Strategies for Improving Ocular Drug Bioavailability and Corneal Wound Healing with Chitosan-Based Delivery Systems
Abstract
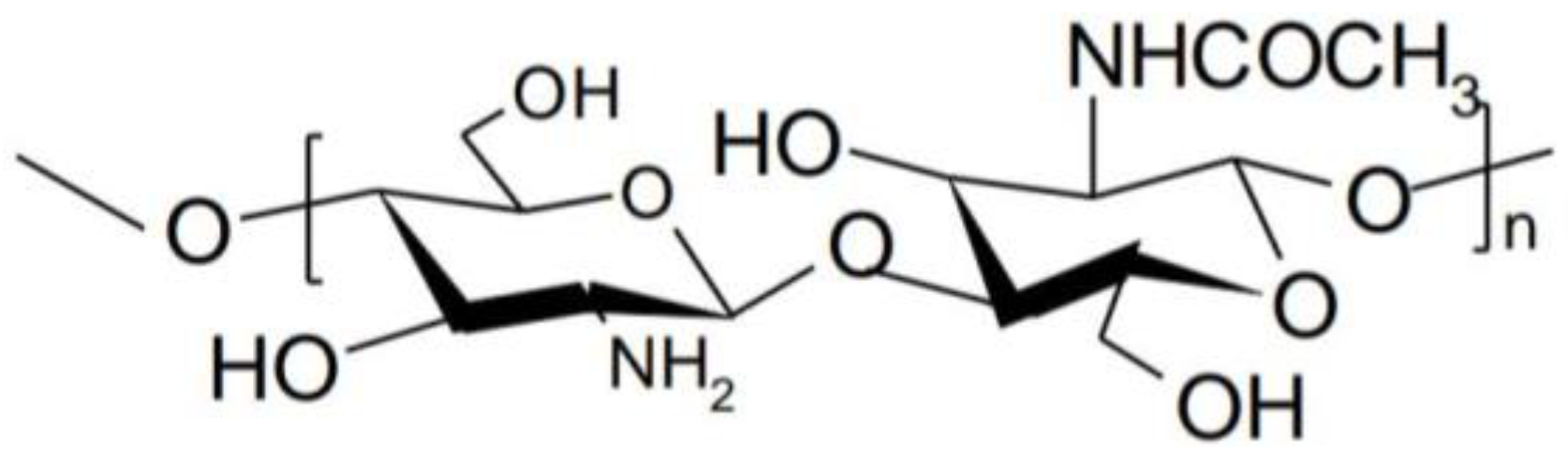
1. Introduction
- Increasing corneal residence time using viscosity enhancers, mucoadhesive agents and in situ gels;
- Increasing corneal permeability using penetration enhancers, prodrugs and colloidal systems such as nanoparticles and liposomes [22].
2. Strategies to Increase Residence Time on the Ocular Surface
2.1. Viscosity Enhancers
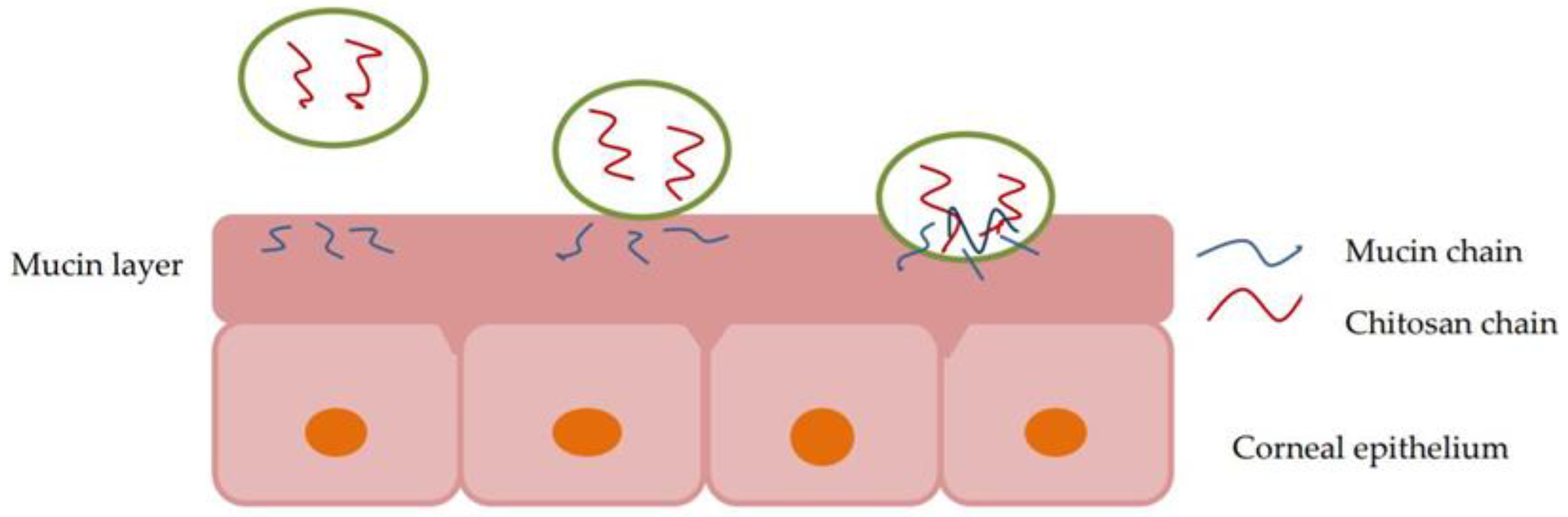
2.2. Mucoadhesive Agents
2.3. Ocular In Situ Gels
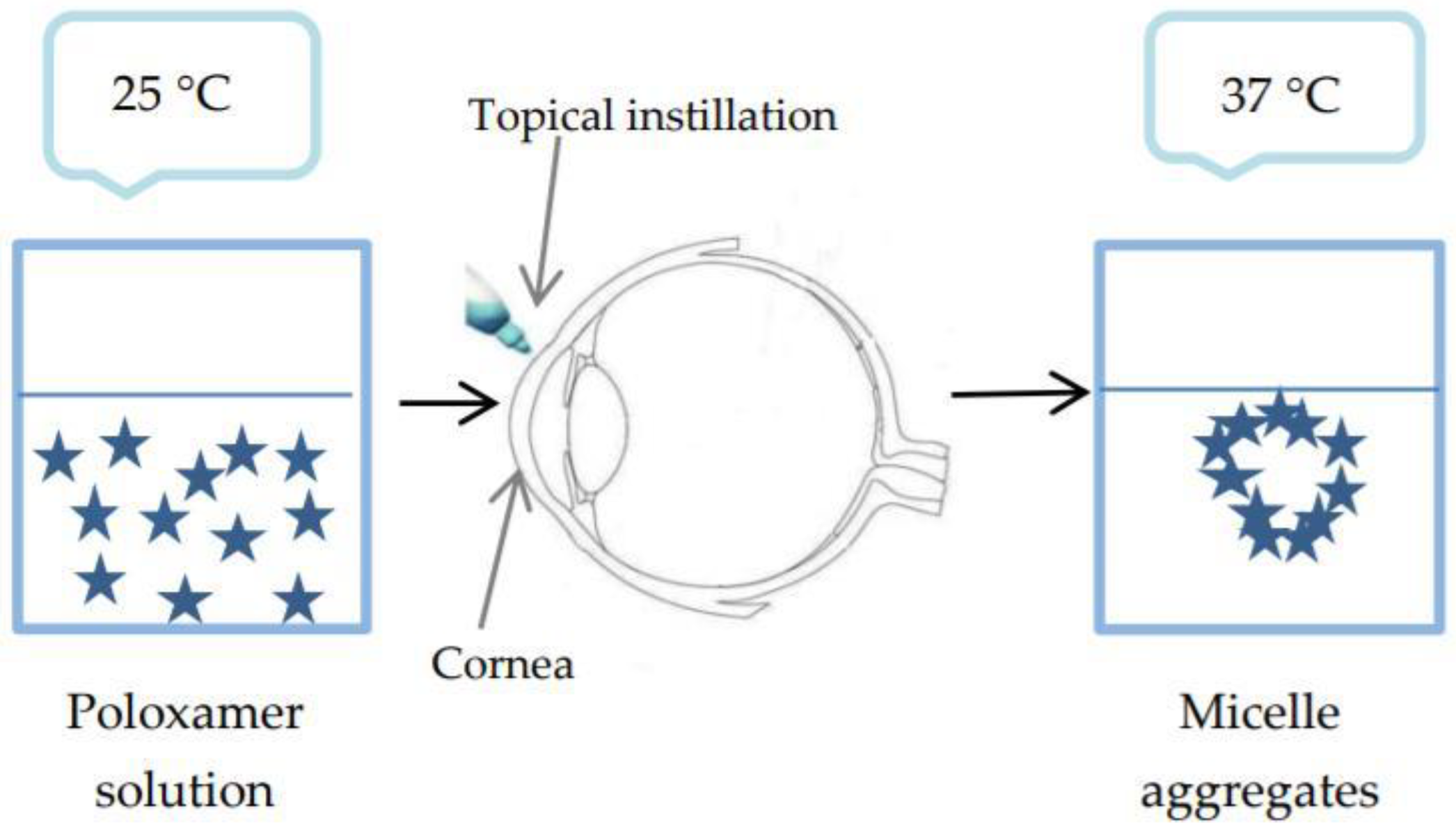
- Temperature-triggered in situ gelling polymers. The phase transition temperature is called the low critical system temperature (LCST). Below this value, the hydrogen bonds between the hydrophilic groups of the polymer and the water molecules improve dissolution of the polymer, and the system is a solution. As the temperature rises, the hydrogen bonds break, hydrophobic interactions appear and sol-gel transition takes place [57].
