The Effect of Reactive Ionic Liquid or Plasticizer Incorporation on the Physicochemical and Transport Properties of Cellulose Acetate Propionate-Based Membranes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Studied Materials
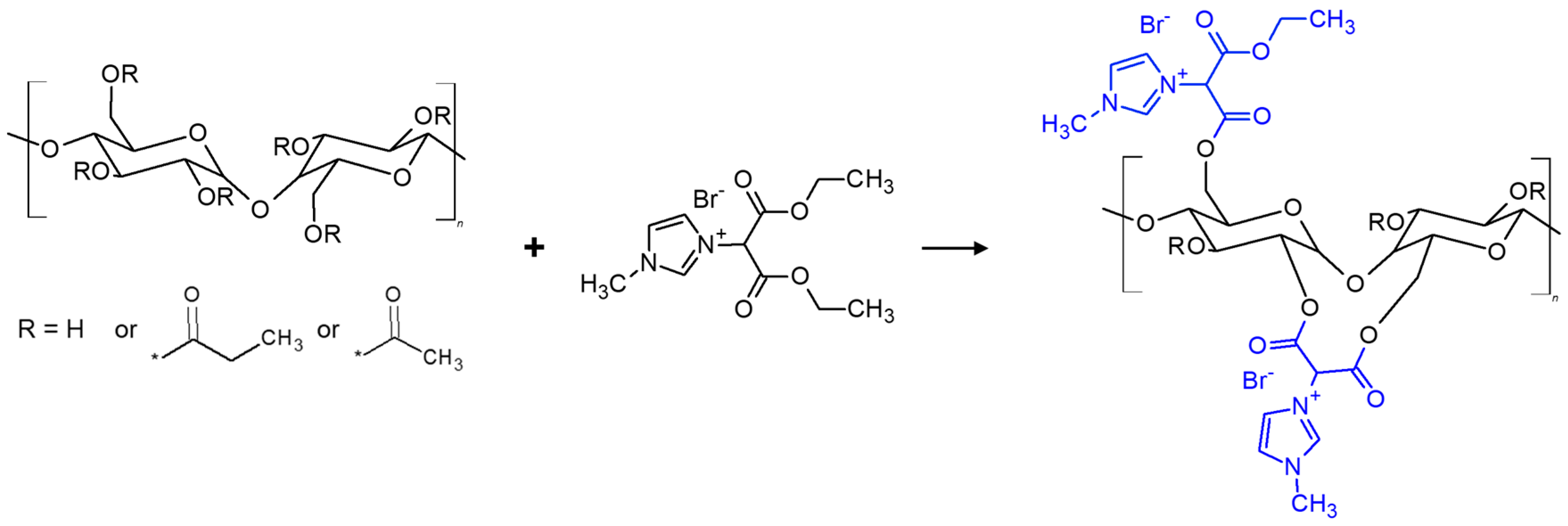
2.2. Synthesis of Reactive Ionic Liquid (RIL)
2.3. Preparation of Cellulose Acetate Propionate-Based Membranes Containing RILs and Plasticizers
2.4. Characterization of Membranes
2.5. Swelling Measurements
2.6. Pervaporation
3. Results and Discussion
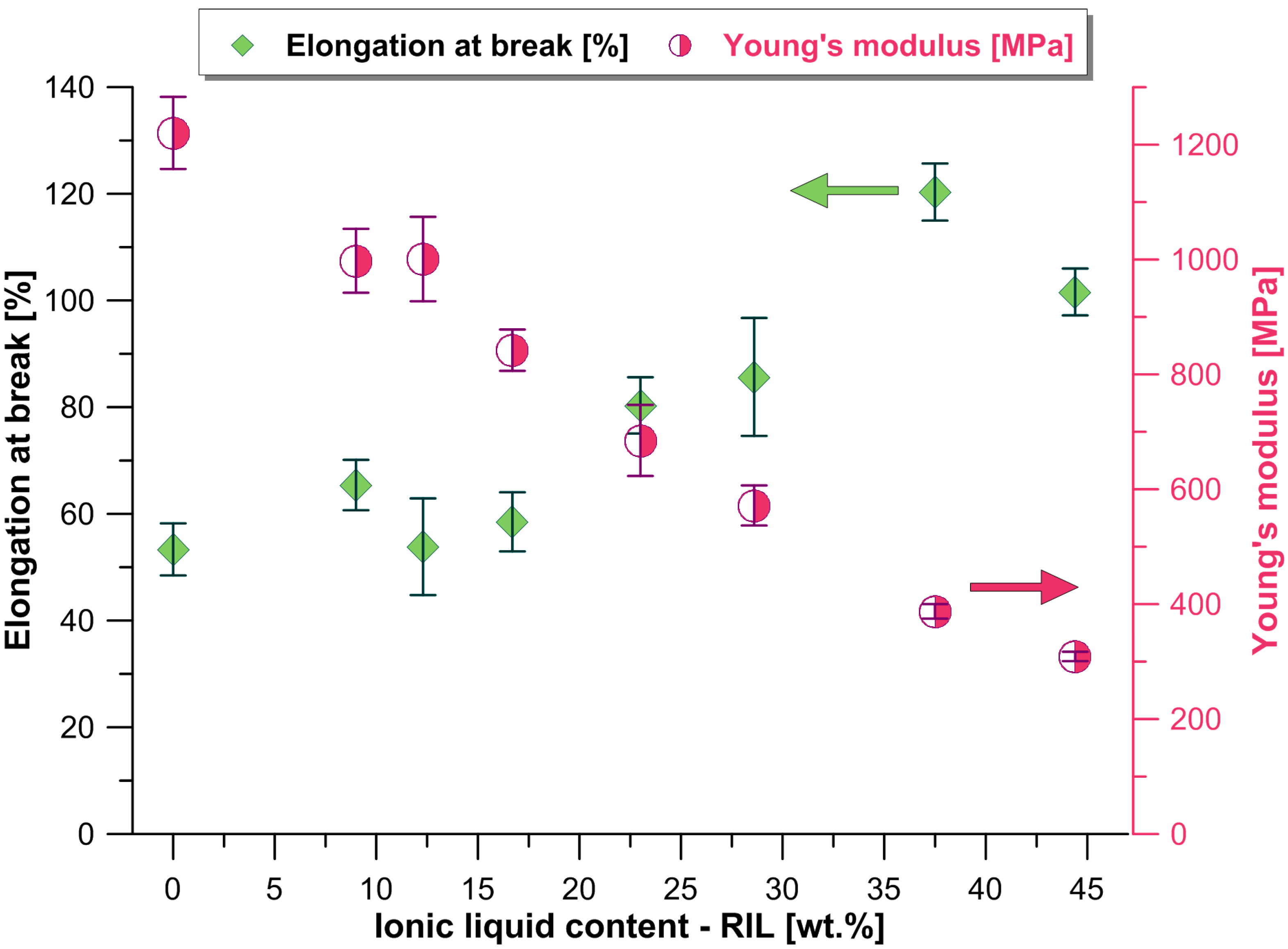
3.1. Mechanical Properties
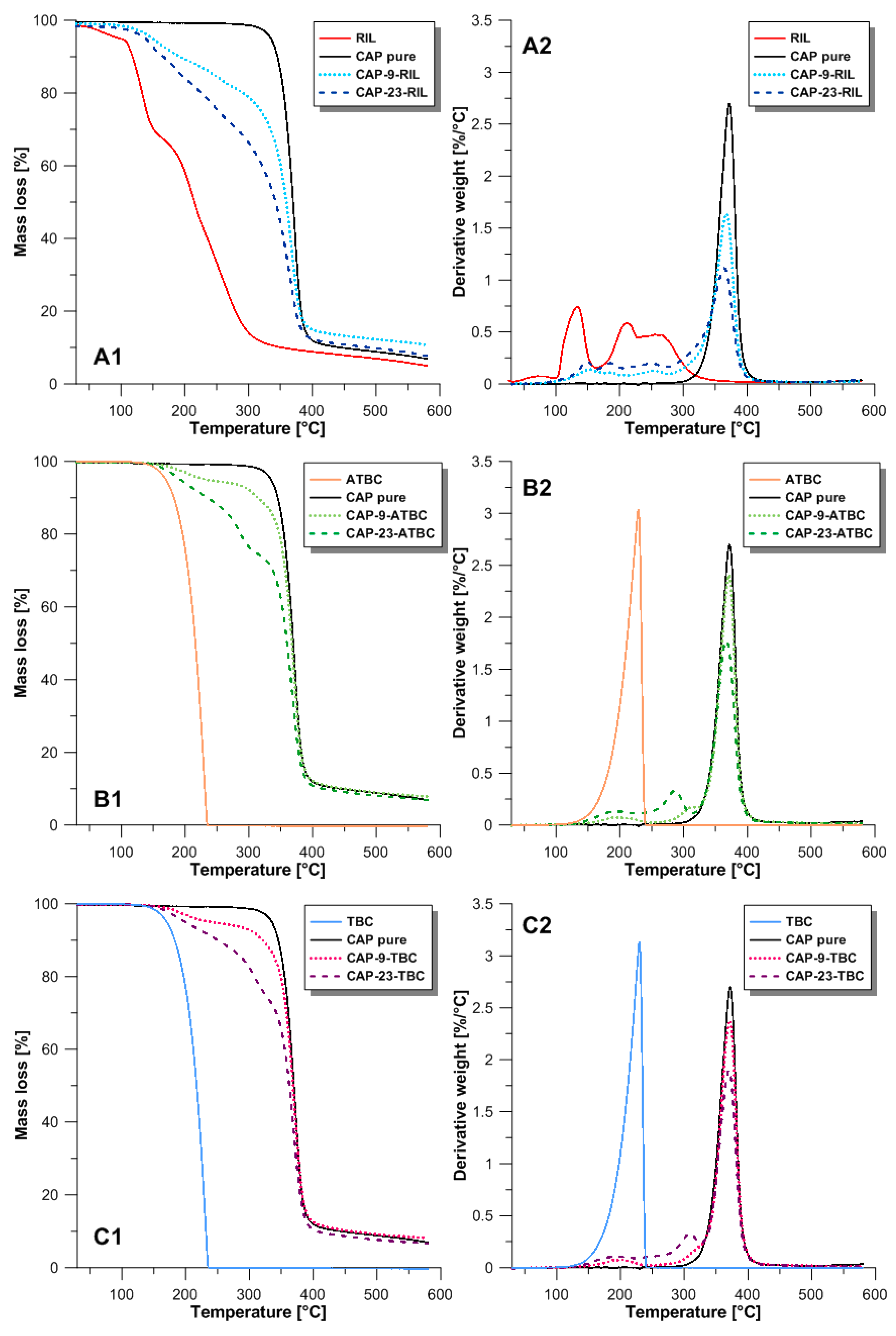
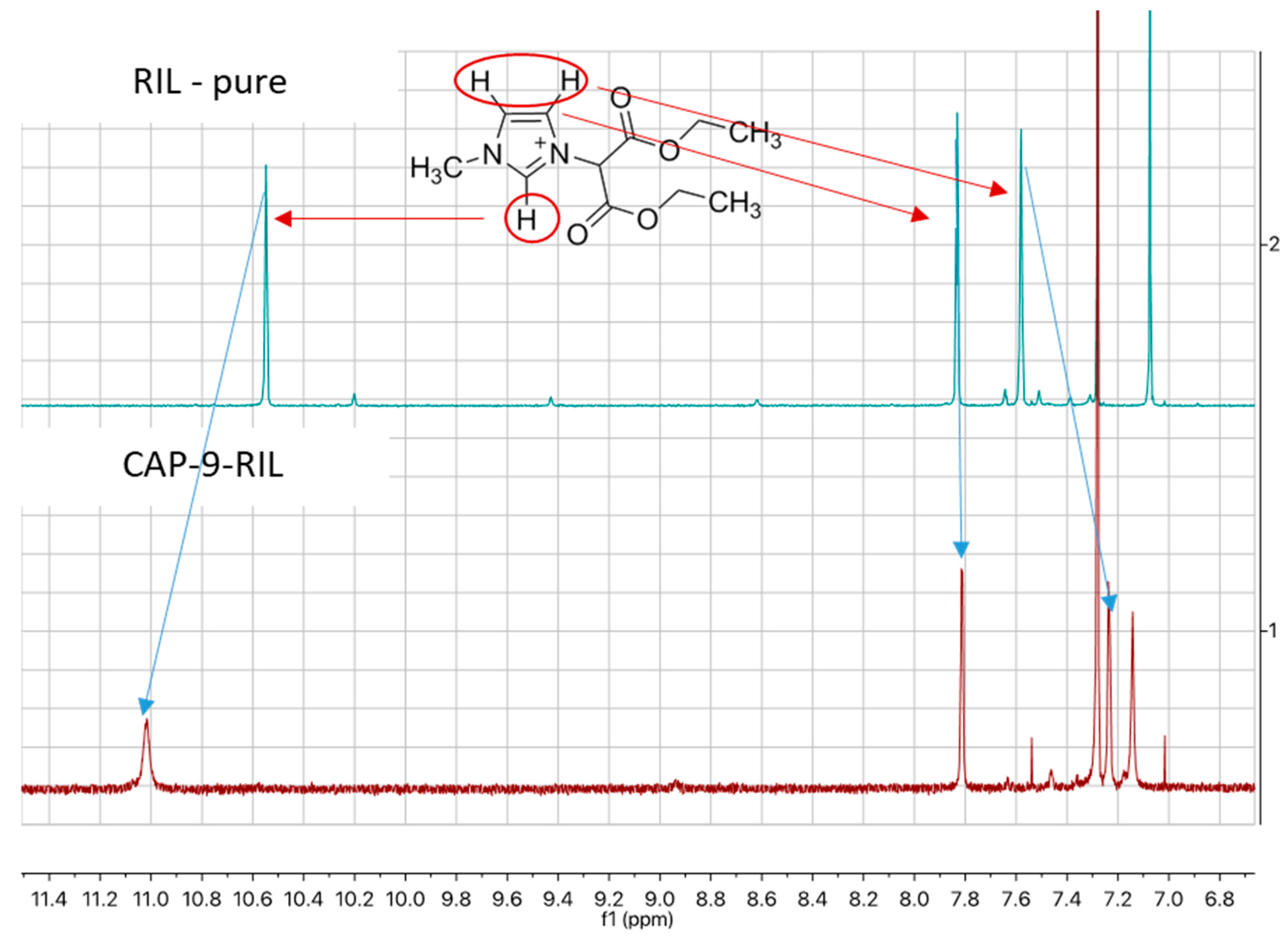
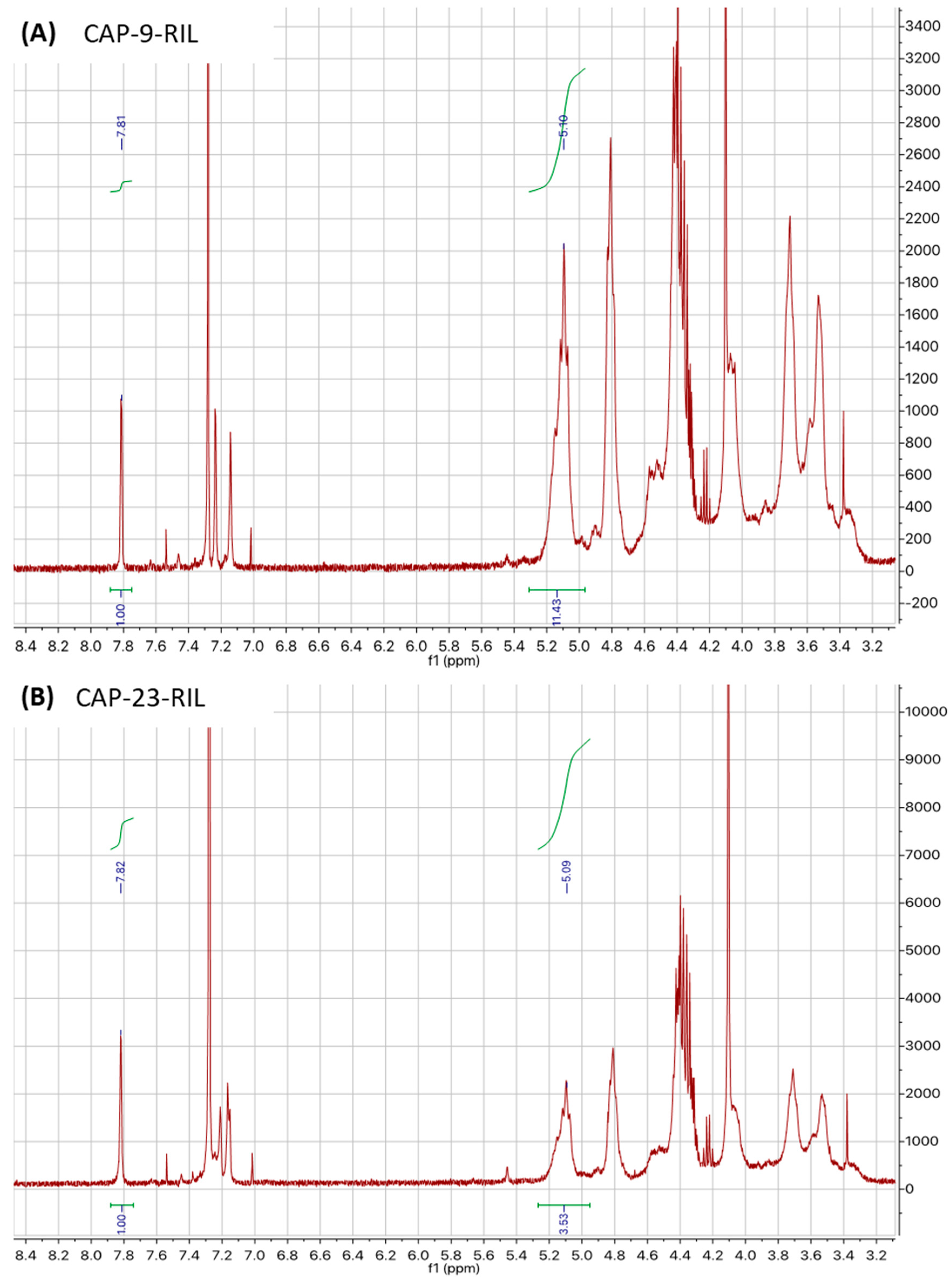
3.2. Thermal Properties
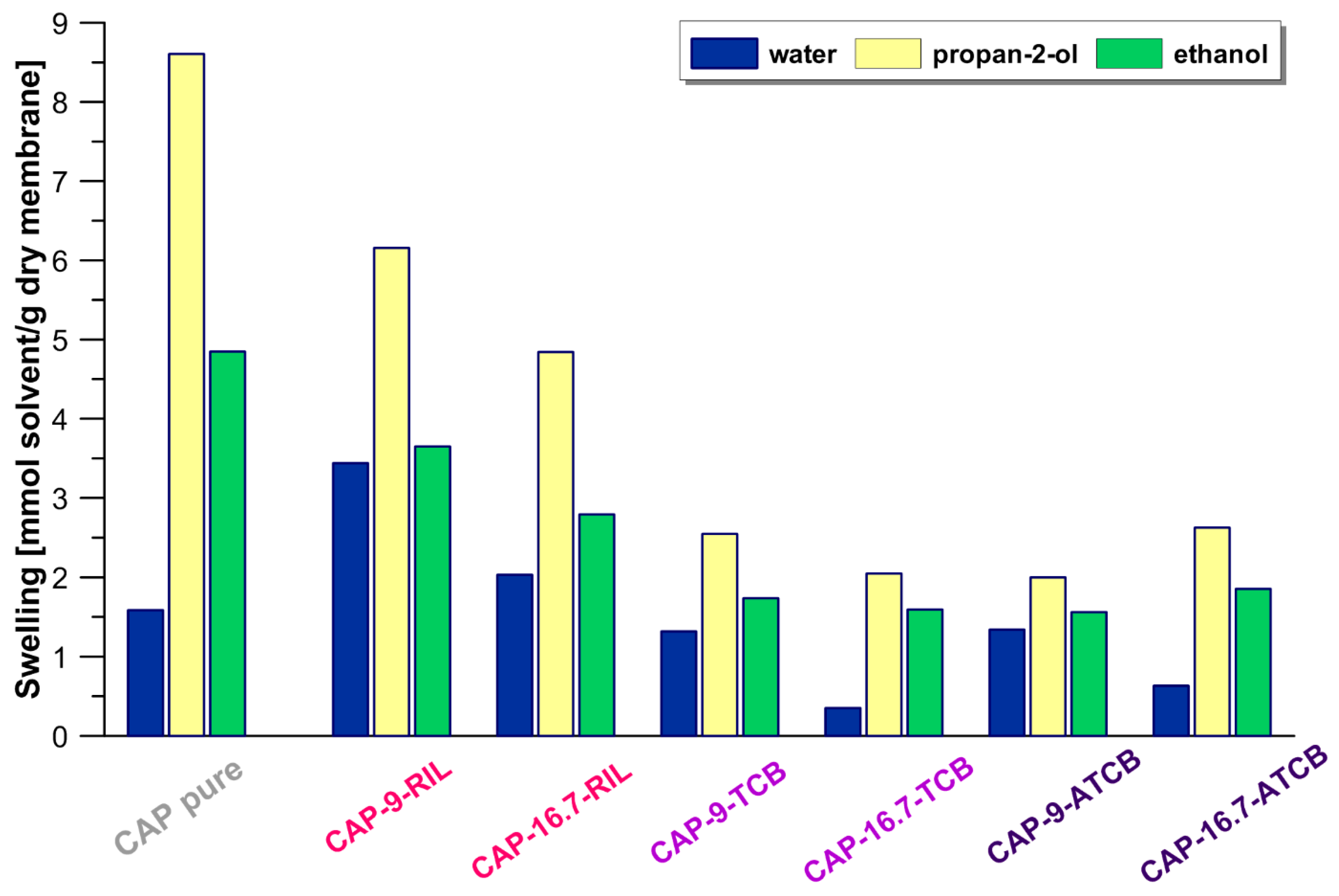
3.3. Equilibrium Properties
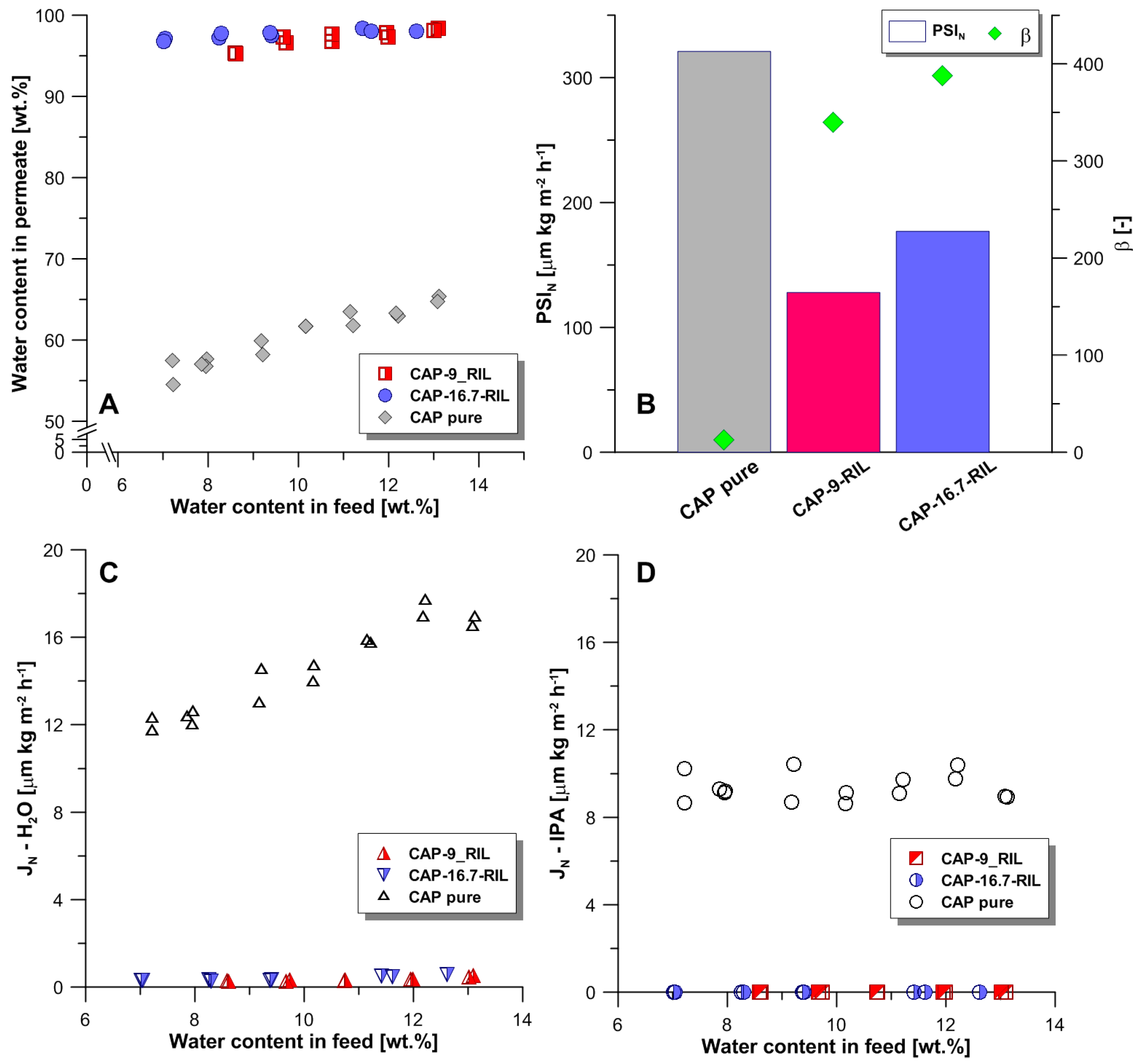
3.4. Transport and Separation Properties in Pervaporation
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Moreau, C.; Villares, A.; Capron, I.; Cathala, B. Tuning supramolecular interactions of cellulose nanocrystals to design innovative functional materials. Ind. Crop. Prod. 2016, 93, 96–107. [Google Scholar] [CrossRef]
- Wang, S.; Lu, A.; Zhang, L. Recent advances in regenerated cellulose materials. Prog. Polym. Sci. 2016, 53, 169–206. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, J.; He, J.; Li, H.; Zhang, Y. Homogeneous Acetylation of Cellulose at Relatively High Concentrations in an Ionic Liquid. Chin. J. Chem. Eng. 2010, 18, 515–522. [Google Scholar] [CrossRef]
- Kosan, B.; Dorn, S.; Meister, F.; Heinze, T. Preparation and Subsequent Shaping of Cellulose Acetates Using Ionic Liquids. Macromol. Mater. Eng. 2010, 295, 676–681. [Google Scholar] [CrossRef]
- Liebert, T.; Heinze, T. Interaction of Ionic Liquids with Polysaccharides. 5. Solvents and Reaction Media for the Modification of Cellulose. Bioresources 2008, 3, 576–601. [Google Scholar]
