The Effect of Substances of Plant Origin on the Thermal and Thermo-Oxidative Ageing of Aliphatic Polyesters (PLA, PHA)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Methods for the Preparation of PLA and PHA Composites with Antioxidants
2.3. Measurement Methods
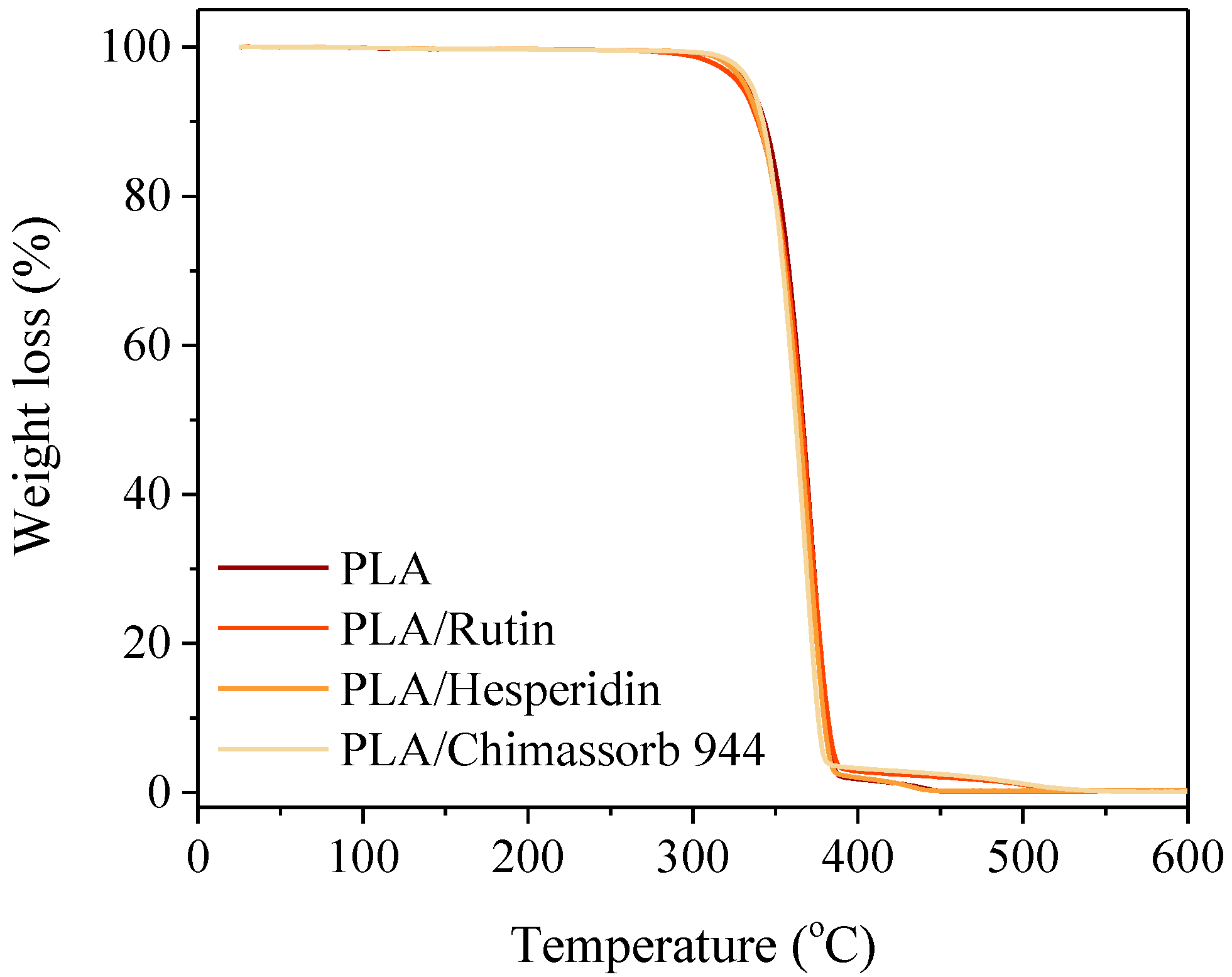
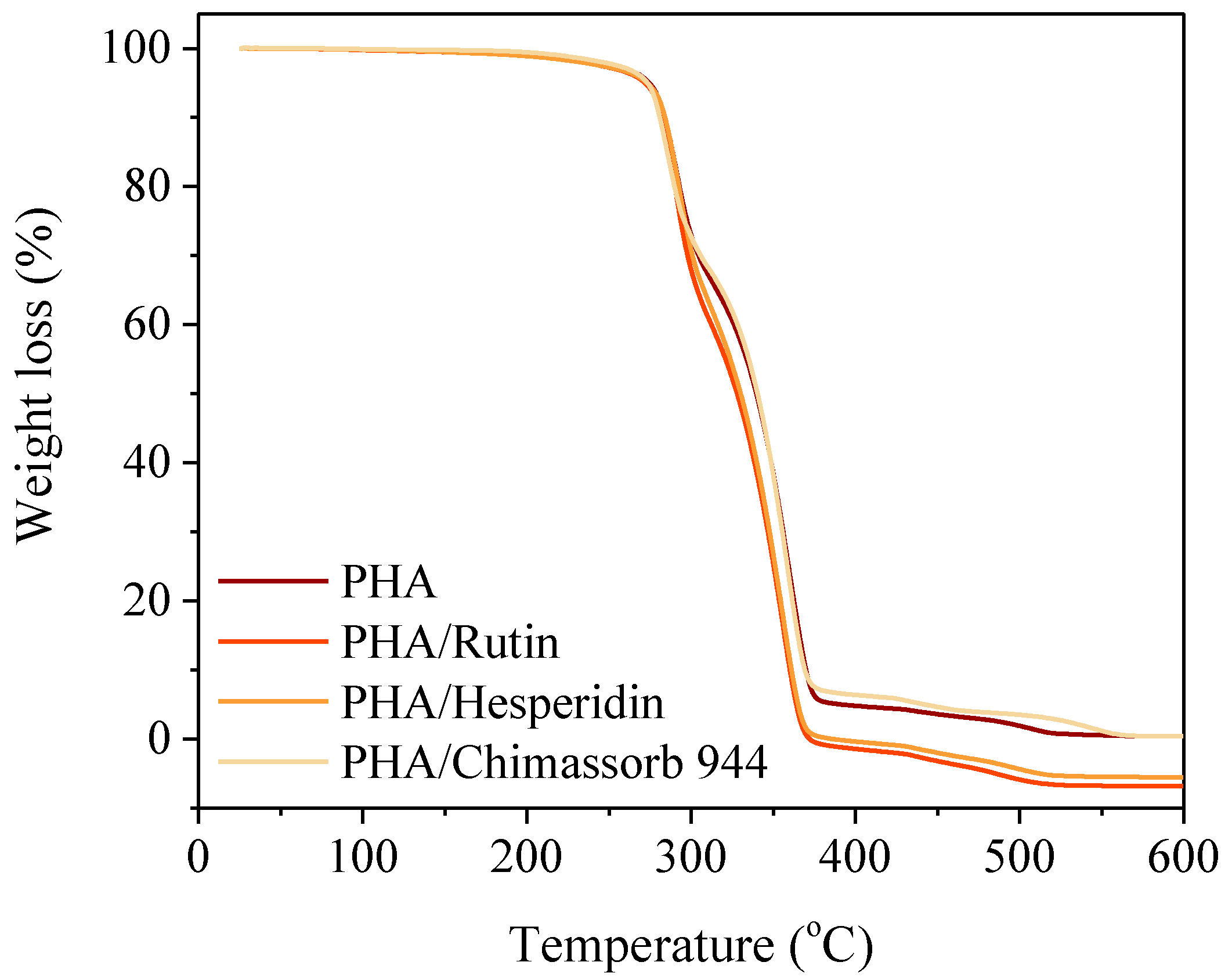
2.3.1. Thermal Analysis of PLA and PHA Composites with Antioxidants
2.3.2. Ageing of PLA and PHA Composites with Antioxidants
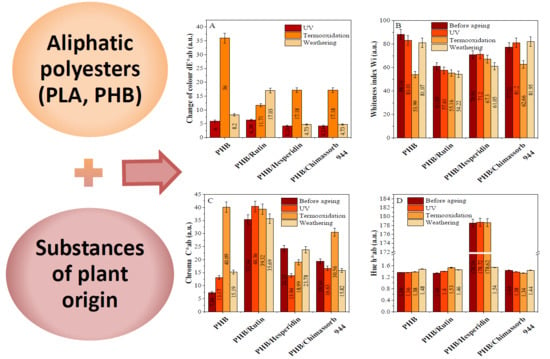
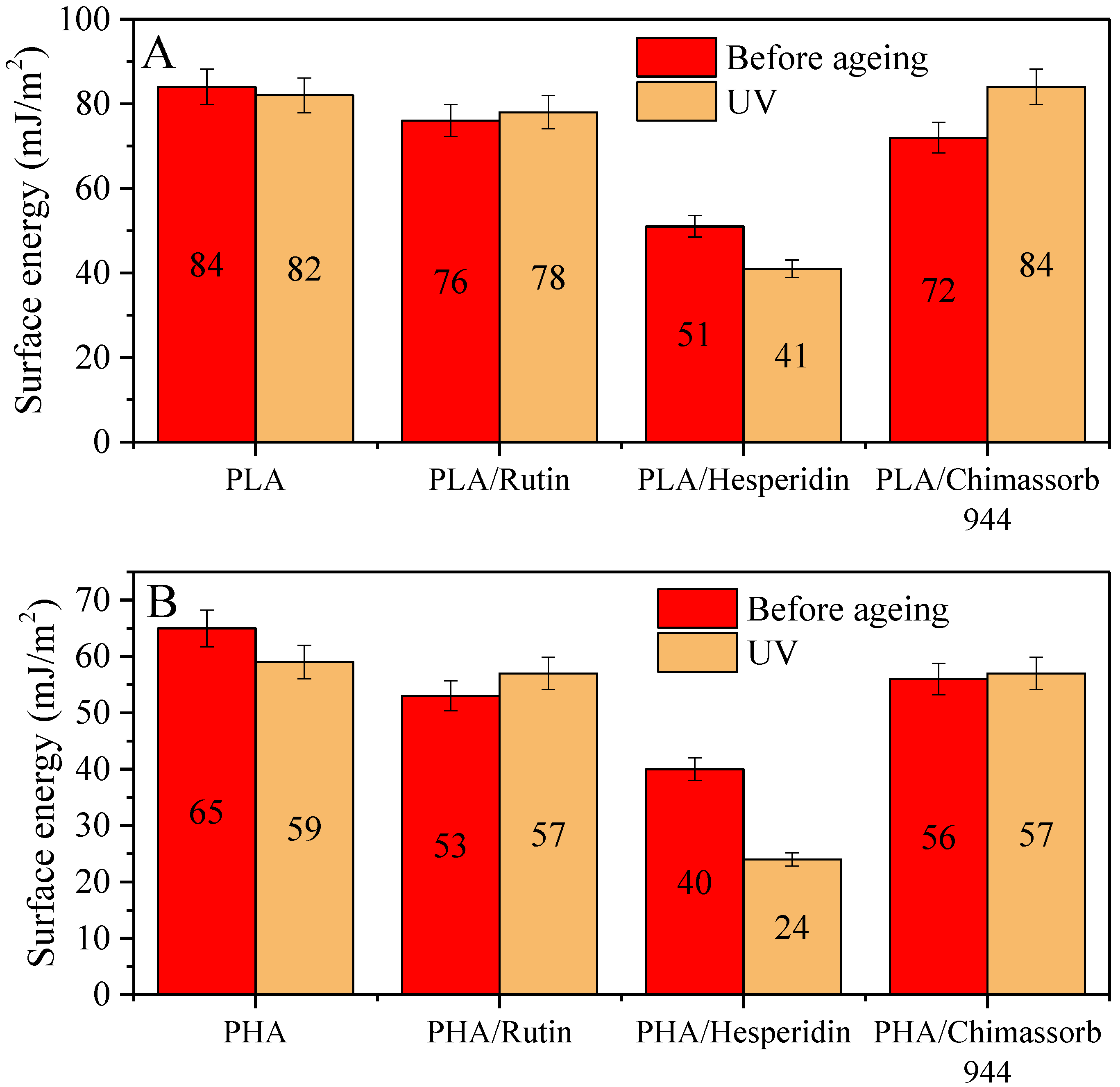
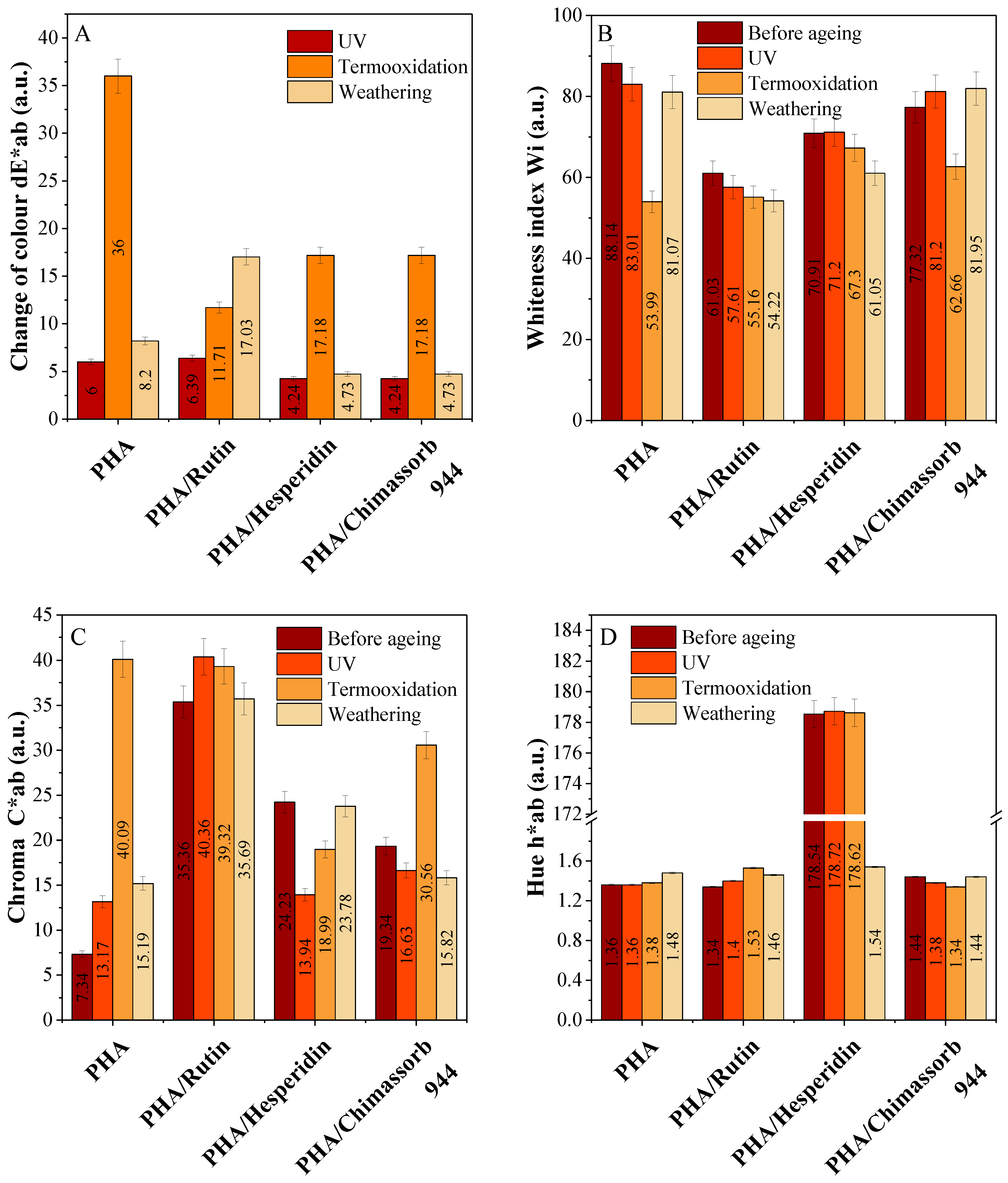
2.3.3. Surface Properties and Color Characteristics of PLA and PHA Composites with Antioxidants
- , = water dispersion component (=21.6 mJ/m2) and water polar component (=51.0 mJ/m2)
- = diiodomethane surface free energy (=50.8 mJ/m2)
- , = diiodomethane dispersion component (=48.5 mJ/m2) and polar component (=2.3 mJ/m2)
3. Results and Discussion
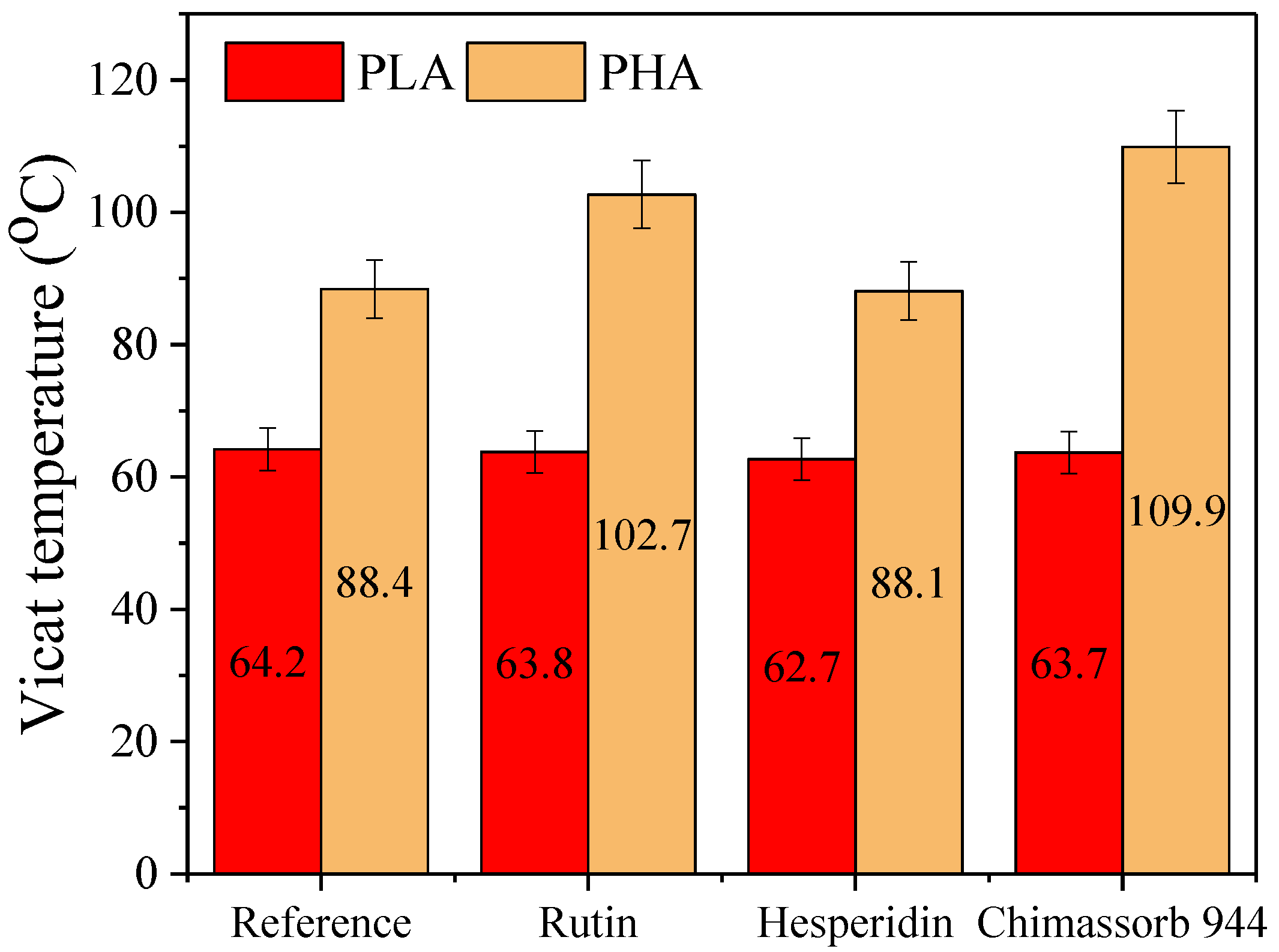
3.1. Thermal Analysis (Differential Scanning Calorimetry and Thermogravimetry) of PLA and PHA Composites with Antioxidants
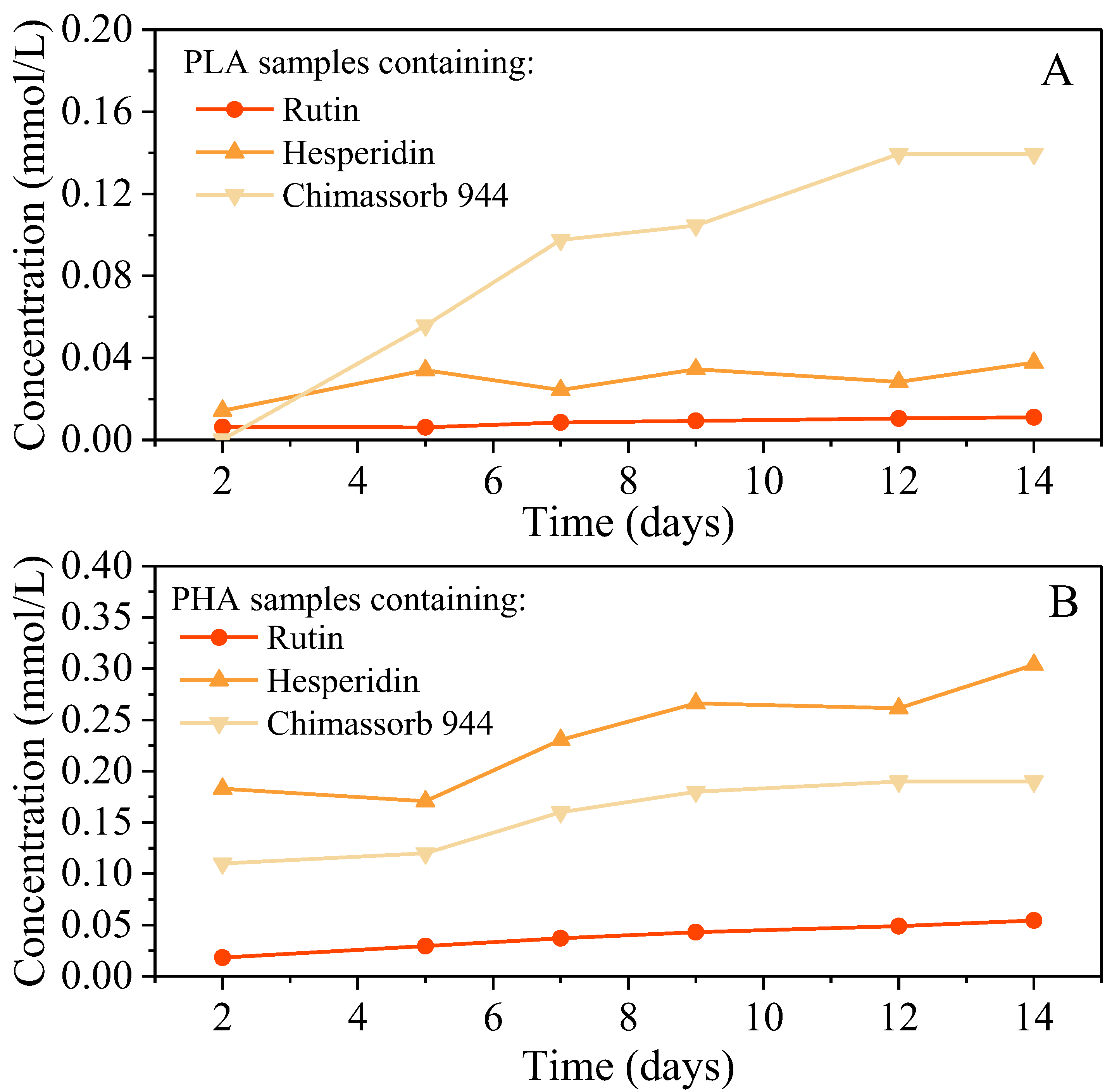
3.2. Physicochemical Properties of PLA and PHA Composites with Antioxidants
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Badia, J.D.; Strömberg, E.; Ribes-Greus, A.; Karlsson, S. Assessing the MALDI-TOF MS sample preparation procedure to analyze the influence of thermo-oxidative ageing and thermo-mechanical degradation on poly (lactide). Eur. Polym. J. 2011, 47, 1416–1428. [Google Scholar] [CrossRef]
- Zhou, Q.; Xanthos, M. Nanosize and microsize clay effects on the kinetics of the thermal degradation of polylactides. Polym. Degrad. Stab. 2009, 94, 327–338. [Google Scholar] [CrossRef]
