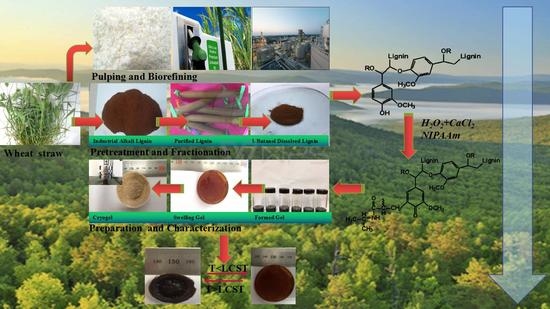
Study on the Effect of 1-Butanol Soluble Lignin on Temperature-Sensitive Gel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials and Chemicals
2.2. Lignin Purification and Fractionation
2.3. Synthesis of Temperature-Sensitive Gel
2.4. Characterization of Lignin
2.5. Characterization of Gel
3. Results and Discussion
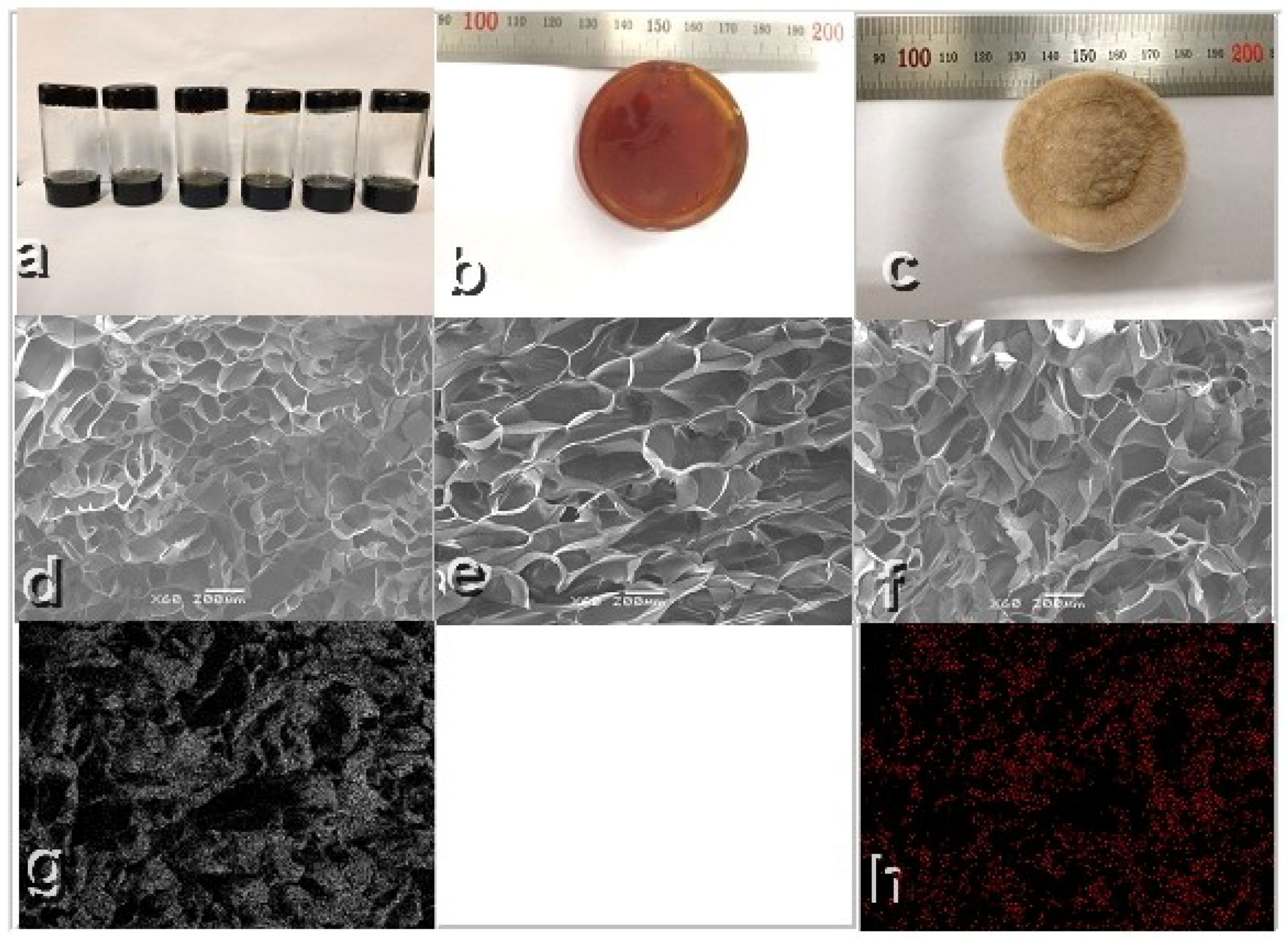
3.1. Lignin Fractionation and Characterization
3.2. Gel Formation and Characterization
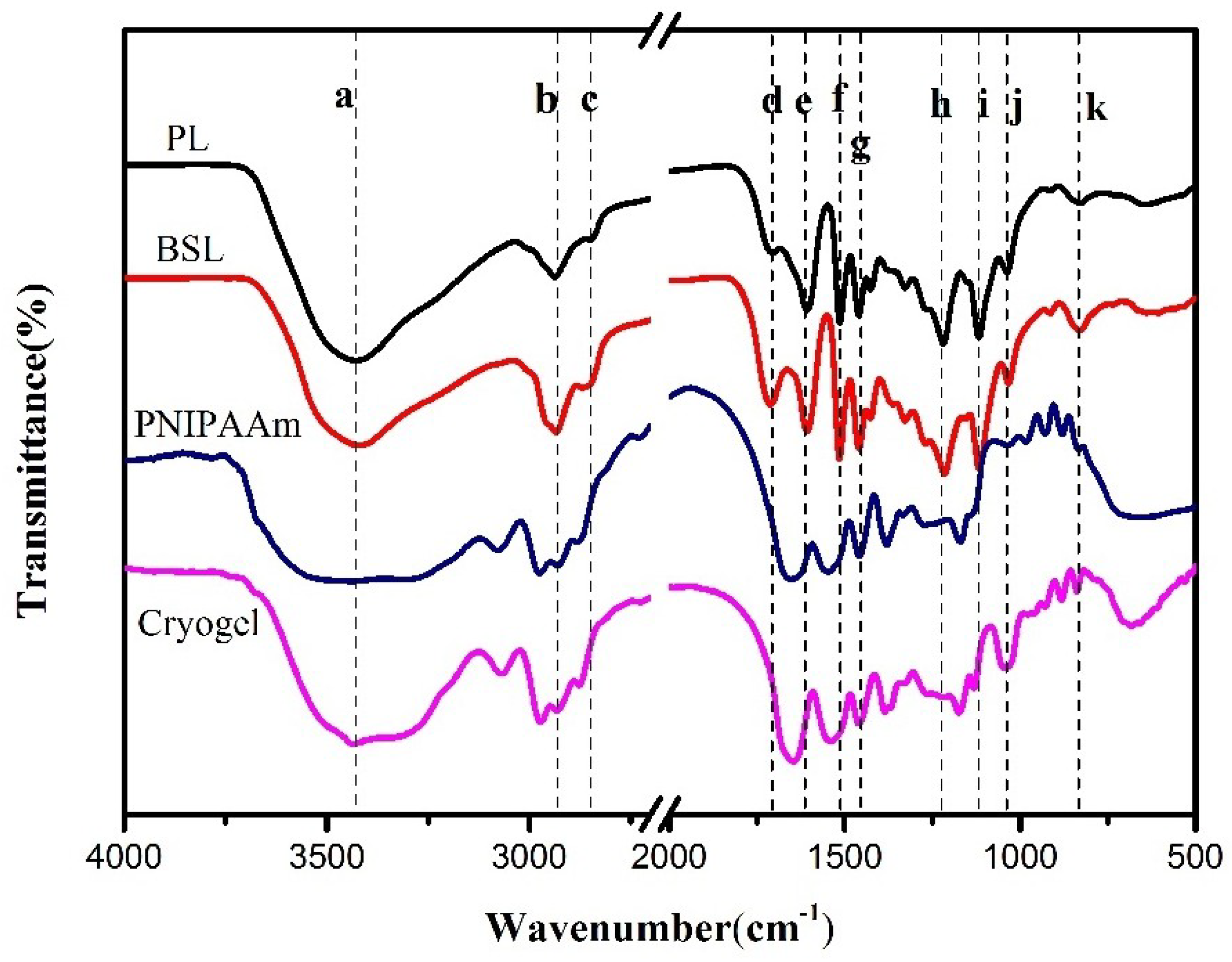
3.3. Fourier Transform Infrared Spectroscopy (FTIR)
3.4. 31P NMR
3.5. Gel Permeation Chromatography (GPC)
3.6. Glass Transition Temperature (Tg)
3.7. Lower Critical Solution Temperature (LCST)
3.8. Elemental Composition of Lignin Fractions and Gel
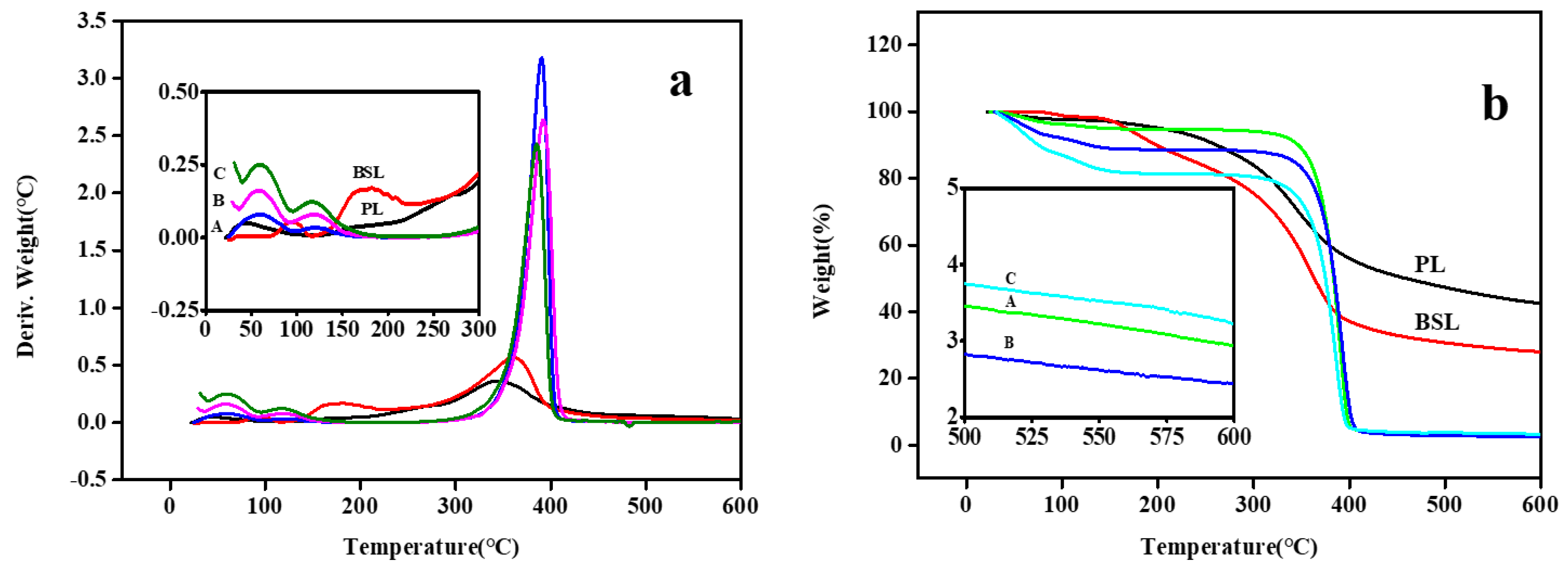
3.9. Thermogravimetric Analysis (TGA)
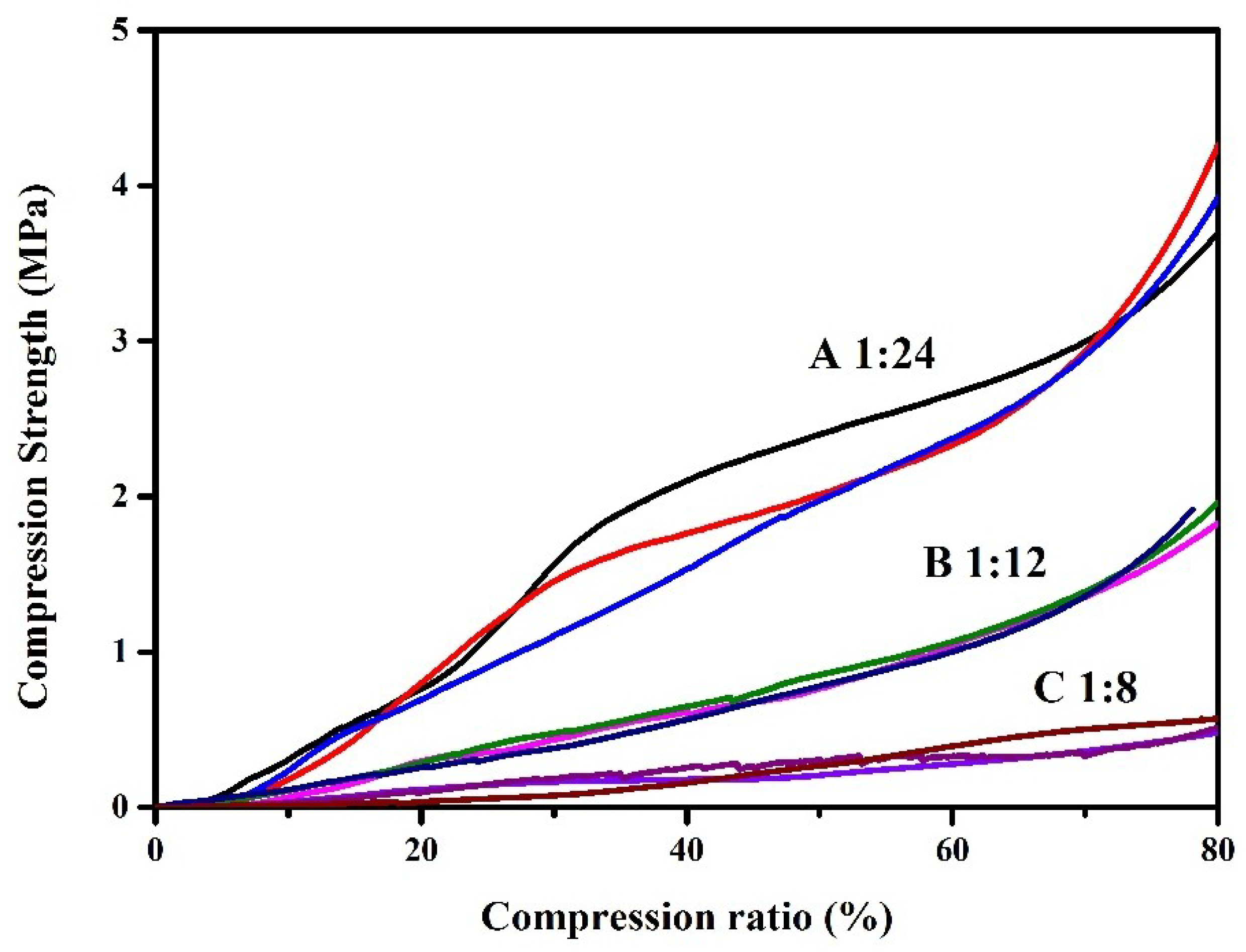
3.10. Interior Morphology and Mechanical Property
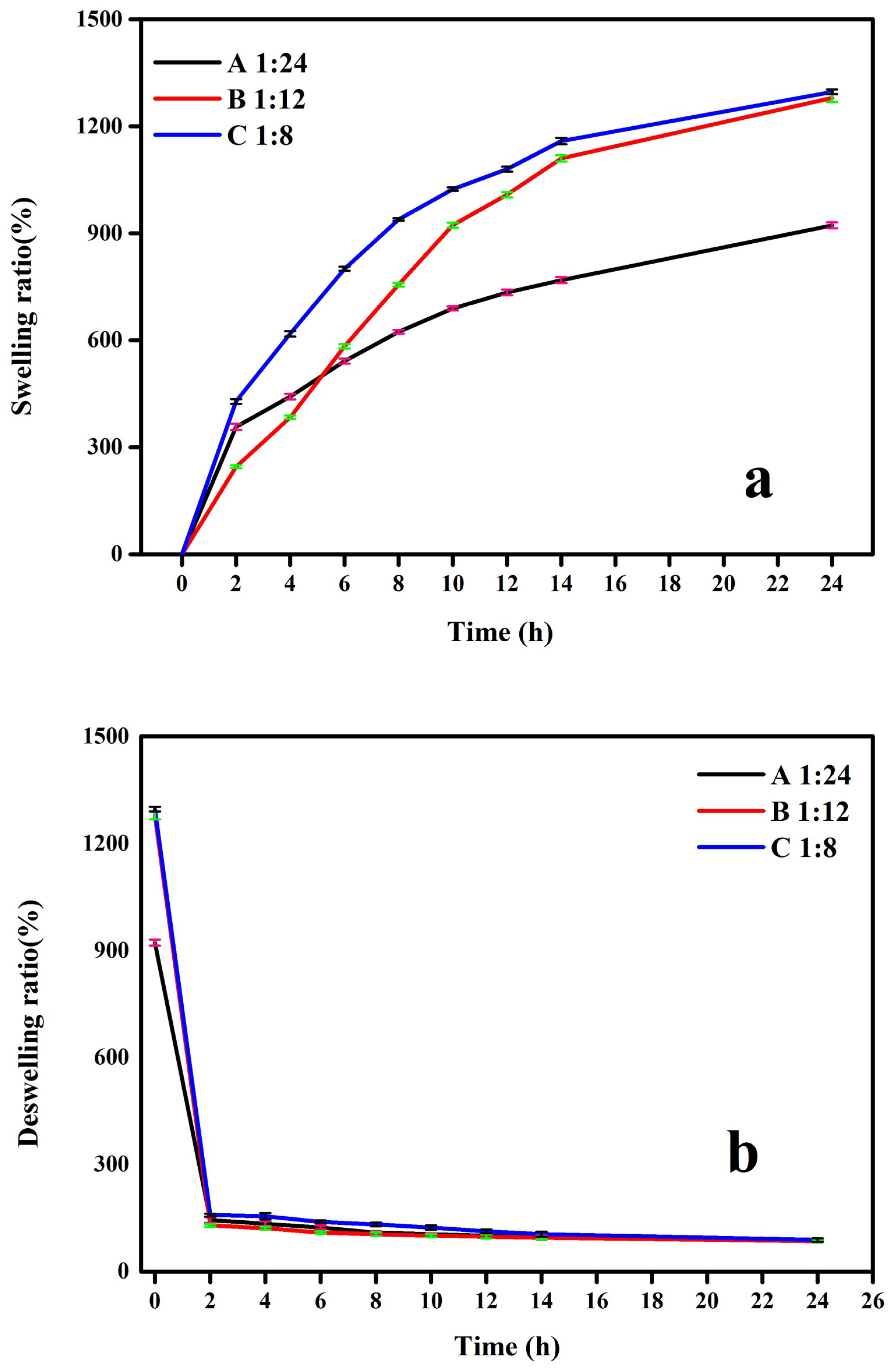
3.11. Water Absorption and Desorption Behavior
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Achyuthan, K.E.; Achyuthan, A.M.; Adams, P.D.; Dirk, S.M.; Harper, J.C.; Simmons, B.A.; Singh, A.K. Supramolecular self-assembled chaos: Polyphenolic lignin’s barrier to cost-effective lignocellulosic biofuels. Molecules 2010, 15, 8641–8688. [Google Scholar] [CrossRef] [PubMed]
- Ragauskas, A.J.; Beckham, G.T.; Biddy, M.J.; Chandra, R.; Chen, F.; Davis, M.F.; Davison, B.H.; Dixon, R.A.; Gilna, P.; Keller, M.; et al. Lignin valorization: Improving lignin processing in the biorefinery. Science 2014, 344, 1246843. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Dutta, T.; Varman, A.M.; Eudes, A.; Manalansan, B.; Loque, D.; Singh, S. Lignin valorization: Two hybrid biochemical routes for the conversion of polymeric lignin into value-added chemicals. Sci. Rep. 2017, 7, 8420. [Google Scholar] [CrossRef] [PubMed]