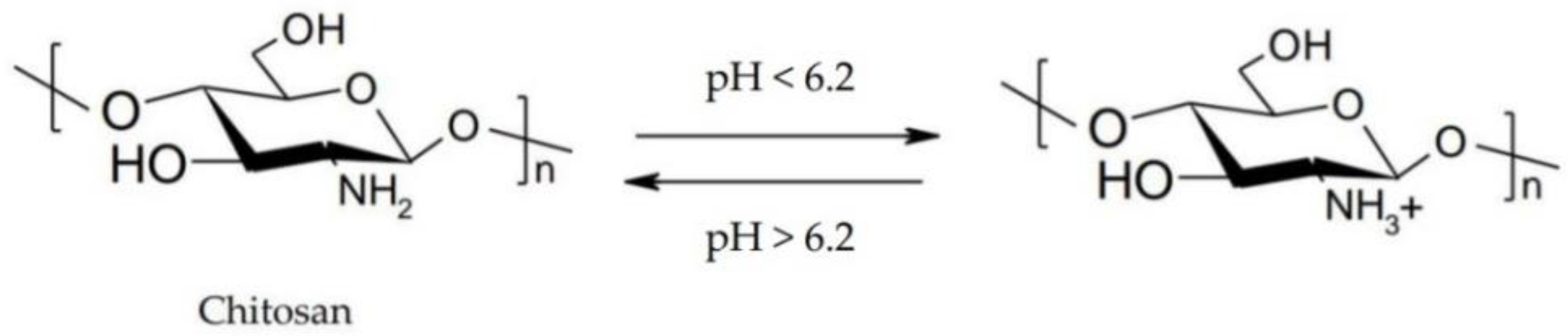
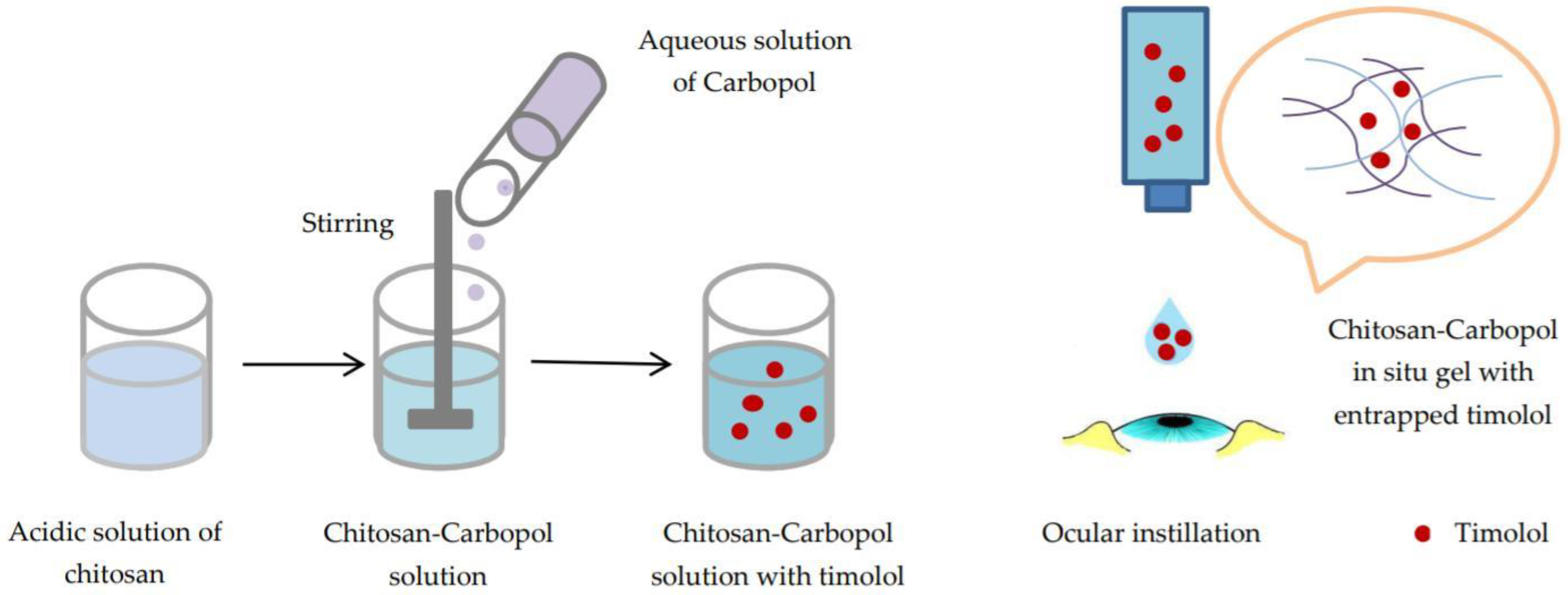
- pH-triggered in situ gelling polymers. pH-responsive polymers contain weak acidic or basic groups that release or accept protons in response to pH changes. Thus, conformational changes occur in the polymer structure that determine its swelling.
- Ion-triggered in situ gelling polymers. Cross-linking of sensitive polymers takes place due to monovalent or divalent cations in the tear film [58].
2.3.1. Temperature-Triggered In Situ Gel Systems Based on Chitosan
2.3.2. pH-Triggered In Situ Gel Systems Based on Chitosan
2.3.3. Ion-Triggered In Situ Gel Systems Based on Chitosan
3. Strategies to Increase Corneal Permeability
3.1. Permeation Enhancers
3.2. Prodrugs
3.3. Colloidal Systems: Nanoparticles and Liposomes
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jothi, M.; Harikumar, S.L.; Aggarwal, G. In-situ ophtalmic gels for the treatment of eye diseases. Int. J. Pharm. Sci. Res. 2012, 3, 1891–1904. [Google Scholar]
- Patel, A.; Cholkar, K.; Agrahari, V.; Mitra, A.K. Ocular drug delivery systems: An overview. World J. Pharmacol. 2013, 2, 47–64. [Google Scholar] [CrossRef] [PubMed]
- Moosa, R.M.; Choonara, Y.E.; du Toit, L.C.; Kumar, P.; Carmichael, T.; Tomar, L.K.; Tyagi, C.; Pillay, V. A review of topically administered mini-tablets for drug delivery to the anterior segment of the eye. J. Pharm. Pharmacol. 2014, 66, 490–506. [Google Scholar] [CrossRef] [PubMed]
- Vaishya, R.D.; Khurana, V.; Patel, S.; Mitra, A.K. Controlled ocular drug delivery with nanomicelles. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2014, 6, 422–437. [Google Scholar] [CrossRef] [PubMed]
- Roesky, C. Overcoming the challenges of ophthalmic delivery using aqueous- free technology: Redefining dry eye disease. ONdrugDelivery 2018, 82, 10–14. [Google Scholar]
- DelMonte, D.W.; Kim, T. Anatomy and physiology of the cornea. J. Cataract Refract. Surg. 2011, 37, 588–598. [Google Scholar] [CrossRef] [PubMed]
- Basaran, E.; Yazan, Y. Ocular application of chitosan. Expert Opin. Drug Deliv. 2012, 9, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Rimpelä, A.K.; Reinisalo, M.; Hellinen, L.; Grazhdankin, E.; Kidron, H.; Urtti, A.; Del Amo, E.M. Implications of melanin binding in ocular drug delivery. Adv. Drug Deliv. Rev. 2018, 126, 23–43. [Google Scholar] [CrossRef] [PubMed]
- AGaudana, R.; Ananthula, H.K.; Parenky, A.; Mitra, A.K. Ocular drug delivery. AAPS J. 2010, 12, 348–360. [Google Scholar] [CrossRef] [PubMed]
- Montilla, A.; Ruiz-Matute, A.I.; Corzo, N. Biological Effects and Extraction Processes Used to Obtain Marine Chitosan. In Bioactive Compounds from Marine Foods: Plant and Animal Sources, 1st ed.; Hernandez-Ledesma, B., Herrero, M., Eds.; John Wiley Sons, Ltd.: Chichester, UK, 2014; Volume 1, pp. 193–218. ISBN 9781118412893. [Google Scholar]
- Ahmed, T.A.; Aljaeid, B.M. Preparation, characterization, and potential application of chitosan, chitosan derivatives, and chitosan metal nanoparticles in pharmaceutical drug delivery. Drug Des. Devel. Ther. 2016, 10, 483–507. [Google Scholar] [CrossRef] [PubMed]
- Kumirska, J.; Weinhold, M.X.; Thöming, J.; Stepnowski, P. Biomedical Activity of Chitin/Chitosan Based Materials—Influence of Physicochemical Properties Apart from Molecular Weight and Degree of N-Acetylation. Polymers 2011, 3, 1875–1901. [Google Scholar] [CrossRef]
- Zhao, D.; Yu, S.; Sun, B.; Gao, S.; Guo, S.; Zhao, K. Biomedical Applications of Chitosan and Its Derivative Nanoparticles. Polymers 2018, 10, 1–17. [Google Scholar] [CrossRef]
- Szymańska, E.; Winnicka, K. Stability of Chitosan—A Challenge for Pharmaceutical and Biomedical Applications. Mar. Drugs 2015, 13, 1819–1846. [Google Scholar] [CrossRef] [PubMed]
- Cheung, R.C.F.; Ng, T.B.; Wong, J.H.; Chan, W.Y. Chitosan: An Update on Potential Biomedical and Pharmaceutical Applications. Mar. Drugs 2015, 13, 5156–5186. [Google Scholar] [CrossRef] [PubMed]
- Peña, A.; Sánchez, N.S.; Calahorra, M. Effects of chitosan on Candida albicans: Conditions for its antifungal activity. Biomed. Res. Int. 2013, 2013, 1–15. [Google Scholar] [CrossRef]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Alonso, M.J.; Sánchez, A. The potential of chitosan in ocular drug delivery. J. Pharm. Pharmacol. 2003, 55, 1451–1463. [Google Scholar] [CrossRef] [PubMed]
- Khare, A.; Grover, K.; Pawar, P.; Singh, I. Mucoadhesive Polymers for Enhancing Retention in Ocular Drug Delivery: A Critical Review. Rev. Adhesion Adhesives 2014, 2, 467–502. [Google Scholar] [CrossRef]
- Cui, R.; Lu, Q.; Teng, Y.; Li, K.; Li, N. Chitosan Promoted the Corneal Epithelial Wound Healing via Activation of ERK Pathway. Curr. Eye Res. 2017, 42, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Baranowski, P.; Karolewicz, B.; Gajda, M.; Pluta, J. Ophthalmic Drug Dosage Forms: Characterisation and Research Methods. Sci. World J. 2014, 2014, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Rupenthal, I.D. Ocular Drug Delivery Technologies: Exciting Times Ahead. ONdrugDelivery 2015, 54, 7–11. [Google Scholar]
- Saettone, M.F. Progress and Problems in Ophthalmic Drug Delivery. In Business Briefing: Pharma Tech, 4th ed.; World Markets Research Center, Ed.; World Markets Research Center: London, UK, 2002; Volume 1, pp. 167–171. ISBN 9781903150603. [Google Scholar]
