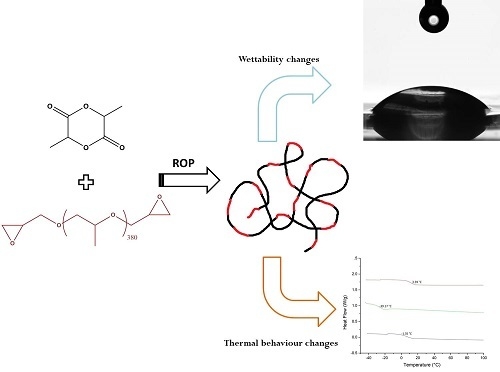
Bulk Modification of Poly(lactide) (PLA) via Copolymerization with Poly(propylene glycol) Diglycidylether (PPGDGE)
Abstract
1. Introduction
2. Materials and Methods
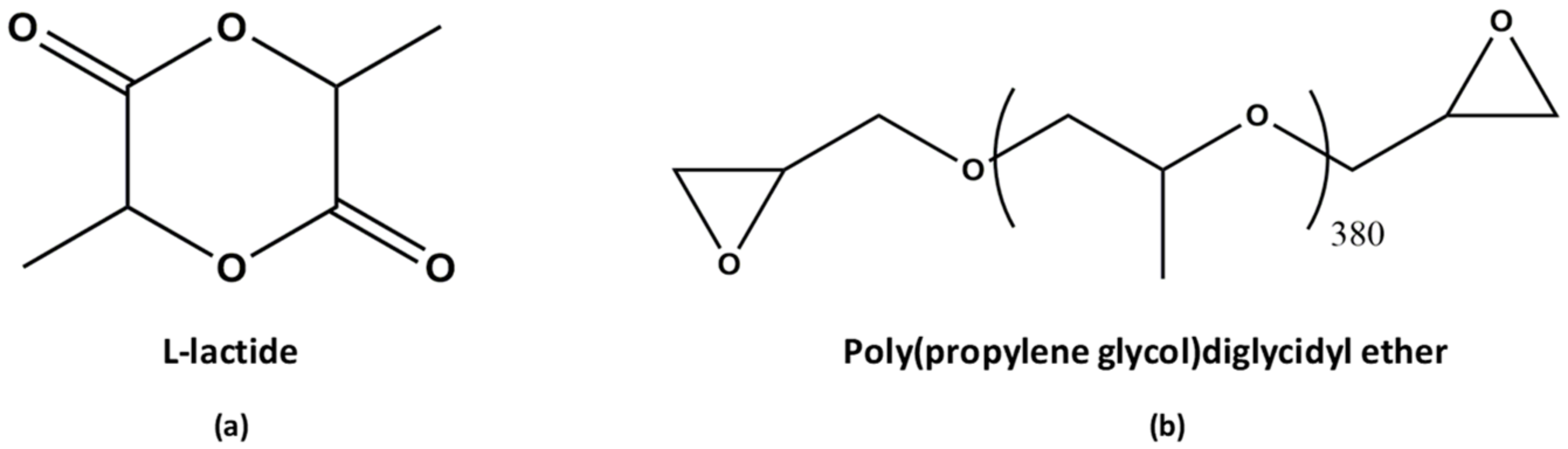
2.1. Materials
2.2. Copolymerization
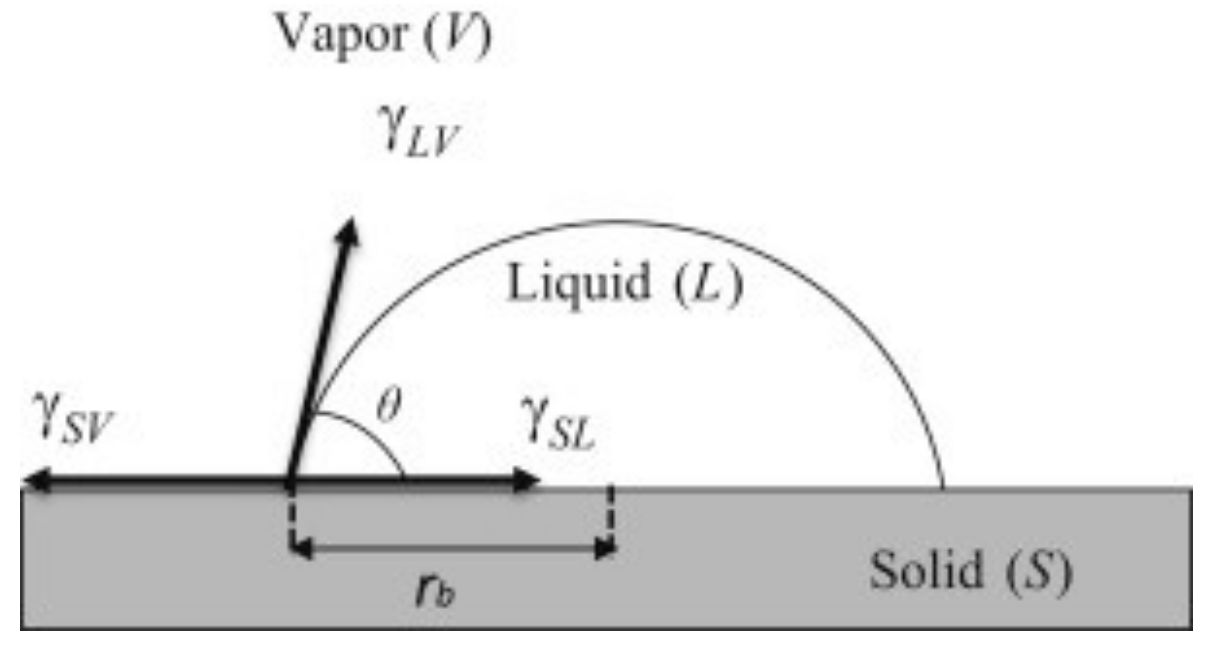
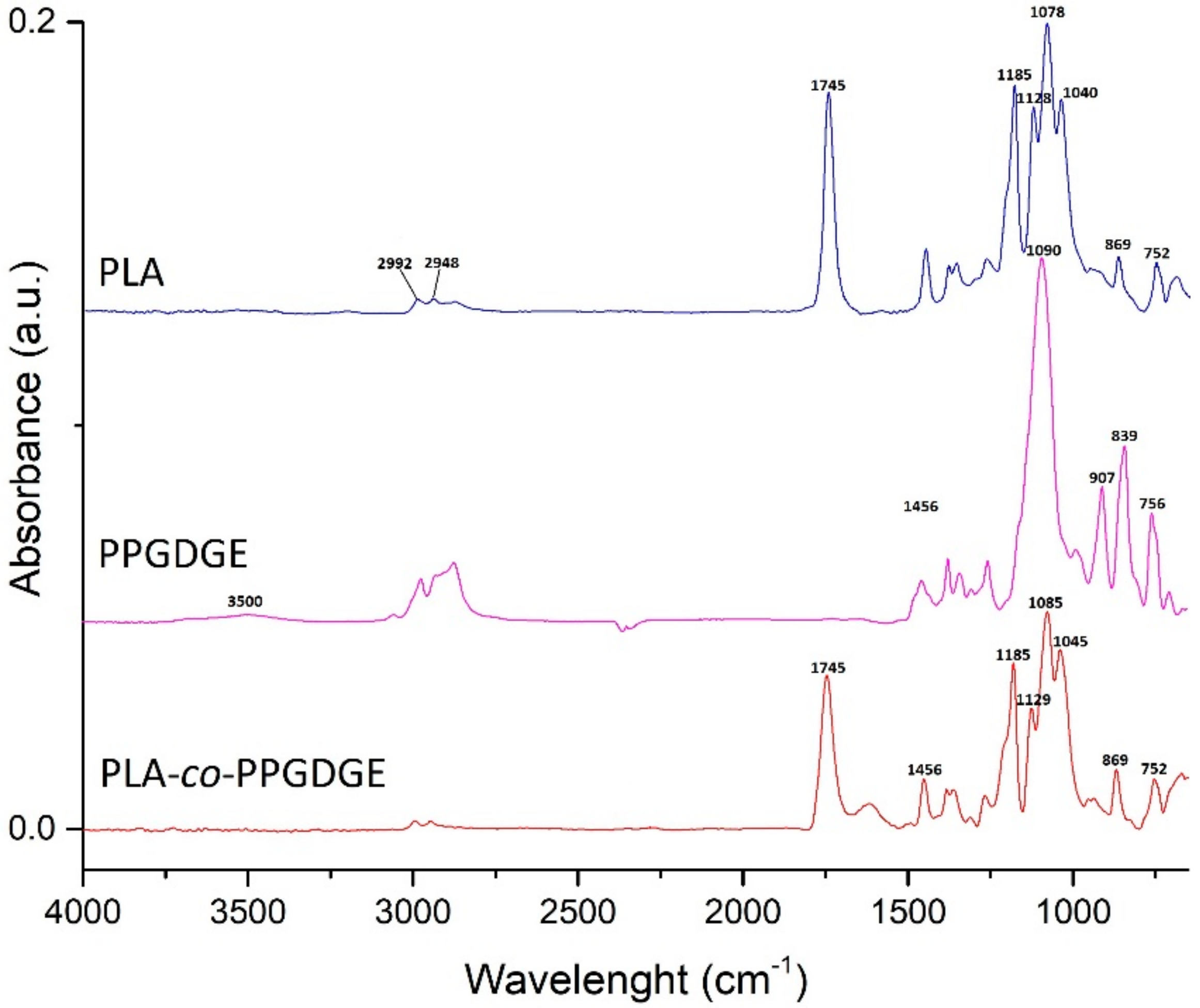
2.3. Characterization
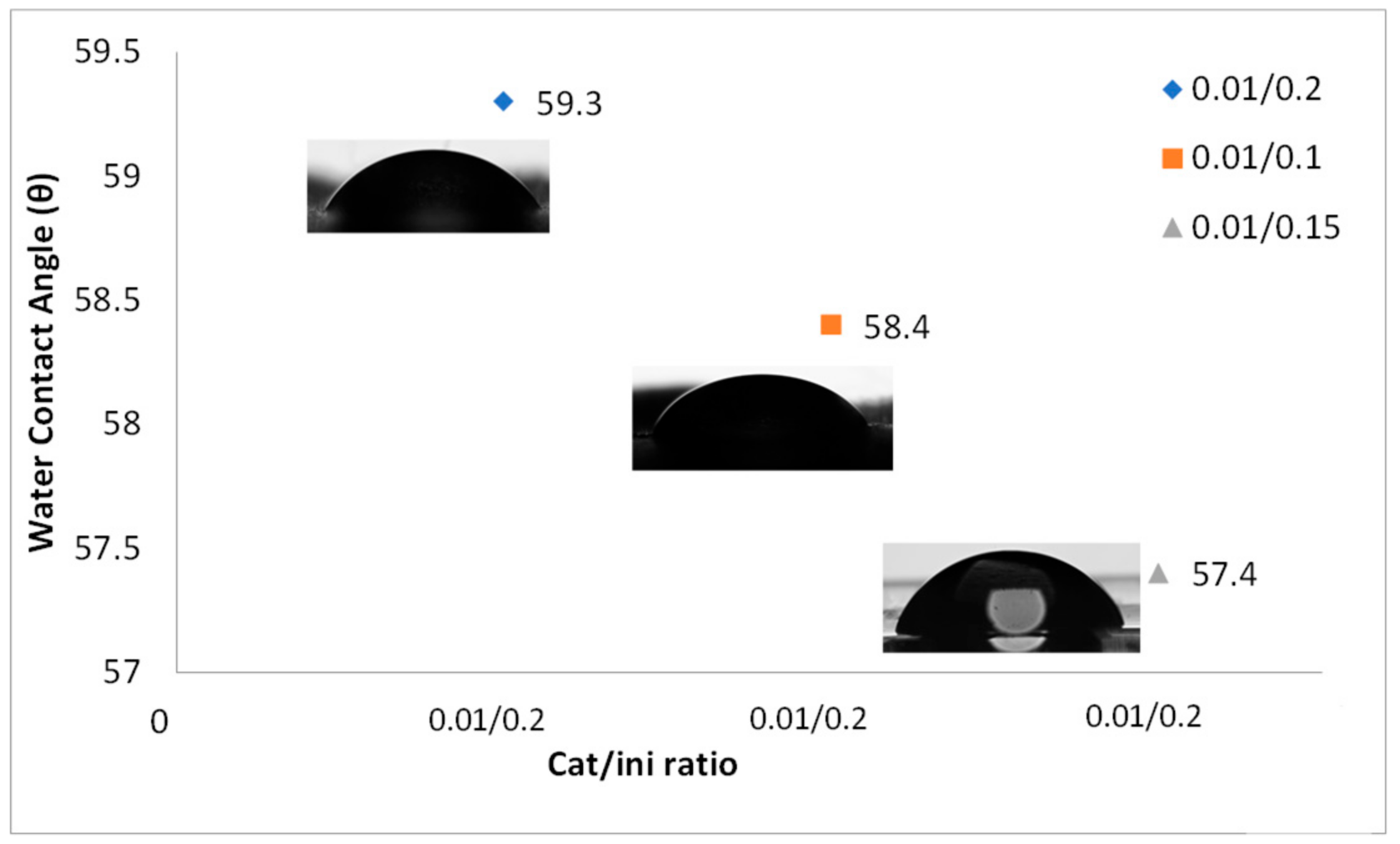
3. Results
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Koronis, G.; Silva, A.; Fontul, M. Green composites: A review of adequate materials for automotive applications. Compos. Part B Eng. 2013, 44, 120–127. [Google Scholar] [CrossRef]
- Hillmyer, M.; Tolman, W. Aliphatic Polyester Block Polymers: Renewable, Degradable, and Sustainable. Acc. Chem. Res. 2014, 47, 2390–2396. [Google Scholar] [CrossRef] [PubMed]
- Malinconico, M.; Cerruti, P.; Santagata, G.; Immirzo, B. Natural Polymers and additives in Commodity and Specialty Applications: A Challenge for the Chemistry of Future. Macromol. Symp. 2014, 337, 124–133. [Google Scholar] [CrossRef]
- Senthil, K.P.; Gunasundari, E. Green Chemistry in Textiles. In Sustainable Innovations in Textile Chemistry and Dyes. Textile Science and Clothing Technology; Muthu, S., Ed.; Springer Nature Singapore: Singapore, 2018. [Google Scholar]
- Yadav, A.; Mangaraj, S.; Singh, R.; Kumar, N.; Simran, A. Biopolymers as packaging material in food and allied industry. Int. J. Chem. Stud. 2018, 6, 2411–2418. [Google Scholar]
- Kale, G.; Auras, R.; Singh, S.P.; Narayan, R. Biodegradability of polylactide bottles in real and simulated composting conditions. Polym. Test. 2007, 26, 1049–1061. [Google Scholar] [CrossRef]
- Maharana, T.; Mohanty, B.; Negi, Y.S. Melt-solid polycondensation of lactic acid and its biodegradability. Prog. Polym. Sci. 2009, 34, 99–124. [Google Scholar] [CrossRef]
- Duigou, A.; Pillin, I.; Bourmaud, A.; Davies, P.; Baley, C. Effect of recycling on mechanical behavior of biocompostable flax/poly(L-lactide) composites. Compos. Part A Appl. Sci. Manuf. 2008, 39, 1471–1478. [Google Scholar] [CrossRef]
