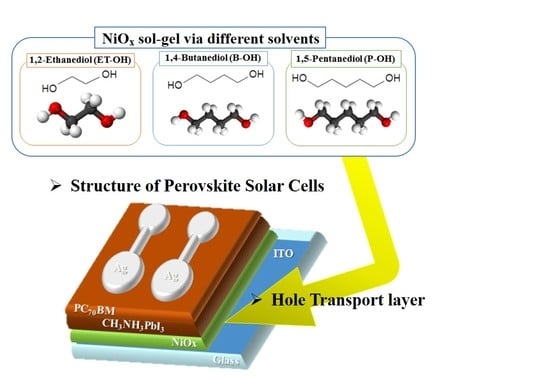
Facile NiOx Sol-Gel Synthesis Depending on Chain Length of Various Solvents without Catalyst for Efficient Hole Charge Transfer in Perovskite Solar Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Preparation
2.2. NiOx Sol-Gel Preparation
2.3. Thin Films and Device Fabrication
2.4. Characterization of the Devices
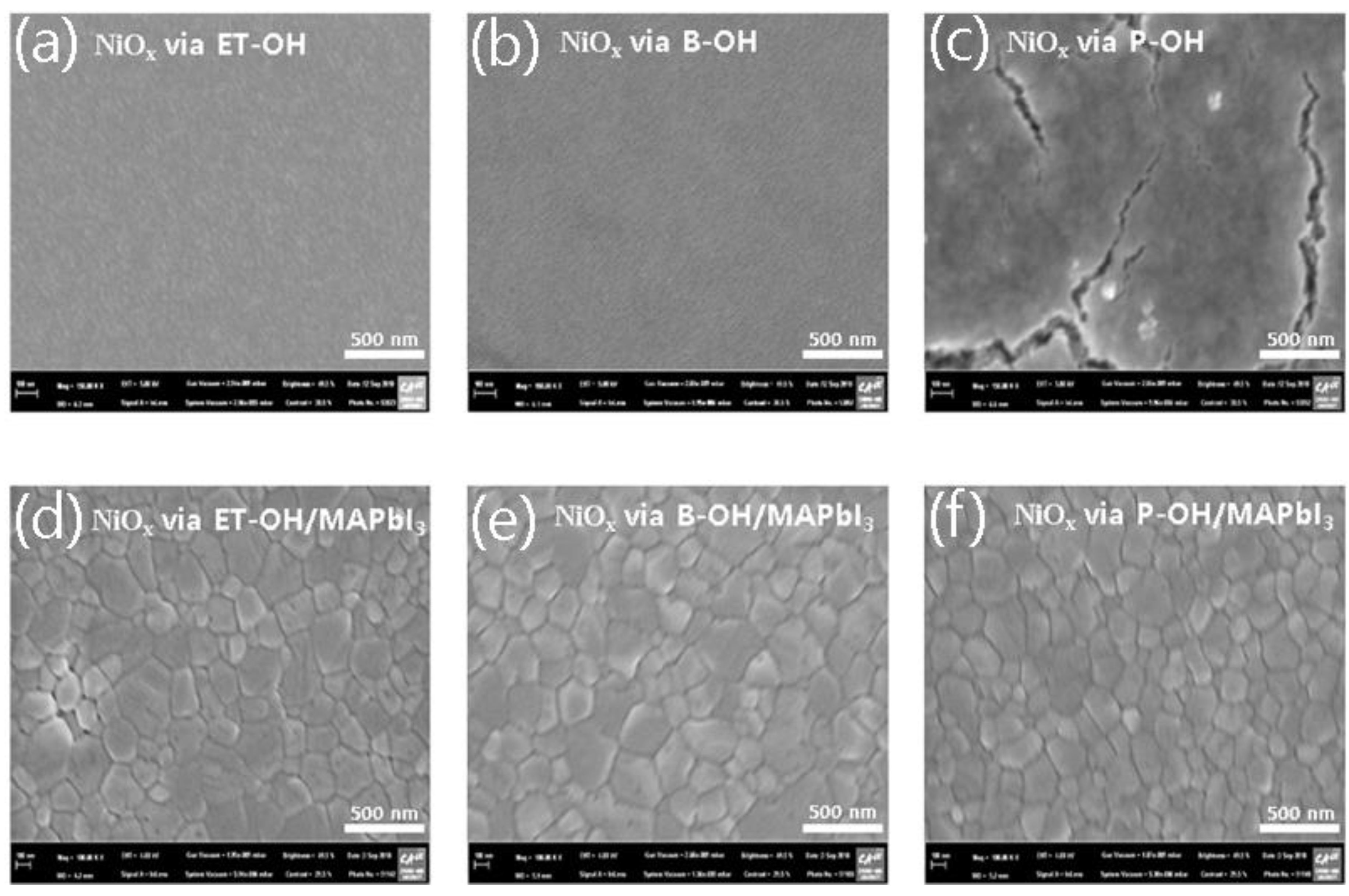
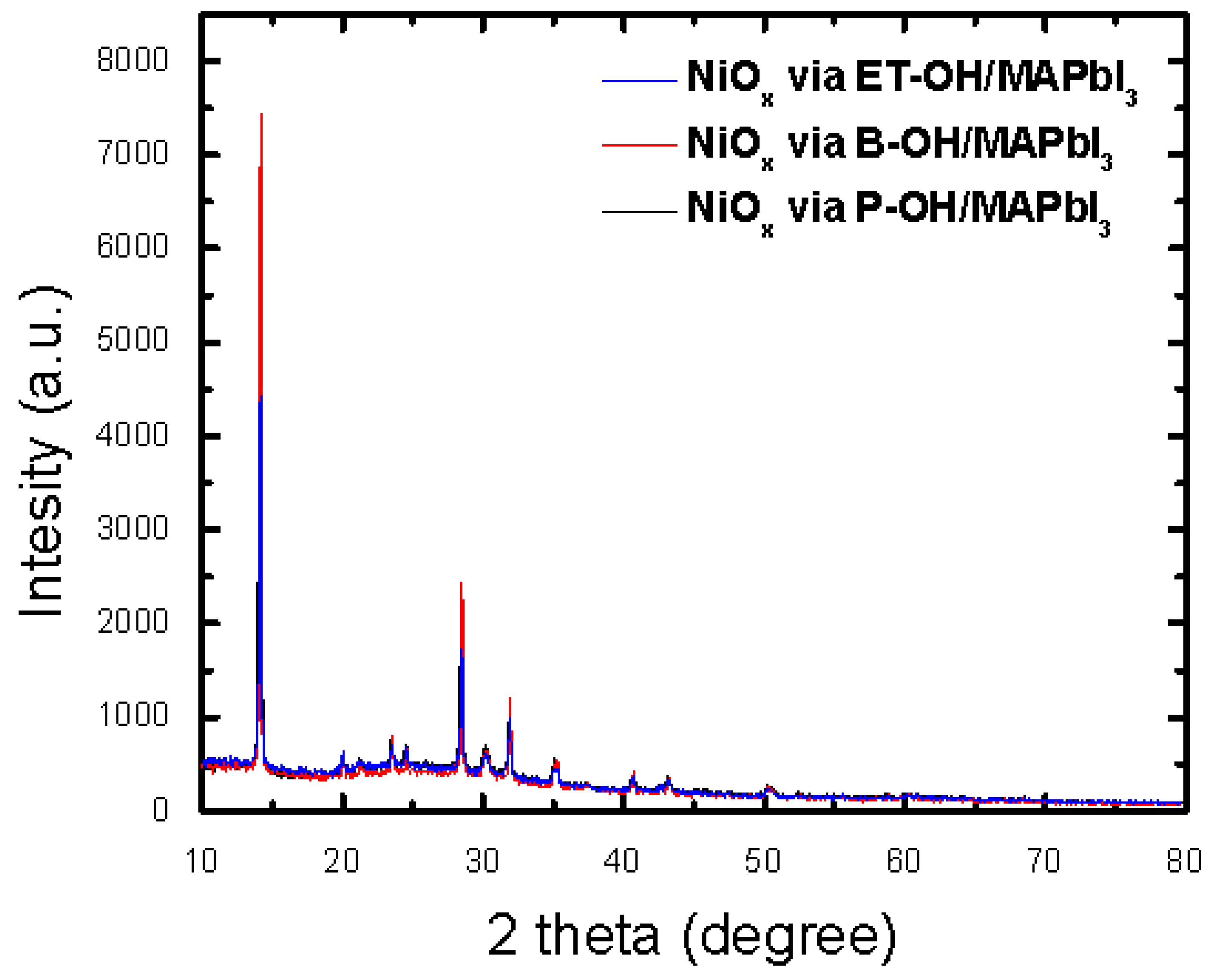
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Uddin, A. Perovskite Solar Cells. In World Scientific Handbook of Organic Optoelectronic Devices; Huang, J., Yuan, Y., Eds.; World Scientific: Munich, Germany, 2018; pp. 285–367. [Google Scholar]
- Di Girolamo, D.; Matteocci, F.; Lamanna, E.; Calabrò, E.; Di Carlo, A.; Dini, D. Inverted perovskite solar cells with transparent hole transporting layer based on semiconducting nickel oxide. AIP Conf. Proc. 2018, 1990, 020011. [Google Scholar] [CrossRef]
- Lee, D.S.; Yun, J.S.; Kim, J.; Soufiani, A.M.; Chen, S.; Cho, Y.; Deng, X.; Seidel, J.; Lim, S.; Huang, S.; et al. Passivation of Grain Boundaries by Phenethylammonium in Formamidinium-Methylammonium Lead Halide Perovskite Solar Cells. ACS Energy Lett. 2018, 3, 647–654. [Google Scholar] [CrossRef]
- NREL. Best Research Cell Efficiency Chart. National Renewable Energy Laboratory: Golden, CO, USA, 2018. Available online: https://www.nrel.gov/pv/assets/pdfs/pv-efficiencies-07-17-2018.pdf (accessed on 17 July 2017).
- Huang, Z.; Wang, D.; Wang, S.; Zhang, T. Highly Efficient and Stable MAPbI3 Perovskite Solar Cell Induced by Regulated Nucleation and Ostwald Recrystallization. Materials 2018, 11, 778. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Zhou, P.; Lei, X.; Fang, Z.; Zhang, M.; Liu, Q.; Chen, T.; Zeng, H.; Ding, L.; Zhu, J.; et al. Phase Engineering of Perovskite Materials for High-Efficiency Solar Cells: Rapid Conversion of CH3 NH3 PbI3 to Phase-Pure CH3 NH3 PbCl3 via Hydrochloric Acid Vapor Annealing Post-Treatment. ACS Appl. Mater. Interfaces 2018, 10, 1897–1908. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Yuan, H.; Li, J.; Xu, X.; Shen, Y.; Lin, H.; Wang, M. Recent progress in efficient hybrid lead halide perovskite solar cells. Sci. Technol. Adv. Mater. 2015, 16, 036004. [Google Scholar] [CrossRef] [PubMed]
- Pang, S.; Li, X.; Dong, H.; Chen, D.; Zhu, W.; Chang, J.; Lin, Z.; Xi, H.; Zhang, J.; Zhang, C.; et al. Efficient Bifacial Semitransparent Perovskite Solar Cells Using Ag/V2 O5 as Transparent Anodes. ACS Appl. Mater. Interfaces 2018, 10, 12731–12739. [Google Scholar] [CrossRef] [PubMed]
- Pang, S.; Chen, D.; Zhang, C.; Chang, J.; Lin, Z.; Yang, H.; Sun, X.; Mo, J.; Xi, H.; Han, G.; et al. Efficient bifacial semitransparent perovskite solar cells with silver thin film electrode. Sol. Energy Mater. Sol. Cells 2017, 170, 278–286. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, J.; Zhang, C.; Chang, J.; Lin, Z.; Chen, D.; Xi, H.; Hao, Y.; Yang, H.; Zhang, J.; et al. Effects of Annealing Conditions on Mixed Lead Halide Perovskite Solar Cells and Their Thermal Stability Investigation. Materials 2017, 10, 837. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhang, C.; Chang, J.; Yang, H.; Xi, H.; Lu, G.; Chen, D.; Lin, Z.; Lu, X.; Zhang, J.; et al. Mixed-solvent-vapor annealing of perovskite for photovoltaic device efficiency enhancement. Nano Energy 2016, 28, 417–425. [Google Scholar] [CrossRef]