- Zhu, H.; Chauhan, A. Effect of viscosity on tear drainage and ocular residence time. Optom. Vis. Sci. 2008, 85, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Morrison, P.W.; Khutoryanskiy, V.V. Advances in ophthalmic drug delivery. Ther. Deliv. 2014, 5, 1297–1315. [Google Scholar] [CrossRef] [PubMed]
- Shastri, D.H.; Shelat, P.K.; Shukla, A.K.; Patel, P.B. Ophthalmic drug delivery system: Challenges and approaches. Syst. Rev. Pharm. 2010, 1, 113–120. [Google Scholar] [CrossRef]
- Silva, C.L.; Pereira, J.C.; Ramalho, A.; Pais, A.A.C.C.; Sousa, J.J.S. Films based on chitosan polyelectrolyte complexes for skin drug delivery. Development and characterization. J. Memb. Sci. 2008, 320, 268–279. [Google Scholar] [CrossRef]
- Bhattarai, N.; Gunn, J.; Zhang, M. Chitosan-based hydrogels for controlled, localized drug delivery. Adv. Drug Deliv. Rev. 2010, 62, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Rafique, A.; Mahmood Zia, K.; Zuber, M.; Tabasum, S.; Rehman, S. Chitosan functionalized poly(vinyl alcohol) for prospects biomedical and industrial applications: A review. Int. J. Biol. Macromol. 2016, 87, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Ricciardi, R.; Mangiapia, G.; Lo Celso, F.; Paduano, L.; Triolo, R.; Auriemma, F.; De Rosa, C.; Lauprêtre, F. Structural Organization of Poly(vinyl alcohol) Hydrogels Obtained by Freezing and Thawing Techniques: A SANS Study. Chem. Mater. 2005, 17, 1183–1189. [Google Scholar] [CrossRef]
- Bhoyar, B.S.; Agnihotrh, V.V.; Bodhankar, M.M. A noval thermoreversible phase transition system with flux enhancers for opthalmic application. Int. J. Pharm. Pharm. Sci. 2011, 3, 367–370. [Google Scholar]
- Seyed, M.A.; Vijayaraghavan, K. Physicochemical Characterizaton and Bioactivity of an Improved Chitosan Scaffold Cross-Linked With Polyvinyl Alcohol for Corneal Tissue Engineering Applications. Annu. Res. Rev. Biol. 2018, 24, 1–16. [Google Scholar] [CrossRef]
- Wagh, V.D.; Inamdar, B.; Samanta, M.K. Polymers used in ocular dosage form and drug delivery systems. Asian J. Pharm. 2008, 2, 12–17. [Google Scholar] [CrossRef]
- Ahmed, T.A.; Aljaeid, B.M. A potential in situ gel formulation loaded with novel fabricated poly(lactide-co-glycolide) nanoparticles for enhancing and sustaining the ophthalmic delivery of ketoconazole. Int. J. Nanomed. 2017, 12, 1863–1875. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.M.; Calado, R.; Marto, J.; Bettencourt, A.; Almeida, A.J.; Gonçalves, L.M.D. Chitosan Nanoparticles as a Mucoadhesive Drug Delivery System for Ocular Administration. Mar. Drugs 2017, 15, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Gharge, V.; Pawar, P. Recent Trends in Chitosan Based Nanotechnology: A Reference to Ocular Drug Delivery System. IJOVS 2017, 2, 98–105. [Google Scholar]
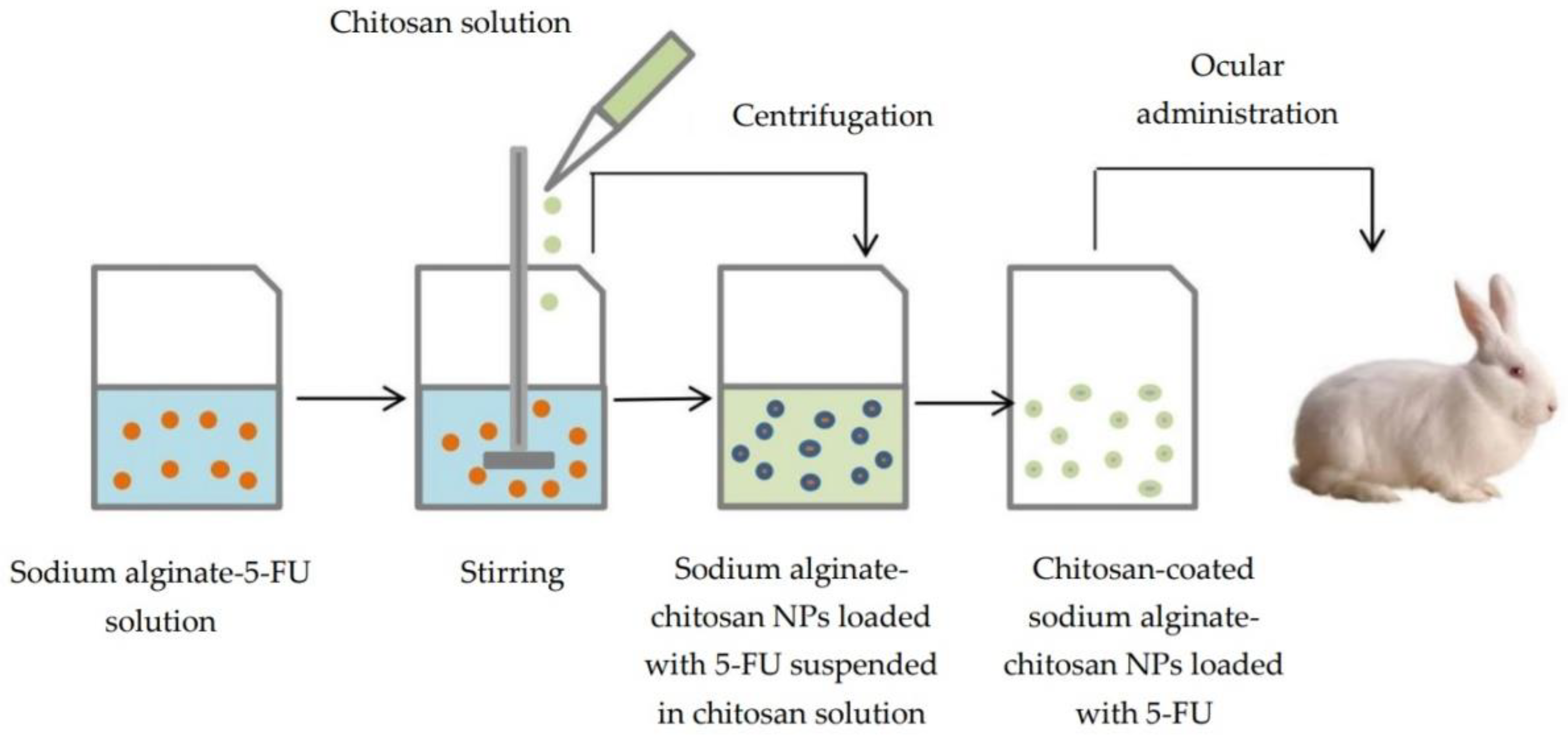
- Nagarwal, R.C.; Kumar, R.; Pandit, J.K. Chitosan coated sodium alginate-chitosan nanoparticles loaded with 5-FU for ocular delivery: In vitro characterization and in vivo study in rabbit eye. Eur. J. Pharm. Sci. 2012, 47, 678–685. [Google Scholar] [CrossRef] [PubMed]
- Ways, T.M.M.; Lau, W.M.L.; Khutoryanskiy, V.V. Chitosan and Its Derivatives for Application in Mucoadhesive Drug Delivery Systems. Polymers 2018, 10, 1–37. [Google Scholar] [CrossRef]
- Conrady, C.D.; Joos, Z.P.; Patel, B.C.K. Review: The lacrimal gland and its role in dry eye. J. Ophthalmol. 2016, 2016, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Boddupalli, B.M.; Mohammed, Z.N.K.; Nath, R.A.; Banji, D. Mucoadhesive drug delivery system: An overview. J. Adv. Pharm. Technol. Res. 2010, 1, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Ueda, K.; Isowaki, A.; Ohtori, A.; Takeuchi, H.; Ohguro, N.; Tojo, K. Mucoadhesive properties of chitosan-coated ophthalmic lipid emulsion containing indomethacin in tear fluid. Biol. Pharm. Bull. 2009, 32, 1266–1271. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Tian, S.; Tao, Q.; Zhao, Y.; Gui, R.; Yang, F.; Zang, L.; Chen, Y.; Ping, Q.; Hou, D. Montmorillonite/chitosan nanoparticles as a novel controlled-release topical ophthalmic delivery system for the treatment of glaucoma. Int. J. Nanomed. 2018, 13, 3975–3987. [Google Scholar] [CrossRef] [PubMed]
- Cook, S.L.; Bull, S.P.; Methwen, L.; Parker, J.K.; Khutoryanskiya, V.V. Mucoadhesion: A food perspective. Food Hydrocolloids 2017, 72, 281–296. [Google Scholar] [CrossRef]
- Shinde, U.A.; Shete, J.N.; Nair, H.A.; Singh, K.H. Design and characterization of chitosan-alginate microspheres for ocular delivery of azelastine. Pharm. Dev. Technol. 2014, 19, 813–823. [Google Scholar] [CrossRef] [PubMed]
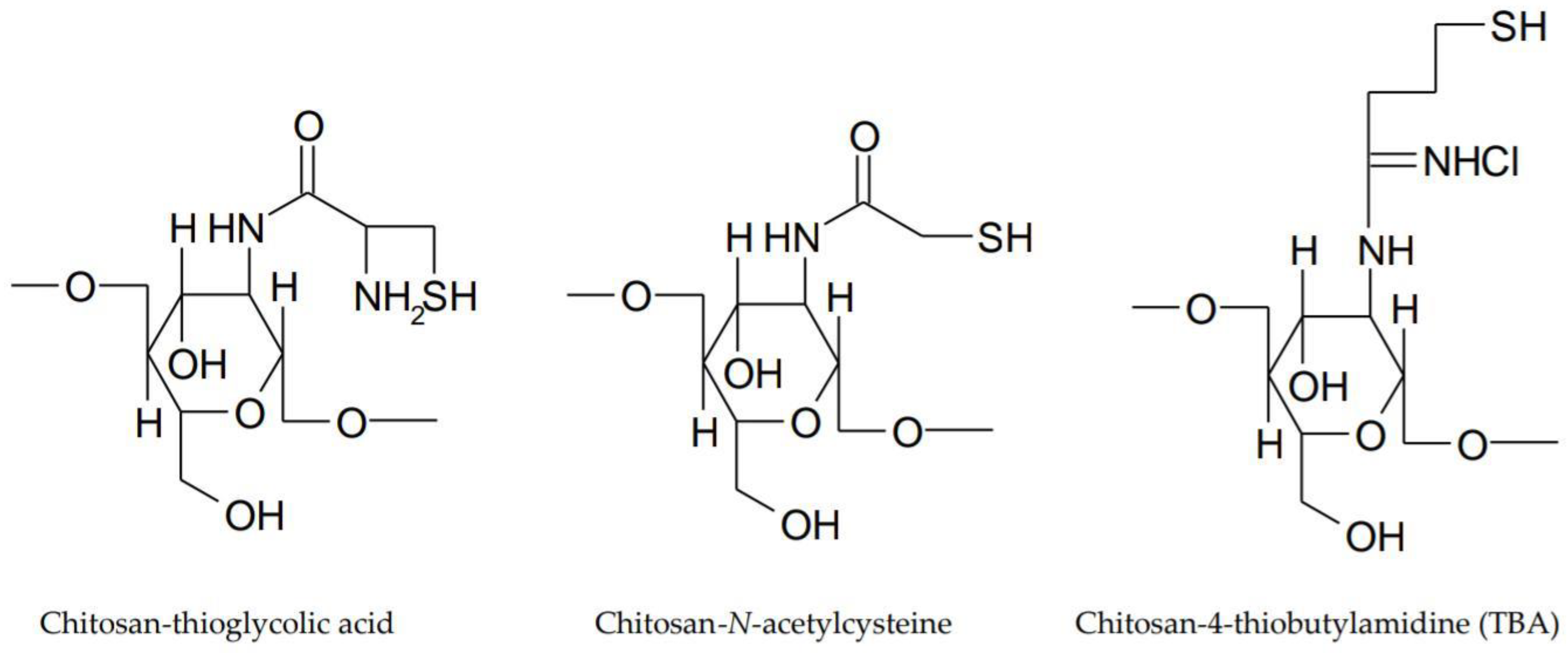
- Shastri, D.H. Thiolated chitosan: A boon to ocular delivery of therapeutics. MOJ Bioequiv. Availab. 2017, 3, 34–37. [Google Scholar] [CrossRef]
- Sreenivas, S.A.; Pai, K.V. Thiolated Chitosans: Novel Polymers for Mucoadhesive Drug Delivery—A Review. Trop. J. Pharm. Res. 2008, 7, 1077–1088. [Google Scholar] [CrossRef]
- Fischak, C.; Klaus, R.; Werkmeister, R.M.; Hohenadl, C.; Prinz, M.; Schmetterer, L.; Garhöfer, G. Effect of Topically Administered Chitosan-N-Acetylcysteine on Corneal Wound Healing in a Rabbit Model. J. Ophthalmol. 2017, 2017, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Su, M.; Tang, S.; Wang, L.; Liang, X.; Meng, F.; Hong, Y.; Xu, Z. Synthesis of thiolated chitosan and preparation nanoparticles with sodium alginate for ocular drug delivery. Mol. Vis. 2012, 18, 1973–1982. [Google Scholar] [PubMed]
