Two Tetranuclear Butterfly-Shaped Co(II) Complexes: Structure, Mass Spectrometric, and Magnetism
Abstract
:1. Introduction
2. Results and Discussion
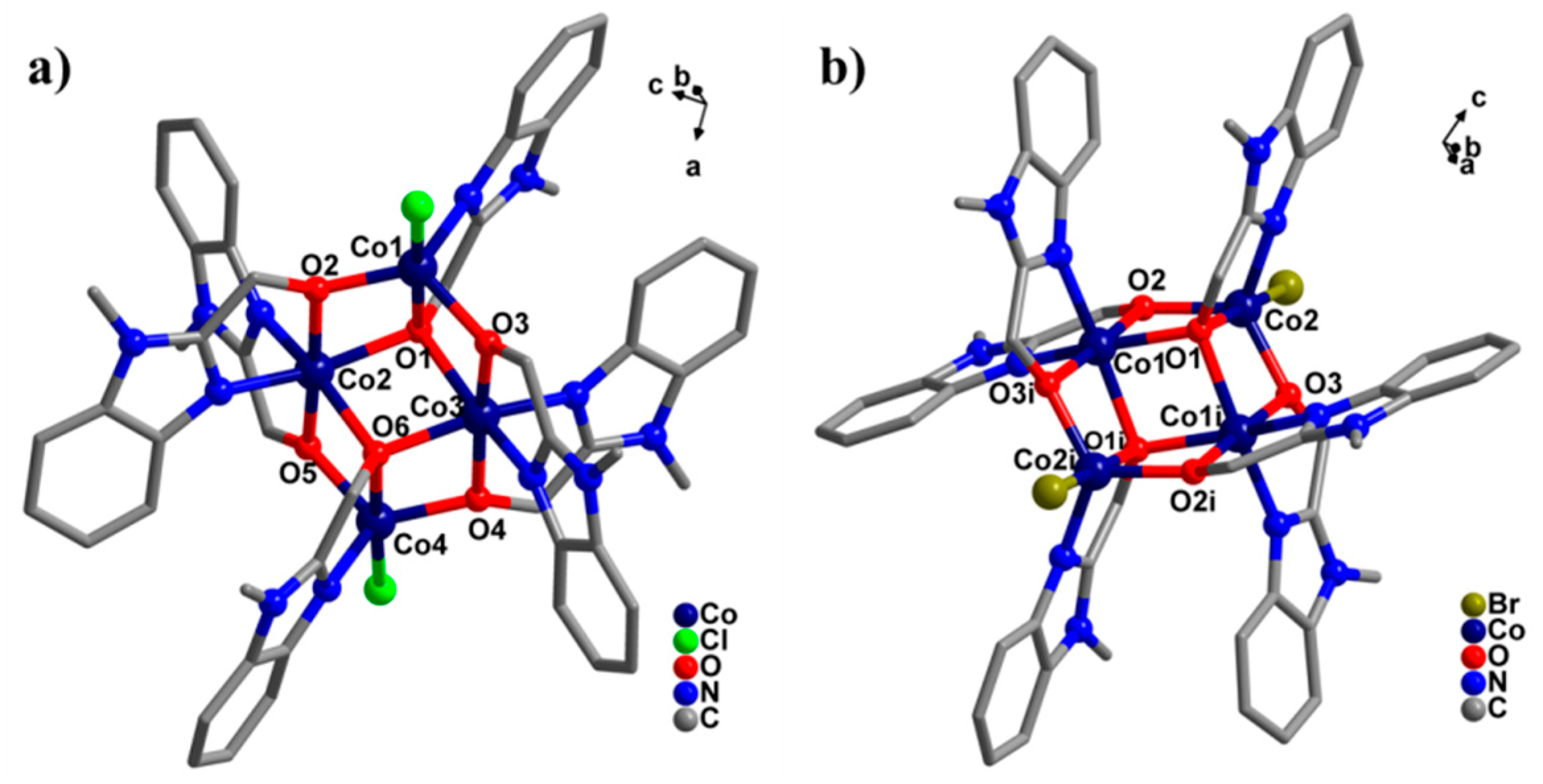
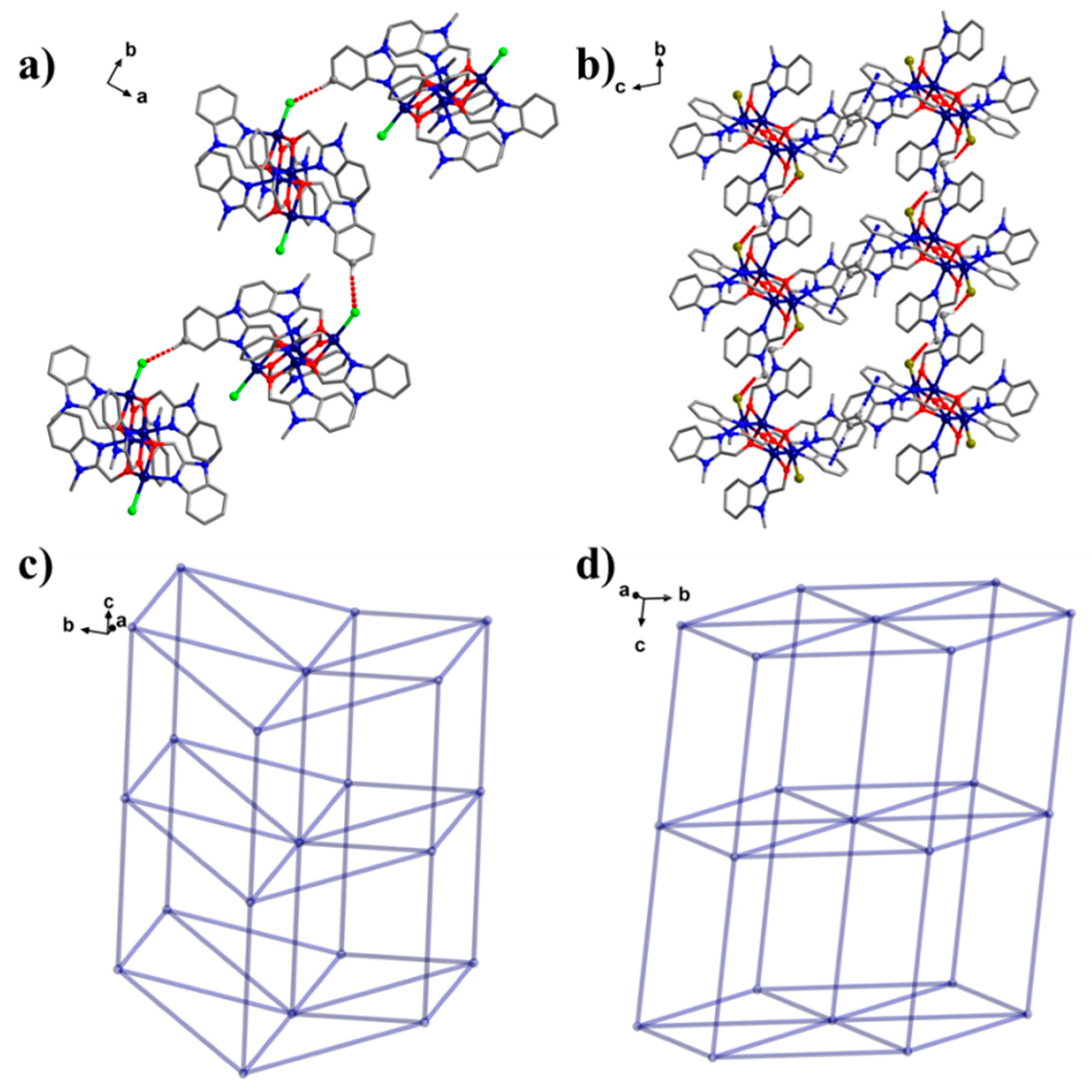
2.1. Crystal Structure
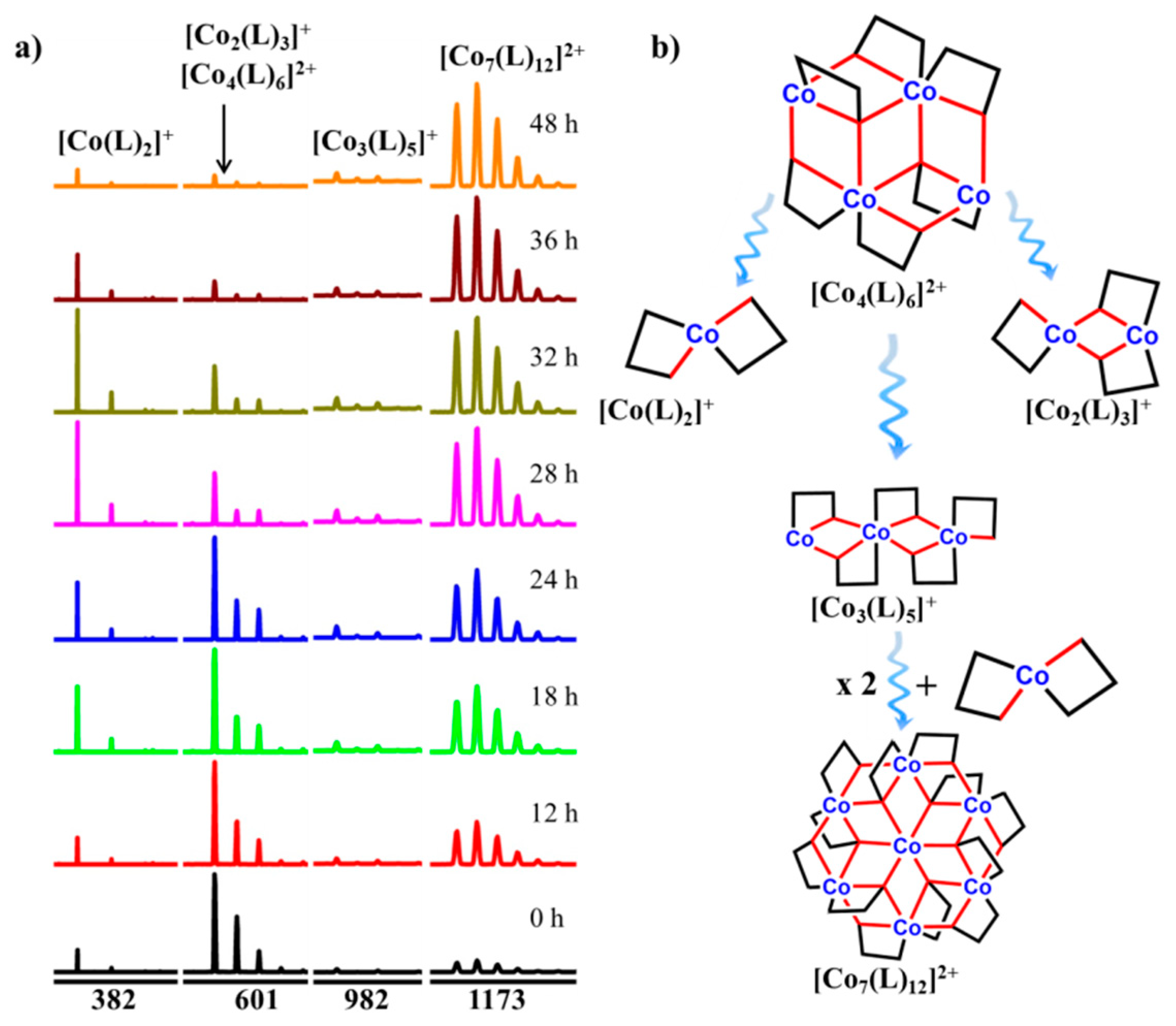
2.2. Electrospray Ionization Mass Spectrometry
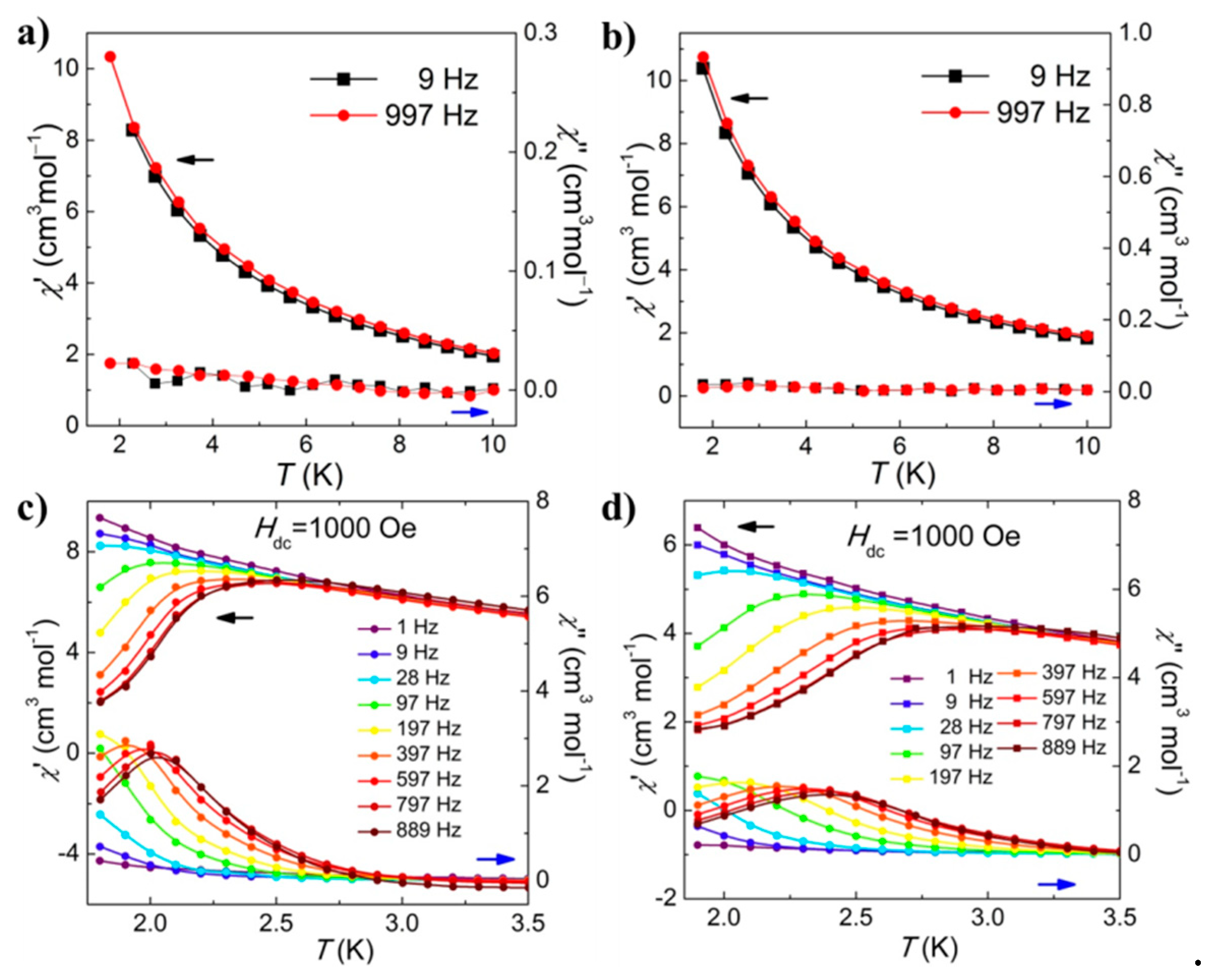
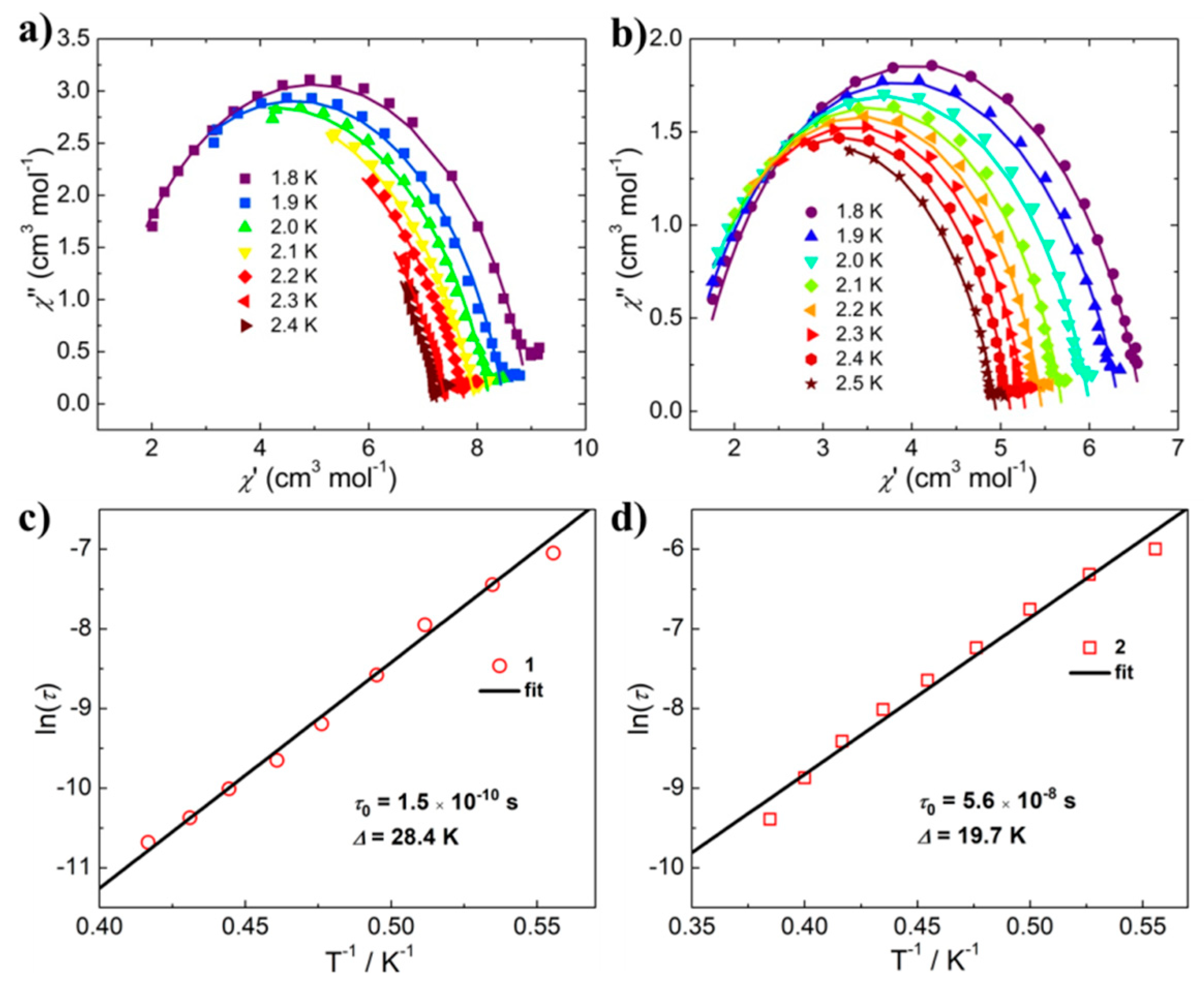
2.3. Magnetic Properties.
3. Conclusions
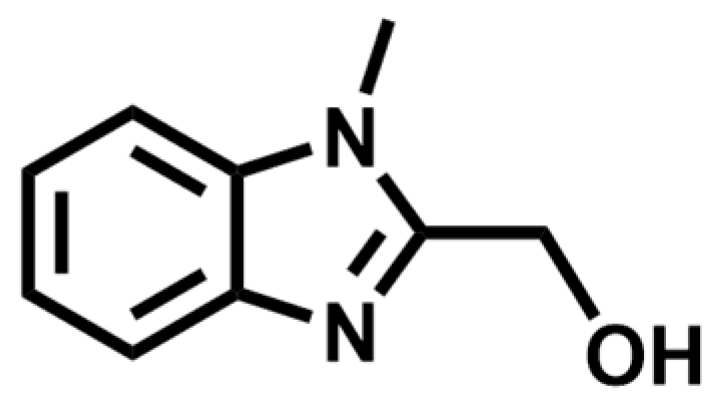
4. Experimental Section
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sessoli, R.; Gatteschi, D.; Caneschi, A.; Novak, M.A. Magnetic bistability in a metal-ion cluster. Nature. 1993, 365, 141–143. [Google Scholar] [CrossRef]
- Murrie, M. Cobalt(II) single-molecule magnets. Chem. Soc. Rev. 2010, 39, 1986–1995. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Guo, Y.N.; Tang, J. Recent advances in dysprosium-based single molecule magnets: Structural overview and synthetic strategies. Coord. Chem. Rev. 2013, 257, 1728–1763. [Google Scholar] [CrossRef]