- Gupta, M.C.; Deshmukh, V.G. Thermal oxidative degradation of poly (lactic acid), Part I: Activation energy of thermal degradation in air. Colloid Polym. Sci. 1982, 260, 308–311. [Google Scholar] [CrossRef]
- Gupta, M.C.; Deshmukh, V.G. Thermal oxidative degradation of poly-lactic acid. Colloid Polym. Sci. 1982, 260, 514–517. [Google Scholar] [CrossRef]
- McNeill, I.C.; Leiper, H.A. Degradation studies of some polyesters and polycarbonates-2. Polylactide: Degradation under isothermal conditions, thermal degradation mechanism and photolysis of the polymer. Polym. Degrad. Stab. 1985, 11, 309–326. [Google Scholar] [CrossRef]
- McNeill, I.C.; Leiper, H.A. Degradation studies of some polyesters and polycarbonates-1. Polylactide: General features of the degradation under programmed heating conditions. Polym. Degrad. Stab. 1985, 11, 267–285. [Google Scholar] [CrossRef]
- Tsuji, H.; Mizuno, A.; Ikada, Y. Blends of aliphatic polyesters. III. Biodegradation of solution-cast blends from poly(l-lactide) and poly(ε-caprolactone). J. Appl. Polym. Sci. 1998, 70, 2259–2268. [Google Scholar] [CrossRef]
- Koller, M. Poly (hydroxyalkanoates) for food packaging: Application and attempts towards implementation. Appl. Food Biotechnol. 2014, 1, 3–15. [Google Scholar]
- Torres, A.; Li, S.; Roussos, S.; Vert, M. Poly (lactic acid) degradation in soil or under controlled conditions. J. Appl. Polym. Sci. 1996, 62, 2295–2302. [Google Scholar] [CrossRef]
- Ho, K.G.; Pometto, A.L. Temperature effects on soil mineralization of polylactic acid plastic in laboratory respirometers. J. Environ. Polym. Degrad. 1999, 7, 101–108. [Google Scholar] [CrossRef]
- Tsuji, H.; Suzuyoshi, K. Environmental degradation of biodegradable polyesters 1. Poly(ε-caprolactone), poly[(R)-3-hydroxybutyrate], and poly(l-lactide) films in controlled static seawater. Polym. Degrad. Stab. 2002, 75, 347–355. [Google Scholar] [CrossRef]