- Mo, J.; Zhang, C.; Chang, J.; Yang, H.; Xi, H.; Chen, D.; Lin, Z.; Lu, G.; Zhang, J.; Hao, Y. Enhanced efficiency of planar perovskite solar cells via a two-step deposition using DMF as an additive to optimize the crystal growth behavior. J. Mater. Chem. A 2017, 5, 13032–13038. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, J.; Zhang, C.; Chang, J.; Lin, Z.; Chen, D.; Sun, X.; Xi, H.; Han, G.; Hao, Y. Effect of polyelectrolyte interlayer on efficiency and stability of p-i-n perovskite solar cells. Sol. Energy 2016, 139, 190–198. [Google Scholar] [CrossRef]
- Liu, T.; Chen, K.; Hu, Q.; Zhu, R.; Gong, Q. Inverted Perovskite Solar Cells: Progresses and Perspectives. Adv. Energy Mater. 2016, 6, 1600457. [Google Scholar] [CrossRef]
- Yang, W.S.; Park, B.-W.; Jung, E.H.; Jeon, N.J.; Kim, Y.C.; Lee, D.U.; Shin, S.S.; Seo, J.; Kim, E.K.; Noh, J.H.; et al. Iodide management in formamidinium-lead-halide-based perovskite layers for efficient solar cells. Science 2017, 356, 1376–1379. [Google Scholar] [CrossRef] [PubMed]
- Rong, Y.; Hu, Y.; Mei, A.; Tan, H.; Saidaminov, M.I.; Seok, S.; McGehee, M.D.; Sargent, E.H.; Han, H. Challenges for commercializing perovskite solar cells. Science 2018, 361, eaat8235. [Google Scholar] [CrossRef] [PubMed]
- Pitchaiya, S.; Natarajan, M.; Santhanam, A.; Asokan, V.; Yuvapragasam, A.; Madurai Ramakrishnan, V.; Palanisamy, S.E.; Sundaram, S.; Velauthapillai, D. A review on the classification of organic/inorganic/carbonaceous hole transporting materials for perovskite solar cell application. Arab. J. Chem. 2018. [Google Scholar] [CrossRef]
- Li, M.-H.; Yum, J.-H.; Moon, S.-J.; Chen, P.; Li, M.-H.; Yum, J.-H.; Moon, S.-J.; Chen, P. Inorganic p-Type Semiconductors: Their Applications and Progress in Dye-Sensitized Solar Cells and Perovskite Solar Cells. Energies 2016, 9, 331. [Google Scholar] [CrossRef]
- Yu, Z.; Sun, L. Inorganic Hole-Transporting Materials for Perovskite Solar Cells. Small Methods 2018, 2, 1700280. [Google Scholar] [CrossRef]
- Arora, N.; Dar, M.I.; Hinderhofer, A.; Pellet, N.; Schreiber, F.; Zakeeruddin, S.M.; Grätzel, M. Perovskite solar cells with CuSCN hole extraction layers yield stabilized efficiencies greater than 20. Science 2017, 358, 768–771. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, E.; Wong, K.K.; Müller, M.; Hu, H.; Ehrenreich, P.; Kohlstädt, M.; Würfel, U.; Mastroianni, S.; Mathiazhagan, G.; Hinsch, A.; et al. Characterization of perovskite solar cells: Towards a reliable measurement protocol. APL Mater. 2016, 4, 091901. [Google Scholar] [CrossRef]
- Chen, B.; Yang, M.; Priya, S.; Zhu, K. Origin of J–V Hysteresis in Perovskite Solar Cells. J. Phys. Chem. Lett. 2016, 7, 905–917. [Google Scholar] [CrossRef] [PubMed]
- Duc Duong, T. Development of High Efficiency Four-Terminal Perovskite-Silicon Tandems. Ph.D. Thesis, The Australian National University, Canberra, Australia, July 2017. [Google Scholar]