- Kadokawa, J.-I. Preparation of Polysaccharide-Based Materials Compatibilized with Ionic Liquids. In Ionic Liquids: Applications and Perspectives; Kokorin, A., Ed.; InTech: Rijeka, Croatia, 2011. [Google Scholar]
- Isik, M.; Sardon, H.; Mecerreyes, D. Ionic Liquids and Cellulose: Dissolution, Chemical Modification and Preparation of New Cellulosic Materials. Int. J. Mol. Sci. 2014, 15, 11922–11940. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-M.; Misra, M.; Drzal, L.T.; Mohanty, A.K. “Green” Nanocomposites from Cellulose Acetate Bioplastic and Clay: Effect of Eco-Friendly Triethyl Citrate Plasticizer. Biomacromolecules 2004, 5, 2281–2288. [Google Scholar] [CrossRef] [PubMed]
- Schilling, M.; Bouchard, M.; Khanjian, H.; Learner, T.; Phenix, A.; Rivenc, R. Application of Chemical and Thermal Analysis Methods for Studying Cellulose Ester Plastics. Acc. Chem. Res. 2010, 43, 888–896. [Google Scholar] [CrossRef] [PubMed]
- Hassan Hassan Abdellatif, F.; Babin, J.; Arnal-Herault, C.; David, L.; Jonquieres, A. Grafting of cellulose acetate with ionic liquids for biofuel purification by a membrane process: Influence of the cation. Carbohydr. Polym. 2016, 147, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Landry, C.J.T.; Lum, K.K.; O’Reilly, J.M. Physical aging of blends of cellulose acetate polymers with dyes and plasticizers. Polymer 2001, 42, 5781–5792. [Google Scholar] [CrossRef]
- Huang, K.L.; Wang, B.; Cao, Y.; Li, H.Q.; Wang, J.S.; Lin, W.J.; Mu, C.S.; Liao, D.K. Homogeneous Preparation of Cellulose Acetate Propionate (CAP) and Cellulose Acetate Butyrate (CAB) from Sugarcane Bagasse Cellulose in Ionic Liquid. J. Agric. Food Chem. 2011, 59, 5376–5381. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.G.; Weissberg, S.G. Film-Forming Properties of Cellulose Acetate Propionates. Effects of Solvents, Diluents, and Plasticizers. Ind. Eng. Chem. 1951, 43, 2088–2098. [Google Scholar] [CrossRef]
- Buchanan, C.M.; Gedon, S.C.; White, A.W.; Wood, M.D. Cellulose acetate propionate and poly(tetramethylene glutarate) blends. Macromolecules 1993, 26, 2963–2967. [Google Scholar] [CrossRef]
