Fabrication, Characterization, and Cytotoxicity of Thermoplastic Polyurethane/Poly(lactic acid) Material Using Human Adipose Derived Mesenchymal Stromal Stem Cells (hASCs)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Polymer Blends Fabrication
2.2. Biomaterials Characterization
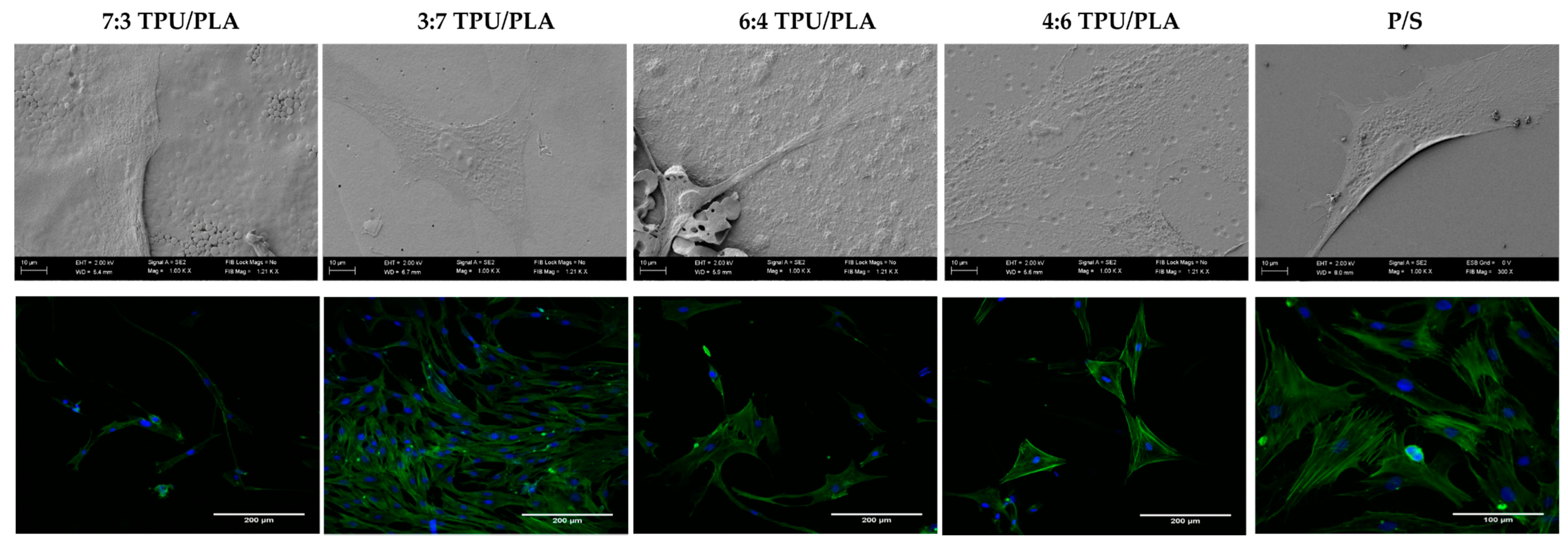
2.2.1. Scanning Electron Microscopy (SEM)
2.2.2. Surface Wettability
2.2.3. Surface Roughness
2.2.4. Mechanical Properties Evaluation
2.2.5. Thermal Degradation Test
2.2.6. Sterilization
2.3. Assessment of Biomaterials Cytocompatibility Using Model of Human Adipose Derived Mesenchymal Stromal Stem Cells (hASCs)
2.3.1. The Characteristics of Human Adipose-derived Stromal Cells (hASCs) Used for the Experiment
2.3.2. The Metabolic Activity and Proliferation of hASCs Cultures on Biomaterials
2.3.3. The Morphology of hASCs Cultures on Biomaterials
2.3.4. The Analysis of Antiapoptotic and Proapoptotic Bcl-2 Proteins
2.4. Statistical Analysis
3. Results
3.1. Fabrication and Microstructure
3.2. Surface Wettability
3.3. Surface Roughness
3.4. Thermal Properties of (TPU+PLA) Blends
3.5. Tensile Test
3.6. The In Vitro Evaluation of Cytotoxicity of Obtained Biomaterial
3.6.1. Proliferation and Metabolic Activity of hASCs in Cultures with Obtained Biomaterials
3.6.2. The Morphology and Growth Pattern of hASCs Cultured on Obtained (TPU+PLA) Films
3.6.3. Evaluation of Bax/Bcl-2 Ratio and Total Bax-α in hASCs Cultured on (TPU+PLA) Blends
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Horch, R.E.; Kneser, U.; Polykandriotis, E.; Schmidt, V.J.; Sun, J.; Arkudas, A. Tissue engineering and regenerative medicine -where do we stand? J. Cell. Mol. Med. 2012, 16, 1157–1165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mao, A.S.; Mooney, D.J. Regenerative medicine: Current therapies and future directions. Proc. Natl. Acad. Sci. USA 2015, 112, 14452–14459. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Link, J.M.; Hu, J.C.Y.; Athanasiou, K.A. The Self-Assembling Process and Applications in Tissue Engineering. Cold Spring Harbor Perspect. Med. 2017. [Google Scholar] [CrossRef] [PubMed]
