The New Generation from Biomembrane with Green Technologies for Wastewater Treatment
Abstract
1. Introduction
2. Experimental
2.1. Materials and Blend Preparation
2.2. Methods
2.2.1. Differential Scanning Calorimetry (DSC)
2.2.2. Mechanical Analysis
2.2.3. Thermogravimetric Analysis (TGA)
2.2.4. Biodegradation Test
2.2.5. Electrospinning Equipment
2.2.6. Scanning Electron Microscopy (SEM)
3. Results and Discussion
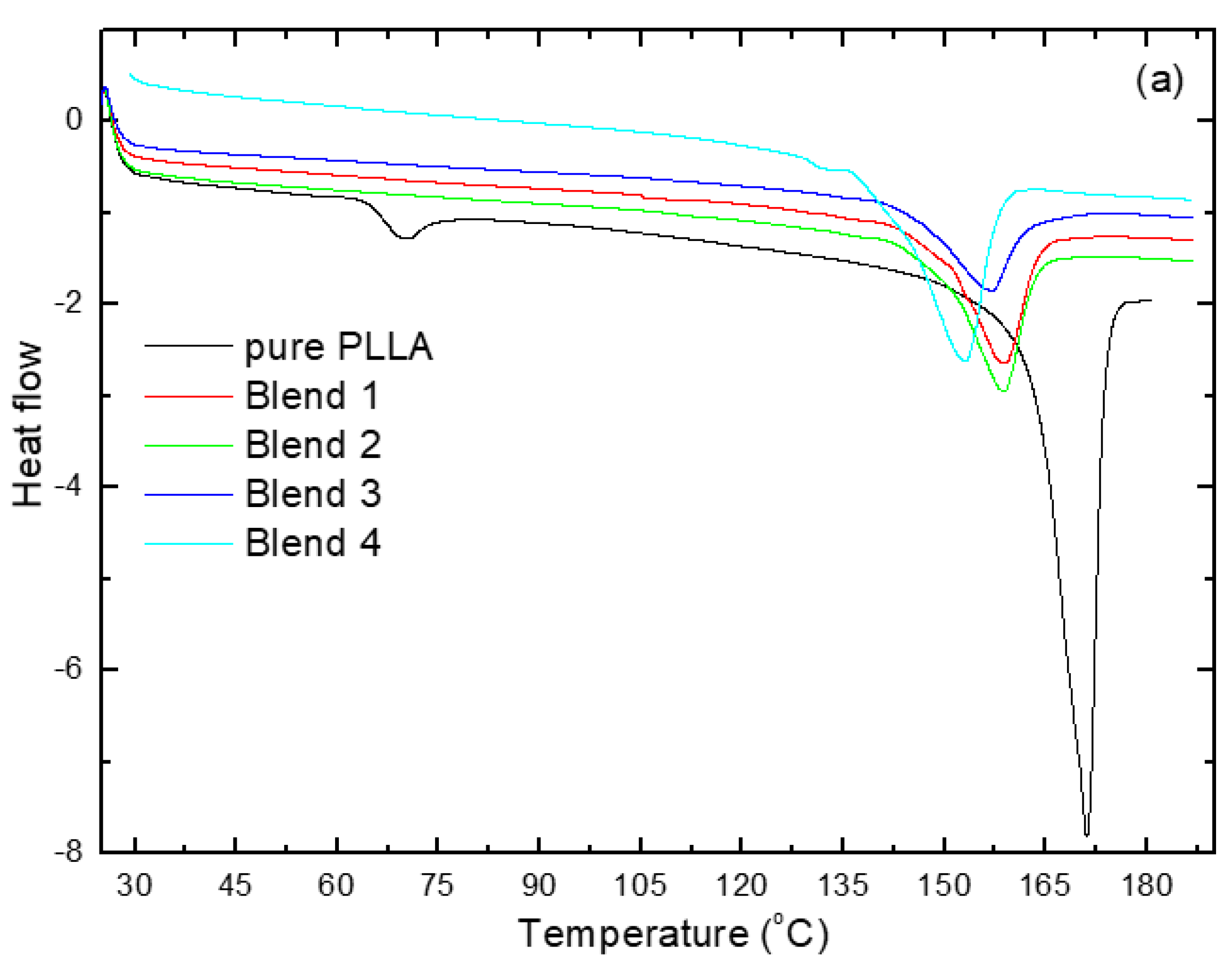
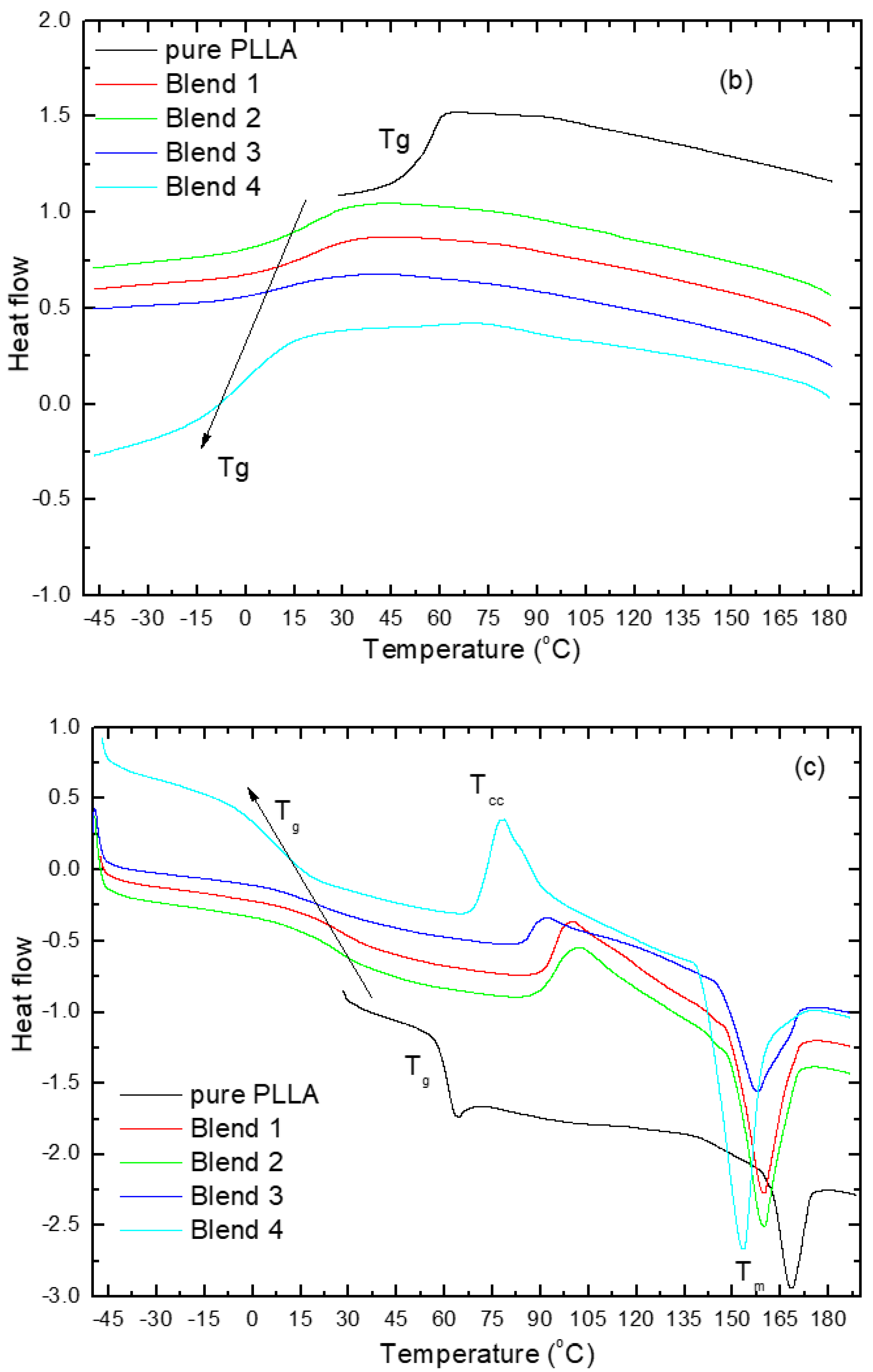