- Zhu, Z.H.; Ma, X.F.; Wang, H.L.; Zou, H.H.; Mo, K.Q.; Zhang, Y.Q.; Yang, Q.Z.; Li, B.; Liang, F.P. A triangular Dy3 single-molecule toroic with high inversion energy barrier: magnetic properties and multiple-step assembly mechanism. Inorg. Chem. Front. 2018, 5, 3155–3162. [Google Scholar] [CrossRef]
- Wang, H.L.; Ma, X.F.; Peng, J.M.; Zhu, Z.H.; Li, B.; Zou, H.H.; Liang, F.P. Tracking the Stepwise Formation of the Dysprosium Cluster (Dy10) with Multiple Relaxation Behavior. Inorg. Chem. 2019, 58, 9169–9174. [Google Scholar] [CrossRef]
- Ma, X.F.; Wang, H.L.; Zhu, Z.H.; Li, B.; Mo, K.Q.; Zou, H.H.; Liang, F.P. Formation of nanocluster {Dy12} containing Dy-exclusive vertex-sharing [Dy4(μ3-OH)4] cubanes via simultaneous multitemplate guided and step-by-step assembly. Dalt. Trans. 2019, 48, 11338–11344. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.L.; Peng, J.M.; Zhu, Z.H.; Mo, K.Q.; Ma, X.F.; Li, B.; Zou, H.H.; Liang, F.P. Step-by-Step and Competitive Assembly of Two Dy(III) Single-Molecule Magnets with Their Performance Tuned by Schiff Base Ligands. Cryst. Growth Des. 2019, 19, 5369–5375. [Google Scholar] [CrossRef]
- Gao, S. Molecular Nanomagnets and Related Phenomena; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Christou, G. Single-molecule magnets: a molecular approach to nanoscale magnetic materials. Polyhedron 2005, 24, 2065–2075. [Google Scholar] [CrossRef]
- Wang, H.L.; Ma, X.F.; Zou, H.H.; Wang, K.; Li, B.; Chen, Z.L.; Liang, F.P. Mixed chelating ligands used to regulate the luminescence of Ln(iii) complexes and single-ion magnet behavior in Dy-based analogues. Dalton. Trans. 2018, 47, 15929–15940. [Google Scholar] [CrossRef]
- Fortier, S.; Le Roy, J.J.; Chen, C.H.; Vieru, V.; Murugesu, M.; Chibotaru, L.F.; Mindiola, D.J.; Caulton, K.G. A Dinuclear Cobalt Complex Featuring Unprecedented Anodic and Cathodic Redox Switches for Single-Molecule Magnet Activity. J. Am. Chem. Soc. 2013, 135, 14670–14678. [Google Scholar] [CrossRef]