- Bhatia, A.; Gupta, R.; Bhattacharya, S.; Choi, H. Compatibility of biodegradable poly (lactic acid) (PLA) and poly (butylene succinate) (PBS) blends for packaging applications. Korea Aust. Rheol. J. 2007, 19, 125–131. [Google Scholar]
- Jandas, P.J.; Mohanty, S.; Nayak, S.K. Surface treated banana fiber reinforced poly (lactic acid) nanocomposites for disposable applications. J. Clean. Prod. 2013, 52, 392–401. [Google Scholar] [CrossRef]
- Nofar, M.; Park, C.B. Poly (lactic acid) foaming. Prog. Polym. Sci. 2014, 39, 1721–1741. [Google Scholar] [CrossRef]
- Gu, J.; Xiao, P.; Zhang, L.; Wang, H.; Dai, L.; Song, L.; Huang, Y.; Zhang, J.; Chen, T. Functionalization of biodegradable PLA non-woven fabric as superoleophilic and superhydrophobic material for efficient oil absorption and oil/water separation. Appl. Mater. Interfaces 2017, 9, 5968–5973. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Shi, G.; Bei, J.; Wang, S.; Cao, Y.; Shang, O.; Yang, G.; Wan, W. Fabrication and surface modification of macroporus poly(lactic acid) and poly(L-lactic-co-glycolic acid) (70/30) cell scaffolds for human skin fibroblast cell culture. J. Biomed. Mater. Res. 2002, 62, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Singha, L.; Kumara, V.; Ratnerb, B.D. Generation of porous microcellular 85/15 poly(dl-lactide-co-glycolide) foams for biomedical applications. Biomaterials 2004, 25, 2611–2617. [Google Scholar] [CrossRef]
- Jeong, S.I.; Ko, E.K.; Yum, J.; Jung, C.H.; Lee, Y.M.; Shin, H. Nanofibrous poly(lactic acids)/hydroxipatite composite scaffolds for guided tissue regeneration. Macromol. Biosci. 2008, 8, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.M.; Moreira, S.; Gonςalves, I.C.; Gama, F.M.; Mendes, A.M.; Magalhaes, F.D. Biocompatibility of poly(lactic acid) with incorporated graphene-based materials. Colloids Surf. B Biointerfaces 2013, 104, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Ramot, Y.; Haim-Zada, M.; Domb, A.J.; Nyska, A. Biocompatibility and safety of PLA and its copolymers. Adv. Drug Deliv. Rev. 2016, 107, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Fishbein, I.; Chorny, M.; Rabinovich, L.; Banai, S.; Gati, I.; Golomb, G. Nanoparticulate delivery system of a tyrphostin for the treatment of restenosis. J. Control. Release 2000, 65, 221–229. [Google Scholar] [CrossRef]
- Matsusue, Y.; Yamamuro, T.; Yoshii, S.; Oka, M.; Ikada, Y.; Hyon, S.; Shikinami, Y. Biodegradable screw fixation of rabbit tibia proximal osteotomies. J. Appl. Biomater. 1991, 2, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Dev, A.; Binulal, N.S.; Anitha, A.; Nair, S.V.; Furuike, T.; Tamura, H.; Jayakumar, R. Preparation of poly(lactic acid)/chitosan nanoparticles for anti-HIV drug delivery applications. Carbohydr. Polym. 2010, 80, 833–838. [Google Scholar] [CrossRef]
- Jain, A.; Kunduru, K.R.; Basu, A.; Mizrahi, B.; Domb, A.J.; Khan, W. Injectable formulations of poly(lactic acid) and its copolymers in clinical use. Adv. Drug Deliv. Rev. 2016, 107, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Cicero, J.A.; Dorgan, J.R.; Garrett, J.; Runt, J.; Lin, J.S. Effects of Molecular Architecture on Two-Step, Melt-Spun Poly(Lactic Acid) Fibers. J. Appl. Polym. Sci. 2002, 86, 2839–2846. [Google Scholar] [CrossRef]
- Shaver, M.P.; Cameron, D.J.A. Tacticity Control in the Synthesis of Poly(lactic acid) Polymer Stars with Dipentaerythritol Cores. Biomacromolecules 2010, 11, 3673–3679. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Long, L.; Shi, Z.; Liu, C.; Qiu, M.; Sheng, J.; Pu, P.; Yuan, X.; Ren, Y.; Kang, C. Star-branched amphiphilic PLA-b-PDMAEMA copolymers for co-delivery of miR-21 inhibitor and doxorubicin to treat glioma. Biomaterials 2014, 35, 2322–2335. [Google Scholar] [CrossRef] [PubMed]
- Ya, L.; Weijun, Z. Preparation and Performance of Poly(Lactic Acid)-γ-Cyclodextrin Inclusion Complex-Poly(Lactic Acid) Multibranched Polymers by the Reactive Extrusion Process. Polym. Plast. Technol. Eng. 2018, 57, 836–849. [Google Scholar]