- Qiu, L.; Ono, L.K.; Qi, Y. Advances and challenges to the commercialization of organic–inorganic halide perovskite solar cell technology. Mater. Today Energy 2018, 7, 169–189. [Google Scholar] [CrossRef]
- Tonui, P.; Oseni, S.O.; Sharma, G.; Mola, G.T.; Oseni, S.O.; Yan, Q.; Tessema Mola, G. Perovskites photovoltaic solar cells: An overview of current status. Renew. Sustain. Energy Rev. 2018, 91, 1025–1044. [Google Scholar] [CrossRef]
- Savva, A.; Papadas, I.T.; Tsikritzis, D.; Armatas, G.S.; Kennou, S.; Choulis, S.A. Room Temperature Nanoparticulate Interfacial Layers for Perovskite Solar Cells via solvothermal synthesis. J. Mater. Chem. A 2017, 5, 20381–20389. [Google Scholar] [CrossRef]
- Fan, J.; Jia, B.; Gu, M. Perovskite-based low-cost and high-efficiency hybrid halide solar cells. Photonics Res. 2014, 2, 111–120. [Google Scholar] [CrossRef]
- Guo, Y.; Lei, H.; Xiong, L.; Li, B.; Fang, G. An integrated organic–inorganic hole transport layer for efficient and stable perovskite solar cells. J. Mater. Chem. A 2018, 6, 2157–2165. [Google Scholar] [CrossRef]
- Chang, C.-C.; Tao, J.-H.; Tsai, C.-E.; Cheng, Y.-J.; Hsu, C.-S. Cross-linked Triarylamine-Based Hole-Transporting Layer for Solution-Processed PEDOT:PSS-Free Inverted Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2018, 10, 21466–21471. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Hu, L.; Yun, J.S.; Zhang, M.; Lau, C.F.J.; Bing, J.; Deng, X.; Ma, Q.; Cho, Y.; Fu, W.; et al. Solution-Processed, Silver-Doped NiOx as Hole Transporting Layer for High-Efficiency Inverted Perovskite Solar Cells. ACS Appl. Energy Mater. 2018, 1, 561–570. [Google Scholar] [CrossRef]
- Pérez-Tomás, A.; Mingorance, A.; Tanenbaum, D.; Lira-Cantú, M. Metal Oxides in Photovoltaics: All-Oxide, Ferroic, and Perovskite Solar Cells. In The Future of Semiconductor Oxides in Next-Generation Solar Cells; Lira-Cantu, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 267–356. [Google Scholar]
- Kumar Elumalai, N.; Vijila, C.; Jose, R.; Uddin, A.; Ramakrishna, S. Metal oxide semiconducting interfacial layers for photovoltaic and photocatalytic applications. Mater. Renew. Sustain. Energy 2015, 4, 11. [Google Scholar] [CrossRef]
- Livage, J.; Ganguli, D. Sol–gel electrochromic coatings and devices: A review. Sol. Energy Mater. Sol. Cells 2001, 68, 365–381. [Google Scholar] [CrossRef]
- Koplick, A.J.; Jenkins, S.M. Preparation of Metal Alkoxides. U.S. Patent 09530858, 6 November 1998. [Google Scholar]
- Omaima, A.M. Metal Alkoxides as Starting Materials for Hydrolysis Processes. Master’s Thesis, Department of Chemistry, Faculty of Education, University of Khartoum, Khartoum State, Sudan, December 1999. [Google Scholar]
- Enggrob, M.; Gydesen, E.; Simonsen, M.E.; Søgaard, E.G. Aalborg Universitet Sol-gel reactions of titanium alkoxides and water: Influence of pH and alkoxy group on cluster formation and properties of the resulting products Sol-gel reactions of titanium alkoxides and water: Influence of pH and alkoxy group on cluster formation and properties of the resulting products. J. Sol-Gel Sci. Technol. 2010, 53, 485–497. [Google Scholar] [CrossRef]
