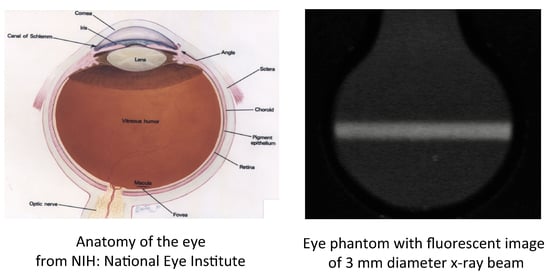
A Polymer-Gel Eye-Phantom for 3D Fluorescent Imaging of Millimetre Radiation Beams
Abstract
1. Introduction
2. Materials and Methods
2.1. RFG Gel Formation
2.2. X-ray Beam Irradiation
2.3. Fluorescence Imaging
3. Results and Discussion
3.1. Test Measurement with a Standard Fluorescent Solution
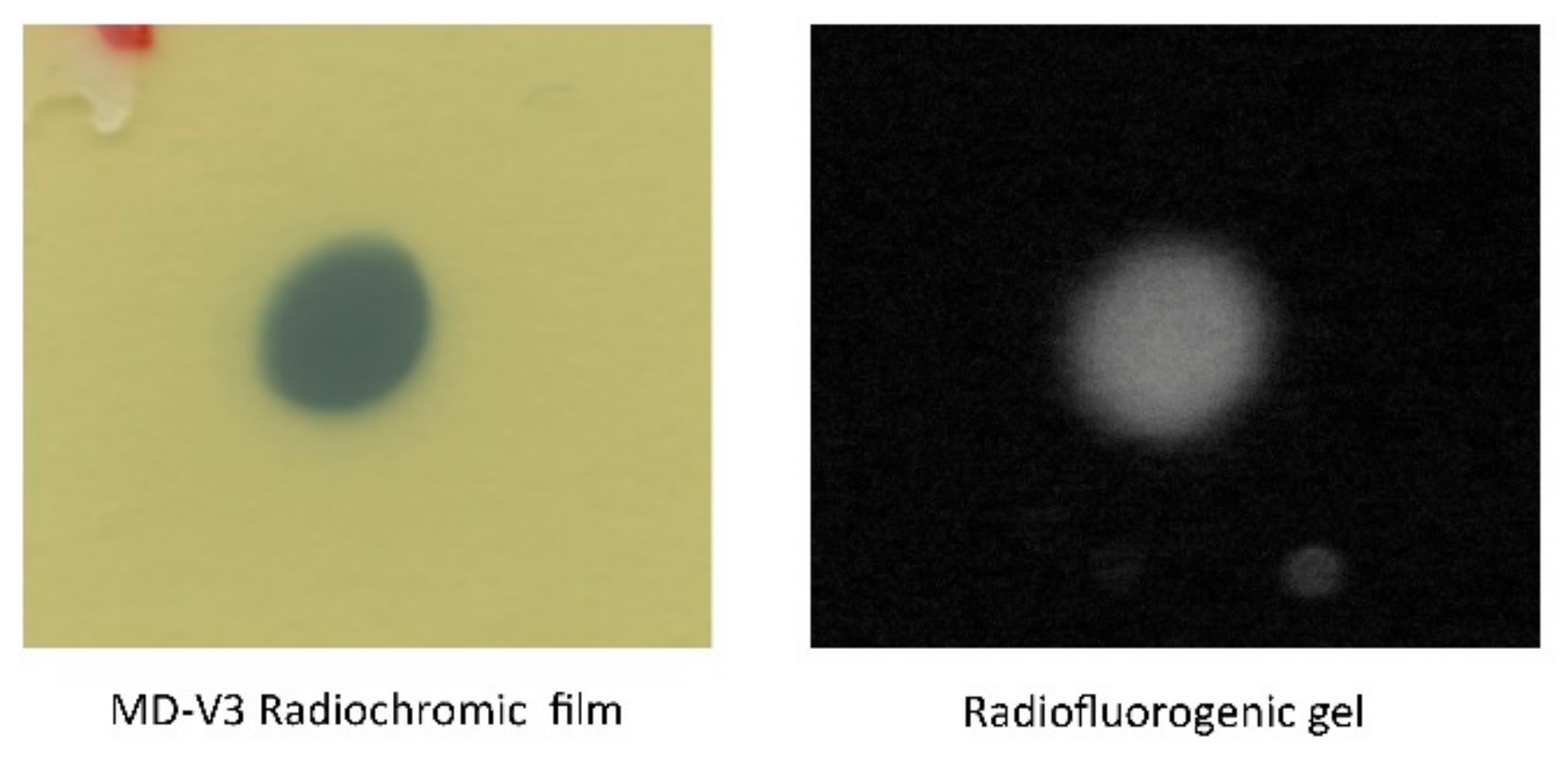
3.2. Radiochromic Film Measurements
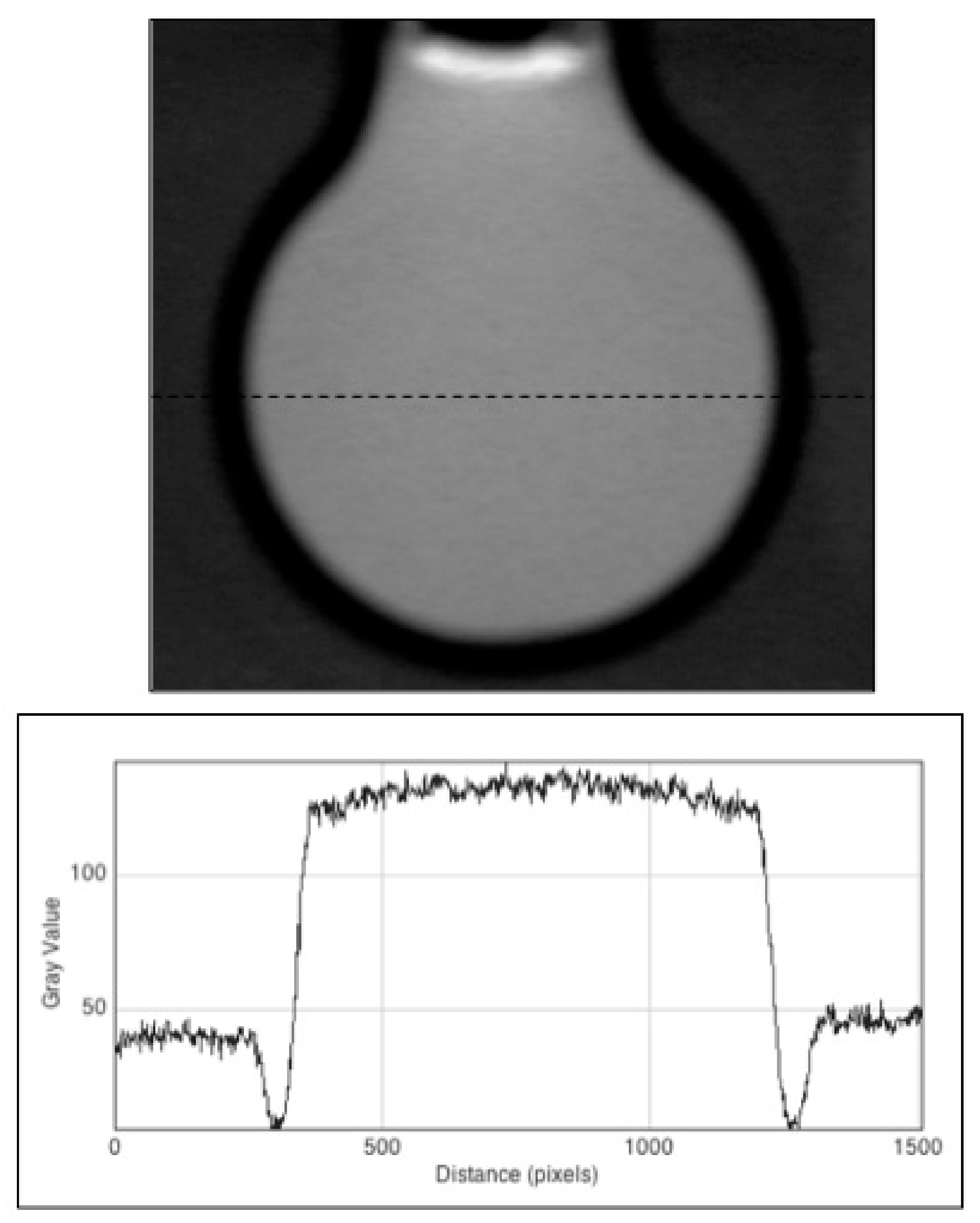
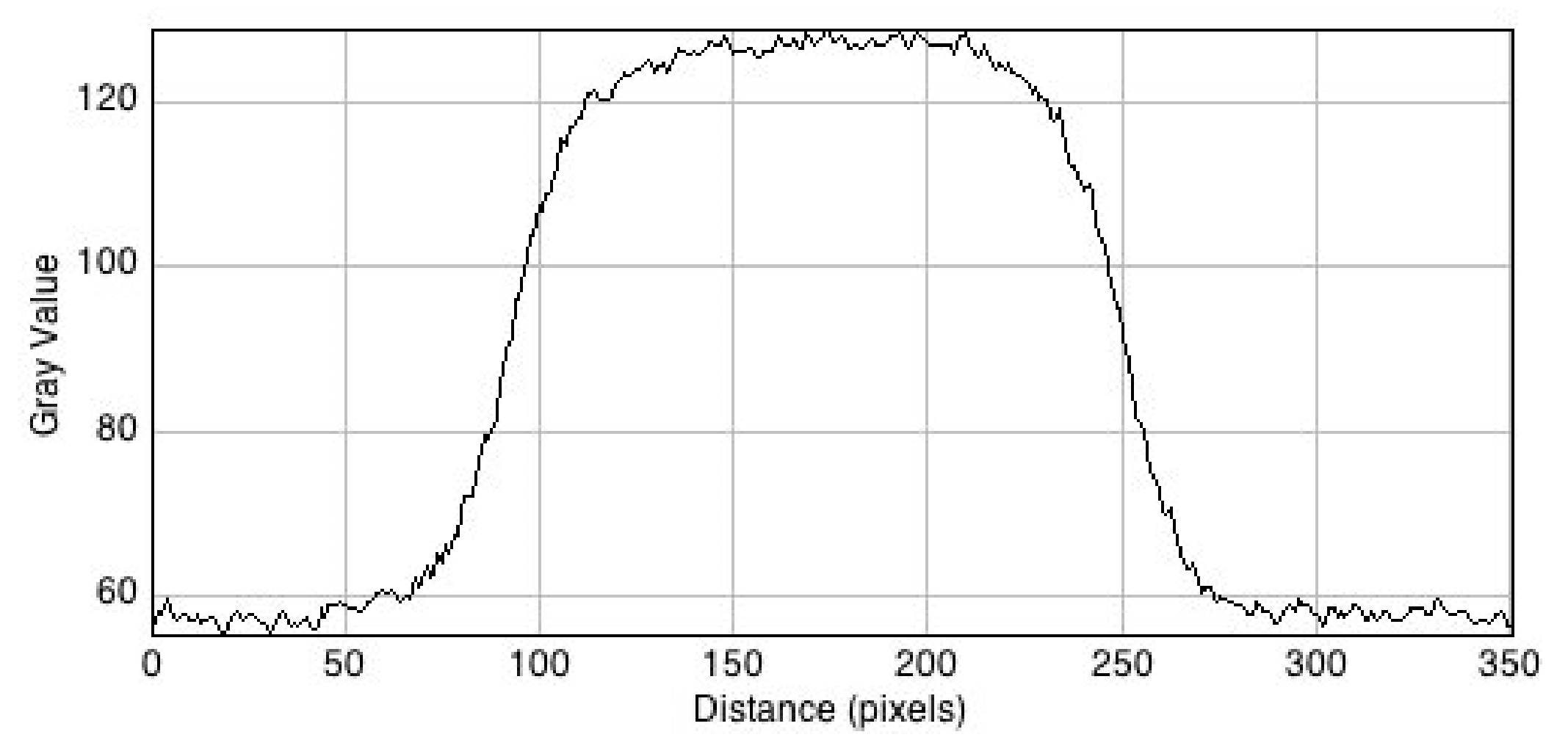
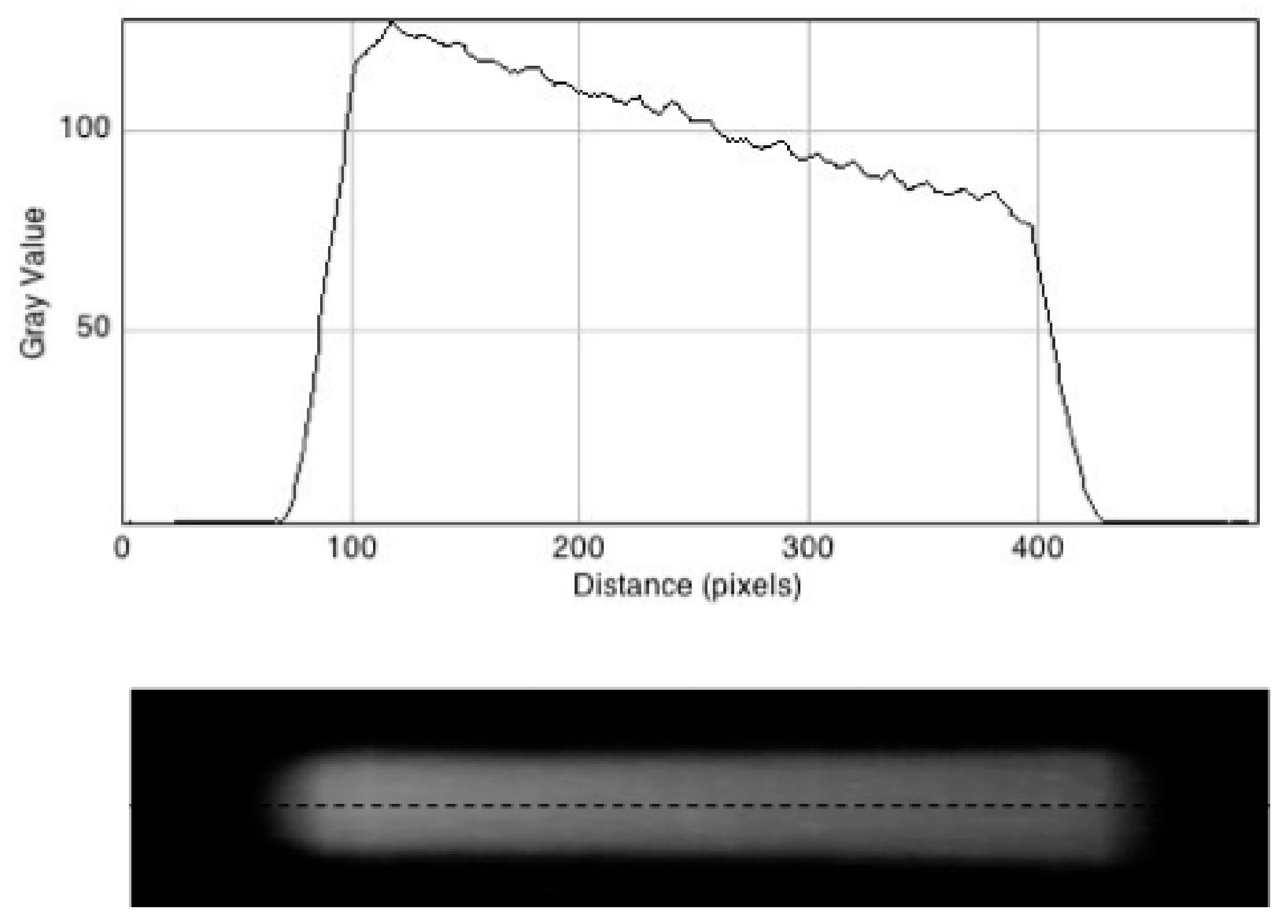
3.3. Fluorescent Images of the Irradiated Gel
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Stannard, C.; Sauerwein, W.; Maree, G.; Lecuona, K. Radiotherapy for ocular tumours. Eye 2013, 27, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Poder, J.; Corde, S. I-125 ROPES eye plaque dosimetry: Validation of a commercial 3D opthalmic brachytherapy treatment planning system and independent dose calculation software with GafChromic EBT3 films. Med. Phys. 2013, 40, 121709. [Google Scholar] [CrossRef] [PubMed]
- Eye Cancer: Treatment Options. Available online: https://www.cancer.net/cancer-types/eye-cancer/treatment-options (accessed on 6 June 2018).