- Tsuji, H.; Suzuyoshi, K. Environmental degradation of biodegradable polyesters 2. Poly(ε-caprolactone), poly[(R)-3-hydroxybutyrate], and poly(l-lactide) films in natural dynamic seawater. Polym. Degrad. Stab. 2002, 75, 357–365. [Google Scholar] [CrossRef]
- Doi, Y.; Kasuya, K.; Abe, H.; Koyama, N.; Ishiwatari, S.; Takagi, K.; Yoshida, Y. Evaluation of biodegradabilities of biosynthetic and chemosynthetic polyesters in river water. Polym. Degrad. Stab. 1996, 51, 281–286. [Google Scholar] [CrossRef]
- Bourbigot, S.; Duquesne, S.; Fontaine, G.; Bellayer, S.; Turf, T.; Samyn, F. Characterization and reaction to fire of polymer nanocomposites with and without conventional flame retardants. Mol. Cryst. Liqid Cryst. 2008, 486, 325–339. [Google Scholar] [CrossRef]
- Solarski, S.; Ferreira, M.; Devaux, E.; Fontaine, G.; Bachelet, P.; Bourbigot, S.; Delobel, R.; Coszach, P.; Murariu, M.; Ferreira, A.D.S.; et al. Designing polylactide/clay nanocomposites for textile applications: Effect of processing conditions, spinning and characterization. J. Appl. Polym. Sci. 2008, 109, 841–851. [Google Scholar] [CrossRef]
- Fontaine, G.; Bourbigot, S. Intumescent polylactide: A non-flammable material. J. Appl. Polym. Sci. 2009, 113, 3860–3865. [Google Scholar] [CrossRef]
- Fontaine, G.; Gallos, A.; Bourbigot, S. Role of montmorillonite for enhancing fire retardancy of intumescent PLA. Fire Saf. Sci. 2014, 11, 808–820. [Google Scholar] [CrossRef] [Green Version]
- Fukushima, K.; Tabuani, D.; Dottori, M.; Armentano, I.; Kenny, J.M.; Camino, G. Effect of temperature and nanoparticle type on hydrolytic degradation of poly(lactic acid) nanocomposites. Polym. Degrad. Stab. 2011, 96, 2120–2129. [Google Scholar] [CrossRef]