- Okamoto, T.; Ichino, R.; Okido, M.; Liu, Z.; Zhang, C. Effect of Ammonia on the Crystal Morphology of Nickel Oxalate Precipitates and their Thermal Decomposition into Metallic Nickel. Mater. Trans. 2005, 46, 171–174. [Google Scholar] [CrossRef]
- Hasan, M.; Drazin, J.; Dey, S.; Castro, R.H.R. Synthesis of stoichiometric nickel aluminate spinel nanoparticles. Am. Mineral. 2015, 100, 652–657. [Google Scholar] [CrossRef]
- He, L.-H.; Zhao, Z.-W.; Zhang, Y.-X. Synthesis of nickel ferrite precursors from low grade nickel matte. Trans. Nonferrous Met. Soc. China 2013, 23, 2422–2430. [Google Scholar] [CrossRef]
- Edward, P.; Ramakumar, A.; Alexandra, N.; Donald, L.S. Nickel Solubility and Precipitation in Soils: A Thermodynamic Study. Clays Clay Miner. 2006, 54, 153–164. [Google Scholar] [CrossRef]
- Okamoto, T.; Yang, J.G.; Kuroda, K.; Ichino, R.; Okido, M. Preparation of Size and Aggregation Controlled Nickel Oxalate Dihydrate Particles From Nickel Hydroxide. Adv. Mater. Res. 2014, 15–17, 581–586. [Google Scholar] [CrossRef]
- Dean, F. Chelation Compositions. U.S. Patent 6870026B1, 22 March 2005. [Google Scholar]
- Mohammadi, E.; Aliofkhazraei, M.; Hasanpoor, M.; Chipara, M. Hierarchical and Complex ZnO Nanostructures by Microwave-Assisted Synthesis: Morphologies, Growth Mechanism and Classification. Crit. Rev. Solid State Mater. Sci. 2018, 43, 475–541. [Google Scholar] [CrossRef]
- Koschnick, T.J.; Haller, W.T. Effects of Copper Chelating Agents on Diquat Activity in Diquat Resistant Landoltia. J. Aquat. Plant Manag. 2006, 44, 125–132. [Google Scholar]
- Zhang, Q.; Sando, D.; Nagarajan, V. Chemical Route derived Bismuth Ferrite Thin films and Nanomaterials. J. Mater. Chem. C 2016, 4, 4092–4124. [Google Scholar] [CrossRef]
- Cabanas-Polo, S.; Ferrari, B.; Sánchez-Herencia, A.J. Colloidal stability of Ni(OH)2 in water and its dispersion into a ceramic matrix from the reaction media to obtain Ni/Al2O3 materials. Bol. Soc. Esp. Ceram. Vidrio 2014, 53, 265–274. [Google Scholar] [CrossRef]
- Munnik, P.; De Jongh, P.E.; De Jong, K.P. Recent Developments in the Synthesis of Supported Catalysts. Chem. Rev. 2015, 115, 6687–6718. [Google Scholar] [CrossRef] [PubMed]
- Majewski, A.J.; Wood, J.; Bujalski, W. Nickel–silica core@shell catalyst for methane reforming. Int. J. Hydrogen Energy 2013, 38, 14531–14541. [Google Scholar] [CrossRef]
- Imran Din, M.; Rani, A. Recent Advances in the Synthesis and Stabilization of Nickel and Nickel Oxide Nanoparticles: A Green Adeptness. Int. J. Anal. Chem. 2016, 2016, 1–14. [Google Scholar] [CrossRef] [PubMed]
- De, S.; Zhang, J.; Luque, R.; Yan, N. Ni-based bimetallic heterogeneous catalysts for energy and environmental applications. Energy Environ. Sci. 2016, 9, 3314–3347. [Google Scholar] [CrossRef]
- Talbot, F.J.; Richard R De, B.; Powell, B.E.; Nobbe, L.B. Boiling Point Determination. U.S. Patent 3216239A, 9 November 1965. [Google Scholar]
- Benham, S. Experiments on Evaporation & Surface Area. Available online: https://sciencing.com/experiments-evaporation-surface-area-10041602.html (accessed on 26 September 2018).
- Intermolecular and Interatomic Forces. Available online: https://intl.siyavula.com/read/science/grade-11/intermolecular-forces/04-intermolecular-forces-01 (accessed on 26 September 2018).