- Radiation Therapy for Eye Cancer. Available online: https://www.cancer.org/cancer/eye-cancer/treating/radiation-therapy.html (accessed on 6 June 2018).
- The Changing Landscape of Cancer Therapy. Available online: https://physicsworld.com/a/the-changing-landscape-of-cancer-therapy/ (accessed on 6 June 2018).
- McAuley, G.A.; Heczko, S.L.; Nguyen, T.; Slater, J.M.; Slater, J.D.; Wroe, A.J. Monte Carlo evaluation of magnetically focused proton beams for radiosurgery. Phys. Med. Biol. 2018, 63, 055010. [Google Scholar] [CrossRef] [PubMed]
- Allen, A.M.; Pawlicki, T.; Dong, L.; Fourkal, E.; Buyyounousli, M.; Cengel, K.; Plastaras, J.; Bucci, M.K.; Yock, Y.I.; Bonilla, L.; et al. An evidence based review of proton beam therapy: The report of ASTRO’s emerging technology committee. Radiother. Oncol. 2012, 103, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Goitein, G. Proton radiation therapy of ocular melonoma. PTCOG 49 Teaching Course. 2010. Available online: http://www.ptcog.ch/archive/conference_p&t&v/PTCOG49/presentationsEW/18-4-3_Eye.pdf (accessed on 4 September 2018).
- Pasler, M.; Hernandez, V.; Jornet, N.; Clark, C.H. Novel methodologies for dosimetry audits: Adapting to advanced radiotherapy techniques. Phys. Imaging Radiat. Oncol. 2018, 5, 76–84. [Google Scholar] [CrossRef]
- Day, M.J.; Stein, G. Chemical effects of ionizing radiation in some gels. Nature 1950, 166, 146–147. [Google Scholar] [CrossRef] [PubMed]
- Baldock, C.; De Deene, Y.; Doran, S.; Ibbott, G.; Jirasek, A.; Lepage, M.; McAuley, K.B.; Oldham, M.; Schreiner, L.J. Polymer gel dosimetry. Phys. Med. Biol. 2010, 55, R1. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Warmington, L.; Gopishankar, N. Three-dimensional radiation dosimetry using polymer gel and solid radiochromic polymer: From basics to clinical applications. World J. Radiol. 2017, 9, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Vandecasteele, J.; De Deene, Y. Evaluation of radiochromic gel dosimetry and polymer gel dosimetry in a clinical dose verification. Phys. Med. Biol. 2013, 58, 6241–6252. [Google Scholar] [CrossRef] [PubMed]
- Schreiner, L.J. True 3D chemical dosimetry (gels, plastics): Development and clinical role. J. Phys. Conf. Ser. 2015, 573, 012003. [Google Scholar] [CrossRef]
- Oldham, M. Radiochromic 3D Detectors. J. Phys. Conf. Ser. 2015, 573, 012006. [Google Scholar] [CrossRef]
- Maryanski, M.J.; Schulz, R.J.; Ibbott, G.S.; Gatenby, J.C.; Xie, J.; Horton, D.; Gore, J.C. Magnetic resonance imaging of radiation dose distributions using a polymer gel dosimeter. Phys. Med. Biol. 1994, 39, 1437–1455. [Google Scholar] [CrossRef] [PubMed]
- Gore, J.C.; Ranade, M.; Maryanski, M.J.; Schulz, R.J. Radiation dose distributions in three dimensions from tomographic optical density scanning of polymer gels: I. Development of an optical scanner. Phys. Med. Biol. 1996, 41, 2695–2704. [Google Scholar] [CrossRef] [PubMed]
- Cameron, M.; Cornelius, I.; Cutajar, D.L.; Davis, J.A.; Rosenfeld, A.B.; Lerch, M.; Guatelli, S. Comparison of phantom materials for use in quality assurance of microbeam radiation therapy. J. Synchrotron Radiat. 2017, 24, 866–876. [Google Scholar] [CrossRef] [PubMed]