- Wojciechowska, P. The Effect of Concentration and Type of Plasticizer on the Mechanical Properties of Cellulose Acetate Butyrate Organic-Inorganic Hybrids. In Recent Advances in Plasticisers; Luqman, D.M., Ed.; InTech: Rijeka, Croatia, 2012; pp. 141–164. [Google Scholar]
- Li, X.; Wang, K.Y.; Helmer, B.; Chung, T.S. Thin-Film Composite Membranes and Formation Mechanism of Thin-Film Layers on Hydrophilic Cellulose Acetate Propionate Substrates for Forward Osmosis Processes. Ind. Eng. Chem. Res. 2012, 51, 10039–10050. [Google Scholar] [CrossRef]
- Mekonnen, T.; Mussone, P.; Khalil, H.; Bressler, D. Progress in bio-based plastics and plasticizing modifications. J. Mater. Chem. A 2013, 1, 13379–13398. [Google Scholar] [CrossRef]
- Ning, W.; Xingxiang, Z.; Haihui, L.; Benqiao, H. 1-Allyl-3-methylimidazolium chloride plasticized-corn starch as solid biopolymer electrolytes. Carbohydr. Polym. 2009, 76, 482–484. [Google Scholar] [CrossRef]
- Liu, P.; Guo, X.; Nan, F.; Duan, Y.; Zhang, J. Modifying Mechanical, Optical Properties and Thermal Processability of Iridescent Cellulose Nanocrystal Films Using Ionic Liquid. ACS Appl. Mater. Interfaces 2017, 9, 3085–3092. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Endo, T. Confinement of Ionic Liquid by Networked Polymers Based on Multifunctional Epoxy Resins. Macromolecules 2008, 41, 6981–6986. [Google Scholar] [CrossRef]
- Jafari, M.; Bayat, A.; Mohammadi, T.; Kazemimoghadam, M. Dehydration of ethylene glycol by pervaporation using gamma alumina/NaA zeolite composite membrane. Chem. Eng. Res. Des. 2013, 91, 2412–2419. [Google Scholar] [CrossRef]
- Schmidt, C.; Glück, T.; Schmidt-Naake, G. Modification of Nafion Membranes by Impregnation with Ionic Liquids. Chem. Eng. Technol. 2008, 31, 13–22. [Google Scholar] [CrossRef]
- Rahman, M.; Brazel, C.S. Ionic liquids: New generation stable plasticizers for poly(vinyl chloride). Polym. Degrad. Stab. 2006, 91, 3371–3382. [Google Scholar] [CrossRef]