- Ho, K.; Pometto, A.L.; Hinz, P.N. Effects of temperature and relative humidity on polylactic acid plastic degradation. J. Environ. Polym. Degrad. 1999, 7, 83–92. [Google Scholar] [CrossRef]
- Tsuji, H.; Echizen, Y.; Nishimura, Y. Photodegradation of biodegradable poly-esters: A comprehensive study on poly(l-lactide) and poly(ε-caprolactone). Polym. Degrad. Stab. 2006, 91, 1128–1137. [Google Scholar] [CrossRef]
- Janorkar, A.V.; Metters, A.T.; Hirt, D.E. Degradation of poly(l-lactide) films under ultraviolet-induced photografting and sterilization conditions. J. Appl. Polym. Sci. 2007, 106, 1042–1047. [Google Scholar] [CrossRef]
- Copinet, A.; Bertrand, C.; Govindin, S.; Coma, V.; Couturier, Y. Effects of ultraviolet light (315 nm), temperature and relative humidity on the degradation of polylactic acid plastic films. Chemosphere 2004, 55, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Sambha’a, E.L.; Lallam, A.; Jada, A. Effect of hydrothermal polylactic acid degradation on polymer molecular weight and surface properties. J. Polym. Environ. 2010, 18, 532–538. [Google Scholar] [CrossRef]
- Koller, M.; Maršálek, L.; de Sousa Dias, M.M.; Braunegg, G. Producing microbial polyhydroxyalkanoate (PHA) biopolyesters in a sustainable manner. New Biotechnol. 2017, 37, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Kourmentza, C.; Plácido, J.; Venetsaneas, N.; Burniol-Figols, A.; Varrone, C.; Gavala, H.N.; Reis, M.A.M. Recent advances and challenges towards sustainable polyhydroxyalkanoate (PHA) production. Bioengineering 2017, 4, 55. [Google Scholar] [CrossRef] [PubMed]
- Shen, R.; Yin, J.; Ye, J.; Xiang, R.; Ning, Z.; Huang, W.; Chen, G. Promoter Engineering for Enhanced P(3HB-co-4HB) Production by Halomonas bluephagenesis. ACS Synth. Biol. 2018, 7, 1897–1906. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, U.; Dhar, P.; Kumar, A.; Katiyar, V. Polyhydroxyalkanoates (PHA)-Cellulose Based Nanobiocomposites for Food Packaging Applications. Food Addit. Packag. 2014, 19, 275–314. [Google Scholar]