3.1. Differential Scanning Calorimetry (DSC) Analysis
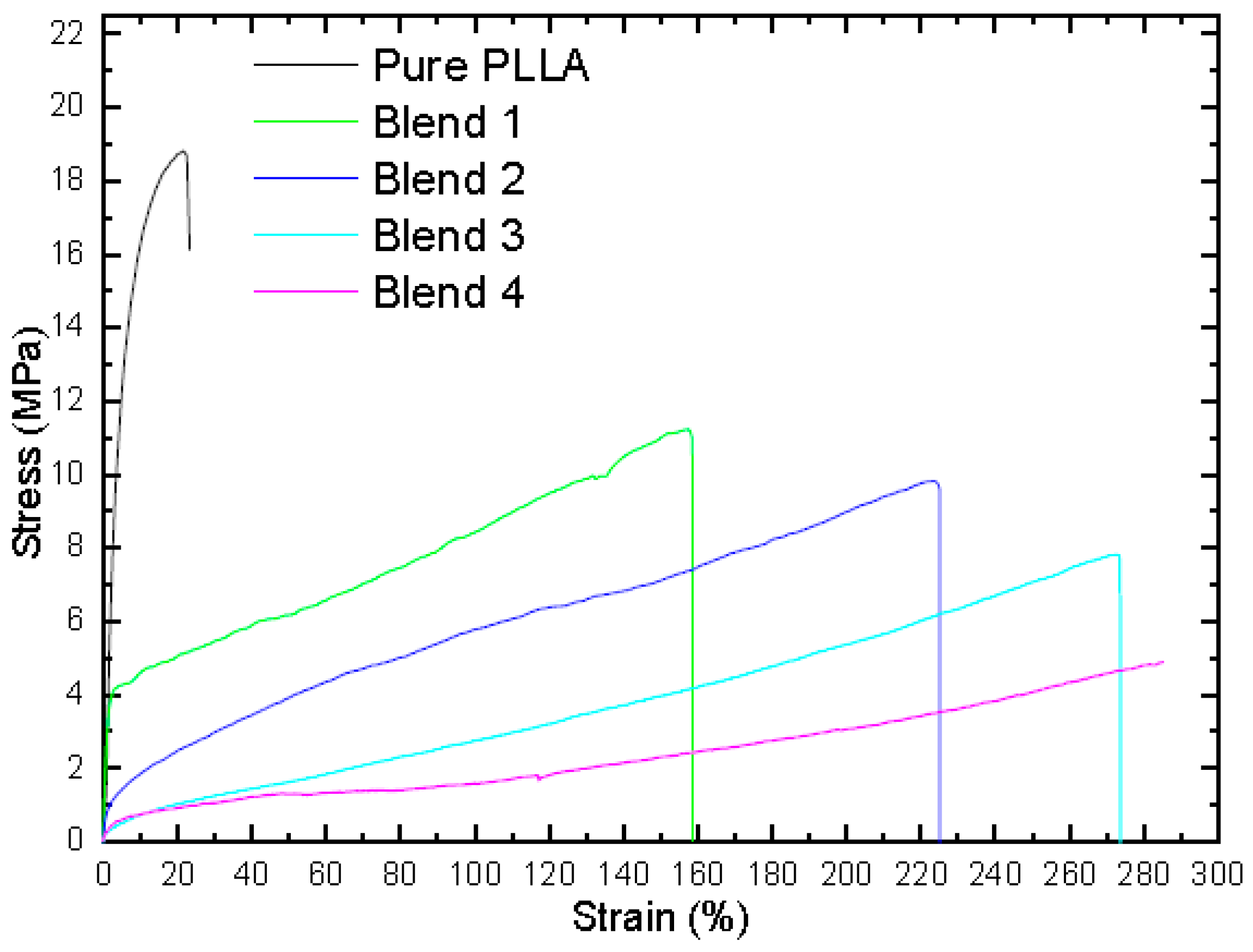
3.2. Mechanical Properties
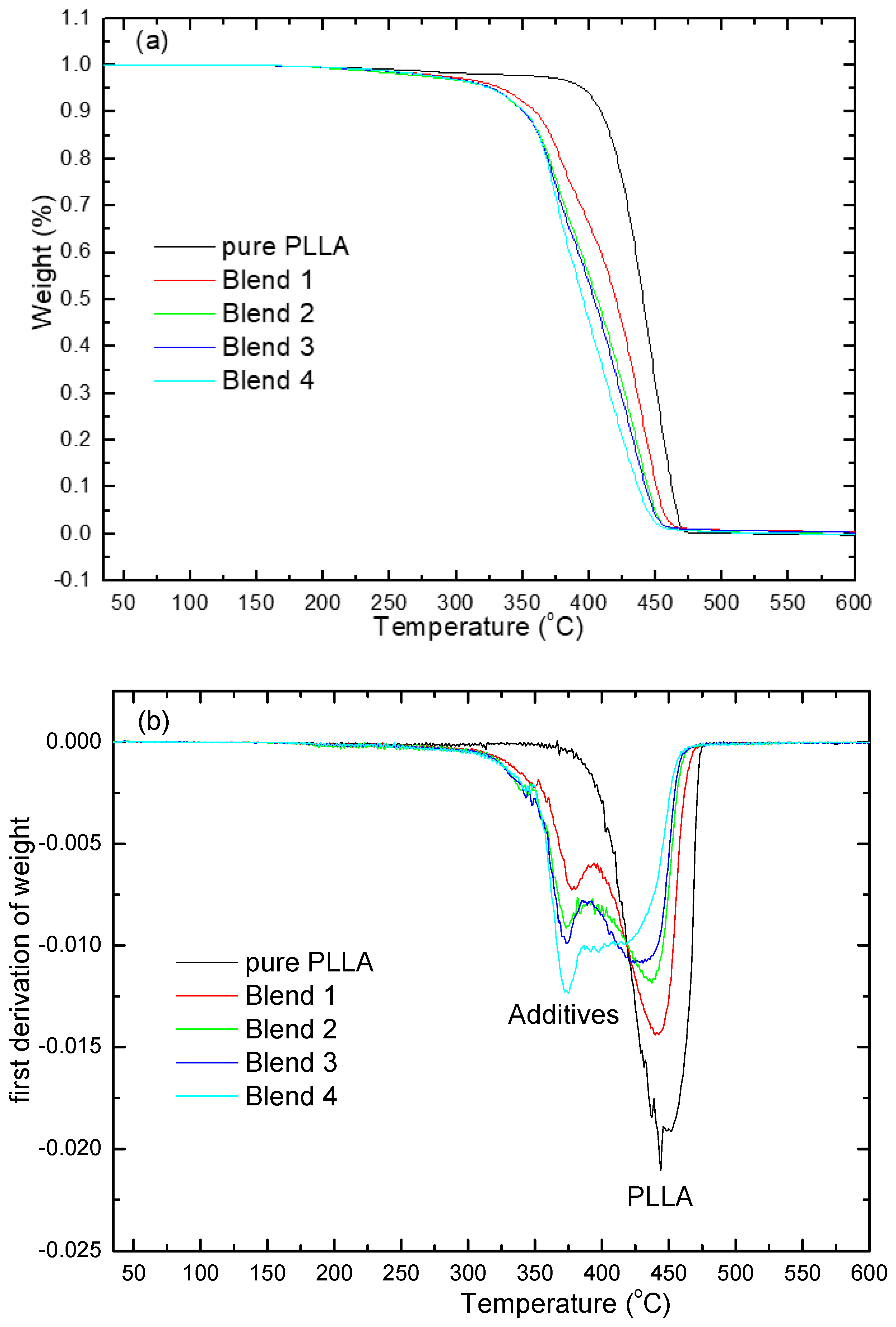
3.3. Thermogravimetric Analysis (TGA)
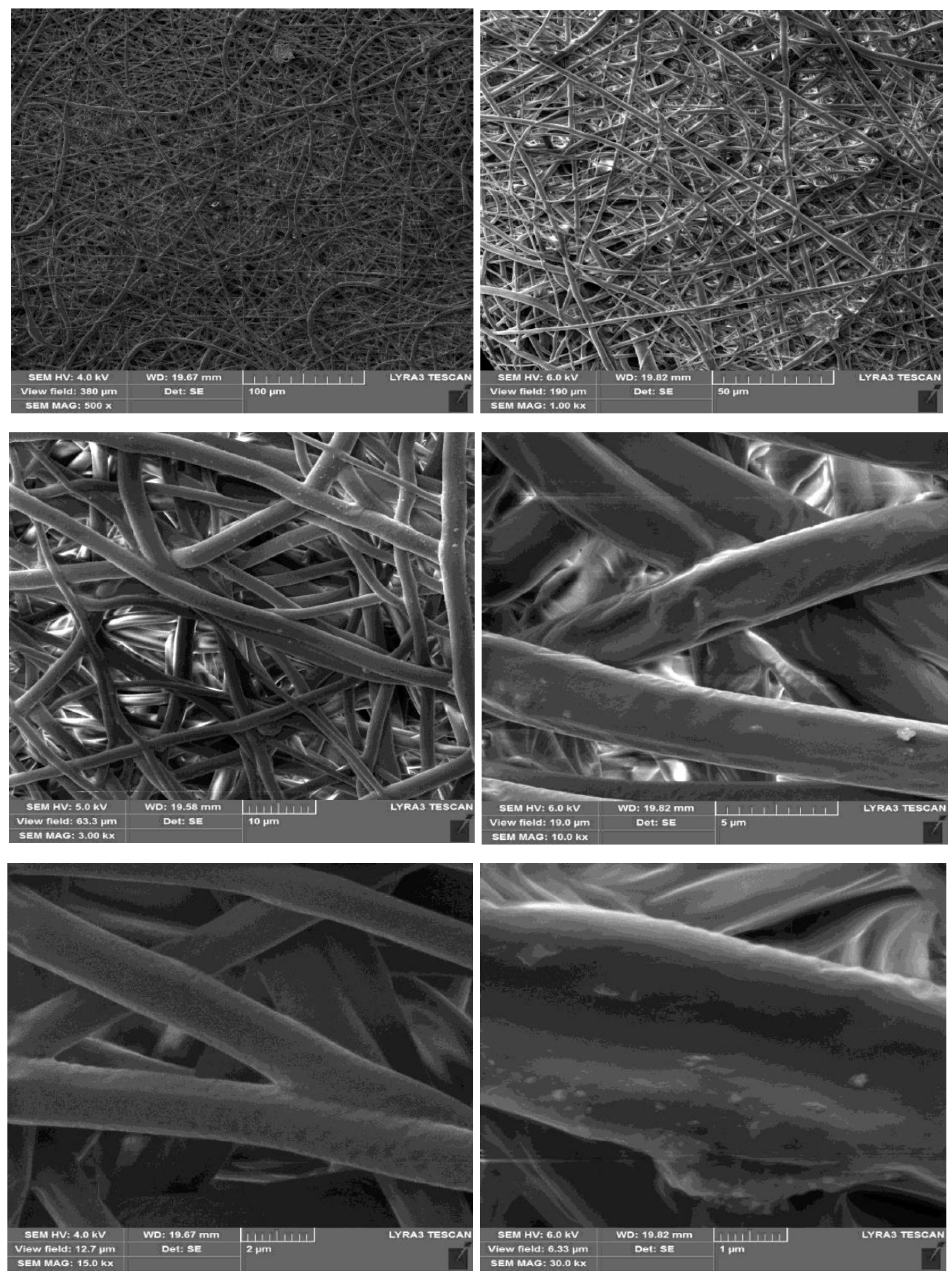
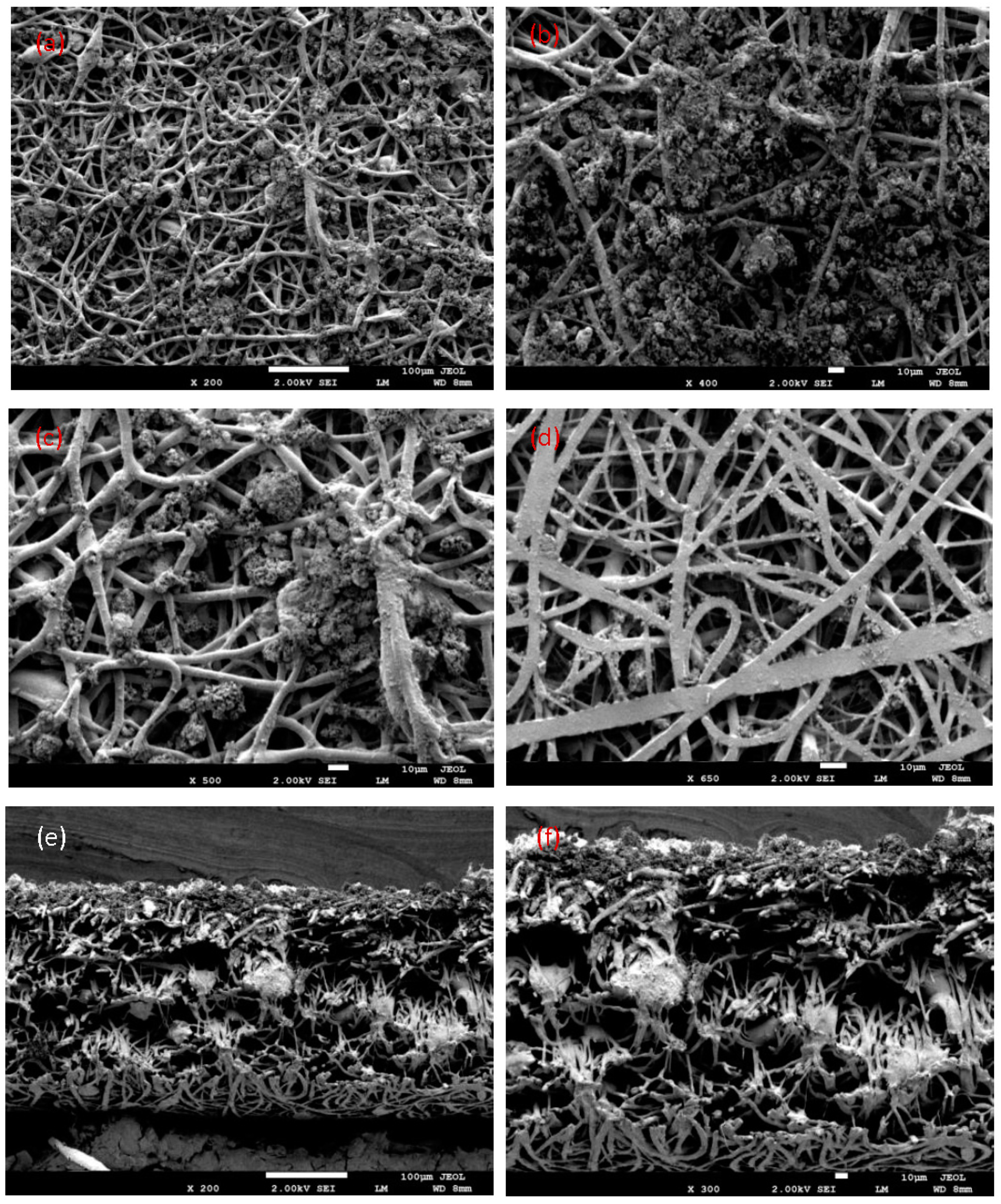