- Boys, A.J.; McCorry, M.C.; Rodeo, S.; Bonassar, L.J.; Estroff, L.A. Next generation tissue engineering of orthopedic soft tissue-to-bone interfaces. MRS Commun. 2017, 7, 289–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mi, H.-Y.; Palumbo, S.; Jing, X.; Turng, L.-S.; Li, W.-J.; Peng, X.-F. Thermoplastic polyurethane/hydroxyapatite electrospun scaffolds for bone tissue engineering: Effects of polymer properties and particle size. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014, 102, 1434–1444. [Google Scholar] [CrossRef] [PubMed]
- Mi, H.-Y.; Salick, M.R.; Jing, X.; Jacques, B.R.; Crone, W.C.; Peng, X.-F.; Turng, L.-S. Characterization of thermoplastic polyurethane/polylactic acid (TPU/PLA) tissue engineering scaffolds fabricated by microcellular injection molding. Mater. Sci. Eng. C 2013, 33, 4767–4776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Zhang, Y.; Yin, G.; Wang, H.; Dong, Z. Electrospun polylactide/silk fibroin-gelatin composite tubular scaffolds for small-diameter tissue engineering blood vessels. J. Appl. Polym. Sci. 2009, 113, 2675–2682. [Google Scholar] [CrossRef]
- Kornicka, K.; Nawrocka, D.; Lis-Bartos, A.; Marędziak, M.; Marycz, K. Polyurethane–polylactide-based material doped with resveratrol decreases senescence and oxidative stress of adipose-derived mesenchymal stromal stem cell (ASCs). RSC Adv. 2017, 7, 24070–24084. [Google Scholar] [CrossRef] [Green Version]
- Khan, F.; Tanaka, M.; Ahmad, S.R. Fabrication of polymeric biomaterials: A strategy for tissue engineering and medical devices. J. Mater. Chem. B 2015, 3, 8224–8249. [Google Scholar] [CrossRef]
- Konta, A.; García-Piña, M.; Serrano, D. Personalised 3D Printed Medicines: Which Techniques and Polymers Are More Successful? Bioengineering 2017, 4, 79. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Ma, P.X. Synthetic biodegradable functional polymers for tissue engineering: A brief review. Sci. China Chem. 2014, 57, 490–500. [Google Scholar] [CrossRef]