- Kai, D.; Loh, X.J. Polyhydroxyalkanoates: Chemical Modifications toward Biomedical Applications. ACS Sustain. Chem. Eng. 2014, 2, 106–119. [Google Scholar] [CrossRef]
- Zaidi, L.; Kaci, M.; Bruzaud, S.; Bourmaud, A.; Grohens, Y. Effect of natural weather on the structure and properties of polylactide/Cloisite 30B nano-composites. Polym. Degrad. Stab. 2010, 95, 1751–1758. [Google Scholar] [CrossRef]
- Belbachir, S.; Zaïri, F.; Ayoub, G.; Maschke, U.; Naït-Abdelaziz, M.; Gloaguen, J.M.; Benguediab, M.; Lefebvre, J.M. Modelling of photodegradation effect on elastic-viscoplastic behaviour of amorphous polylactic acid films. J. Mech. Phys. Solids 2010, 58, 241–255. [Google Scholar] [CrossRef]
- De Oliveira, H.P.; Rieumont, J.; Nogeuiras, C.; Souza, D.; Sanchez, R. Thermal degradation behavior of a carboxylic acid-terminated amphiphilic block copolymer poly(ethylene)-b-poly(ethylene oxide). J. Therm. Anal. Calorim. 2011, 103, 443–451. [Google Scholar] [CrossRef]
- Vidovic, E.; Faraguna, F.; Jukic, A. Influence of inorganic fillers on PLA crystallinity and thermal properties. J. Therm. Anal. Calorim. 2017, 127, 371–380. [Google Scholar] [CrossRef]
- Scotter, M. The chemistry and analysis of annatto food colouring: A review. Food. Addit. Contam. Part A Chem. Anal. Control Expo Risk Assess. 2009, 26, 1123–1145. [Google Scholar] [CrossRef]
- Boon, C.S.; Mc Clements, D.J.; Weiss, J.; Decker, E.A. Factors in fluencing the chemical stability of carotenoids in foods. Crit. Rev. Food Sci. Nutr. 2010, 50, 515–532. [Google Scholar] [CrossRef] [PubMed]