- Duval, A.; Vilaplana, F.; Crestini, C.; Lawoko, M. Solvent screening for the fractionation of industrial kraft lignin. Holzforschung 2016, 7, 11–20. [Google Scholar] [CrossRef]
- Laurichesse, S.; Avérous, L. Chemical modification of lignins: Towards biobased polymers. Prog. Polym. Sci. 2014, 39, 1266–1290. [Google Scholar] [CrossRef]
- Passoni, V.; Scarica, C.; Levi, M.; Turri, S.; Griffini, G. Fractionation of industrial softwood kraft lignin: Solvent selection as a tool for tailored material properties. ACS Sustain. Chem. Eng. 2016, 4, 2232–2242. [Google Scholar] [CrossRef]
- Chatterjee, S.; Saito, T. Lignin-derived advanced carbon materials. ChemSusChem 2015, 8, 3941–3958. [Google Scholar] [CrossRef] [PubMed]
- Jääskeläinen, A.S.; Liitiä, T.; Mikkelson, A.; Tamminen, T. Aqueous organic solvent fractionation as means to improve lignin homogeneity and purity. Ind. Crops Prod. 2017, 103, 51–58. [Google Scholar] [CrossRef]
- Alekhina, M.; Ershova, O.; Ebert, A.; Heikkinen, S.; Sixta, H. Softwood kraft lignin for value-added applications: Fractionation and structural characterization. Ind. Crops Prod. 2015, 66, 220–228. [Google Scholar] [CrossRef]
- Arshanitsa, A.; Ponomarenko, J.; Dizhbite, T.; Andersone, A.; Gosselink, R.J.A.; van der Putten, J.; Lauberts, M.; Telysheva, G. Fractionation of technical lignins as a tool for improvement of their antioxidant properties. J. Anal. Appl. Pyrolysis 2013, 103, 78–85. [Google Scholar] [CrossRef]
- Dodd, A.P.; Kadla, J.F.; Straus, S.K. Characterization of fractions obtained from two industrial softwood kraft lignins. ACS Sustain. Chem. Eng. 2014, 3, 103–110. [Google Scholar] [CrossRef]