- Murphy, C.; Collins, M. Microcrystalline Cellulose Reinforced Polylactic acid Biocomposite Filaments for 3D Printing. Polym. Compos. 2018, 39, 1311–1320. [Google Scholar] [CrossRef]
- Kao, C.; Lin, C.; Chen, Y.; Yeh, C.; Fang, H.; Shie, M. Poly(dopamine coating of 3D printed poly(lactic acid) scaffolds for bone tissue engineering. Mater. Sci. Eng. C. Mater. Biol. Appl. 2015, 56, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ma, P. Polymeric Scaffolds for Bone Tissue Engineering. Ann. Biomed. Eng. 2004, 32, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Plackett, D.V.; Holm, V.K.; Johansen, P.; Ndomi, S.; Nielsen, P.V.; Sipilainen-Malm, T.; Södergard, A.; Verstichel, S. Characterization of L-Polylactide and L-Polylactide-Polycaprolactone Co-Polymer Films for use in Cheese-Packaging Applications. Packag. Technol. Sci. 2006, 19, 1–24. [Google Scholar] [CrossRef]
- Wang, Y.F.; Guo, H.F.; Ying, D.J. Multilayer scaffold of electrospun PLA-PCL-collagen nanofibers as a dural substitute. J. Biomed. Mater. Res. B Appl. Biomater. 2013, 101, 1359–1366. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Liu, S.; Zhou, G.; Huang, Y.; Xie, Z.; Jing, X. Electrospinning of polymeric nanofibers for drug delivery applications. J. Control. Release 2014, 185, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Rosen, T.; Goldeberg, I.; Navarra, G.; Venditto, V.; Kol, M. Block-Steeoblock Copolymers of Poly(Ɛ-Capolactone) and Poly(Lactic Acid). Angew. Chem. Int. Engl. 2018, 57, 7191–7195. [Google Scholar] [CrossRef] [PubMed]
- Pisani, S.; Dorati, B.; Modena, T.; Bruni, G.; Genta, I. Design of copolymer PLA-PCL electrospun matrix for biomedical applications. React. Funct. Polym. 2018, 124, 77–89. [Google Scholar] [CrossRef]
- Grayson, A.; Voskerician, G.; Lynn, A.; Anderson, J.M.; Cima, M.J.; Langer, R. Differential degradation rates in vivo and in vitro of biocompatible poly(lactic acid) and poly(glycolic acid) homo- and co-polymers for a polymeric drug-delivery microchip. J. Biomater. Sci. Polym. Ed. 2004, 15, 1281–1304. [Google Scholar] [CrossRef] [PubMed]