- Cerruti, P.; Malinconico, M.; Rychly, J.; Matisova-Rychla, L.; Carfagna, C. Effect of natural antioxidants on the stability of polypropylene films. Polym. Degrad. Stab. 2009, 94, 2095–2100. [Google Scholar] [CrossRef]
- Abdel-Razik, E.A. Aspects of degradation and stability of ABS copolymers. I. Effect of b-carotene as antioxidant. J. Polym. Sci. Part A Polym. Chem. 1989, 27, 343–355. [Google Scholar] [CrossRef]
- Lopez-Rubio, A.; Lagaron, J.M. Improvement of UV stability and mechanical properties of biopolyesters through the addition of b–carotene. Polym. Degrad. Stab. 2010, 95, 2162–2168. [Google Scholar] [CrossRef]
- Ahmed, J.; Zhang, J.X.; Song, Z.; Varshney, S.K. Thermal properties of polylactides. Effect of molecular mass and nature of lactide isomer. J. Therm. Anal. Calorim. 2009, 95, 957–964. [Google Scholar] [CrossRef]
- Saad, G.R.; Elsawy, M.A.; Aziz, M.S.A. Nonisothermal crystallization behavior and molecular dynamics of poly(lactic acid) plasticized with jojoba oil. J. Therm. Anal. Calorim. 2017, 128, 211–223. [Google Scholar] [CrossRef]
- Lv, S.; Zhao, X.; Shi, L.; Zhang, G.; Wang, S.; Kang, W.; Zhuang, X. Preparation and Properties of sc-PLA/PMMA Transparent Nanofiber Air Filter. Polymers 2018, 10, 996. [Google Scholar] [CrossRef]
- Wang, F.; Zhou, S.; Yang, M.; Chen, Z.; Ran, S. Thermo-Mechanical Performance of Polylactide Composites Reinforced with Alkali-Treated Bamboo Fibers. Polymers 2018, 10, 401. [Google Scholar] [CrossRef]
- Salehiyan, R.; Ray, S.S.; Bandyopadhyay, J.; Ojijo, V. The Distribution of Nanoclay Particles at the Interface and Their Influence on the Microstructure Development and Rheological Properties of Reactively Processed Biodegradable Polylactide/Poly(butylene succinate) Blend Nanocomposites. Polymers 2017, 9, 350. [Google Scholar] [CrossRef]





| Sample | Tg (°C) | ΔHcc (J/g) | Tcc (°C) | ΔHm (J/g) | Tm (°C) | ΔH0 (J/g) | T0 (°C) |