- Miyasaka, H.; Yamashita, M. A look at molecular nanosized magnets from the aspect of inter-molecular interactions. Dalton Trans. 2007, 399–406. [Google Scholar] [CrossRef]
- Yang, E.C.; Hendrickson, D.N.; Wernsdorfer, W.; Nakano, M.; Zakharov, L.N.; Sommer, R.D.; Rheingold, A.L.; Ledezma-Gairaud, M.; Christou, G. Cobalt single-molecule magnet. J. Appl. Phys. 2002, 91, 7382–7384. [Google Scholar] [CrossRef]
- Kostakis, G.E.; Perlepes, S.P.; Blatov, V.A.; Proserpio, D.M.; Powell, A.K. High-nuclearity cobalt coordination clusters: Synthetic, topological and magnetic aspects. Coord. Chem. Rev. 2012, 256, 1246–1278. [Google Scholar] [CrossRef] [Green Version]
- Klinke, F.J.; Das, A.; Demeshko, S.; Dechert, S.; Meyer, F. Inducing Single Molecule Magnetic Behavior in a [Co4O4] Cubane via a Pronounced Solvatomagnetic Effect. Inorg. Chem. 2014, 53, 2976–2982. [Google Scholar] [CrossRef]
- Rechkemmer, Y.; Breitgoff, F.D.; van der Meer, M.; Atanasov, M.; Hakl, M.; Orlita, M.; Neugebauer, P.; Neese, F.; Sarkar, B.; van Slageren, J. A four-coordinate cobalt (II) single-ion magnet with coercivity and a very high energy barrier. Nat. Commun. 2016, 7, 10467. [Google Scholar] [CrossRef] [PubMed]
- Shankar, K.; Mondal, A.; Li, Y.; Journaux, Y.; Baruah, J.B. Hydroxide-Bridged Mixed-Valence Tetranuclear Cobalt 4-Nitrophenol Inclusion Complex Showing Single Molecule Magnet Property. ChemistrySelect 2017, 2, 7792–7798. [Google Scholar] [CrossRef]
- Plaul, D.; Böhme, M.; Ostrovsky, S.; Tomkowicz, Z.; Görls, H.; Haase, W.; Plass, W. Modeling Spin Interactions in a Triangular Cobalt (II) Complex with Triaminoguanidine Ligand Framework: Synthesis, Structure, and Magnetic Properties. Inorg. Chem. 2018, 57, 106–119. [Google Scholar] [CrossRef] [PubMed]
- Espin, J.; Zarzuela, R.; Statuto, N.; Juanhuix, J.; Maspoch, D.; Imaz, I.; Chudnovsky, E.; Tejada, J. Narrowing the Zero-Field Tunneling Resonance by Decreasing the Crystal Symmetry of Mn12 Acetate. J. Am. Chem. Soc. 2016, 138, 9065–9068. [Google Scholar] [CrossRef]