- Sadeghifar, H.; Wells, T.; Le, R.K.; Sadeghifar, F.; Yuan, J.S.; Jonas Ragauskas, A. Fractionation of organosolv lignin using acetone:Water and properties of the obtained fractions. ACS Sustain. Chem. Eng. 2016, 5, 580–587. [Google Scholar] [CrossRef]
- Feng, Q.; Chen, F.; Wu, H. Preparation and characterization of a temperature-sensitive lignin-based hydrogel. Bioresources 2011, 6, 4942–4952. [Google Scholar]
- Feng, Q.; Chen, F.; Zhou, X. Preparation of thermo-sensitive hydrogels from acrylated lignin and n-isopropylacrylamide through photocrosslinking. J. Biobased Mater. Bioenergy 2012, 6, 336–342. [Google Scholar] [CrossRef]
- Gao, C.; Ren, J.; Zhao, C.; Kong, W.; Dai, Q.; Chen, Q.; Liu, C.; Sun, R. Xylan-based temperature/ph sensitive hydrogels for drug controlled release. Carbohydr. Polym. 2016, 151, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Ren, S.; Ma, Y.; Fang, G.; Jiang, G. Preparation and properties of kraft lignin-n-isopropyl acrylamide hydrogel. Bioresources 2015, 10, 3507–3519. [Google Scholar] [CrossRef]
- Qian, Y.; Zhang, Q.; Qiu, X.; Zhu, S. CO2-responsive diethylaminoethyl-modified lignin nanoparticles and their application as surfactant for CO2/N2–switchable pickering emulsions. Green Chem. 2014, 16, 4963–4968. [Google Scholar] [CrossRef]
- Yuan, Z.L.; Chen, H.S.; Li, Z.Y.; Li, H.Y. Synthesis of ph-responsive hydrogel based on bagasse lignin and controlled release of protein. Guihaia 2017, 37, 248–254. [Google Scholar]
- Wang, H.; Chen, Q.; Zhou, S. Carbon-based hybrid nanogels: A synergistic nanoplatform for combined biosensing, bioimaging, and responsive drug delivery. Chem. Soc. Rev. 2018, 47, 4198–4232. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.A.; Takafuji, M.; Ihara, H. Silica nanoparticle-crosslinked thermosensitive hybrid hydrogels as potential drug-release carriers. Polym. J. 2014, 46, 293–300. [Google Scholar] [CrossRef]