- Dhandayuthapani, B.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Polymeric Scaffolds in Tissue Engineering Application: A Review. Int. J. Polym. Sci. 2011, 2011. [Google Scholar] [CrossRef]
- Yu, F.; Huang, H.-X. Simultaneously toughening and reinforcing poly(lactic acid)/thermoplastic polyurethane blend via enhancing interfacial adhesion by hydrophobic silica nanoparticles. Polym. Test. 2015, 45, 107–113. [Google Scholar] [CrossRef]
- Feng, F.; Ye, L. Morphologies and mechanical properties of polylactide/thermoplastic polyurethane elastomer blends. J. Appl. Polym. Sci. 2011, 119, 2778–2783. [Google Scholar] [CrossRef]
- Navarro, M.; Michiardi, A.; Castano, O.; Planell, J. Biomaterials in orthopaedics. J. R. Soc. Interface 2008, 5, 1137–1158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, F.; Tanaka, M. Designing Smart Biomaterials for Tissue Engineering. Int. J. Mol. Sci. 2017, 19, 17. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Yang, M.; Yu, T.; Ren, T.; Ren, J. Synthesis and characterization of biodegradable lactic acid-based polymers by chain extension. Polym. Int. 2008, 57, 982–986. [Google Scholar] [CrossRef]
- Tabatabaei Qomi, R.; Sheykhhasan, M. Adipose-derived stromal cell in regenerative medicine: A review. World J. Stem Cells 2017, 9, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Locke, M.; Feisst, V.; Meidinger, S. From bench to bedside: Use of human adipose-derived stem cells. Stem Cells Cloning Adv. Appl. 2015, 8, 149. [Google Scholar] [CrossRef] [PubMed]
- Ullah, I.; Subbarao, R.B.; Rho, G.J. Human mesenchymal stem cells–current trends and future prospective. Biosci. Rep. 2015, 35. [Google Scholar] [CrossRef] [PubMed]
- Golonka, P.; Szklarz, M.; Kusz, M.; Marędziak, M.; Houston, J.M.I.; Marycz, K. Subchondral bone cyst surgical treatment using the application of stem progenitor cells combined with alginate hydrogel in small joints in horses. Pol. J. Vet. Sci. 2018, 21, 307–316. [Google Scholar]