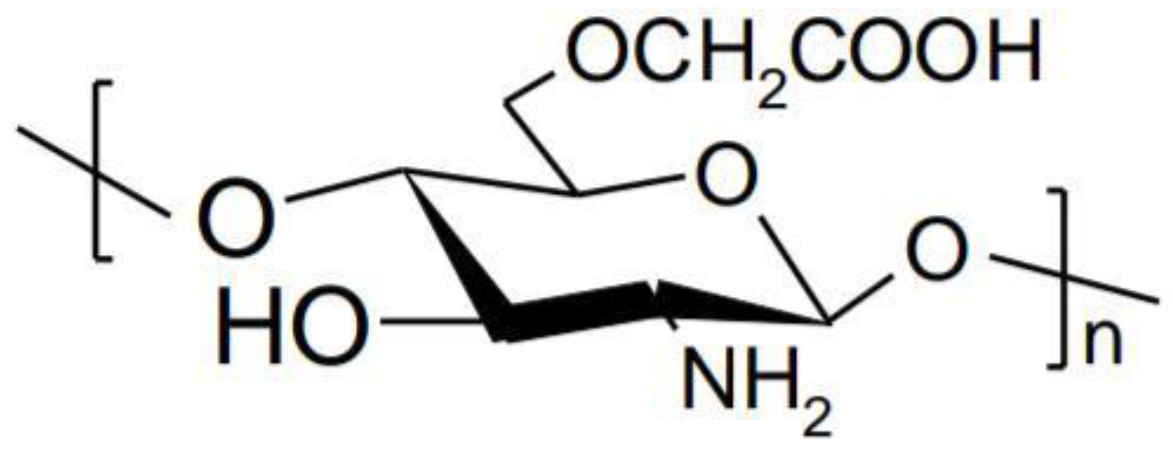
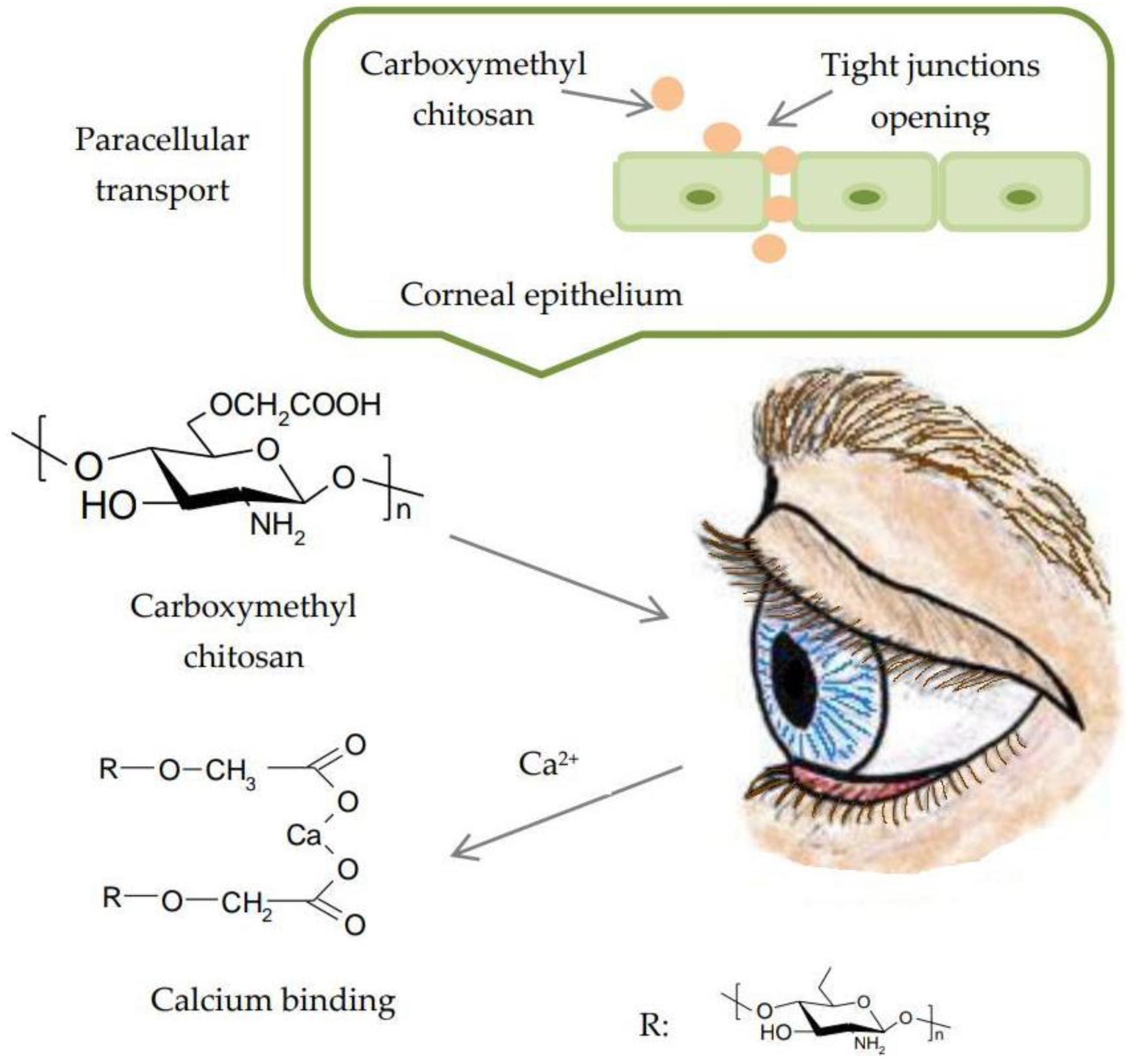
- Fonseca-Santos, B.; Chorilli, M. An overview of carboxymethyl derivatives of chitosan: Their use as biomaterials and drug delivery systems. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 77, 1349–1362. [Google Scholar] [CrossRef] [PubMed]
- Farag, R.K.; Mohamed, R.R. Synthesis and characterization of carboxymethyl chitosan nanogels for swelling studies and antimicrobial activity. Molecules 2012, 18, 190–203. [Google Scholar] [CrossRef] [PubMed]
- Shinde, U.; Ahmed, M.H.; Singh, K. Development of Dorzolamide Loaded 6-O-Carboxymethyl Chitosan Nanoparticles for Open Angle Glaucoma. J. Drug Deliv. 2013, 2013, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Salzillo, R.; Schiraldi, C.; Corsuto, L.; D’Agostino, A.; Filosa, R.; De Rosa, M.; La Gatta, A. Optimization of hyaluronan-based eye drop formulations. Carbohydr. Polym. 2016, 153, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Wang, Z.; Liu, Y.; Wang, L.; Jiang, Z.; Li, T.; Zhang, W.; Liang, Y. Carboxymethyl chitosan/gelatin/hyaluronic acid blended-membranes as epithelia transplanting scaffold for corneal wound healing. Carbohydr. Polym. 2018, 192, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Makwana, S.B.; Patel, V.A.; Parmar, S.J. Development and characterization of in situ gel for ophthalmic formulation containing ciprofloxacin hydrochloride. Results Pharma Sci. 2015, 6, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Mali, M.N.; Hajare, A.A. In situ gel-forming systems for sustained ocular drug delivery. Eur. Ind. Pharm. 2010, 5, 17–20. [Google Scholar]
- Kumar, V.; Rajput, R.; Singh, S. The use of in situ hydrogel in ocular drug delivery. IJPPR 2016, 7, 1320–1325. [Google Scholar]
- Agrawal, A.K.; Das, M.; Jain, S. In situ gel systems as “smart” carriers for sustained ocular drug delivery. Expert Opin. Drug Deliv. 2012, 9, 383–402. [Google Scholar] [CrossRef] [PubMed]
- Jain, D.; Kumar, V.; Singh, S.; Mullertz, A.; Bar-Shalon, D. Newer trends in in situ gelling systems for controlled ocular drug delivery. J. Anal. Pharm. Res. 2016, 2, 1–16. [Google Scholar] [CrossRef]
- Cho, J.; Heuzey, M.C.; Bégin, A.; Carreau, P.J. Physical Gelation of Chitosan in the Presence of β-Glycerophosphate: The Effect of Temperature. Biomacromolecules 2005, 6, 3267–3275. [Google Scholar] [CrossRef] [PubMed]
- Carreira, A.S.; Gonçalves, A.M.M.; Mendonça, P.V.; Gil, M.H.; Coelho, J.F.J. Temperature and pH responsive polymers based on chitosan: Applications and new graft copolymerization strategies based on living radical polymerization. Carbohydr. Polym. 2010, 80, 618–630. [Google Scholar] [CrossRef]
- Almeida, H.; Amaral, M.H.; Lobão, P.; Lobo, J.M. In situ gelling systems: A strategy to improve the bioavailability of ophthalmic pharmaceutical formulations. Drug Discov. Today. 2014, 19, 400–412. [Google Scholar] [CrossRef] [PubMed]
- Florea, M.; Monciu, C.M.; Ilie, M. Determination of nimesulide by ion pair high-performance liquid chromatography using tetrabutylammonium as the counterion. Anal. Lett. 2015, 48, 328–339. [Google Scholar] [CrossRef]
- Racine, L.; Texier, I.; Auzély-Velty, R. Chitosan-based hydrogels: Recent design concepts to tailor properties and functions. Polym. Int. 2017, 66, 981–998. [Google Scholar] [CrossRef]
- Ahmadi, F.; Oveisi, Z.; Samani, S.M.; Amoozgar, Z. Chitosan based hydrogels: Characteristics and pharmaceutical applications. Res. Pharm. Sci. 2015, 10, 1–16. [Google Scholar] [PubMed]
- Al-Kinani, A.A.; Zidan, G.; Elsaid, N.; Seyfoddin, A.; Alani, A.W.G.; Alany, R.G. Ophthalmic gels: Past, present and future. Adv. Drug Deliv. Rev. 2018, 126, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Tuğcu Demiröz, F. Development of in situ poloxamer-chitosan hydrogels for vaginal drug delivery of benzydamine hydrochloride: Textural, mucoadhesive and in vitro release properties. Marmara Pharm. J. 2017, 21, 762–770. [Google Scholar] [CrossRef]
- Bachhav, H.D.; Bachhav, A.; Bachhav, R.; Derle, D. Development of poloxamer based thermosensitive in situ ocular gel of betaxolol hydrochloride. Int. J. Pharm. Pharm. Sci. 2015, 7, 287–291. [Google Scholar]
- Qian, Y.; Wang, F.; Li, R.; Zhang, Q.; Xu, Q. Preparation and evaluation of in situ gelling ophthalmic drug delivery system for methazolamide. Drug Dev. Ind. Pharm. 2010, 36, 1340–1347. [Google Scholar] [CrossRef] [PubMed]
- Kadam, A.T.; Jadhav, R.I.; Salunke, P.B.; Kadam, S.S. Design and evaluation of modified chitosan based in situ gel for ocular drug delivery. Int. J. Pharm. Pharm. Sci. 2017, 9, 87–91. [Google Scholar] [CrossRef]
- Fabiano, A.; Bizzarri, R.; Zambito, Y. Thermosensitive hydrogel based on chitosan and its derivatives containing medicated nanoparticles for transcorneal administration of 5-fluorouracil. Int. J. Nanomed. 2017, 12, 633–643. [Google Scholar] [CrossRef] [PubMed]
- del Valle, L.J.; Diaz, A.; Puiggali, J. Hydrogels for Biomedical Applications: Cellulose, Chitosan, and Protein/Peptide Derivatives. Gels 2017, 3, 1–27. [Google Scholar] [CrossRef]