- Rynkowska, E.; Fatyeyeva, K.; Kujawski, W. Application of polymer-based membranes containing ionic liquids in membrane separation processes: A critical review. Rev. Chem. Eng. 2017. [Google Scholar] [CrossRef]
- Mohsen-Nia, M.; Amiri, H.; Jazi, B. Dielectric Constants of Water, Methanol, Ethanol, Butanol and Acetone: Measurement and Computational Study. J. Solut. Chem. 2010, 39, 701–708. [Google Scholar] [CrossRef]
- Mulder, M. Basic Principles of Membrane Technology, 2nd ed.; Springer Science & Business Media: Berlin, Germany, 1995. [Google Scholar]
- Albo, J.; Wang, J.; Tsuru, T. Gas transport properties of interfacially polymerized polyamide composite membranes under different pre-treatments and temperatures. J. Membr. Sci. 2014, 449, 109–118. [Google Scholar] [CrossRef]
- Albo, J.; Hagiwara, H.; Yanagishita, H.; Ito, K.; Tsuru, T. Structural Characterization of Thin-Film Polyamide Reverse Osmosis Membranes. Ind. Eng. Chem. Res. 2014, 53, 1442–1451. [Google Scholar] [CrossRef]
- Albo, J.; Wang, J.; Tsuru, T. Application of interfacially polymerized polyamide composite membranes to isopropanol dehydration: Effect of membrane pre-treatment and temperature. J. Membr. Sci. 2014, 453, 384–393. [Google Scholar] [CrossRef]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Rynkowska, E.; Kujawa, J.; Chappey, C.; Fatyeyeva, K.; Karpenko-Jereb, L.; Kelterer, A.-M.; Marais, S.; Kujawski, W. Effect of the polar–nonpolar liquid mixtures on pervaporative behavior of perfluorinated sulfonic membranes in lithium form. J. Membr. Sci. 2016, 518, 313–327. [Google Scholar] [CrossRef]
- Rynkowska, E.; Dzieszkowski, K.; Lancien, A.; Fatyeyeva, K.; Szymczyk, A.; Kujawa, J.; Koter, S.; Marais, S.; Wolan, A.; Kujawski, W. Physicochemical properties and pervaporation performance of dense membranes based on cellulose acetate propionate (CAP) and containing polymerizable ionic liquid (PIL). J. Membr. Sci. 2017, 544, 243–251. [Google Scholar] [CrossRef]