- Paquet, C.; Lacelle, T.; Liu, X.; Deore, B.; Kell, A.J.; Lafrenière, S.; Malenfant, P.R.L. The role of amine ligands in governing film morphology and electrical properties of copper films derived from copper formate-based molecular inks. Nanoscale 2018, 10, 6911–6921. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Kim, S.; Kim, H.; Lee, J. Solvent-dependent performance of solution-processed small-molecule organic field-effect transistors. Org. Electron. 2018, 52, 184–189. [Google Scholar] [CrossRef]
- Lasprilla-Botero, J.; Álvarez-Láinez, M.; Lagaron, J.M. The influence of electrospinning parameters and solvent selection on the morphology and diameter of polyimide nanofibers. Mater. Today Commun. 2018, 14, 1–9. [Google Scholar] [CrossRef]
- Carey, V.P. Liquid-Vapor Phase-Change Phenomena: An Introduction to the Thermophysics of Vaporization and Condensation Processes in Heat Transfer Equipment; Taylor and Francis Group, LLC: New York, NY, USA, 2008; ISBN 1351434861. [Google Scholar]
- Mugheirbi, N.A.; Mosquera-Giraldo, L.I.; Borca, C.H.; Slipchenko, L.V.; Taylor, L.S. Phase Behavior of Drug-Hydroxypropyl Methylcellulose Amorphous Solid Dispersions Produced from Various Solvent Systems: Mechanistic Understanding of the Role of Polymer using Experimental and Theoretical Methods. Mol. Pharm. 2018, 15, 3236–3251. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Chiang, S.W.; Chu, X.; Du, H.; Li, J.; Gan, L.; Xu, C.; Yao, Y.; He, Y.; Li, B.; et al. Effects of solvent on structures and properties of electrospun poly(ethylene oxide) nanofibers. J. Appl. Polym. Sci. 2018, 135, 45787. [Google Scholar] [CrossRef]
- Steirer, K.X.; Richards, R.E.; Sigdel, A.K.; Garcia, A.; Ndione, P.F.; Hammond, S.; Baker, D.; Ratcliff, E.L.; Curtis, C.; Furtak, T.; et al. Nickel oxide interlayer films from nickel formate–ethylenediamine precursor: Influence of annealing on thin film properties and photovoltaic device performance. J. Mater. Chem. A 2015, 3, 10949–10958. [Google Scholar] [CrossRef]
- Garcia-Miquel, J.; Zhang, Q.; Allen, S.; Rougier, A.; Blyr, A.; Davies, H.; Jones, A.; Leedham, T.; Williams, P.; Impey, S. Nickel oxide sol–gel films from nickel diacetate for electrochromic applications. Thin Solid Films 2003, 424, 165–170. [Google Scholar] [CrossRef]
- Sumida, K.; Liang, K.; Reboul, J.; Ibarra, I.A.; Furukawa, S.; Falcaro, P. Sol–Gel Processing of Metal–Organic Frameworks. Chem. Mater. 2017, 29, 2626–2645. [Google Scholar] [CrossRef]
- Liu, F.; Wu, J.; Chen, K.; Xue, D. Morphology Study by Using Scanning Electron Microscopy. Microsc. Sci. Technol. Appl. Educ. 2010, 1781–1792. [Google Scholar]
- Eugene, A.; Mariagoretti, U.; Samuel, A. A Review on Synthetic Methods of Nanostructured Materials. Chem. Res. J. 2017, 2, 97–123. [Google Scholar]
- Wang, N.; Sun, X. Perovskite Thin Films Having Large Crystalline Grains. U.S. Patent 20160254472A1, 1 September 2016. [Google Scholar]