- Tsai, C.Y.; Woung, L.C.; Yen, J.C.; Tseng, P.C.; Chiou, S.H.; Sung, Y.J.; Liu, K.T.; Cheng, Y.H. Thermosensitive chitosan-based hydrogels for sustained release of ferulic acid on corneal wound healing. Carbohydr. Polym. 2016, 135, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Nilsen-Nygaard, J.; Strand, S.; Varum, K.M.; Draget, K.I.; Nordgard, C.T. Chitosan: Gels and Interfacial Properties. Polymers 2015, 7, 552–579. [Google Scholar] [CrossRef]
- Song, Y.; Nagai, N.; Saijo, S.; Kaji, H.; Nishizawa, M.; Abe, T. In situ formation of injectable chitosan-gelatin hydrogels through double crosslinking for sustained intraocular drug delivery. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 88, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Chien, Y.; Liao, Y.W.; Liu, D.M.; Lin, H.L.; Chen, S.J.; Chen, H.L.; Peng, C.H.; Liang, C.M.; Mou, C.Y.; Chiou, S.H. Corneal repair by human corneal keratocyte-reprogrammed iPSCs and amphiphatic carboxymethyl-hexanoyl chitosan hydrogel. Biomaterials 2012, 33, 8003–8016. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Liu, Y.; Li, X.; Dereje, K.; Zhang, B.; Ren, J.; Lu, J.; Li, J.; Du, S.; Liu, Z. Research progress of in-situ gelling ophthalmic drug delivery system. AJPS 2018, in press. [Google Scholar] [CrossRef]
- Gupta, S.; Vyas, S.P. Carbopol/Chitosan Based pH Triggered In Situ Gelling System for Ocular Delivery of Timolol Maleate. Sci. Pharm. 2010, 78, 959–976. [Google Scholar] [CrossRef] [PubMed]
- Zaki, R.; Khames, A.; Hosny, K.M.; Abd-Elbary, A. Ketorolac tromethamine in situ ocular hydrogel; preparation, characterization and in vivo evaluation. Arjournals 2011, 3, 535–545. [Google Scholar]
- Coviello, T.; Matricardi, P.; Marianecci, C.; Alhaique, F. Polysaccharide hydrogels for modified release formulations. J. Control. Release 2007, 119, 5–24. [Google Scholar] [CrossRef] [PubMed]
- Gupta, H.; Aqil, M.; Khar, R.K.; Ali, A.; Bhatnagar, A.; Mittal, G. An alternative in situ gel-formulation of levofloxacin eye drops for prolonged ocular retention. J. Pharm. Bioallied Sci. 2015, 7, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Weng, Y.; Xu, L.; Chen, H. Sustained release of Avastin® from polysaccharides cross-linked hydrogels for ocular drug delivery. Int. J. Biol. Macromol. 2013, 60, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Gupta, H.; Velpandian, T.; Jain, S. Ion- and pH-activated novel in-situ gel system for sustained ocular drug delivery. J. Drug Target. 2010, 18, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Achouri, D.; Alhanout, K.; Piccerelle, P.; Andrieu, V. Recent advances in ocular drug delivery. Drug Dev. Ind. Pharm. 2013, 39, 1599–1617. [Google Scholar] [CrossRef] [PubMed]
- Morrison, P.W.J.; Khutoryanskiy, V.V. Enhancement in corneal permeability of riboflavin using calcium sequestering compounds. Int. J. Pharm. 2014, 472, 56–64. [Google Scholar] [CrossRef]
- Yeh, T.H.; Hsu, L.W.; Tseng, M.T.; Lee, P.L.; Sonjae, K.; Ho, Y.C.; Sung, H.W. Mechanism and consequence of chitosan-mediated reversible epithelial tight junction opening. Biomaterials 2011, 32, 6164–6173. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, S.; Hippalgaonkar, K.; Repka, M.A. Effect of chitosan, benzalkonium chloride and ethylenediaminetetraacetic acid on permeation of acyclovir across isolated rabbit cornea. Int. J. Pharm. 2008, 348, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.A.; El-Feky, G.S.; Kamel, R.; Awad, G.E. Chitosan/sulfobutylether-β-cyclodextrin nanoparticles as a potential approach for ocular drug delivery. Int. J. Pharm. 2011, 413, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Liu, X.; Hu, W.; Bai, Y.; Zhang, L. Preparation and evaluation of naringenin-loaded sulfobutylether-β-cyclodextrin/chitosan nanoparticles for ocular drug delivery. Carbohydr. Polym. 2016, 149, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Caramella, C.; Ferrari, F.; Bonferoni, M.C.; Rossi, S.; Sandri, G. Chitosan and its derivatives as drug penetration enhancers. J. Drug Del. Sci. Technol. 2010, 20, 5–13. [Google Scholar] [CrossRef]
- Zambito, Y.; Di Colo, G. Chitosan and its derivatives as intraocular penetration enhancers. J. Drug Deliv. Sci. Technol. 2010, 20, 45–52. [Google Scholar] [CrossRef]
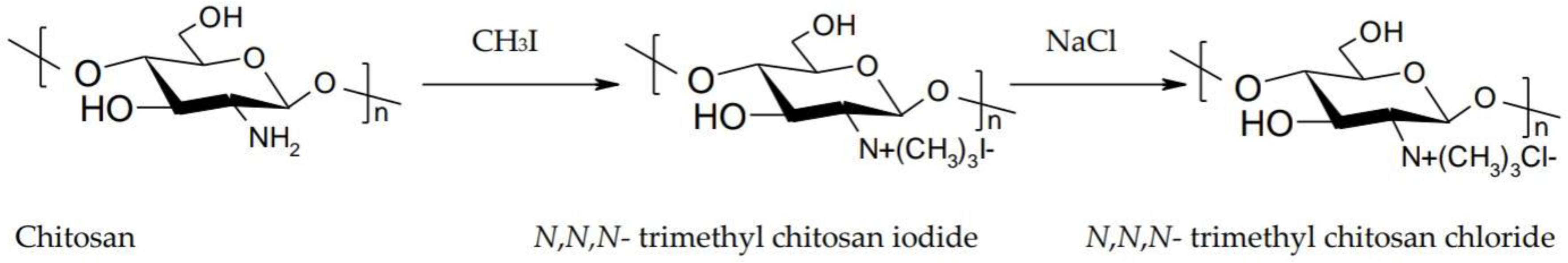
- Asasutjarit, R.; Theerachayanan, T.; Kewsuwan, P.; Veeranodha, S.; Fuongfuchat, A.; Ritthidej, G.C. Development and Evaluation of Diclofenac Sodium Loaded-N-Trimethyl Chitosan Nanoparticles for Ophthalmic Use. AAPS PharmSciTech 2015, 16, 1013–1024. [Google Scholar] [CrossRef] [PubMed]
- Di Colo, G.; Burgalassi, S.; Zambito, Y.; Monti, D.; Chetoni, P. Effects of different N-trimethyl chitosans on in vitro/in vivo ofloxacin transcorneal permeation. J. Pharm. Sci. 2004, 93, 2851–2862. [Google Scholar] [CrossRef] [PubMed]
- Kean, T.; Thanou, M. Biodegradation, biodistribution and toxicity of chitosan. Adv. Drug Deliv. Rev. 2010, 62, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Zubareva, A.; Shagdarova, B.; Varlamov, V.; Kashirina, E.; Svirshchevskaya, E. Penetration and toxicity of chitosan and its derivatives. Eur. Polym. J. 2017, 93, 743–749. [Google Scholar] [CrossRef]
- Rizwan, M.; Yahya, R.; Hassan, A.; Yar, M.; Azzahari, A.D.; Selvanathan, V.; Sonsudin, F.; Abouloula, C.N. pH sensitive hydrogels in drug delivery: Brief history, properties, swelling and release mechanism, material selection and applications. Polymers 2017, 9, 1–37. [Google Scholar] [CrossRef]