- Pak, J.; Lee, J.H.; Park, K.S.; Park, M.; Kang, L.-W.; Lee, S.H. Current use of autologous adipose tissue-derived stromal vascular fraction cells for orthopedic applications. J. Biomed. Sci. 2017, 24, 9. [Google Scholar] [CrossRef] [PubMed]
- Raposio, E.; Bonomini, S.; Calderazzi, F. Isolation of autologous adipose tissue-derived mesenchymal stem cells for bone repair. Orthop. Traumatol. Surg. Res. 2016, 102, 909–912. [Google Scholar] [CrossRef] [PubMed]
- Mohammadzadeh, A.; Pourfathollah, A.A.; Shahrokhi, S.; Hashemi, S.M.; Moradi, S.L.A.; Soleimani, M. Immunomodulatory effects of adipose-derived mesenchymal stem cells on the gene expression of major transcription factors of T cell subsets. Int. Immunopharmacol. 2014, 20, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Marędziak, M.; Marycz, K.; Lewandowski, D.; Siudzińska, A.; Śmieszek, A. Static magnetic field enhances synthesis and secretion of membrane-derived microvesicles (MVs) rich in VEGF and BMP-2 in equine adipose-derived stromal cells (EqASCs)—A new approach in veterinary regenerative medicine. In Vitro Cell. Dev. Biol.–Anim. 2015, 51, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Śmieszek, A.; Szydlarska, J.; Mucha, A.; Chrapiec, M.; Marycz, K. Enhanced cytocompatibility and osteoinductive properties of sol–gel-derived silica/zirconium dioxide coatings by metformin functionalization. J. Biomater. Appl. 2017, 32, 570–586. [Google Scholar] [CrossRef] [PubMed]
- Marycz, K.; Pazik, R.; Zawisza, K.; Wiglusz, K.; Maredziak, M.; Sobierajska, P.; Wiglusz, R.J. Multifunctional nanocrystalline calcium phosphates loaded with Tetracycline antibiotic combined with human adipose derived mesenchymal stromal stem cells (hASCs). Mater. Sci. Eng. C 2016, 69, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Marycz, K.; Krzak, J.; Urbański, W.; Pezowicz, C. In Vitro and In Vivo Evaluation of Sol-Gel Derived TiO2 Coatings Based on a Variety of Precursors and Synthesis Conditions. J. Nanomater. 2014, 2014, 1–14. [Google Scholar] [CrossRef]