- Ogundana, I.J.; Foo, S.Y. Improving the Morphology of the Perovskite Absorber Layer in Hybrid Organic/Inorganic Halide Perovskite MAPbI3 Solar Cells. J. Sol. Energy 2017, 2017, 1–9. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, W.; Yue, Y.; Liu, J.; Bi, E.; Yang, X.; Islam, A.; Han, L. Consecutive Morphology Controlling Operations for Highly Reproducible Mesostructured Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2015, 7, 20707–20713. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Chen, S.; Dong, B.; Gao, X.; Xiao, X.; Zhou, J.; Hu, J.; Tang, S.; Yan, K.; Hu, H.; et al. Electrical Heating-Assisted Multiple Coating Method for Fabrication of High-Performance Perovskite Fiber Solar Cells by Thickness Control. Adv. Mater. Interfaces 2017, 4, 1700833. [Google Scholar] [CrossRef]
- Ulkuniemi, M. Influence of Processing Conditions on the Performance of Perovskite Solar Cells. Master’s Thesis, University of Oulu, Oulu, Finland, November 2017. [Google Scholar]
- Fachbereich, I.; Kirill, M.S.; Aus, Z. Solution-Processed Charge Extraction Interlayers and Electrodes for Organic Solar Cells. Master’s Thesis, The University of Wuppertal, Wuppertal, Germany, July 2014. [Google Scholar]
- Wing, D. The Selection and Optimization of Transparent Conducting Oxides for Electrodes in Polymer Solar Cells. Research Thesis, Israel Institute of Technology, Haifa, Israel, May 2014. [Google Scholar]
- Ferguson, T.P. Jianmin Qu Moisture and temperature effects on the reliability of interfacial adhesion of a polymer/metal interface. In Proceedings of the 54th Electronic Components and Technology Conference, Las Vegas, NV, USA, 4–4 June 2004; pp. 1752–1758. [Google Scholar]
- Chen, L.-C.; Lin, Y.-S.; Tseng, Z.-L.; Wu, C.; Kao, F.-S.; Chen, S.-H.; Chen, L.-C.; Lin, Y.-S.; Tseng, Z.-L.; Wu, C.; et al. Overcoming the Intrinsic Difference between Hydrophilic CH3NH3PbI3 and Hydrophobic C60 Thin Films to Improve the Photovoltaic Performance. Nanomaterials 2017, 7, 166. [Google Scholar] [CrossRef] [PubMed]
- Murali, B.; Yengel, E.; Yang, C.; Peng, W.; Alarousu, E.; Bakr, O.M.; Mohammed, O.F. The Surface of Hybrid Perovskite Crystals: A Boon or Bane. ACS Energy Lett. 2017, 2, 846–856. [Google Scholar] [CrossRef]
- Xu, X.; Liu, Z.; Zuo, Z.; Zhang, M.; Zhao, Z.; Shen, Y.; Zhou, H.; Chen, Q.; Yang, Y.; Wang, M. Hole Selective NiO Contact for Efficient Perovskite Solar Cells with Carbon Electrode. Nano Lett. 2015, 15, 2402–2408. [Google Scholar] [CrossRef] [PubMed]
- Song, T.-B.; Chen, Q.; Zhou, H.; Jiang, C.; Wang, H.-H.; Yang, Y.; Liu, Y.; You, J.; Yang, Y. Perovskite solar cells: Film formation and properties. J. Mater. Chem. A 2015, 3, 9032–9050. [Google Scholar] [CrossRef]
- Hettiarachchi, C.L. Organolead Halide Perovskite Solar Absorbers and Ferroelectric Nanocomposites for Harvesting Solar Energy Perovskite Solar Cells View Project Quantum Dot Solar Cells View Project. Ph.D. Thesis, University of South Florida, Tampa, FL, USA, April 2018. [Google Scholar]