- Erler, P.; Schmitt, P.; Barth, N.; Irmler, A.; Bouvron, S.; Huhn, T.; Groth, U.; Pauly, F.; Gragnaniello, L.; Fonin, M. Highly Ordered Surface Self-Assembly of Fe4 Single Molecule Magnets. Nano Lett. 2015, 15, 4546–4552. [Google Scholar] [CrossRef]
- Zaleski, C.M.; Depperman, E.C.; Dendrinou-Samara, C.; Alexiou, M.; Kampf, J.W.; Kessissoglou, D.P.; Kirk, M.L.; Pecoraro, V.L. Metallacryptate Metallacryptate single-molecule magnets: Effect of lower molecular symmetry on blocking temperature. J. Am. Chem. Soc. 2005, 127, 12862–12872. [Google Scholar] [CrossRef]
- Kirchon, A.; Feng, L.; Drake, H.F.; Joseph, E.A.; Zhou, H.C. From fundamentals to applications: a toolbox for robust and multifunctional MOF materials. Chem. Soc. Rev. 2018, 47, 8611–8638. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.F.; Guo, L.Y.; Xu, J.H.; Jagodič, M.; Jagličić, Z.; Wang, W.-G.; Zhuang, G.L.; Wang, Z.; Tung, C.H.; Sun, D. Multifaceted Bicubane Co4 Clusters: Magnetism, Photocatalytic Oxygen Evolution, and Electrical Conductivity. Eur. J. Inorg. Chem. 2016, 3253–3261. [Google Scholar] [CrossRef]