- Grzesiak, J.; Marycz, K.; Szarek, D.; Bednarz, P.; Laska, J. Polyurethane/polylactide-based biomaterials combined with rat olfactory bulb-derived glial cells and adipose-derived mesenchymal stromal cells for neural regenerative medicine applications. Mater. Sci. Eng. C 2015, 52, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Marycz, K.; Sobierajska, P.; Smieszek, A.; Maredziak, M.; Wiglusz, K.; Wiglusz, R.J. Li+ activated nanohydroxyapatite doped with Eu3+ ions enhances proliferative activity and viability of human stem progenitor cells of adipose tissue and olfactory ensheathing cells. Further perspective of nHAP:Li+, Eu3+ application in theranostics. Mater. Sci. Eng. C 2017, 78, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Kornicka, K.; Marycz, K.; Tomaszewski, K.A.; Marędziak, M.; Śmieszek, A. The Effect of Age on Osteogenic and Adipogenic Differentiation Potential of Human Adipose Derived Stromal Stem Cells (hASCs) and the Impact of Stress Factors in the Course of the Differentiation Process. Oxid. Med. Cell. Longev. 2015, 2015, 309169. [Google Scholar] [CrossRef] [PubMed]
- Mirsaidi, A.; Genelin, K.; Vetsch, J.R.; Stanger, S.; Theiss, F.; Lindtner, R.A.; von Rechenberg, B.; Blauth, M.; Müller, R.; Kuhn, G.A.; et al. Therapeutic potential of adipose-derived stromal cells in age-related osteoporosis. Biomaterials 2014, 35, 7326–7335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kornicka, K.; Walczak, R.; Mucha, A.; Marycz, K. Released from ZrO2/SiO2 coating resveratrol inhibits senescence and oxidative stress of human adipose-derived stem cells (ASC). Open Chem. 2018, 16, 481–495. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Zimoch-Korzycka, A.; Śmieszek, A.; Jarmoluk, A.; Nowak, U.; Marycz, K. Potential Biomedical Application of Enzymatically Treated Alginate/Chitosan Hydrosols in Sponges—Biocompatible Scaffolds Inducing Chondrogenic Differentiation of Human Adipose Derived Multipotent Stromal Cells. Polymers 2016, 8, 320. [Google Scholar] [CrossRef]