- Wang, H.; Ke, F.; Mararenko, A.; Wei, Z.; Banerjee, P.; Zhou, S. Responsive polymer-fluorescent carbon nanoparticle hybrid nanogels for optical temperature sensing, near-infrared light-responsive drug release, and tumor cell imaging. Nanoscale 2014, 6, 7443–7452. [Google Scholar] [CrossRef] [PubMed]
- Ju, H.O.; Hong, S.Y.; Park, H.; Sang, W.J.; Yu, R.J.; Oh, S.Y.; Yun, J.; Lee, H.; Kim, J.W.; Ha, J.S. Fabrication of high-sensitivity skin-attachable temperature sensors with bioinspired microstructured adhesive. ACS Appl. Mater. Interfaces 2018, 10, 7263–7270. [Google Scholar]
- Zhou, T.; Li, X.; Li, G.; Tian, T.; Lin, S.; Shi, S.; Liao, J.; Cai, X.; Lin, Y. Injectable and thermosensitive tgf-beta1-loaded pcec hydrogel system for in vivo cartilage repair. Sci. Rep. 2017, 7, 10553. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xiong, Y.; Wang, J.; Zhang, X. Ultrasonic-assisted fabrication of montmorillonite-lignin hybrid hydrogel: Highly efficient swelling behaviors and super-sorbent for dye removal from wastewater. Colloids Surf. A 2017, 520, 903–913. [Google Scholar] [CrossRef]
- Lu, N.; Liu, J.; Li, J.; Zhang, Z.; Weng, Y.; Yuan, B.; Yang, K.; Ma, Y. Tunable dual-stimuli response of a microgel composite consisting of reduced graphene oxide nanoparticles and poly(n-isopropylacrylamide) hydrogel microspheres. J. Mater. Chem. B 2014, 2, 3791–3798. [Google Scholar] [CrossRef]