- Kujawska, A.; Knozowska, K.; Kujawa, J.; Kujawski, W. Influence of downstream pressure on pervaporation properties of PDMS and POMS based membranes. Sep. Purif. Technol. 2016, 159, 68–80. [Google Scholar] [CrossRef]
- Baker, R.W.; Wijmans, J.G.; Huang, Y. Permeability, permeance and selectivity: A preferred way of reporting pervaporation performance data. J. Membr. Sci. 2010, 348, 346–352. [Google Scholar] [CrossRef]
- Kujawski, J.; Rozicka, A.; Bryjak, M.; Kujawski, W. Pervaporative removal of acetone, butanol and ethanol from binary and multicomponent aqueous mixtures. Sep. Purif. Technol. 2014, 132, 422–429. [Google Scholar] [CrossRef]
- Chen, J.; Guo, Q.; Li, D.; Tong, J.; Li, X. Properties improvement of SPEEK based proton exchange membranes by doping of ionic liquids and Y2O3. Prog. Nat. Sci. 2012, 22, 26–30. [Google Scholar] [CrossRef]
- Shuto, Y.; Taniguchi, H. Cellulose Acetate Propionate. U.S. Patent 5,977,347, 2 November 1999. [Google Scholar]
- Brydson, J.A. Plastics Materials, 7th ed.; Butterworth-Heinemann: Oxford, UK, 1999. [Google Scholar]
- Bastos, M.D.S.R.; Laurentino, L.D.S.; Canuto, K.M.; Mendes, L.G.; Martins, C.M.; Silva, S.M.F.; Furtado, R.F.; Kim, S.; Biswas, A.; Cheng, H.N. Physical and mechanical testing of essential oil-embedded cellulose ester films. Polym. Test. 2016, 49, 156–161. [Google Scholar] [CrossRef]
- Manaf, M.E.A.; Nitta, K.-H.; Yamaguchi, M. Mechanical properties of plasticized cellulose ester films at room and high temperatures. J. Eng. Appl. Sci. 2016, 11, 2354–2358. [Google Scholar]
- White, A.W.; Buschanan, C.M.; Pearcy, B.G.; Wood, M.D. Mechanical properties of cellulose acetate propionate/aliphatic polyester blends. J. Appl. Polym. Sci. 1994, 52, 525–530. [Google Scholar] [CrossRef]
- Weber, R.L.; Ye, Y.; Banik, S.M.; Elabd, Y.A.; Hickner, M.A.; Mahanthappa, M.K. Thermal and ion transport properties of hydrophilic and hydrophobic polymerized styrenic imidazolium ionic liquids. J. Polym. Sci. Part B Polym. Phys. 2011, 49, 1287–1296. [Google Scholar] [CrossRef]