- Tamura, H.; Burghardt, I. Ultrafast charge separation in organic photovoltaics enhanced by charge delocalization and vibronically hot exciton dissociation. J. Am. Chem. Soc. 2013, 135, 16364–16367. [Google Scholar] [CrossRef] [PubMed]
- Jankovi´c, V.J.; Vukmirovi´cvukmirovi´c, N. Identification of Ultrafast Photophysical Pathways in Photoexcited Organic Heterojunctions. J. Am. Chem. Soc. 2017, 121, 19602–19618. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, M.; Qiu, H.; Yao, X.; Lao, X.; Xu, S.; Lin, Z.; Sun, L.; Shao, J. Engineering the Exciton Dissociation in Quantum-Confined 2D CsPbBr3 Nanosheet Films. Adv. Funct. Mater. 2018, 28, 1705908. [Google Scholar] [CrossRef]
- Dimitrov, S.D.; Nielsen, C.B.; Shoaee, S.; Shakya Tuladhar, P.; Du, J.; McCulloch, I.; Durrant, J.R. Efficient Charge Photogeneration by the Dissociation of PC70 BM Excitons in Polymer/Fullerene Solar Cells. J. Phys. Chem. Lett. 2012, 3, 140–144. [Google Scholar] [CrossRef]
- Paulke, A.; Stranks, S.D.; Kniepert, J.; Kurpiers, J.; Wolff, C.M.; Schön, N.; Snaith, H.J.; Brenner, T.J.K.; Neher, D. Charge carrier recombination dynamics in perovskite and polymer solar cells; Charge carrier recombination dynamics in perovskite and polymer solar cells. Appl. Phys. Lett. 2016, 108, 103102. [Google Scholar] [CrossRef]
- Ponseca, C.S.; Tian, Y.; Sundström, V.; Scheblykin, I.G. Excited state and charge-carrier dynamics in perovskite solar cell materials. Nanotechnology 2016, 27, 082001. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Chen, Y.; Zheng, K.; Pullerits, T.; Liang, Z. Insights into charge carrier dynamics in organo-metal halide perovskites: From neat films to solar cells. Chem. Soc. Rev. 2017, 46, 5714–5729. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Adinolfi, V.; Comin, R.; Yuan, M.; Alarousu, E.; Buin, A.; Chen, Y.; Hoogland, S.; Rothenberger, A.; Katsiev, K.; et al. Low trap-state density and long carrier diffusion in organolead trihalide perovskite single crystals. Science 2015, 347, 519–522. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Fuentes-Hernandez, C.; Shim, J.; Meyer, J.; Giordano, A.J.; Li, H.; Winget, P.; Papadopoulos, T.; Cheun, H.; Kim, J.; et al. A universal method to produce low-work function electrodes for organic electronics. Science 2012, 336, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Stranks, S.D.; Eperon, G.E.; Grancini, G.; Menelaou, C.; Alcocer, M.J.P.; Leijtens, T.; Herz, L.M.; Petrozza, A.; Snaith, H.J. Electron-hole diffusion lengths exceeding 1 micrometer in an organometal trihalide perovskite absorber. Science 2013, 342, 341–344. [Google Scholar] [CrossRef] [PubMed]
- Galatopoulos, F.; Savva, A.; Papadas, I.T.; Choulis, S.A. The Effect of Hole Transporting Layer in Charge Accumulation Properties of p-i-n Perovskite Solar Cells. APL Mater. 2017, 5, 076102. [Google Scholar] [CrossRef]