- Schuerer, N.; Stein, E.; Inic-Kanada, A.; Ghasemian, E.; Stojanovic, M.; Montanaro, J.; Bintner, N.; Hohenadl, C.; Sachsenhofer, R.; Barisani-Asenbauer, T. Effects of chitosan and chitosan N-acetylcysteine solutions on conjunctival epithelial cells. J. EuCornea 2018, 1, 12–18. [Google Scholar] [CrossRef]
- Liu, D.; Li, J.; Pan, H.; He, F.; Liu, Z.; Wu, Q.; Bai, C.; Yu, S.; Yang, X. Potential advantages of a novel chitosan-N-acetylcysteine surface modified nanostructured lipid carrier on the performance of ophthalmic delivery of curcumin. Sci. Rep. 2016, 6, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Tan, G.; Cheng, B.; Liu, D.; Pan, W. Transport mechanism of chitosan-N-acetylcysteine, chitosan oligosaccharides or carboxymethyl chitosan decorated coumarin-6 loaded nanostructured lipid carriers across the rabbit ocular. Eur. J. Pharm. Biopharm. 2017, 120, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Ye, T.; Yuan, K.; Zhang, W.; Song, S.; Chen, F.; Yang, X.; Wang, S.; Bi, J.; Pan, W. Prodrugs incorporated into nanotechnology-based drug delivery systems for possible improvement in bioavailability of ocular drugs delivery. AJPS 2013, 8, 207–217. [Google Scholar] [CrossRef]
- Barot, M.; Bagui, M.; Gokulgandhi, M.R.; Mitra, A.K. Prodrug Strategies in Ocular Drug Delivery. Med. Chem. 2012, 8, 753–768. [Google Scholar] [CrossRef] [PubMed]
- Kapanigowda, U.G.; Nagaraja, S.H.; Ramaiah, B.; Boggarapu, P.R. Improved intraocular bioavailability of ganciclovir by mucoadhesive polymer based ocular microspheres: Development and simulation process in Wistar rats. Daru 2015, 23, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.H.; Hung, K.H.; Tsai, T.H.; Lee, C.J.; Ku, R.Y.; Chiu, A.W.; Chiou, S.H.; Liu, C.J. Sustained delivery of latanoprost by thermosensitive chitosan-gelatin-based hydrogel for controlling ocular hypertension. Acta Biomater. 2014, 10, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Li, J.; Wang, J.; Yin, Z.; Zhu, Y.; Liu, W. Development of Timolol-Loaded Galactosylated Chitosan Nanoparticles and Evaluation of Their Potential for Ocular Drug Delivery. AAPS PharmSciTech 2017, 18, 997–1008. [Google Scholar] [CrossRef] [PubMed]
- Taskar, P.; Tatke, A.; Majumdar, S. Advances in the use of prodrugs for drug delivery to the eye. Expert Opin. Drug Deliv. 2017, 14, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Li, Q.; Li, Y.; Wang, H.; Liu, D.; Yang, X.; Pan, W. A novel hydrogel with dual temperature and pH responsiveness loading nanostructured lipid carriers as an ophthalmic delivery system: Enhanced trans-corneal permeability and bioavailability of nepafenac. New J. Chem. 2017, 41, 1–11. [Google Scholar] [CrossRef]
- Barar, J.; Aghanejad, A.; Fathi, M.; Omidi, Y. Advanced drug delivery and targeting technologies for the ocular diseases. Bioimpacts 2016, 6, 49–67. [Google Scholar] [CrossRef] [PubMed]
- Paolicelli, P.; de la Fuente, M.; Sánchez, A.; Seijo, B.; Alonso, M.J. Chitosan nanoparticles for drug delivery to the eye. Expert Opin. Drug Deliv. 2009, 6, 239–253. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.-Y.; Hao, J.-L.; Wang, S.; Zheng, Y.; Zhang, W.-S. Nanoparticles in the ocular drug delivery. Int. J. Ophthalmol. 2013, 6, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Silva, N.C.; Silva, S.; Sarmento, B.; Pintado, M. Chitosan nanoparticles for daptomycin delivery in ocular treatment of bacterial endophthalmitis. Drug Deliv. 2015, 22, 885–893. [Google Scholar] [CrossRef] [PubMed]
- Badawi, A.A.; El-Laithy, H.M.; El Qidra, R.K.; El Mofty, H.; El dally, M. Chitosan based nanocarriers for indomethacin ocular delivery. Arch. Pharm. Res. 2008, 31, 1040–1049. [Google Scholar] [CrossRef] [PubMed]
- .Kaskoos, R.A. Investigation of moxifloxacin loaded chitosan–dextran nanoparticles for topical instillation into eye: In-vitro and ex-vivo evaluation. Int. J. Pharm. Investig. 2014, 4, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Ruiz, L.; de la Fuente, M.; Párraga, J.E.; López-García, A.; Fernández, I.; Seijo, B.; Sánchez, A.; Calonge, M.; Diebold, Y. Intracellular trafficking of hyaluronic acid-chitosan oligomer-based nanoparticles in cultured human ocular surface cells. Mol. Vis. 2011, 17, 279–290. [Google Scholar] [PubMed]
- Roy, K.; Cheung, C.H.; Kanwar, R.K.; Sandhir, R.; Kanwar, J.R. Topical Ophthalmic Formulation of Trichostatin A and SurR9-C84A for Quick Recovery Post-alkali Burn of Corneal Haze. Front. Pharmacol. 2017, 8, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, T.A.; Ibrahim, N.J.; Warsi, M.H. Chondroitin sulfate-chitosan nanoparticles for ocular delivery of bromfenac sodium: Improved permeation, retention, and penetration. Int. J. Pharm. Investig. 2016, 6, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Başaran, E.; Yenilmez, E.; Berkman, M.S.; Büyükköroğlu, G.; Yazan, Y. Chitosan nanoparticles for ocular delivery of cyclosporine A. J. Microencapsul. 2014, 31, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Ameeduzzafar; Ali, J.; Bhatnagar, A.; Kumar, N.; Ali, A. Chitosan nanoparticles amplify the ocular hypotensive effect of cateolol in rabbits. Int. J. Biol. Macromol. 2014, 65, 479–491. [Google Scholar] [CrossRef] [PubMed]