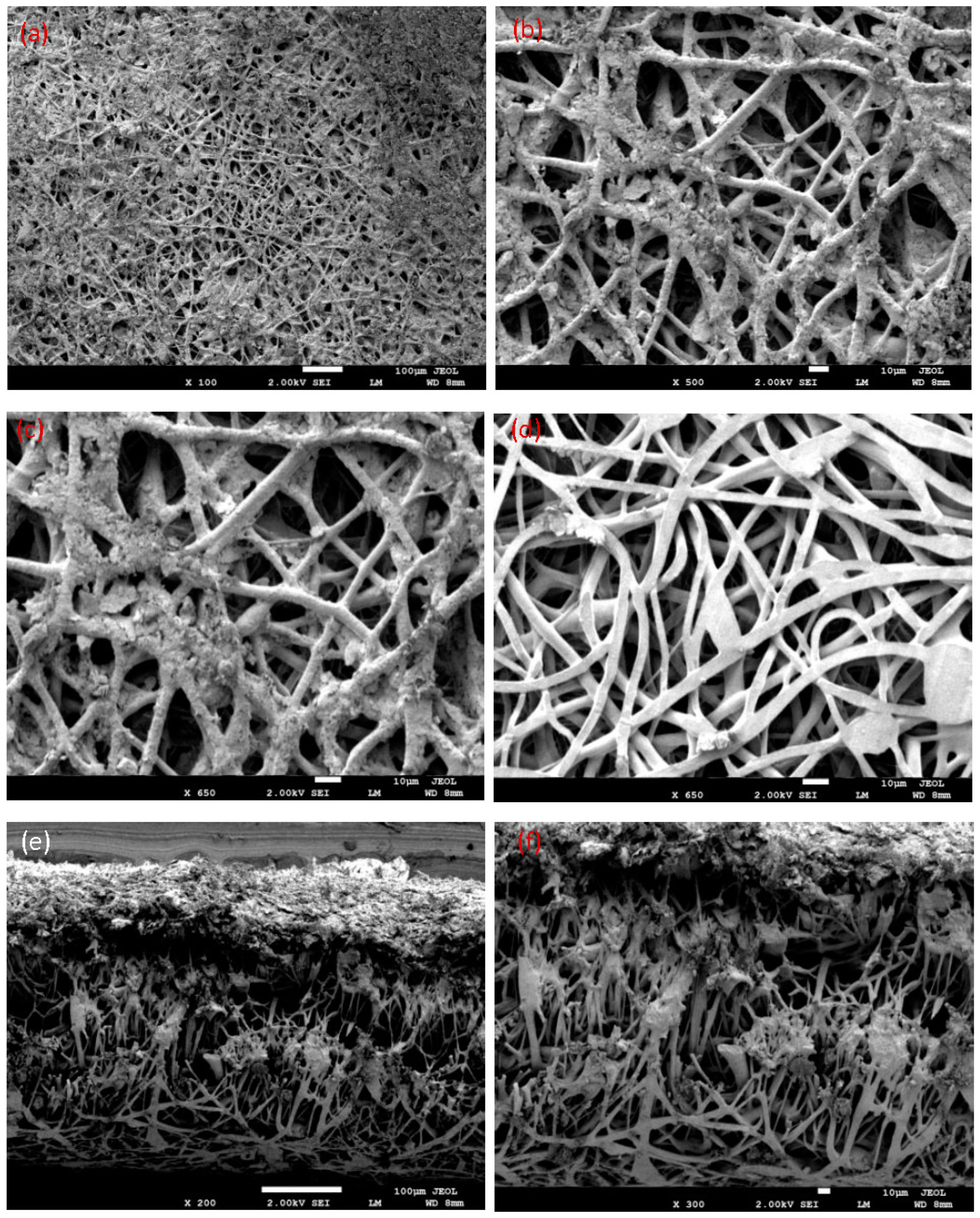
3.4. Fiber Morphology by Scanning Electron Microscopy (SEM)
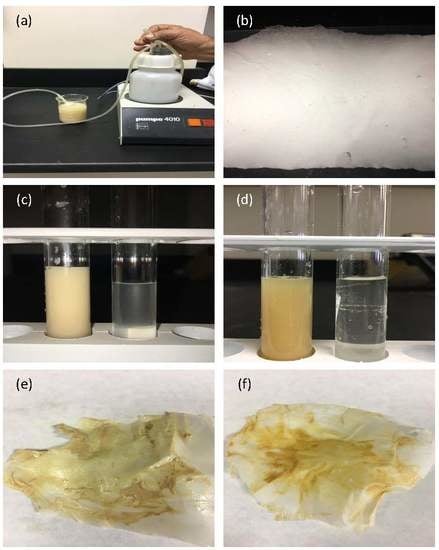
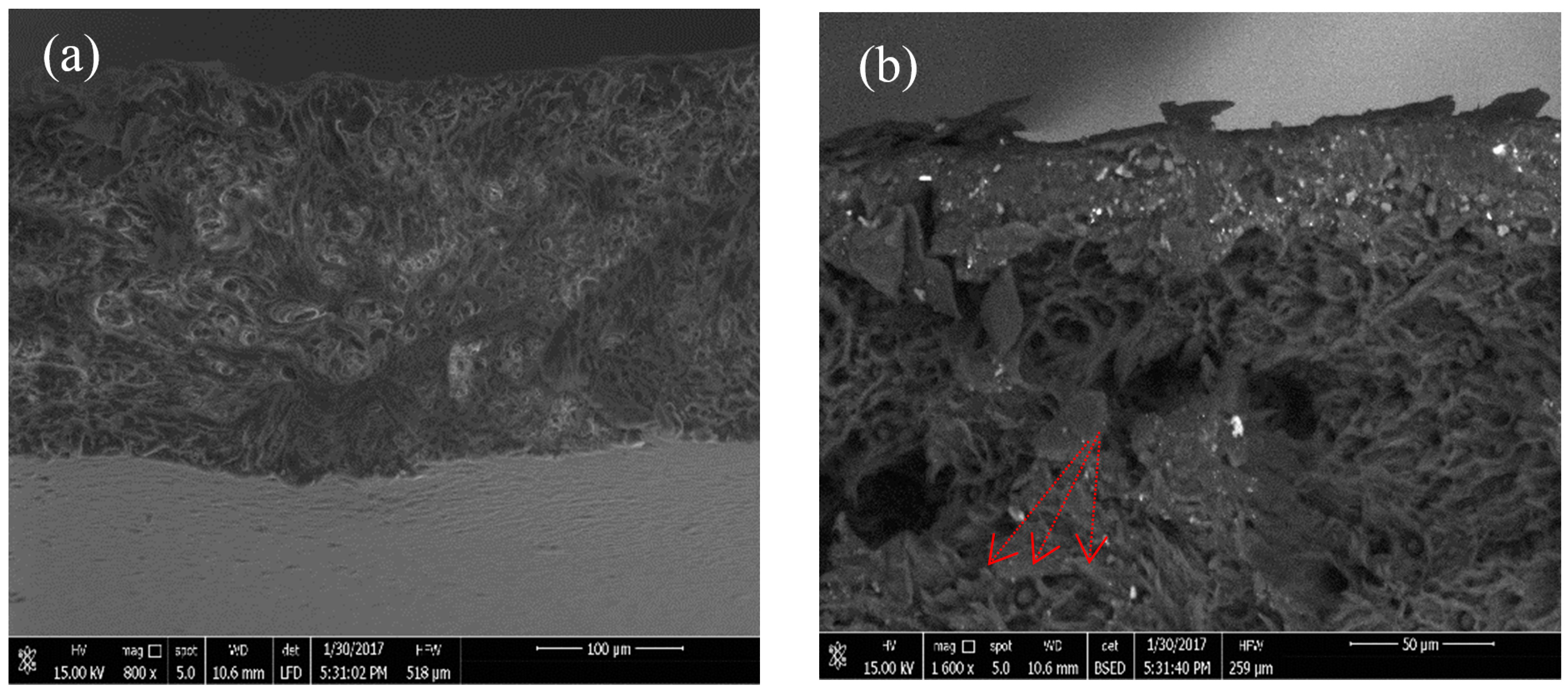
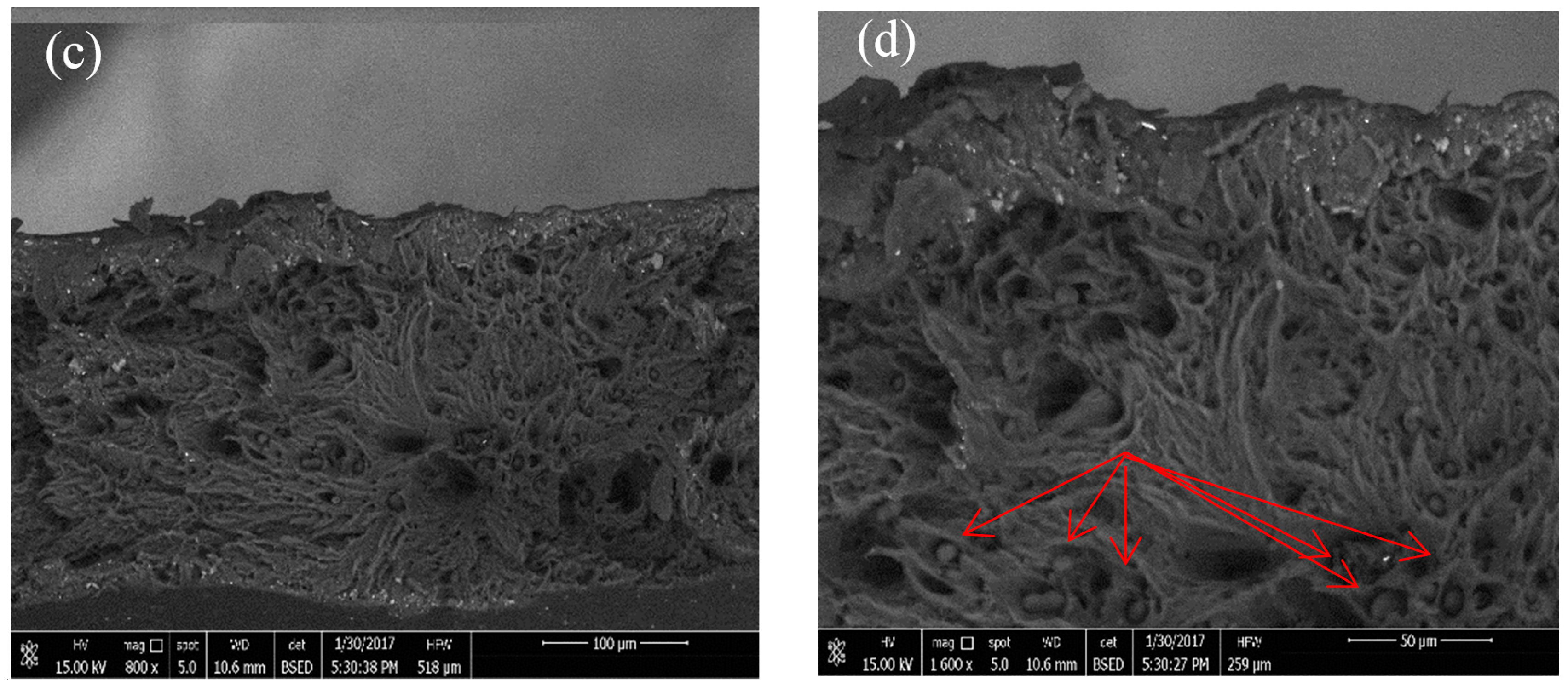
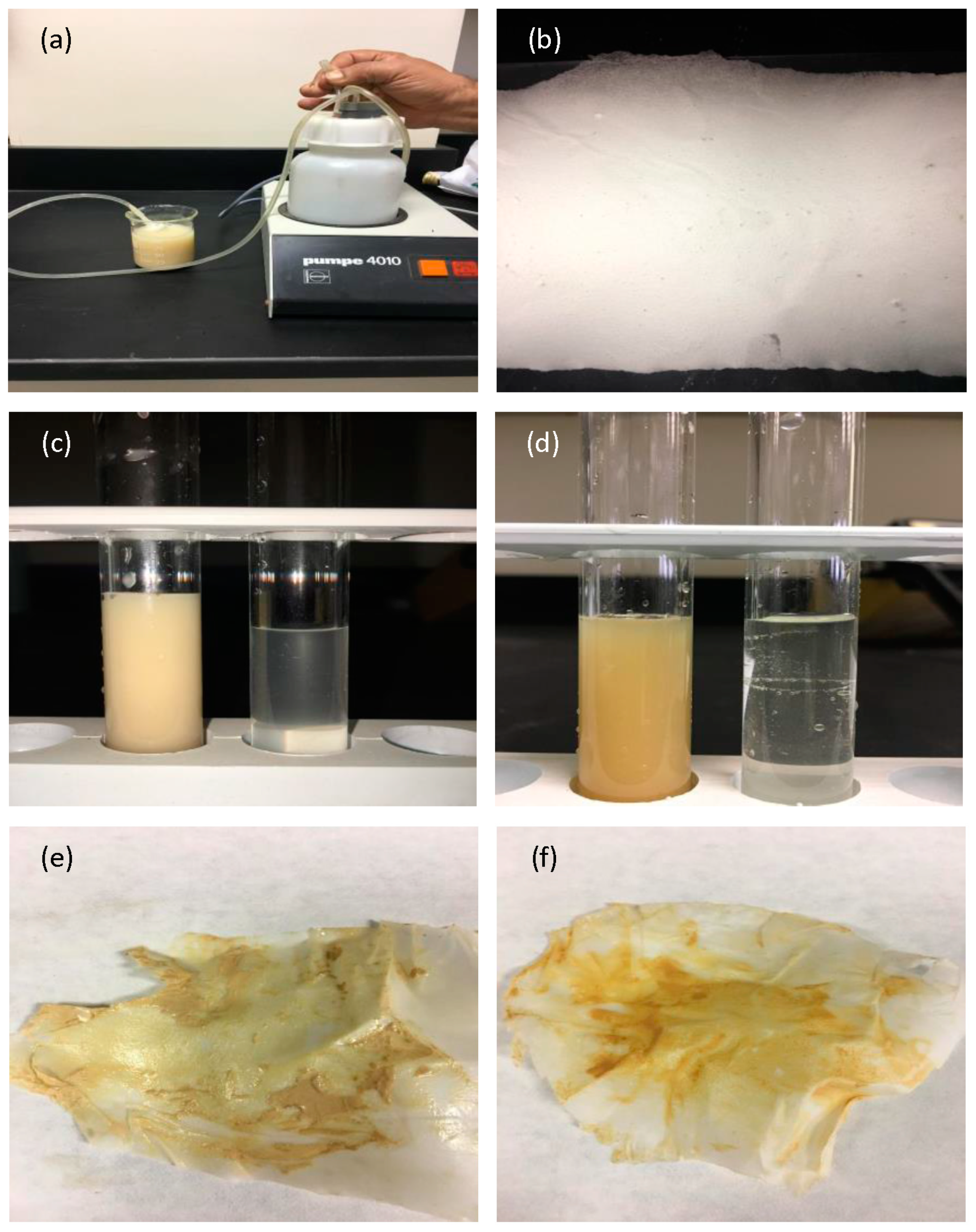
3.5. Biodegradability Test in Wastewater
3.6. Filtration Test
4. Conclusions
- The addition of PPC, PHB, and TEC led to an improvement in the elongation at break, therefore reducing the tensile strength of the films. The fine structure of many PPC particles combined in the PLLA matrix improved the mechanical properties with very large stretching deformation in the PLLA blends (285%) when compared with pure PLLA (6%). The additives led to changes in the Tg, Tcc, and Tm; therefore, the chain mobility increased.