- Gui, H.; Li, Y.; Chen, S.; Xu, P.; Zheng, B.; Ding, Y. Effects of biodegradable imidazolium-based ionic liquid with ester group on the structure and properties of PLLA. Macromol. Res. 2014, 22, 583–591. [Google Scholar] [CrossRef]
- Ohtani, H.; Ishimura, S.; Kumai, M. Thermal Decomposition Behaviors of Imidazolium-type Ionic Liquids Studied by Pyrolysis-Gas Chromatography. Anal. Sci. 2008, 24, 1335–1340. [Google Scholar] [CrossRef] [PubMed]
- Dantas, P.A.; Botaro, V.R. Synthesis and Characterization of a New Cellulose Acetate-Propionate Gel: Crosslinking Density Determination. Open J. Polym. Chem. 2012, 2, 144–151. [Google Scholar] [CrossRef]
- Maiza, M.; Benaniba, M.T.; Quintard, G.; Massardier-Nageotte, V. Biobased additive plasticizing Polylactic acid (PLA). Polimeros 2015, 25, 581–590. [Google Scholar] [CrossRef]
- Sanz, M.T.; Gmehling, J. Study of the Dehydration of Isopropanol by a Pervaporation-Based Hybrid Process. Chem. Eng. Technol. 2006, 29, 473–480. [Google Scholar] [CrossRef]
- Carvalho, E.S.; Sánchez, R.J.; Tavares, M.I.B.; Lamônica, Á.C. Characterization and Properties of Hydrophilic Cellulose Acetate Propionate Derivative. J. Polym. Environ. 2010, 18, 661–667. [Google Scholar] [CrossRef]
- Pereira, M.M.; Kurnia, K.A.; Sousa, F.L.; Silva, N.J.O.; Lopes-da-Silva, J.A.; Coutinhoa, J.A.P.; Freire, M.G. Contact angles and wettability of ionic liquids on polar and non-polar surfaces. Phys. Chem. Chem. Phys. 2015, 17, 31653–31661. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.-S.; Rick, J.; Hwang, B.-J. Ionic liquid polymer electrolytes. J. Mater. Chem. A 2013, 1, 2719–2743. [Google Scholar] [CrossRef]
- Mecerreyes, D. Polymeric ionic liquids: Broadening the properties and applications of polyelectrolytes. Prog. Polym. Sci. 2011, 36, 1629–1648. [Google Scholar] [CrossRef]
- Anjali Devi, D.; Smitha, B.; Sridhar, S.; Aminabhavi, T.M. Pervaporation separation of isopropanol/water mixtures through crosslinked chitosan membranes. J. Membr. Sci. 2005, 262, 91–99. [Google Scholar] [CrossRef]