- Kron, T.; Lehmann, J.; Greer, P. Dosimetry of ionising radiation in modern radiation oncology. Phys. Med. Biol. 2016, 61, R167–R205. [Google Scholar] [CrossRef] [PubMed]
- Newton, J.; Oldham, M.; Thomas, A.; Li, Y.; Adamovics, J.; Kirsch, D.G.; Das, S. Commissioning a small-field biological irradiator using point, 2D, and 3D dosimetry techniques. Med. Phys. 2011, 38, 6754–6762. [Google Scholar] [CrossRef] [PubMed]
- Warman, J.M.; Luthjens, L.H.; de Haas, M.P. High-energy radiation monitoring based on radio-fluorogenic co-polymerization II: Fixed fluorescent images of collimated X-ray beams using an RFCP gel. Phys. Med. Biol. 2011, 56, 1487–1508. [Google Scholar] [CrossRef] [PubMed]
- Warman, J.M.; de Haas, M.P.; Luthjens, L.H.; Denkova, A.G.; Yao, T. A radio-fluorogenic polymer-gel makes fixed fluorescent images of complex radiation fields. Polymers 2018, 10, 685. [Google Scholar] [CrossRef]
- Yao, T.; Gasparini, A.; de Haas, M.P.; Luthjens, L.H.; Denkova, A.G.; Warman, J.M. A tomographic UV-sheet scanning technique for producing 3D fluorescence images of x-ray beams in a radio-fluorogenic gel. Biomed. Phys. Eng. Express 2017, 3, 027004. [Google Scholar] [CrossRef]
- Warman, J.M.; de Haas, M.P.; Luthjens, L.H.; Denkova, A.G.; Kavatsyuk, O.; van Gothem, M.-J.; Kiewiet, H.H.; Brandenburg, S. Fixed fluorescent images of an 80 MeV proton pencil beam. Rad. Phys. Chem. 2013, 85, 179–183. [Google Scholar] [CrossRef]
- Yao, T.; Luthjens, L.H.; Gasparini, A.; Warman, J.M. A study of four radiochromic films currently used for (2D) radiation dosimetry. Rad. Phys. Chem. 2017, 133, 37–44. [Google Scholar] [CrossRef]
- Yao, T. 3D Radiation Dosimetry using a Radio-Fluorogenic Gel. Ph.D. Thesis, Technische Universiteit Delft, Mekelweg, Delft, The Netherlands, January 2017. [Google Scholar]
- Hubbell, J.H.; Seltzer, S.M. Tables of X-ray Mass Attenuation Coefficients and Mass Energy-Absorption Coefficients from 1 keV to 20 MeV for Elements Z = 1 to 92 and 48 Additional Substances of Dosimetric Interest; PLM: Gaithersburg, MD, USA, 1995; NISTIR-5632. [Google Scholar]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luthjens, L.H.; Yao, T.; Warman, J.M. A Polymer-Gel Eye-Phantom for 3D Fluorescent Imaging of Millimetre Radiation Beams. Polymers 2018, 10, 1195. https://doi.org/10.3390/polym10111195
Luthjens LH, Yao T, Warman JM. A Polymer-Gel Eye-Phantom for 3D Fluorescent Imaging of Millimetre Radiation Beams. Polymers. 2018; 10(11):1195. https://doi.org/10.3390/polym10111195
Chicago/Turabian StyleLuthjens, Leonard H., Tiantian Yao, and John M. Warman. 2018. "A Polymer-Gel Eye-Phantom for 3D Fluorescent Imaging of Millimetre Radiation Beams" Polymers 10, no. 11: 1195. https://doi.org/10.3390/polym10111195
APA StyleLuthjens, L. H., Yao, T., & Warman, J. M. (2018). A Polymer-Gel Eye-Phantom for 3D Fluorescent Imaging of Millimetre Radiation Beams. Polymers, 10(11), 1195. https://doi.org/10.3390/polym10111195