- Li, Y.; Ding, B.; Chu, Q.-Q.; Yang, G.-J.; Wang, M.; Li, C.-X.; Li, C.-J. Ultra-high open-circuit voltage of perovskite solar cells induced by nucleation thermodynamics on rough substrates. Sci. Rep. 2017, 7, 46141. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, T.; Liu, L.; Hu, M.; Yang, Y.; Mei, A.; Han, H. The effect of carbon counter electrodes on fully printable mesoscopic perovskite solar cells. J. Mater. Chem. A 2015, 3, 9165–9170. [Google Scholar] [CrossRef]
- Yadav, P.; Prochowicz, D.; Saliba, M.; Boix, P.; Zakeeruddin, S.; Grätzel, M.; Yadav, P.; Prochowicz, D.; Saliba, M.; Boix, P.P.; et al. Interfacial Kinetics of Efficient Perovskite Solar Cells. Crystals 2017, 7, 252. [Google Scholar] [CrossRef]

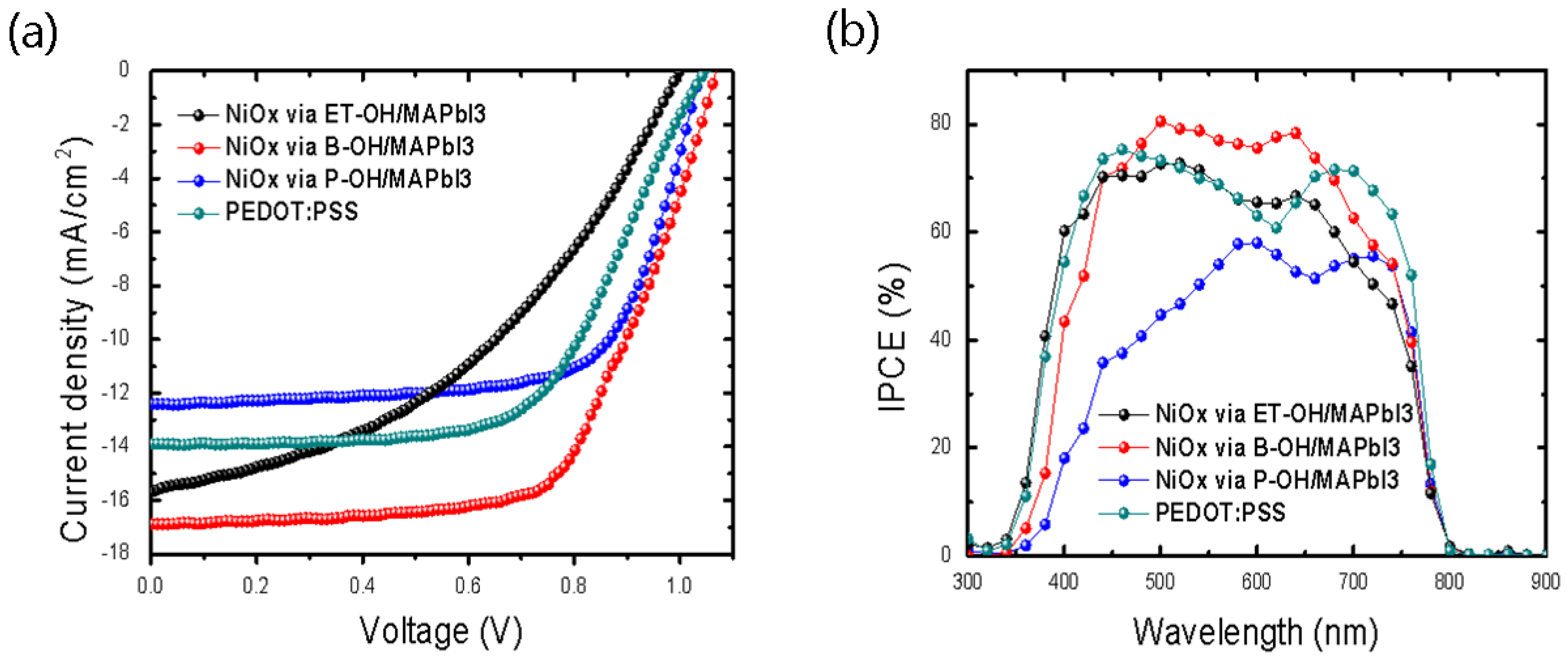
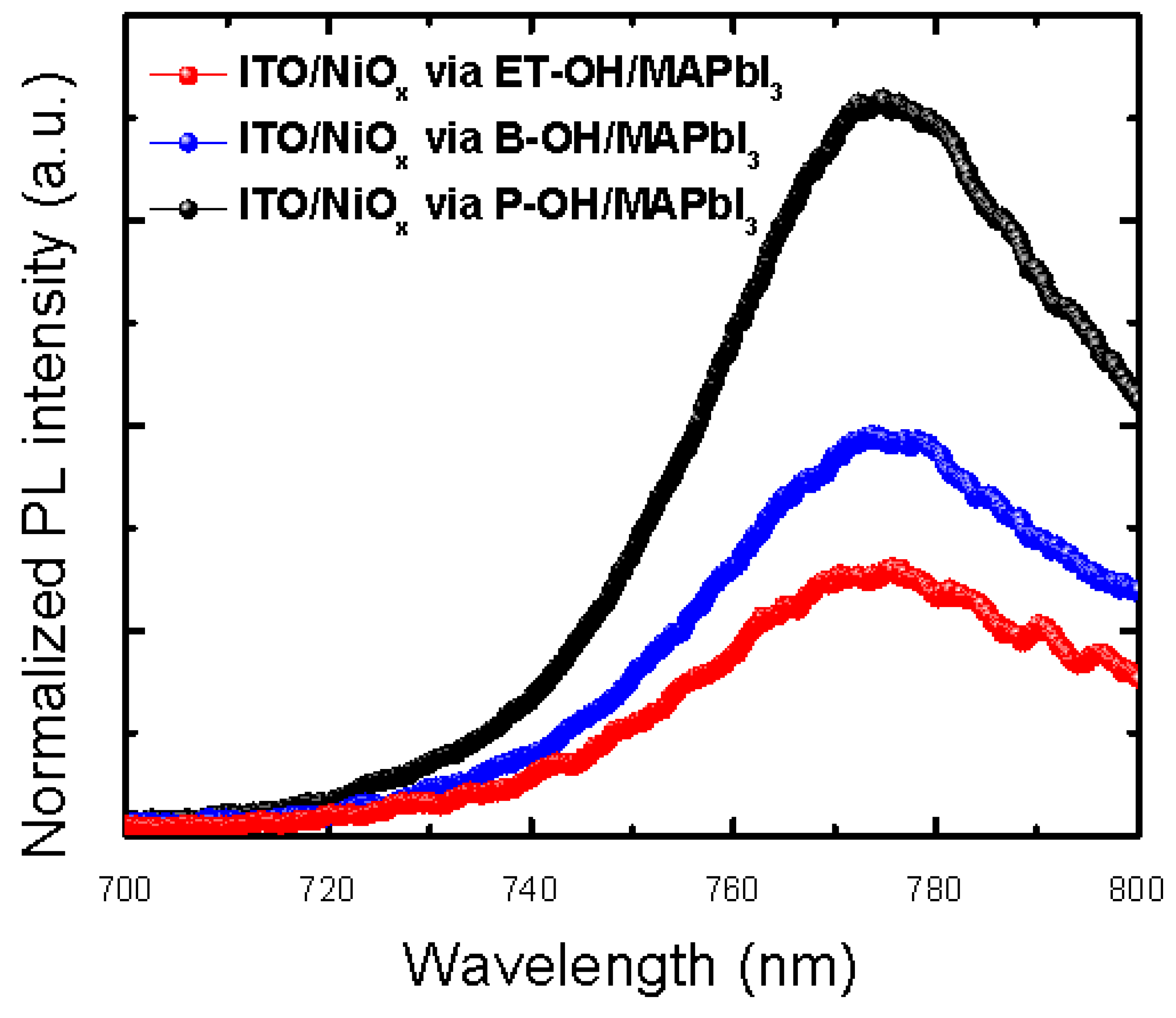
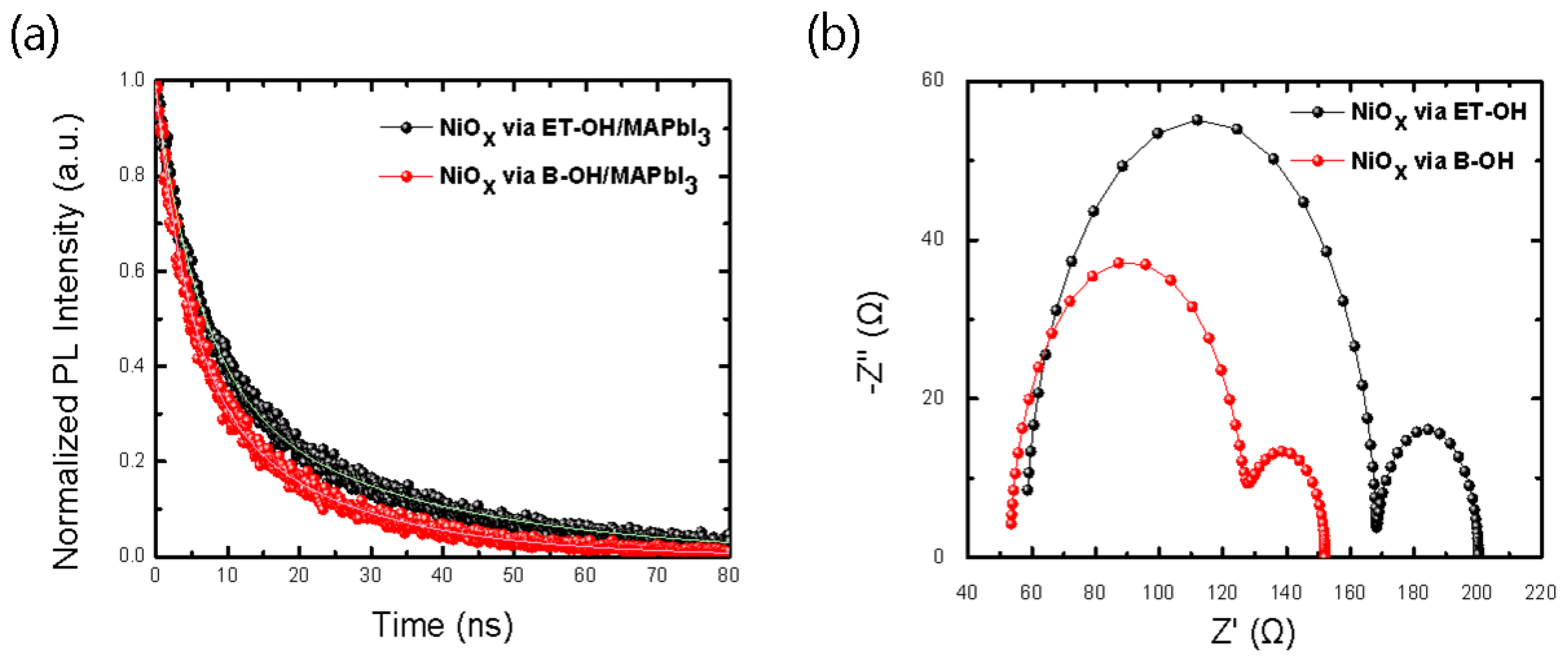
| HTLs/Parameter | Voc | Jsc | Jsc_IPCE | FF | PCE% |
|---|---|---|---|---|---|
| NiOx via ET-OH | 0.999 | 15.68 | 15.41 | 0.42 | 6.56 |
| NiOx via B-OH | 1.069 | 16.89 | 16.79 | 0.62 | 11.22 |
| NiOx via P-OH | 1.039 | 12.42 | 11.78 | 0.69 | 8.91 |
| PEDOT:PSS | 0.969 | 16.18 | 16.53 | 0.62 | 9.74 |
| Sample Condition | FWHM | C. Size (Å) |
|---|---|---|
| ITO/NiOx via ET-OH | 0.285 | 324.8 |
| ITO/NiOx via B-OH | 0.248 | 373.5 |
| ITO/NiOx via P-OH | 0.258 | 358.8 |
| NiOx via ET-OH/MAPbI3 | 0.252 | 353.6 |
| NiOx via B-OH/MAPbI3 | 0.103 | 860.6 |
| NiOx via P-OH/MAPbI3 | 0.167 | 533.6 |
| NiOx via Solvent | τ1 (ns) | τ2 (ns) | τavg (ns) | Fraction 1 (%) | Fraction 2 (%) | Rs1 (Ω) | Rs2 (Ω) |
|---|---|---|---|---|---|---|---|
| ET-OH | 6.42 | 31.91 | 24.9 | 0.72 | 0.38 | 1.10 × 102 | 3.20 × 101 |
| B-OH | 4.67 | 20.41 | 16.07 | 0.68 | 0.41 | 7.45 × 101 | 2.43 × 101 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, B.G.; Jang, W.; Wang, D.H. Facile NiOx Sol-Gel Synthesis Depending on Chain Length of Various Solvents without Catalyst for Efficient Hole Charge Transfer in Perovskite Solar Cells. Polymers 2018, 10, 1227. https://doi.org/10.3390/polym10111227
Kim BG, Jang W, Wang DH. Facile NiOx Sol-Gel Synthesis Depending on Chain Length of Various Solvents without Catalyst for Efficient Hole Charge Transfer in Perovskite Solar Cells. Polymers. 2018; 10(11):1227. https://doi.org/10.3390/polym10111227
Chicago/Turabian StyleKim, Byung Gi, Woongsik Jang, and Dong Hwan Wang. 2018. "Facile NiOx Sol-Gel Synthesis Depending on Chain Length of Various Solvents without Catalyst for Efficient Hole Charge Transfer in Perovskite Solar Cells" Polymers 10, no. 11: 1227. https://doi.org/10.3390/polym10111227
APA StyleKim, B. G., Jang, W., & Wang, D. H. (2018). Facile NiOx Sol-Gel Synthesis Depending on Chain Length of Various Solvents without Catalyst for Efficient Hole Charge Transfer in Perovskite Solar Cells. Polymers, 10(11), 1227. https://doi.org/10.3390/polym10111227