ossiculoplasty E Medicine. Available online: http://www.emedicine.com/ENT/topic219.html (accessed on 1 February 2003).
- Ziąbka, M.; Menaszek, E.; Tarasiuk, J.; Wroński, S. Biocompatible nanocomposite implant with silver nanoparticles for otology—In vivo evaluation. Nanomaterials 2018, 8, 764. [Google Scholar] [CrossRef] [PubMed]
- Polish Norm PN-EN ISO 527-1. Plastics. Determination of Mechanical Properties at Static Stretching. General Rules; International Organization for Standardization: Geneva, Switzerland, 2012. [Google Scholar]
- Ziąbka, M.; Mertas, A.; Król, W. Poly(lactide-co-glycolide) composites containing antibacterial silver nanoparticles—In vitro examination. Compos. Theor. Pract. 2014, 14, 155–162. [Google Scholar]
- Ziąbka, M.; Dziadek, M.; Menaszek, E.; Banasiuk, R.; Królicka, A. Middle ear prosthesis with bactericidal efficacy—In vitro investigation. Molecules 2017, 22, 1681. [Google Scholar] [CrossRef] [PubMed]
- Cooper, L.F.; Zhou, Y.; Takebe, J.; Guo, J.; Abron, A.; Holmén, A.; Ellingsen, J.E. Fluoride modification effects on osteoblast behavior and bone formation at TiO2 grit-blasted c.p. titanium endosseous implants. Biomaterials 2006, 27, 926–936. [Google Scholar] [CrossRef] [PubMed]
- Zareidoost, A.; Yousefpour, M.; Ghaseme, B.; Amanzadeh, A. The relationship of surface roughness and cell response of chemical surface modification of titanium. J. Mater. Sci. Mater. Med. 2012, 23, 1479–1488. [Google Scholar] [CrossRef] [PubMed]
- Webster, T.J.; Ergun, C.; Doremus, R.H.; Siegel, R.W.; Bizios, R. Specific proteins mediate enhanced osteoblast adhesion on nanophase ceramics. J. Biomed. Mater. Res. 2000, 51, 475–483. [Google Scholar] [CrossRef]
- Puckett, S.; Pareta, R.; Webster, T.J. Nano rough micron patterned titanium for directing osteoblast morphology and adhesion. Int. J. Nanomed. 2008, 3, 229–241. [Google Scholar]
- Kim, P.; Kim, D.H.; Kim, B. Fabrication of nanostructures of polyethylene glycol for applications to protein adsorption and cell adhesion. Nanotechnology 2005, 16, 2420–2426. [Google Scholar] [CrossRef] [PubMed]
- Khang, D.; Kim, S.Y.; Liu-Synder, P.; Palmore, G.T.; Durbin, S.M.; Webster, T.J. Enhanced fibronectin adsorption on carbon nanotube/poly(carbonate) urethane: Independent role of surface nano-roughness and associated surface energy. Biomaterials 2007, 28, 4756–4768. [Google Scholar] [CrossRef] [PubMed]
- Maschhoff, P.M.; Geilich, B.M.; Webster, T.J. Greater fibroblast proliferation on an ultrasonicated ZnO/PVC nanocomposite material. Int. J. Nanomed. 2014, 9, 257–263. [Google Scholar]
- Zhao, F.; Koike, T.; Wang, J.; Sienz, H.; Meredith, R. Finite element analysis of the middle ear transfer functions and related pathologies. Med. Eng. Phys. 2009, 31, 907–916. [Google Scholar] [CrossRef] [PubMed]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ziąbka, M.; Dziadek, M.; Menaszek, E. Biocompatibility of Poly(acrylonitrile-butadiene-styrene) Nanocomposites Modified with Silver Nanoparticles. Polymers 2018, 10, 1257. https://doi.org/10.3390/polym10111257
Ziąbka M, Dziadek M, Menaszek E. Biocompatibility of Poly(acrylonitrile-butadiene-styrene) Nanocomposites Modified with Silver Nanoparticles. Polymers. 2018; 10(11):1257. https://doi.org/10.3390/polym10111257
Chicago/Turabian StyleZiąbka, Magdalena, Michał Dziadek, and Elżbieta Menaszek. 2018. "Biocompatibility of Poly(acrylonitrile-butadiene-styrene) Nanocomposites Modified with Silver Nanoparticles" Polymers 10, no. 11: 1257. https://doi.org/10.3390/polym10111257
APA StyleZiąbka, M., Dziadek, M., & Menaszek, E. (2018). Biocompatibility of Poly(acrylonitrile-butadiene-styrene) Nanocomposites Modified with Silver Nanoparticles. Polymers, 10(11), 1257. https://doi.org/10.3390/polym10111257