- Sunkireddy, P.; Kanwar, R.K.; Kanwar, J.R. Ultra-small algal chitosan ocular nanoparticles with iron-binding milk protein prevents the toxic effects of carbendazim pesticide. Nanomedicine 2015, 11, 495–511. [Google Scholar] [CrossRef] [PubMed]
- Jain, K.; Kumar, R.S.; Sood, S.; Dhyanandhan, G. Betaxolol Hydrochloride Loaded Chitosan Nanoparticles for Ocular Delivery and their Anti-glaucoma Efficacy. Curr. Drug Deliv. 2013, 10, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Ameeduzzafar; Imam, S.S.; Abbas Bukhari, S.N.; Ahmad, J.; Ali, A. Formulation and optimization of levofloxacin loaded chitosan nanoparticle for ocular delivery: In-vitro characterization, ocular tolerance and antibacterial activity. Int. J. Biol. Macromol. 2018, 108, 650–659. [Google Scholar] [CrossRef] [PubMed]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mishra, G.P.; Bagui, M.; Tamboli, V.; Mitra, A.K. Recent Applications of Liposomes in Ophthalmic Drug Delivery. J. Drug Deliv. 2011, 2011, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Mehanna, M.M.; Elmaradny, H.A.; Samaha, M.W. Mucoadhesive liposomes as ocular delivery system: Physical, microbiological, and in vivo assessment. Drug Dev. Ind. Pharm. 2010, 36, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhuang, C.Y.; Wang, M.; Sui, C.G.; Pan, W.S. Low molecular weight chitosan-coated liposomes for ocular drug delivery: In vitro and in vivo studies. Drug Deliv. 2012, 19, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, T.; Hironaka, K.; Fujisawa, T.; Yamaguchi, D.; Tahara, K.; Tozuka, Y.; Takeuchi, H. Preparation of bromfenac-loaded liposomes modified with chitosan for ophthalmic drug delivery and evaluation of physicochemical properties and drug release profile. AJPS 2013, 8, 104–109. [Google Scholar] [CrossRef]
- Abdelbary, G. Ocular ciprofloxacin hydrochloride mucoadhesive chitosan-coated liposomes. Pharm. Dev. Technol. 2011, 16, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Pan, H.; Li, P.; Wang, H.; Wang, X.; Pan, W.; Yuan, Y. The potential use of novel chitosan-coated deformable liposomes in an ocular drug delivery system. Colloids Surf. B Biointerfaces 2016, 143, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.; Yu, S.; Pan, H.; Li, J.; Liu, D.; Yuan, K.; Yang, X.; Pan, W. Bioadhesive chitosan-loaded liposomes: A more efficient and higher permeable ocular delivery platform for timolol maleate. Int. J. Biol. Macromol. 2017, 94, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, S. Topical use of Coenzyme Q10-loaded liposomes coated with trimethyl chitosan: Tolerance, precorneal retention and anti-cataract effect. Int. J. Pharm. 2009, 372, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhuang, C.; Wang, M.; Sun, X.; Nie, S.; Pan, W. Liposome coated with low molecular weight chitosan and its potential use in ocular drug delivery. Int. J. Pharm. 2009, 379, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Deng, L.; Cai, Z.; Zhang, S.; Wang, K.; Li, L.; Ding, S.; Zhou, C. Liposomes coated with thiolated chitosan as drug carriers of curcumin. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 80, 156–164. [Google Scholar] [CrossRef] [PubMed]

| Drug | Main Excipient(s) | Major Findings | Clinical Indications | Ref. |
|---|---|---|---|---|
| Daptomycin | Chitosan and sodium tripolyphosphate (TPP) nanoparticles | This nanoparticulate system could arise as a possible way to deliver the antibiotic directly to the site of action and enhance its residence time in the eye. | Bacterial endophthalmitis | [109] |
| Indomethacin | Chitosan and sodium tripolyphosphate (TPP) nanoparticles and nanoemulsion | In vivo studies and histopathological examination revealed that rabbits’ eyes treated with nanoemulsion showed healing of corneal chemical ulcer with moderate inhibition of polymorph nuclear leukocytic infiltration (PMNLs) compared with nanoparticles. | Post-operative inflammation, healing of corneal ulcers | [110] |
| Moxifloxacin | Chitosan-dextran sulfate nanoparticles | Formulation exhibited biphasic release profile with an initial fast release followed by sustained release in next 24 h. Moxifloxacin loaded nanoparticles exhibited a higher transcorneal permeation as well as significantly higher corneal retention compared to solution. | Ocular infections | [111] |
| Plasmid DNA | Hyaluronic acid-chitosan oligomer nanoparticles (HA-CSO NPs) | HA-CSO NPs had no effect on cell viability. The transfection efficiency of the model plasmid was significantly higher in NP treated cells than in controls. | Ocular surface disorders | [112] |
| Trichostatin A, Dominant negative survivin protein (SurR9-C84A) | Ultra-small chitosan nanoparticles (USC-NPs) | A combination of TSA with SurR9-C8A worked in synergy and showed a promising healing and anti-inflammatory effect in alkali burnt cornea. | Corneal wound healing | [113] |
| Bromfenac sodium | Chondroitin sulfate (ChS)-chitosan (CS)-nanoparticles (NPs) | Significantly high transcorneal permeation (1.62-fold) and corneal retention (1.92-fold) of bromfenac was observed through ChS-CS-NPs when compared with marketed eye drops. | Ocular inflammation | [114] |
| Cyclosporine A | Nanoparticles containing three types of chitosan with different molecular weights | CsA could be detected in both aqueous and vitreous humor samples up to 72 h. In vivo release profiles indicated prolonged release of active agent from nanoparticles containing chitosan with medium molecular weight. | Ocular inflammatory diseases | [115] |