- Ong, Y.T.; Tan, S.H. Pervaporation separation of a ternary azeotrope containing ethyl acetate, ethanol and water using a buckypaper supported ionic liquid membrane. Chem. Eng. Res. Des. 2016, 109, 116–126. [Google Scholar] [CrossRef]
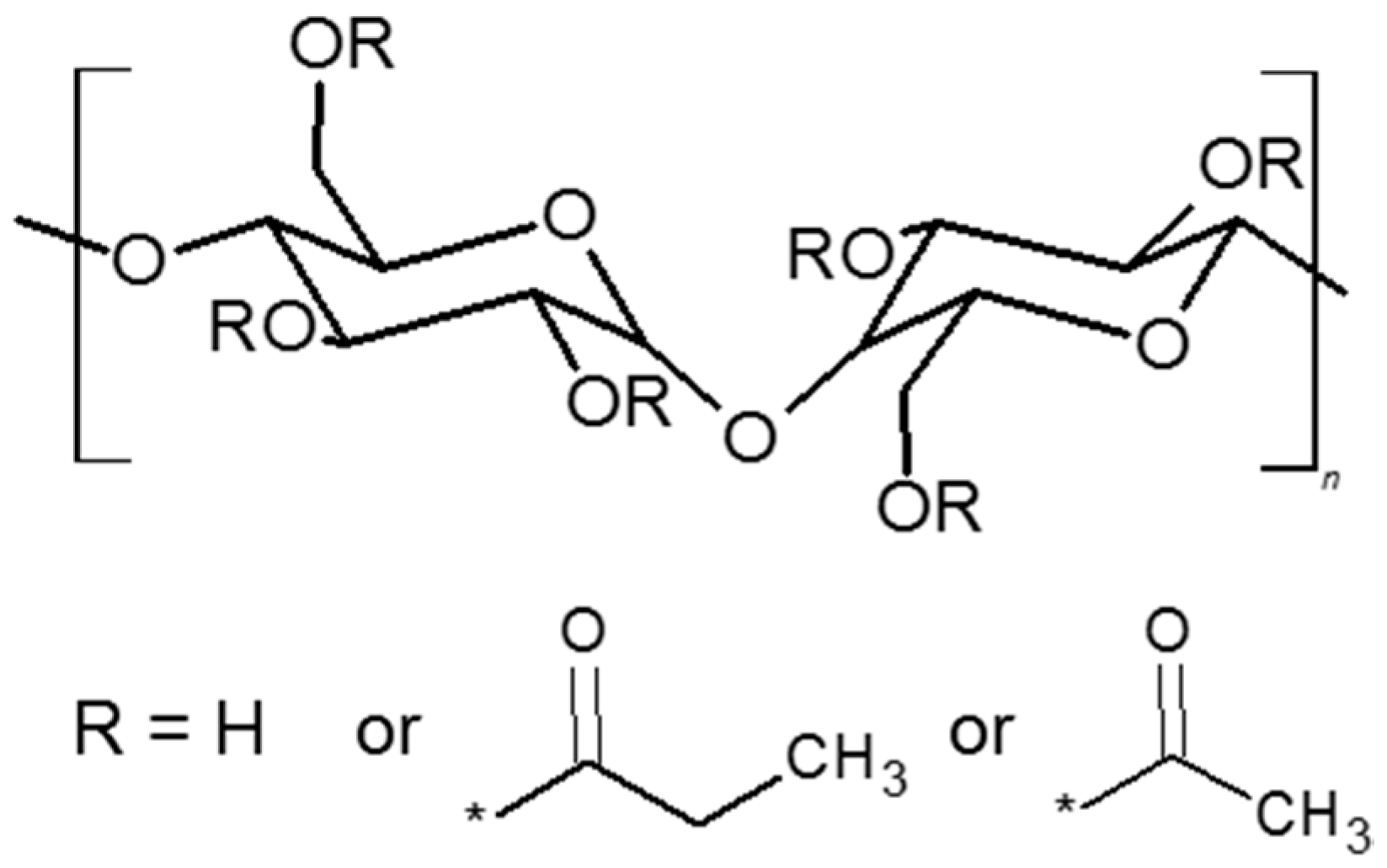
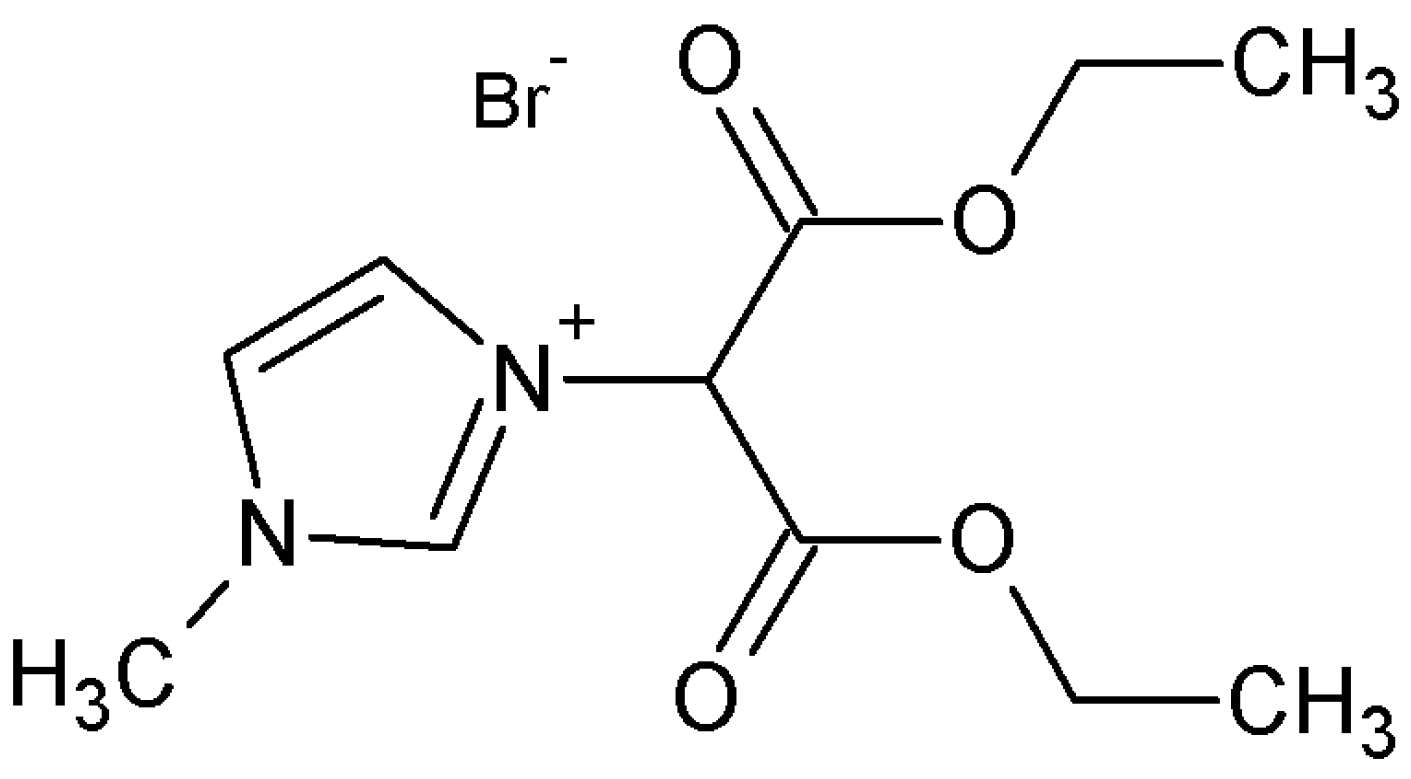
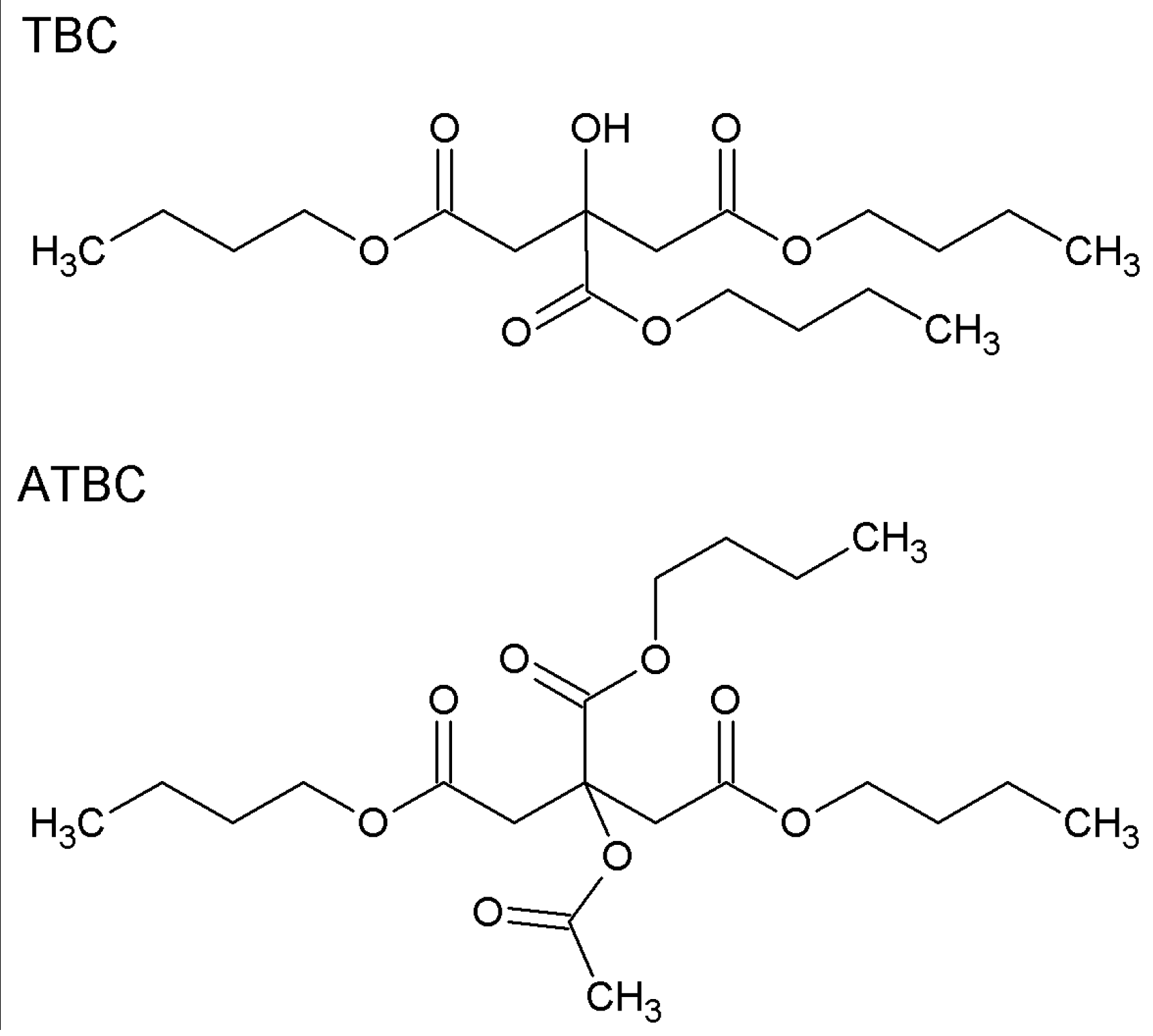

| Solvent | Molar Volume (at 293.15 K) | Molar Mass | Boiling Temperature | Relative Permittivity (at 293.15 K) | Density (at 293.15 K) |
|---|---|---|---|---|---|
| Vm | M | T | ε | d | |
| [cm3·mol−1] | [g·mol−1] | [°C] | [-] | [g·cm−3] | |
| Water | 18.1 | 18.0 | 100 | 80.20 [25] | 0.9982 |
| Ethanol | 56.9 | 46.1 | 78 | 25.16 [25] | 0.81 * |
| Propan-2-ol | 77.1 | 60.1 | 82 | 20.18 * | 0.78 * |
| Type of Plasticizer | Elongation at Break (εmax) | Stress at Break (σmax) | Young’s Modulus (YM) | References |
|---|---|---|---|---|
| [%] | [MPa] | [MPa] | ||
| CAP pure | 2 ± 1 | 50 ± 3 | 1710 ± 64 | This work |
| CAP-9-RIL | 65 ± 5 | 39 ± 01 | 998 ± 56 | This work |
| CAP-9-ATBC | 8 ± 6 | 34 ± 11 | 1329 ± 226 | This work |
| CAP-9-TBC | 9 ± 6 | 28 ± 6 | 1162 ± 44 | This work |
| CAP-23-RIL | 80 ± 5 | 28 ± 2 | 685 ± 62 | This work |
| CAP-23-ATBC | 61 ± 4 | 32 ± 2 | 721 ± 22 | This work |
| CAP-23-TBC | 50 ± 8 | 20 ± 3 | 571 ± 20 | This work |
| CAB-30-TBC | 30.2 ± 6.9 | 21.8 ± 2.3 * | - | [15] |
| CAB/TEOS-30-TBC | 40.9 ± 13.6 | 25.3 ± 2.8 * | - | [15] |
| CAB-30-DOP | 34.3 ± 2.0 | 28.3 ± 1.8 * | - | [15] |
| CAB/TEOS-30-DOP | 52.1 ± 1.5 | 31.1 ± 1.2 * | - | [15] |
| CAP pure | 1 ± 0 | 34 ± 2 * | 2624 ± 169 | [39] |
| CAP-10-Lemongrass oil | 2 ± 1 | 25 ± 6 * | 1632 ± 54 | [39] |
| CAP-20-Lemongrass oil | 2 ± 1 | 25 ± 8 * | 1973 ± 246 | [39] |
| CAP-10-Basil oil | 1 ± 0 | 15 ± 4 * | 1640 ± 52 | [39] |
| CAP-20-Basil oil | - | 35 ± 0 * | 1603 ± 35 | [39] |
| CAP pure | 0.65 | ca. 70 | - | [40] |
| CAP-10-TCP | 0.6 | ca. 48 | - | [40] |