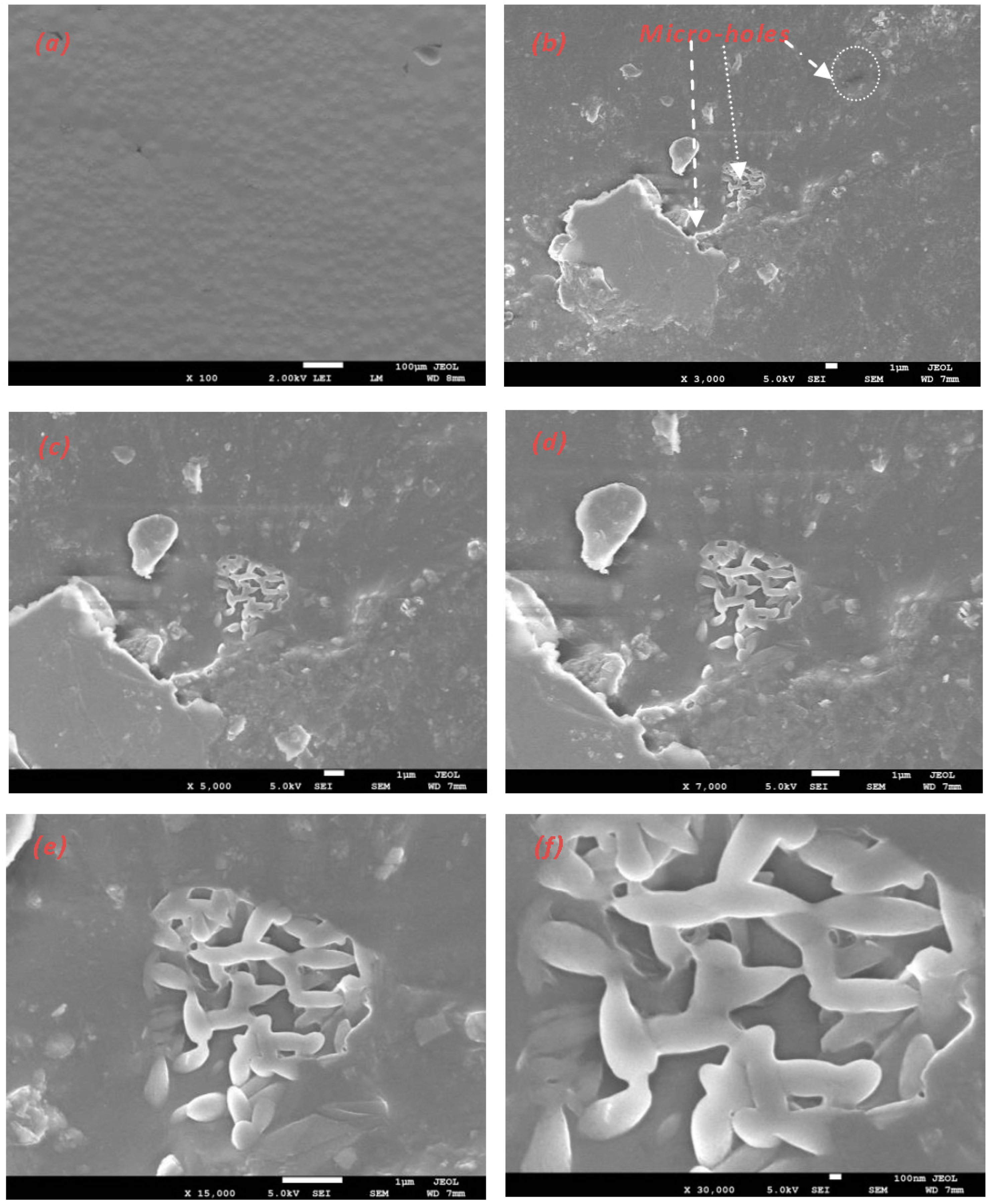
- The biodegradability test of the PLLA blend film was investigated using SEM in wastewater. The degradation at the surface began after more than two months. Degraded surfaces were observed, and some pores with different sizes were found.
- The obtained fibers had a uniform, smooth morphology with a diameter between 500 nm and 3 µm, very small pores, and a fiber structure without beads. The electrospun nanofiber membranes can filter the nanosize and microsize element suspensions in wastewater. Using this method, liquid waste from the industry or home can be disposed of in a sustainable and economical way. Therefore, the PLLA biomembrane is a new solution to confirm clean water and preserve a sustainable environment in the future.
Author Contributions
Funding
Conflicts of Interest
References
- Hao, M.; Wu, H.; Qiu, F.; Wang, X. Interface Bond Improvement of Sisal Fibre Reinforced Polylactide Composites with Added Epoxy Oligomer. Materials 2018, 11, 398. [Google Scholar] [CrossRef] [PubMed]
- Restrepo, I.; Medina, C.; Meruane, V.; Akbari-Fakhrabadi, A.; Flores, P.; Rodríguez-Llamazares, S. The effect of molecular weight and hydrolysis degree of poly(vinyl alcohol) (PVA) on the thermal and mechanical properties of poly(lactic acid)/PVA blends. Polímeros 2018, 28, 169–177. [Google Scholar] [CrossRef]
- Navarro-Baena, I.; Sessini, V.; Dominici, F.; Torre, L.; Kenny, J.M.; Peponi, L. Design of biodegradable blends based on PLA and PCL: From morphological, thermal and mechanical studies to shape memory behavior. Polym. Degrad. Stab. 2016, 132, 97–108. [Google Scholar] [CrossRef]
- Wu, D.-D.; Li, W.; Zhao, Y.; Deng, Y.-J.; Zhang, H.-L.; Zhang, H.-X.; Dong, L.-S. Thermal, Mechanical and Rheological Properties of Biodegradable Poly(propylene carbonate) and Poly(butylene carbonate) Blends. Chin. J. Polym. Sci. 2015, 33, 444–455. [Google Scholar] [CrossRef]
- Fonseca, C.; Ochoa, A.; Ulloa, M.T.; Alvarez, E.; Canales, D.; Zapata, P.A. Poly(lactic acid)/TiO2 nanocomposites as alternative biocidal and antifungal materials. Mater. Sci. Eng. C 2015, 57, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.-Z.; Zhou, L.; Tang, C.-Y.; Tsui, C.-P. Crystalline properties of poly(l-lactic acid) composites filled with nanometer calcium carbonate. Compos. Part B 2013, 45, 1646–1650. [Google Scholar] [CrossRef]
- Anzlovar, A.; Krzan, A.; Zagar, E. Degradation of PLA/ZnO and PHBV/ZnO composites prepared by melt processing. Arab. J. Chem. 2018, 11, 343–352. [Google Scholar] [CrossRef]
- El-Hadi, A.M. Development of Novel Biopolymer Blends Based on Poly(l-lactic acid), Poly((R)-3-hydroxybutyrate), and Plasticizer. Polym. Eng. Sci. 2014, 54, 1394–1402. [Google Scholar] [CrossRef]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread applications A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef] [PubMed]
- El-Hadi, A.M. The effect of annealing treatments on spherulitic morphology and physical ageing on glass transition of poly lactic acid (PLLA). Mater. Sci. Appl. 2011, 2, 439–443. [Google Scholar]
- El-Hadi, A.M. The Effect of Additives Interaction on the Miscibility and Crystal Structure of Two Immiscible Biodegradable Polymers. Polímeros 2014, 24, 9–16. [Google Scholar] [CrossRef]
- Utracki, L.A. Economics of polymer blends. Polym. Eng. Sci. 1982, 28, 1166–1175. [Google Scholar] [CrossRef]
- Vroman, I.; Tighzert, L. Biodegradable Polymers. Materials 2009, 2, 307–344. [Google Scholar] [CrossRef]
- Maiza, M.; Benaniba, M.T.; Quintard, G.; Massardier-Nageotte, V. Biobased additive plasticizing Polylactic acid (PLA). Polímeros 2015, 25, 581–590. [Google Scholar] [CrossRef]
- White, J.L.; Bumm, S.H. Polymer Blend Compounding and Processing, in Encyclopedia of Polymer Blends; Isayev, A.L., Palsule, S., Eds.; Wiley-VCH: Weinheim, Germany, 2011; pp. 1–26. [Google Scholar]
- Muthuraj, R.; Misra, M.; Mohanty, A.K. Biodegradable compatibilized polymer blends for packaging applications: A literature review. Appl. Polym. Sci. 2018, 135, 45726. [Google Scholar] [CrossRef]
- Suthapakti, K.; Molloy, R.; Punyodom, W.; Nalampang, K.; Leejarkpai, T.; Topham, P.D.; Tighe, B.J. Biodegradable Compatibilized Poly(l-lactide)/Thermoplastic Polyurethane Blends: Design, Preparation and Property Testing. J. Polym. Environ. 2018, 26, 1818–1830. [Google Scholar] [CrossRef]
- Kang, H.; Li, Y.; Gong, M.; Guo, Y.; Guo, Z.; Fang, Q.; Li, X. An environmentally sustainable plasticizer toughened polylactide. RSC Adv. 2018, 8, 11643–11651. [Google Scholar] [CrossRef]