| Carteolol | Chitosan nanoparticles (CS-NP) | In vitro release studies displayed a sustained release for 24 h as compared to drug solution. Ex vivo studies showed good permeation and safe nature for NP. | Glaucoma | [116] |
| Bovine lactoferrin (bLf) | Ultra-small algal chitosan nanoparticles (US CS NPs) | The in vivo and ex vivo biodistribution results suggested that the ultra-small CS NPs efficiently internalized into the ocular tissues within 1 h after administration. Ultra-small algal nanocarriers with bLf protein exhibited potential in inhibiting carbendazim-induced human lens cell apoptosis and oxidative stress. | To prevent carbendazim-induced toxicity | [117] |
| Betaxolol hydrochloride | Chitosan nanoparticles | The in vitro release studies in simulated tear fluid exhibited biphasic release pattern with an initial burst followed by sustained release up to 12 h. The developed nanoparticles showed significant decrease in intraocular pressure (IOP) compared to marketed formulation. | Glaucoma | [118] |
| Levofloxacin | Chitosan nanoparticles | Hen’s egg-chorioallantoic membrane test (HET-CAM test) and histopathology of cornea demonstrated that the formulation was non-irritant and safe for ocular administration. The antimicrobial study revealed higher antibacterial activity against P. aeruginosa, and S. aureus. | Ocular infections | [119] |
| Drug | Main Excipient(s) | Major Findings | Clinical Indication | Ref. |
|---|---|---|---|---|
| Cyclosporine A (CsA) | Low molecular weight chitosan coated liposomes (LCHL) | In vitro drug release measurement showed that LCHL had a delayed release profile compared with non-coated liposomes. In vivo study in rabbits showed that the concentrations of CsA in cornea, conjunctiva, and sclera were remarkably increased by LCHL. | Ocular inflammatory diseases | [123] |
| Bromfenac (BRF) | Chitosan-coated liposomes | Release of BRF from liposomes was sustained for several hours depending on lipid concentration, inner water phase, initial drug amounts and surface properties. | Retinal and choroidal neovascularization, cystoid macular edema | [124] |
| Ciprofloxacin hydrochloride (CPX) | Chitosan-coated liposomes | Results showed an alteration in release rate and encapsulation efficiency of CPX from liposomal formulae upon varying the molar ratios of the lipid bilayer composition. | Ocular infections | [125] |
| Flurbiprofen (FP) | Chitosan-coated deformable liposomes (DL-CS) | The apparent permeability coefficient of FP-DL-0.1% CS evaluated using isolated rabbit corneas was 1.29-, 1.95- and 4.59-fold greater than that of uncoated FP-DL, conventional liposomes and FP solution. | Ocular inflammations | [126] |
| Timolol maleate ™ | Chitosan coated liposomes (TM-CHL) | The TM-CHL exhibited significant mucin adhesion compared to commercial eye drops. TM-CHL produced a 3.18-fold increase in the apparent permeability coefficient resulting in a significant enhancement of corneal permeation. | Glaucoma | [127] |
| Coenzyme Q10 | Trimethyl chitosan (TMC)-coated liposomes | A 4.8-fold increase in the precorneal residence time was achieved in the presence of TMC with a higher Mw compared with the control solution. The Draize test demonstrated the excellent ocular tolerance of TMC for topical administration. | Selenite-induced cataract | [128] |
| Diclofenac sodium | Low molecular weight chitosan (LCH)-coated liposomes | The LCH coating displayed a potential penetration enhancing effect for transcorneal drug delivery. In the ocular tolerance study, no irritation or toxicity was observed by continual administration of LCH- coated liposome in 7 days. | Ocular inflammatory diseases | [129] |
| Curcumin | Thiol derivatized chitosan (CSSH) coated liposomes | The CSSH coated curcumin liposomes (Cur-Lip-CSSH) showed slower in vitro release than Cur-Lip at pH 5.5 and pH 7.4. Treatment of MCF-7 cells with curcumin and Cur-Lip-CSSH showed dose and time dependent cytotoxicity. | Posterior ocular diseases | [130] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Irimia, T.; Ghica, M.V.; Popa, L.; Anuţa, V.; Arsene, A.-L.; Dinu-Pîrvu, C.-E. Strategies for Improving Ocular Drug Bioavailability and Corneal Wound Healing with Chitosan-Based Delivery Systems. Polymers 2018, 10, 1221. https://doi.org/10.3390/polym10111221
Irimia T, Ghica MV, Popa L, Anuţa V, Arsene A-L, Dinu-Pîrvu C-E. Strategies for Improving Ocular Drug Bioavailability and Corneal Wound Healing with Chitosan-Based Delivery Systems. Polymers. 2018; 10(11):1221. https://doi.org/10.3390/polym10111221
Chicago/Turabian StyleIrimia, Teodora, Mihaela Violeta Ghica, Lăcrămioara Popa, Valentina Anuţa, Andreea-Letiţia Arsene, and Cristina-Elena Dinu-Pîrvu. 2018. "Strategies for Improving Ocular Drug Bioavailability and Corneal Wound Healing with Chitosan-Based Delivery Systems" Polymers 10, no. 11: 1221. https://doi.org/10.3390/polym10111221
APA StyleIrimia, T., Ghica, M. V., Popa, L., Anuţa, V., Arsene, A.-L., & Dinu-Pîrvu, C.-E. (2018). Strategies for Improving Ocular Drug Bioavailability and Corneal Wound Healing with Chitosan-Based Delivery Systems. Polymers, 10(11), 1221. https://doi.org/10.3390/polym10111221