|---|---|---|---|---|---|---|---|
| Before Ageing | |||||||
| PLA | 59.66 | 18.94 | 108.10 | 16.82 | 148.68 | 21.69 | 229.41 298.22 |
| PLA/Rutin | 59.15 | 12.20 | 111.33 | 12.54 | 148.97 | 11.85 | 245.79 305.38 |
| PLA/Hesperidin | 59.40 | 19.45 | 107.25 | 19.40 | 148.35 | 8.38 | 256.29 308.72 |
| PLA/Chimassorb 944 | 59.81 | 8.13 | 114.90 | 6.07 | 151.04 | 26.52 | 246.22 19.73 |
| After Thermo-Oxidation Ageing | |||||||
| PLA | 58.37 | 27.59 | 107.76 | 23.67 | 148.43 | 19.08 | 223.76 290.52 |
| PLA/Rutin | 59.58 | 26.83 | 108.43 | 21.42 | 148.94 | 2.36 | 242.58 271.04 |
| PLA/Hesperidin | 58.51 | 24.34 | 108.27 | 21.44 | 148.32 | 0.94 | 248.57 250.67 |
| PLA/Chimassorb 944 | 58.67 | 7.57 | 111.92 | 5.61 | 150.63 | 7.03 | 306.02 313.25 |
| After Weathering Ageing | |||||||
| PLA | 58.50 | 27.92 | 107.92 | 23.03 | 149.04 | 26.45 | 259.74 293.48 |
| PLA/Rutin | 58.43 | 30.56 | 105.52 | 25.06 | 147.79 | 10.84 | 237.57 300.43 |
| PLA/Hesperidin | 42.02 | 34.63 | 92.66 | 37.73 | 139.99 | 17.14 | 230.06 270.01 |
| PLA/Chimassorb 944 | 58.44 | 11.04 | 111.85 | 8.79 | 150.46 | 15.72 | 271.96 316.19 |
| Sample | Tg (°C) | ΔHcc (J/g) | Tcc (°C) | ΔHm (J/g) | Tm (°C) | ΔH0 (J/g) | T0 (°C) |
|---|---|---|---|---|---|---|---|
| Before Ageing | |||||||
| PHA | 36.79 | 15.36 | 76.75 | (1) 6.53 (2) 33.93 | (1) 127.85 (2) 156. 67 | 9.66 | 199.27 260.33 |
| PHA/Rutin | 39.67 | 13.37 | 81.51 | (1) 5.76 (2) 28.93 | (1) 128.52 (2) 156.59 | 1.31 | 235.12 255.89 |
| PHA/Hesperidin | 36.57 | 13.87 | 77.35 | (1) 6.15 (2) 32.57 | (1) 128.41 (2) 157. 36 | 20.63 | 243.57 275.98 |
| PHA/Chimassorb 944 | 37.37 | 16.66 | 78.47 | (1) 3.86 (2) 31.50 | (1) 126.52 (2) 156. 49 | 1.71 | 229.18 251.18 |
| After Thermo-Oxidation Ageing | |||||||