- Bardajee, G.R.; Hooshyar, Z.; Farsi, M.; Mobini, A.; Sang, G. Synthesis of a novel thermo/ph sensitive nanogel based on salep modified graphene oxide for drug release. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 72, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Lu, W.; Yang, X.; He, J.; Le, X.; Wang, L.; Zhang, J.; Serpe, M.J.; Huang, Y.; Chen, T. Bioinspired anisotropic hydrogel actuators with on–off switchable and color-tunable fluorescence behaviors. Adv. Funct. Mater. 2018, 28, 1704568. [Google Scholar] [CrossRef]
- Song, P.; Qin, H.; Gao, H.-L.; Cong, H.-P.; Yu, S.-H. Self-healing and superstretchable conductors from hierarchical nanowire assemblies. Nat. Commun. 2018, 9, 2786. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yi, J.; Velado, D.; Yu, Y.; Zhou, S. Immobilization of carbon dots in molecularly imprinted microgels for optical sensing of glucose at physiological ph. ACS Appl. Mater. Interfaces 2015, 7, 15735–15745. [Google Scholar] [CrossRef] [PubMed]
- García, A.; Toledano, A.; Serrano, L.; Egüés, I.; González, M.; Marín, F.; Labidi, J. Characterization of lignins obtained by selective precipitation. Sep. Purif. Technol. 2009, 68, 193–198. [Google Scholar] [CrossRef]
- Pu, Y.; Cao, S.; Ragauskas, A.J. Application of quantitative (31)p nmr in biomass lignin and biofuel precursors characterization. Energy Environ. Sci. 2011, 4, 3154–3166. [Google Scholar] [CrossRef]
- Ni, Y.; Hu, Q. Alcell® lignin solubility in ethanol–water mixtures. J. Appl. Polym. Sci. 1995, 57, 1441–1446. [Google Scholar] [CrossRef]
- Figueiredo, P.; Lintinen, K.; Hirvonen, J.T.; Kostiainen, M.A.; Santos, H.A. Properties and chemical modifications of lignin: Towards lignin-based nanomaterials for biomedical applications. Prog. Mater. Sci. 2018, 93, 233–269. [Google Scholar] [CrossRef]
- Meister, J.J.; Patil, D.R. Solvent effects and initiation mechanisms for graft polymerization on pine lignin. Macromolecules 1985, 18, 1559–1564. [Google Scholar] [CrossRef]