- Marycz, K.; Mierzejewska, K.; Śmieszek, A.; Suszynska, E.; Malicka, I.; Kucia, M.; Ratajczak, M.Z. Endurance Exercise Mobilizes Developmentally Early Stem Cells into Peripheral Blood and Increases Their Number in Bone Marrow: Implications for Tissue Regeneration. Stem Cells Int. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Kalinski, K.; Marycz, K.; Czogala, J.; Serwa, E.; Janeczek, W. An application of scanning electron microscopy combined with roentgen microanalysis (SEM-EDS) in canine urolithiasis. J. Electron Microsc. 2012, 61, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Witek-Krowiak, A.; Podstawczyk, D.; Chojnacka, K.; Dawiec, A.; Marycz, K. Modelling and optimization of chromiumIII biosorption on soybean meal. Open Chem. 2013, 11, 1505–1517. [Google Scholar] [CrossRef] [Green Version]
- Chomzynski, P.; Sacchi, N. Single-Step Method of RNA Isolation by Acid Guanidinium Thiocyanate–Phenol–Chloroform Extraction. Anal. Biochem. 1987, 162, 156–159. [Google Scholar] [CrossRef] [PubMed]
- Śmieszek, A.; Giezek, E.; Chrapiec, M.; Murat, M.; Mucha, A.; Michalak, I.; Marycz, K. The Influence of Spirulina platensis Filtrates on Caco-2 Proliferative Activity and Expression of Apoptosis-Related microRNAs and mRNA. Mar. Drugs 2017, 15, 65. [Google Scholar] [CrossRef] [PubMed]
- Suszynska, M.; Poniewierska-Baran, A.; Gunjal, P.; Ratajczak, J.; Marycz, K.; Kakar, S.S.; Kucia, M.; Ratajczak, M.Z. Expression of the erythropoietin receptor by germline-derived cells–further support for a potential developmental link between the germline and hematopoiesis. J. Ovarian Res. 2014, 7, 66. [Google Scholar] [CrossRef] [PubMed]
- Marycz, K.; Maredziak, M.; Grzesiak, J.; Lis, A.; Smieszek, A. Biphasic Polyurethane/Polylactide Sponges Doped with Nano-Hydroxyapatite (nHAp) Combined with Human Adipose-Derived Mesenchymal Stromal Stem Cells for Regenerative Medicine Applications. Polymers 2016, 8, 339. [Google Scholar] [CrossRef]