| CAP pure | 11 | 60 * | - | [41] |
| CAP-12-PTG | 9 | 55 * | - | [41] |
| CAP-25-PTG | 20 | 42 * | - | [41] |
| CAP-8.7-PTS | 9 | 58 * | - | [41] |
| CAP-27-PTS | 29 | 41 * | - | [41] |
| CAP-12-DOA | 27 | 33 * | - | [41] |
| Membrane Name | Tonset [°C] |
|---|---|
| CAP pure | 351.5 ± 1.5 |
| CAP-9-RIL | 123.2 ± 1.5 |
| CAP-12.3-RIL | 120.0 ± 1.5 |
| CAP-16.7-RIL | 124.5 ± 1.5 |
| CAP-23.0-RIL | 123.8 ± 1.5 |
| CAP-28.6-RIL | 126.9± 1.5 |
| CAP-37.5-RIL | 122.9 ± 1.5 |
| CAP-44.4-RIL | 121.7 ± 1.5 |
| CAP-9-ATBC | 158.8 ± 1.5 |
| CAP-23-ATBC | 154.8 ± 1.5 |
| CAP-9-TBC | 161.4 ± 1.5 |
| CAP-23-TBC | 153.4 ± 1.5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rynkowska, E.; Fatyeyeva, K.; Kujawa, J.; Dzieszkowski, K.; Wolan, A.; Kujawski, W. The Effect of Reactive Ionic Liquid or Plasticizer Incorporation on the Physicochemical and Transport Properties of Cellulose Acetate Propionate-Based Membranes. Polymers 2018, 10, 86. https://doi.org/10.3390/polym10010086
Rynkowska E, Fatyeyeva K, Kujawa J, Dzieszkowski K, Wolan A, Kujawski W. The Effect of Reactive Ionic Liquid or Plasticizer Incorporation on the Physicochemical and Transport Properties of Cellulose Acetate Propionate-Based Membranes. Polymers. 2018; 10(1):86. https://doi.org/10.3390/polym10010086
Chicago/Turabian StyleRynkowska, Edyta, Kateryna Fatyeyeva, Joanna Kujawa, Krzysztof Dzieszkowski, Andrzej Wolan, and Wojciech Kujawski. 2018. "The Effect of Reactive Ionic Liquid or Plasticizer Incorporation on the Physicochemical and Transport Properties of Cellulose Acetate Propionate-Based Membranes" Polymers 10, no. 1: 86. https://doi.org/10.3390/polym10010086
APA StyleRynkowska, E., Fatyeyeva, K., Kujawa, J., Dzieszkowski, K., Wolan, A., & Kujawski, W. (2018). The Effect of Reactive Ionic Liquid or Plasticizer Incorporation on the Physicochemical and Transport Properties of Cellulose Acetate Propionate-Based Membranes. Polymers, 10(1), 86. https://doi.org/10.3390/polym10010086