- Diao, B.; Zhang, Z.; Zhu, J.; Li, J. Biomass-based thermogelling copolymers consisting of lignin and grafted poly(n-isopropylacrylamide), poly(ethylene glycol), and poly(propylene glycol). RSC Adv. 2014, 4, 42996–43003. [Google Scholar] [CrossRef]
- Wang, C.; Venditti, R.A. Uv cross-linkable lignin thermoplastic graft copolymers. ACS Sustain. Chem. Eng. 2015, 3, 1839–1845. [Google Scholar] [CrossRef]
- Wang, J.; Yao, K.; Korich, A.L.; Li, S.; Ma, S.; Ploehn, H.J.; Iovine, P.M.; Wang, C.; Chu, F.; Tang, C. Combining renewable gum rosin and lignin: Towards hydrophobic polymer composites by controlled polymerization. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 3728–3738. [Google Scholar] [CrossRef]
- Dominguez-Robles, J.; Sanchez, R.; Diaz-Carrasco, P.; Espinosa, E.; Garcia-Dominguez, M.T.; Rodriguez, A. Isolation and characterization of lignins from wheat straw: Application as binder in lithium batteries. Int. J. Biol. Macromol. 2017, 104, 909–918. [Google Scholar] [CrossRef] [PubMed]
- Ghaffar, S.H.; Fan, M. Structural analysis for lignin characteristics in biomass straw. Biomass Bioenergy 2013, 57, 264–279. [Google Scholar] [CrossRef]
- García, A.; Spigno, G.; Labidi, J. Antioxidant and biocide behaviour of lignin fractions from apple tree pruning residues. Ind. Crops Prod. 2017, 104, 242–252. [Google Scholar] [CrossRef]
- Xu, X.; Zhou, J.; Nagaraju, D.H.; Jiang, L.; Marinov, V.R.; Lubineau, G.; Alshareef, H.N.; Oh, M. Flexible, highly graphitized carbon aerogels based on bacterial cellulose/lignin: Catalyst-free synthesis and its application in energy storage devices. Adv. Funct. Mater. 2015, 25, 3193–3202. [Google Scholar] [CrossRef]