| PHA | 35.14 | 14.73 | 75.02 | (1) 6.09 (2) 35.57 | (1) 123.17 (2) 156.37 | 2.12 | 209.36 251.44 |
| PHA/Rutin | 36.93 | 17.50 | 76.49 | (1) 9.48 (2) 36.05 | (1) 132.57 (2) 156. 43 | 3.68 | 195.74 255.11 |
| PHA/Hesperidin | 35.30 | 16.50 | 73.33 | (1) 4.95 (2) 36.27 | (1) 128.93 (2) 155.68 | 19.00 | 201.67 264.40 |
| PHA/Chimassorb 944 | 37.12 | 15.85 | 76.17 | (1) 4.44 (2) 34.74 | (1) 125.25 (2) 155.88 | 1.61 | 207.06 243.30 |
| After Weathering Ageing | |||||||
| PHA | 27.85 | 13.54 | 71.15 | (1) 4.56 (2) 34.16 | (1) 129.94 (2) 153.67 | 4.23 | 185.68 243.37 |
| PHA/Rutin | 26.24 | 14.41 | 71.94 | (1) 4.44 (2) 35.67 | (1) 126.63 (2)154.26 | 9.52 | 204.71 262.03 |
| PHA/Hesperidin | 35.38 | 11.44 | 63.90 | (1) 0.12 (2) 30.33 | (1) 100.29 (2) 130.63 | 7.36 | 200.69 249.40 |
| PHA/Chimassorb 944 | 30.35 | 8.29 | 67.09 | (1) 3.08 (2) 28.94 | (1) 123.08 (2) 153.01 | 8.13 | 204.04 251.20 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masek, A.; Latos-Brozio, M. The Effect of Substances of Plant Origin on the Thermal and Thermo-Oxidative Ageing of Aliphatic Polyesters (PLA, PHA). Polymers 2018, 10, 1252. https://doi.org/10.3390/polym10111252
Masek A, Latos-Brozio M. The Effect of Substances of Plant Origin on the Thermal and Thermo-Oxidative Ageing of Aliphatic Polyesters (PLA, PHA). Polymers. 2018; 10(11):1252. https://doi.org/10.3390/polym10111252
Chicago/Turabian StyleMasek, Anna, and Malgorzata Latos-Brozio. 2018. "The Effect of Substances of Plant Origin on the Thermal and Thermo-Oxidative Ageing of Aliphatic Polyesters (PLA, PHA)" Polymers 10, no. 11: 1252. https://doi.org/10.3390/polym10111252
APA StyleMasek, A., & Latos-Brozio, M. (2018). The Effect of Substances of Plant Origin on the Thermal and Thermo-Oxidative Ageing of Aliphatic Polyesters (PLA, PHA). Polymers, 10(11), 1252. https://doi.org/10.3390/polym10111252