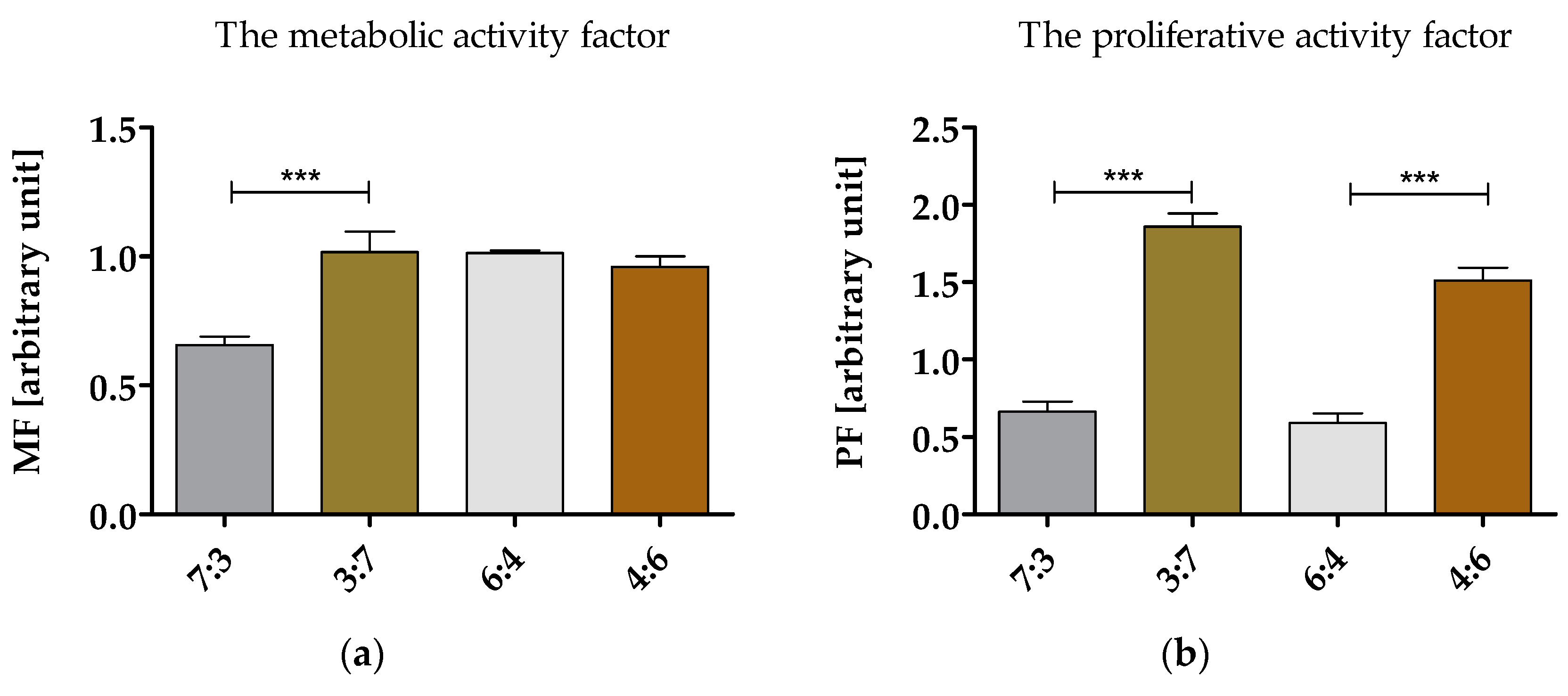
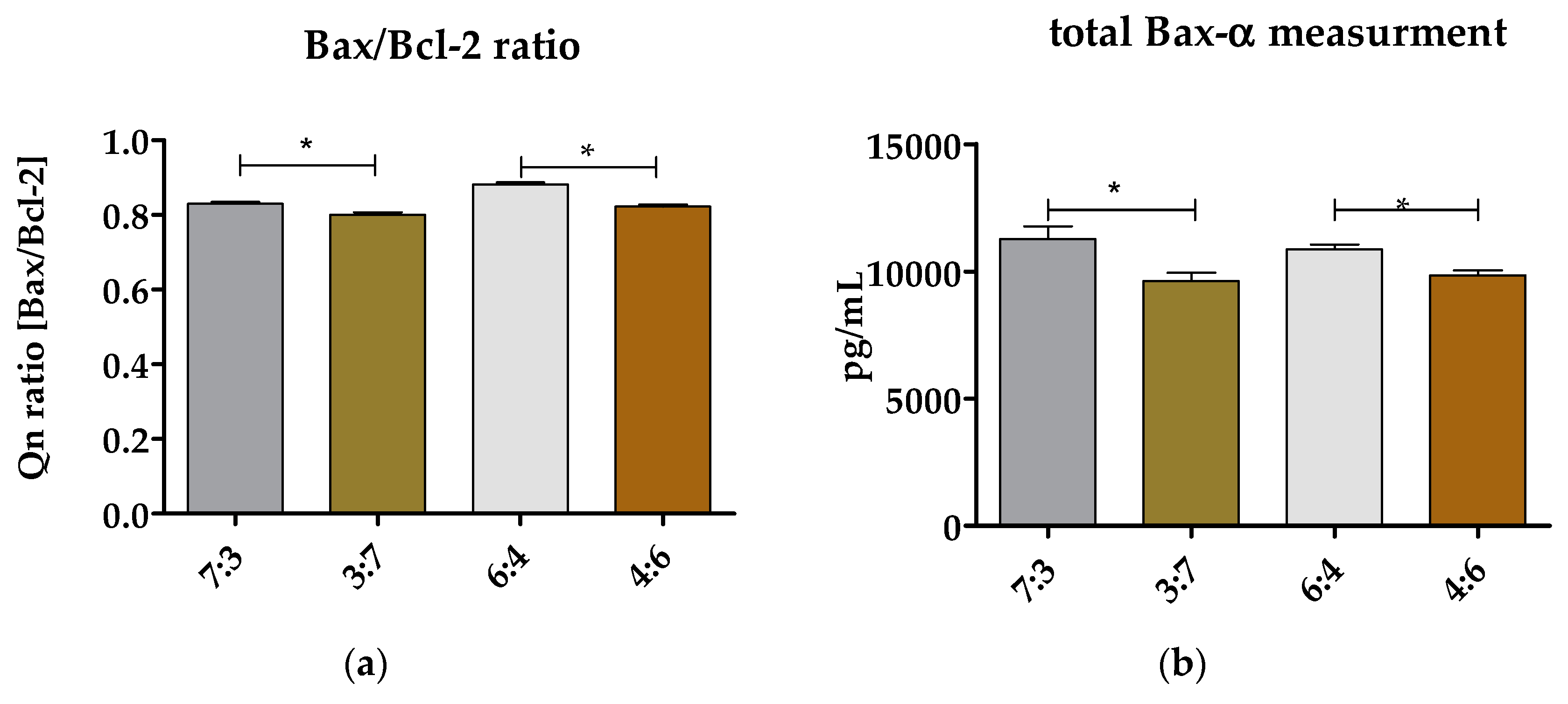
| TPU 100% (control) | (TPU+PLA) 7:3 | (TPU+PLA) 6:4 |
 |  |  |
| (TPU+PLA) 4:6 | (TPU+PLA) 3:7 | PLA 100% (control) |
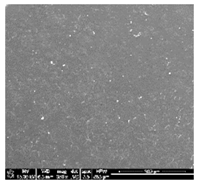 | 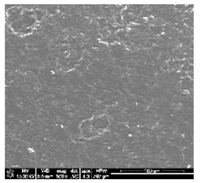 | 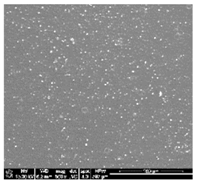 |
| TPU 100% (control) | (TPU+PLA) 7:3 | (TPU+PLA) 6:4 |
 |  |  |
| θ = 123.2 ± 1.8 [°] | θ = 118.9 ± 2.3 [°] | θ = 96.3 ± 1.3 [°] |
| Ra = 72.1 ± 1.2 [µm] | Ra = 86.1 ± 1.7 [µm] | Ra = 83.1 ± 2.1 [µm] |
| (TPU+PLA) 4:6 | (TPU+PLA) 3:7 | PLA 100% (control) |
 |  |  |
| θ = 87.7 ± 1.8 [°] | θ = 86.9 ± 2.6 [°] | θ = 79.9 ± 1.1 [°] |
| Ra = 97.4 ± 1.9 [µm] | Ra = 102.5 ± 1.6 [µm] | Ra = 115.8 ± 2.3 [µm] |
| Polymer Composition | Tensile Young’s Modulus for Thin Films [MPa] |
|---|---|
| TPU 100% (control) | 29.6 ± 0.6 |
| (TPU+PLA) 7:3 | 84.5 ± 0.5 |
| (TPU+PLA) 6:4 | 162.4 ± 1.1 |
| (TPU+PLA) 4:6 | 215.6 ± 1.9 |
| (TPU+PLA) 3:7 | 237.0 ± 3.2 |
| PLA 100% (control) | 3.44 ± 2.5 [GPa] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lis-Bartos, A.; Smieszek, A.; Frańczyk, K.; Marycz, K. Fabrication, Characterization, and Cytotoxicity of Thermoplastic Polyurethane/Poly(lactic acid) Material Using Human Adipose Derived Mesenchymal Stromal Stem Cells (hASCs). Polymers 2018, 10, 1073. https://doi.org/10.3390/polym10101073
Lis-Bartos A, Smieszek A, Frańczyk K, Marycz K. Fabrication, Characterization, and Cytotoxicity of Thermoplastic Polyurethane/Poly(lactic acid) Material Using Human Adipose Derived Mesenchymal Stromal Stem Cells (hASCs). Polymers. 2018; 10(10):1073. https://doi.org/10.3390/polym10101073
Chicago/Turabian StyleLis-Bartos, Anna, Agnieszka Smieszek, Kinga Frańczyk, and Krzysztof Marycz. 2018. "Fabrication, Characterization, and Cytotoxicity of Thermoplastic Polyurethane/Poly(lactic acid) Material Using Human Adipose Derived Mesenchymal Stromal Stem Cells (hASCs)" Polymers 10, no. 10: 1073. https://doi.org/10.3390/polym10101073