- Saito, T.; Perkins, J.H.; Vautard, F.; Meyer, H.M.; Messman, J.M.; Tolnai, B.; Naskar, A.K. Methanol fractionation of softwood kraft lignin: Impact on the lignin properties. ChemSusChem 2014, 7, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Hatakeyama, H.; Nakamura, K.; Hatakeyama, T. Studies on factors affecting the molecular motion of lignin and lignin-related polystyrene derivatives. CPPA Trans. Tech. Sect. 1980, 6, 105–110. [Google Scholar]
- Hatakeyama, T.; Hirose, S.; Hatakeyama, H. Differential scanning calorimetric studies on bound water in 1,4-dioxane acidolysis lignin. Macromol. Chem. Phys. 1983, 184, 1265–1274. [Google Scholar] [CrossRef]
- Hatakeyama, H.; Iwashita, K.; Meshitsuka, G.; Nakano, J. Effect of molecular weight on glass transition temperature of lignin. Mokuzai Gakkaishi 1975, 21, 618–623. [Google Scholar]
- Faris, A.H.; Rahim, A.A.; Mohamad Ibrahim, M.N.; Hussin, M.H.; Alkurdi, A.M.; Salehabadi, A. Investigation of oil palm based kraft and auto-catalyzed organosolv lignin susceptibility as a green wood adhesives. Int. J. Adhes. Adhes. 2017, 74, 115–122. [Google Scholar] [CrossRef]
- Heitner, C.; Dimmel, D.R.; Schmidt, J.A. Lignin and Lignans: Advances in Chemistry; Taylor & Francis: Boca Raton, FL, USA, 2010; pp. 305–316. [Google Scholar]
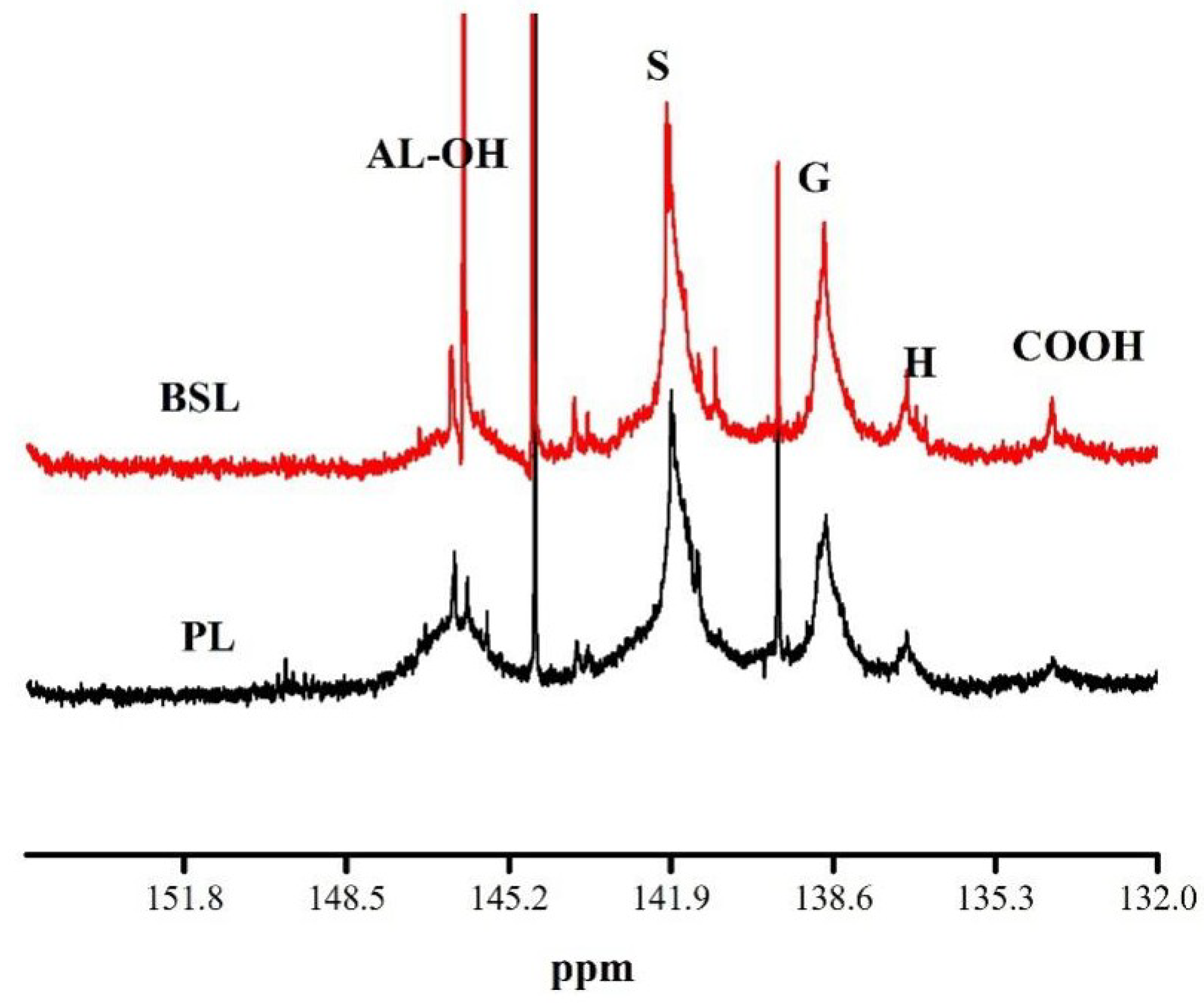


| Samples | Aliphatic OH (mmol/g) | Phenols C5-Substituted (mmol/g) | Phenols Guaiacyl (mmol/g) | Phenols P–Hydroxyphenyl (mmol/g) | Carboxylic Acid OH (mmol/g) | Mw | Mn | PDI |
|---|---|---|---|---|---|---|---|---|
| 145.4–150.0 (ppm) | 140.0–144.5 (ppm) | 139.0–140.2 (ppm) | 137.0~137.8 (ppm) | 133.6–136.0 (ppm) | ||||
| PL | 0.075 | 0.236 | 0.245 | 0.029 | - | 4876 | 1953 | 2.50 |
| BSL | 0.590 | 0.731 | 0.431 | 0.041 | 0.016 | 3342 | 1825 | 1.83 |
| Samples | N (wt %) | C (wt %) | H (wt %) | S (wt %) | C/N | C/H |
|---|---|---|---|---|---|---|
| PL | 0.625 ± 0.02 | 62.725 ± 0.20 | 5.781 ± 0.06 | 0.556 ± 0.02 | 100.005 | 10.851 |