- Cantón, I.; Mckean, R.; Charnley, M.; Blackwood, A.; Fiorica, C.; Ryan, A.J.; MacNeil, S. Development of an Ibuprofen-Releasing Biodegradable PLA/PGA Electrospun Scaffold for Tissue Regeneration. Biotechnol. Bioeng. 2010, 105, 396–408. [Google Scholar] [CrossRef] [PubMed]
- Mannaa, S.; Donnellb, A.M.; Kavalb, N.; Al-Rjouba, M.F.; Augsburgerc, J.; Banerjee, R.K. Improved design and characterization of PLGA/PLA-coated Chitosan based micro-implants for controlled release of hydrophilic drugs. Int. J. Pharm. 2018, 547, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Tian, H.; Zhang, P.; Sun, J.; Chen, X.; Jing, X. Synthesis and Characterization of RGD Peptide Grafted Poly(ethylene glycol)-b-Poly(L-lactide)-b-Poly(L-glutamic acid) Triblock Copolymer. Biomacromolecules 2006, 7, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Pandita, D.; Kumar, S.; Lathe, V. Hibrid poly(lactic-co-glycolic acid) nanoparticles: Design and delivery prospectives. Drug Discov. Today 2015, 20, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Chien-Hsin, C.; Ming-Chien, Y.; Da-Guang, Y.; Chi-Hsiung, J. Effect of immobilization of poly(γ-glutamic acid) on the biocompatibility of electrospun poly (L-lactide) mats. J. Polym. Res. 2018, 25, 92. [Google Scholar] [CrossRef]
- Gonzáles, M.; Cabanelas, J.C.; Baselga, J. Applications of FTIR on Epoxy Resins—Identification, Monitoring the Curing Process, Phase Separation and Water Uptake. In Infrared Spectroscopy-Materials Science, Engineering and Technology; Theophile, P.T., Ed.; InTech: London, UK, 2012. [Google Scholar]
- De Vos, S.; Jansen, P.; Biochem, P. Industrial-Scale PLA Production from PURAC Lactides. Available online: https://www.researchgate.net/profile/Sicco_De_Vos/publication/228836284_IndustrialScale_PLA_Production_from_PURAC_Lactides/links/563efbb508ae8d65c01460a0.pdf (accessed on 22 September 2018).