- Roth, T.; Morningstar, M.L.; Boyer, P.L.; Hughes, S.H.; Buckheit, R.W., Jr.; Michejda, C.J. Synthesis and biological activity of novel nonnucleoside inhibitors of HIV-1 reverse transcriptase. 2-Aryl-substituted benzimidazoles. J. Med. Chem. 1997, 40, 4199–4207. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SHELXT: Integrated space-group and crystalstructure Determination. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]

| Complex | 1 | 2 |
|---|---|---|
| Formula | C54H49Cl2Co4N12O6 | C57H66Br2Co4N12O9 |
| Formula weight | 1268.67 | 1456.08 |
| T (K) | 293(2) | 293(2) |
| Crystal system | Monoclinic | Triclinic |
| Space group | P21 | P-1 |
| a (Å) | 12.5470(6) | 11.7411(10) |
| b (Å) | 19.9240(9) | 12.3673(10) |
| c (Å) | 12.6993(5) | 12.7465(8) |
| α (°) | 90.00 | 98.212(8) |
| β (°) | 103.403(4) | 93.842(6) |
| γ (°) | 90.00 | 117.605(9) |
| V (Å3) | 3088.2(2) | 1604.5(3) |
| Z | 2 | 4 |
| Dc (g cm−3) | 1.364 | 1.488 |
| μ (mm−1) | 1.197 | 9.915 |
| F(000) | 1294.0 | 728.0 |
| Reflns coll. | 17333 | 8924 |
| Unique reflns | 10017 | 5199 |
| Rint | 0.0419 | 0.0866 |
| aR1 [I ≥ 2σ(I)] | 0.0875 | 0.1101 |
| bwR2(all data) | 0.2678 | 0.3229 |
| GOF | 1.037 | 1.072 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, Q.-J.; Chen, M.; Chen, D.-C.; Chen, C.-A. Two Tetranuclear Butterfly-Shaped Co(II) Complexes: Structure, Mass Spectrometric, and Magnetism. Crystals 2019, 9, 477. https://doi.org/10.3390/cryst9090477
Deng Q-J, Chen M, Chen D-C, Chen C-A. Two Tetranuclear Butterfly-Shaped Co(II) Complexes: Structure, Mass Spectrometric, and Magnetism. Crystals. 2019; 9(9):477. https://doi.org/10.3390/cryst9090477
Chicago/Turabian StyleDeng, Qian-Jun, Min Chen, Dong-Chu Chen, and Chang-Ai Chen. 2019. "Two Tetranuclear Butterfly-Shaped Co(II) Complexes: Structure, Mass Spectrometric, and Magnetism" Crystals 9, no. 9: 477. https://doi.org/10.3390/cryst9090477