| BSL | 0.365 ± 0.01 | 65.66 ± 0.33 | 6.397 ± 0.08 | 0.379 ± 0.01 | 179.130 | 10.265 |
| A 1:24 | 11.235 ± 0.09 | 58.64 ± 0.25 | 9.647 ± 0.15 | 0.824 ± 0.03 | 5.220 | 6.079 |
| B 1:12 | 9.705 ± 0.10 | 52.06 ± 0.24 | 8.802 ± 0.05 | 1.405 ± 0.02 | 5.365 | 5.913 |
| C 1:8 | 9.775 ± 0.11 | 54.05 ± 0.28 | 9.103 ± 0.10 | 3.022 ± 0.05 | 5.530 | 5.938 |
| Sample | Temperature at 50% Weight Loss (°C) | Onset Temperature (°C) | Char Residue at 600 °C (wt %) | Compression Strength (MPa) | Surface Area (m2/g) | Pore Size (nm) | Gel Yield (wt %) |
|---|---|---|---|---|---|---|---|
| PL | 458.86 | 344.39 | 42.46 | - | - | - | |
| BSL | 363.49 | 360.57 | 27.94 | - | - | - | |
| A | 383.62 | 390.36 | 2.94 | 3.96 ± 0.23 | 17.18 | 2.35 | 93.5 ± 3.9 |
| B | 384.36 | 392.28 | 2.44 | 1.90 ± 0.06 | 13.96 | 2.44 | 86.6 ± 4.8 |
| C | 374.99 | 385.47 | 3.225 | 0.52 ± 0.03 | 19.11 | 2.46 | 80.5 ± 3.4 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, P.; Cheng, Y.; Yu, S.; Lu, J.; Wang, H. Study on the Effect of 1-Butanol Soluble Lignin on Temperature-Sensitive Gel. Polymers 2018, 10, 1109. https://doi.org/10.3390/polym10101109
Jiang P, Cheng Y, Yu S, Lu J, Wang H. Study on the Effect of 1-Butanol Soluble Lignin on Temperature-Sensitive Gel. Polymers. 2018; 10(10):1109. https://doi.org/10.3390/polym10101109
Chicago/Turabian StyleJiang, Pan, Yi Cheng, Sheng Yu, Jie Lu, and Haisong Wang. 2018. "Study on the Effect of 1-Butanol Soluble Lignin on Temperature-Sensitive Gel" Polymers 10, no. 10: 1109. https://doi.org/10.3390/polym10101109
APA StyleJiang, P., Cheng, Y., Yu, S., Lu, J., & Wang, H. (2018). Study on the Effect of 1-Butanol Soluble Lignin on Temperature-Sensitive Gel. Polymers, 10(10), 1109. https://doi.org/10.3390/polym10101109