- Qin, J.; Lin, L.; Wang, S.; Ye, S.; Luo, W.; Xiao, M.; Han, D.; Meng, Y. Multiblock copolymers of PPC with oligomeric PBS: With low brittle–toughness transition temperature. RSC Adv. 2018, 8, 14722–14731. [Google Scholar] [CrossRef]
- El-hadi, A.M.; Mohan, S.D.; Davis, F.J.; Mitchell, G.R. Enhancing the crystallization and orientation of electrospinning poly (lactic acid) (PLLA) by combining with additives. J. Polym. Res. 2014, 21, 605. [Google Scholar] [CrossRef]
- Mitchell, G.R.; Mohan, D.S.; Davis, J.F.; Al-Azab, M.; El Hadi, A.; Elliott, D.; Nagarajan, A.; Nazhipkyzy, M. Structure Development in Electrospun Fibres. RSC Polym. Chem. Ser. 2015, 14, 136–171. [Google Scholar]
- El-Hadi, A.M. Improvement the miscibility by combination of poly(3-hydroxy butyrate) PHB and poly(propylene carbonate) PPC with additives. J. Polym. Environ. 2017, 25, 728–738. [Google Scholar] [CrossRef]
- El-hadi, A.M.; Al-Jabri, F.Y. Influence of Electrospinning Parameters on Fiber Diameter and Mechanical Properties of Poly(3-Hydroxybutyrate) (PHB) and Polyanilines (PANI) Blends. Polymers 2016, 8, 97. [Google Scholar] [CrossRef]
- El-hadi, A.M. Increase the elongation at break of poly (lactic acid) composites for use in food packaging films. Scientific Reports. Sci. Rep. 2017, 7, 46767. [Google Scholar] [CrossRef] [PubMed]
- Bolong, N.; Ismail, A.F.; Salim, M.R.; Matsuura, T. A review of the effects of emerging contaminants in wastewater and options for their removal. Desalination 2009, 239, 229–246. [Google Scholar] [CrossRef]
- Min, L.-L.; Zhong, L.-B.; Zheng, Y.-M.; Liu, Q.; Yuan, Z.-H.; Yang, L.-M. Functionalized chitosan electrospun nanofiber for effective removal of trace arsenate from water. Sci. Rep. 2016, 6, 32480. [Google Scholar] [CrossRef] [PubMed]
- Sundarrajan, S.; Tan, K.L.; Lim, S.H.; Ramakrishna, S. electrospun Nanofibers for Air Filtration Applications. Procedia Eng. 2014, 75, 159–163. [Google Scholar] [CrossRef]
- Buruga, K.; Kalathia, J.T.; Kim, K.-H.; Ok, Y.S.; Danil, B. Polystyrene-halloysite nano tube membranes for water purification. J. Ind. Eng. Chem. 2018, 61, 169–180. [Google Scholar] [CrossRef]
- Keogh, M.B.; Elmusharaf, K.; Borde, P.; Mc Guigan, K.G. Evaluation of the natural coagulant Moringa oleifera as a pretreatment for SODIS in contaminated turbid water. Sol. Energy 2017, 158, 448–454. [Google Scholar] [CrossRef]
- Fischer, E.W.; Sterzel, H.J.; Wegner, G. Investigation of the structure of solution grown crystals of lactide copolymers by means of chemical reaction. Kolloid-Z. Z. Polym. 1973, 251, 980–990. [Google Scholar] [CrossRef]
- Silverajah, V.S.; Ibrahim, N.A.; Zainuddin, N.; Yunus, W.M.Z.W.; Hassan, H.A. Mechanical, Thermal and Morphological Properties of Poly(lactic acid)/Epoxidized Palm Olein Blend. Molecules 2012, 17, 11729–11747. [Google Scholar] [CrossRef] [PubMed]
- Gopal, R.; Kaur, S.; Ma, Z.; Chan, C.; Ramakrishna, S.; Matsuura, T. Electrospun nanofibrous filtration membrane. J. Membr. Sci. 2006, 281, 581–586. [Google Scholar] [CrossRef]
| PLLA | PPC | PHB | TEC | ||
|---|---|---|---|---|---|
| 1 | blend 1 | 55 | 20 | 10 | 15 |
| 2 | blend 2 | 50 | 20 | 10 | 20 |
| 3 | blend 3 | 45 | 20 | 10 | 25 |
| 4 | blend 4 | 40 | 20 | 10 | 30 |
| Pure PLLA | Blend 1 | Blend 2 | Blend 3 | Blend 4 | |
|---|---|---|---|---|---|
| Tg (°C) | 61 | 27 | 27 | 21 | 7 |
| Tcc (°C) | 135 | 100 | 102 | 92 | 78 |
| Tm (°C) from S.H. | 168 | 160 | 160 | 158 | 153 |
| Tm (°C) from F.H. | 171 | 158.8 | 158.7 | 156.8 | 152.7 |
| ΔHm (J/g) | 7.1 | 29 | 24 | 25 | 22 |
| ΔHcc (J/g) | - | 14 | 11 | 7 | 9 |
| Χ (%) | 8 | 23 | 20 | 30 | 25 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamed El-hadi, A.; Alamri, H.R. The New Generation from Biomembrane with Green Technologies for Wastewater Treatment. Polymers 2018, 10, 1174. https://doi.org/10.3390/polym10101174
Mohamed El-hadi A, Alamri HR. The New Generation from Biomembrane with Green Technologies for Wastewater Treatment. Polymers. 2018; 10(10):1174. https://doi.org/10.3390/polym10101174
Chicago/Turabian StyleMohamed El-hadi, Ahmed, and Hatem Rashad Alamri. 2018. "The New Generation from Biomembrane with Green Technologies for Wastewater Treatment" Polymers 10, no. 10: 1174. https://doi.org/10.3390/polym10101174
APA StyleMohamed El-hadi, A., & Alamri, H. R. (2018). The New Generation from Biomembrane with Green Technologies for Wastewater Treatment. Polymers, 10(10), 1174. https://doi.org/10.3390/polym10101174