- Kowalski, A.; Duda, A.; Penczec, S. Mechanism of Cyclyc Ester Polymerization Initiated with Tin (II) Octoate. 2. Macromolecules Fitted with Tin(II) Alkoxide Species Observed Directly in MALDI-TOF Spectra. Macomolecules 2000, 33, 689–695. [Google Scholar] [CrossRef]
- Kricheldorf, H.R. Tin-initiated Polymerizations of Lactones: Mechanistic and Preparative Aspects. Macromol. Symp. 2000, 153, 55–65. [Google Scholar] [CrossRef]
- Ryner, M.; Stridberg, K.; Albertsson, A. Mechanism of Ring-Opening Polymerization of 1,5-Dioxepan-2-one and L-Lactide with Stannous 2-Ethylhexanoate. A Theoretical Study. Macromolecules 2001, 34, 3877–3881. [Google Scholar] [CrossRef]
- Pack, J.W.; Kim, S.H.; Park, S.Y.; Lee, Y.W.; Kim, Y.H. Kinetic and mechanistic studies of L-Lactide Polymerization in Supercritical Chlorodifluoromethane. Macromolecules 2003, 36, 8923–8930. [Google Scholar] [CrossRef]
- Alli, A.; Hazer, B.; Menceloflu, Y.; Süzer, Ş. Synthesis, characterization and surface properties of amphiphilic polystyrene-b-polypropylene glycol block copolymers. Eur. Polym. J. 2006, 42, 740–750. [Google Scholar] [CrossRef]
- Xiao, C.S.; Wang, Y.C.; Du, J.Z.; Chen, X.S.; Wang, J. Kinetics and Mechanism of 2-Ethoxy-2-oxo 1,3,2-dioxaphospholane Polymerization Initiated by Stannous Octoate. Macromolecules 2006, 39, 6825–6831. [Google Scholar] [CrossRef]

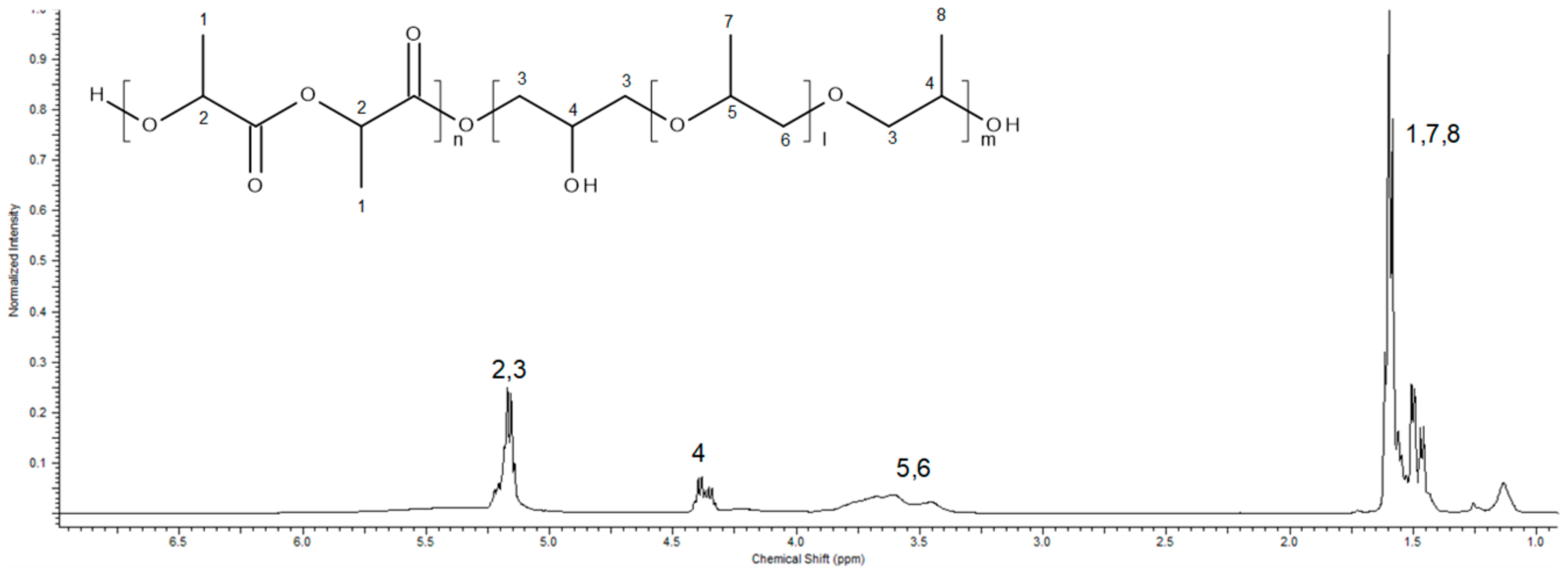
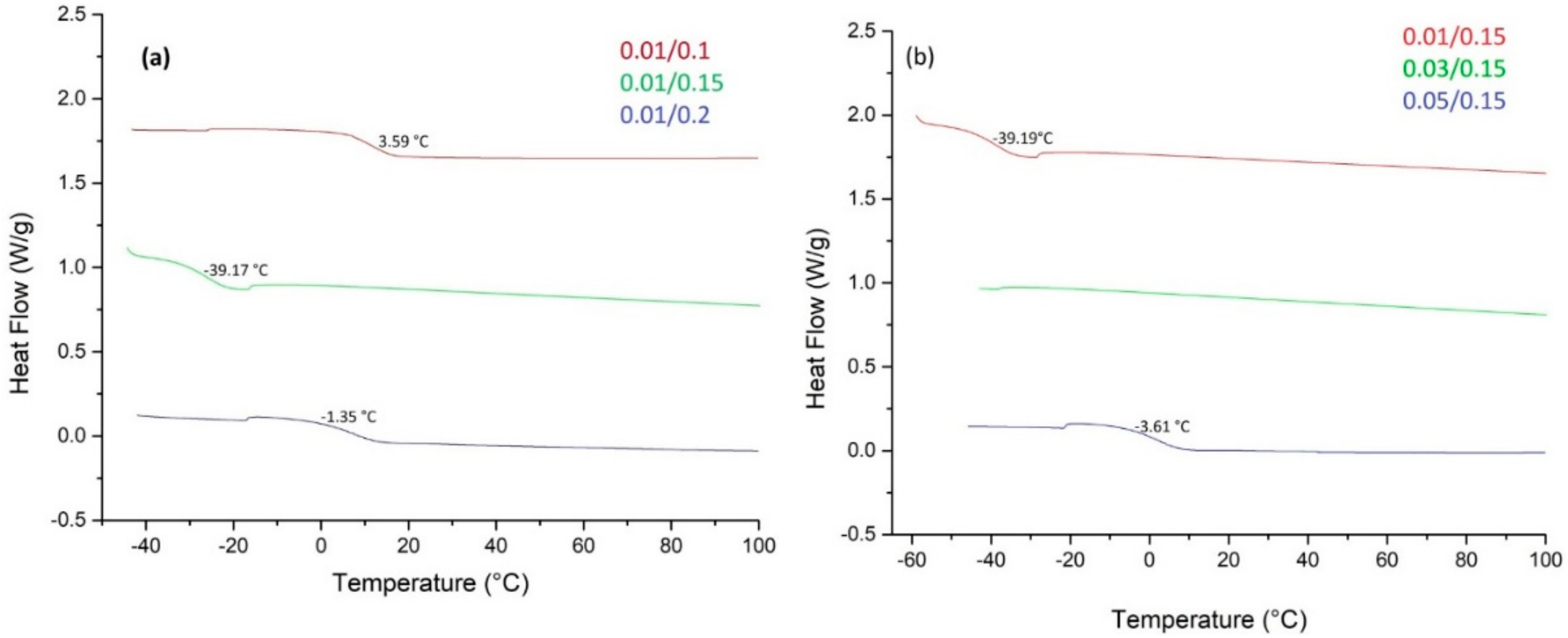
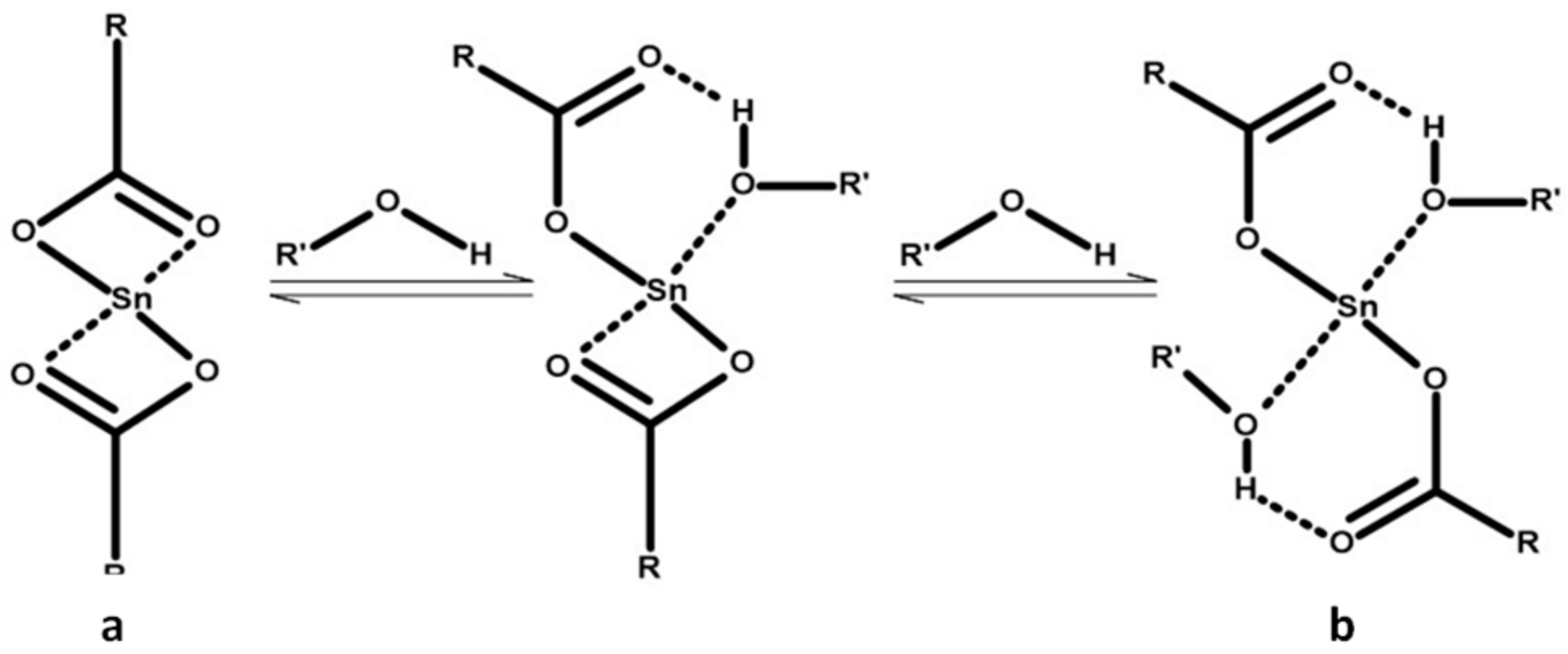
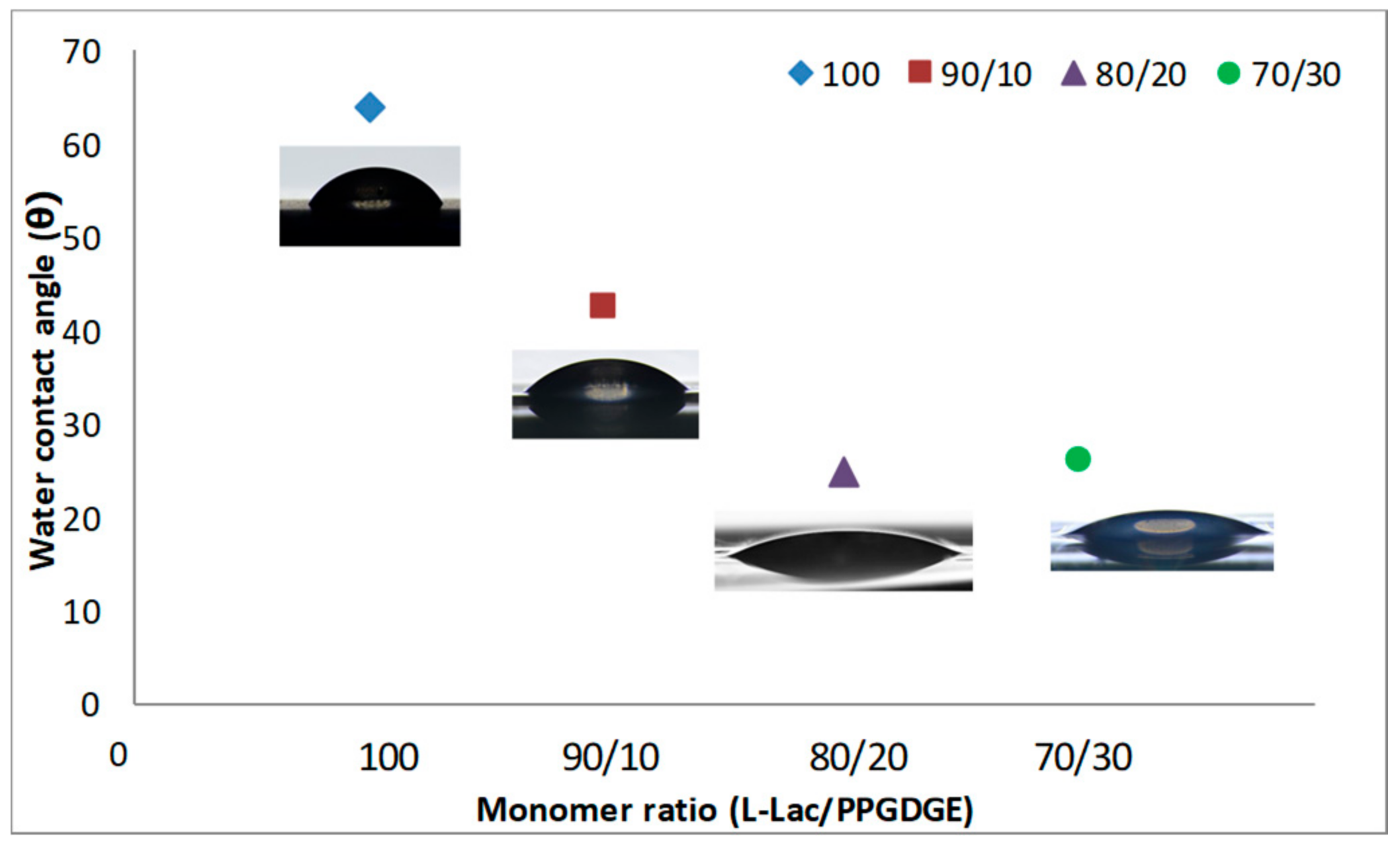
| Sample | Cat/Ini (wt %) | Tg (°C) |
|---|---|---|
| 1 | 0.01/0.1 | 3.59 |
| 2 | 0.01/0.15 | −39.19 |
| 3 | 0.01/0.2 | −1.35 |
| 4 | 0.03/0.15 | ------ |
| 5 | 0.05/0.15 | -3.61 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castillejos, S.; Cerna, J.; Meléndez, F.; Castro, M.E.; Aguilar, R.; Márquez-Beltrán, C.; González, M. Bulk Modification of Poly(lactide) (PLA) via Copolymerization with Poly(propylene glycol) Diglycidylether (PPGDGE). Polymers 2018, 10, 1184. https://doi.org/10.3390/polym10111184
Castillejos S, Cerna J, Meléndez F, Castro ME, Aguilar R, Márquez-Beltrán C, González M. Bulk Modification of Poly(lactide) (PLA) via Copolymerization with Poly(propylene glycol) Diglycidylether (PPGDGE). Polymers. 2018; 10(11):1184. https://doi.org/10.3390/polym10111184
Chicago/Turabian StyleCastillejos, Sandra, Jorge Cerna, Francisco Meléndez, María Eugenia Castro, Rocío Aguilar, César Márquez-Beltrán, and Maykel González. 2018. "Bulk Modification of Poly(lactide) (PLA) via Copolymerization with Poly(propylene glycol) Diglycidylether (PPGDGE)" Polymers 10, no. 11: 1184. https://doi.org/10.3390/polym10111184
APA StyleCastillejos, S., Cerna, J., Meléndez, F., Castro, M. E., Aguilar, R., Márquez-Beltrán, C., & González, M. (2018). Bulk Modification of Poly(lactide) (PLA) via Copolymerization with Poly(propylene glycol) Diglycidylether (PPGDGE). Polymers, 10(11), 1184. https://doi.org/10.3390/polym10111184