Photomechanical Azobenzene Crystals
Abstract
1. Introduction to Photomechanical Materials
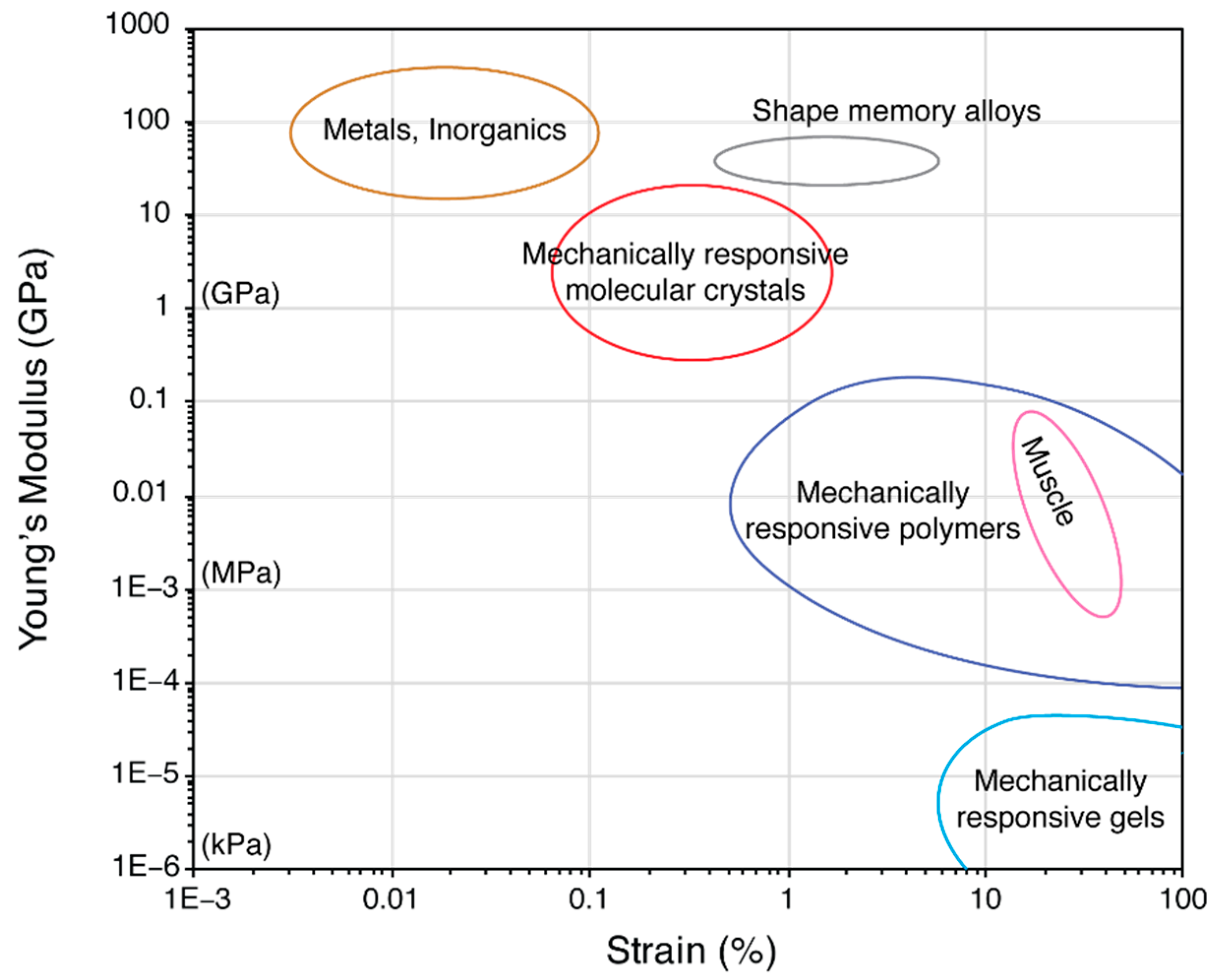
1.1. Actuator Materials
1.2. Azobenzene Compounds for Actuator Materials
1.3. Photomechanical Crystals
2. Development of Photomechanical Azobenzene Crystals
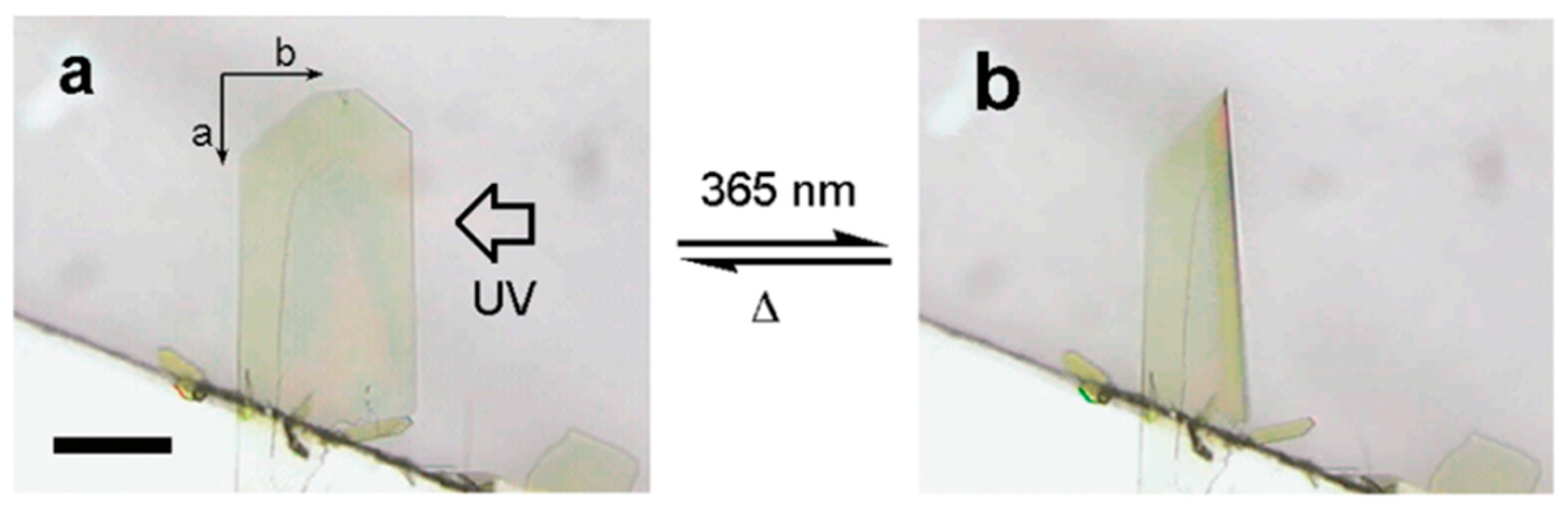
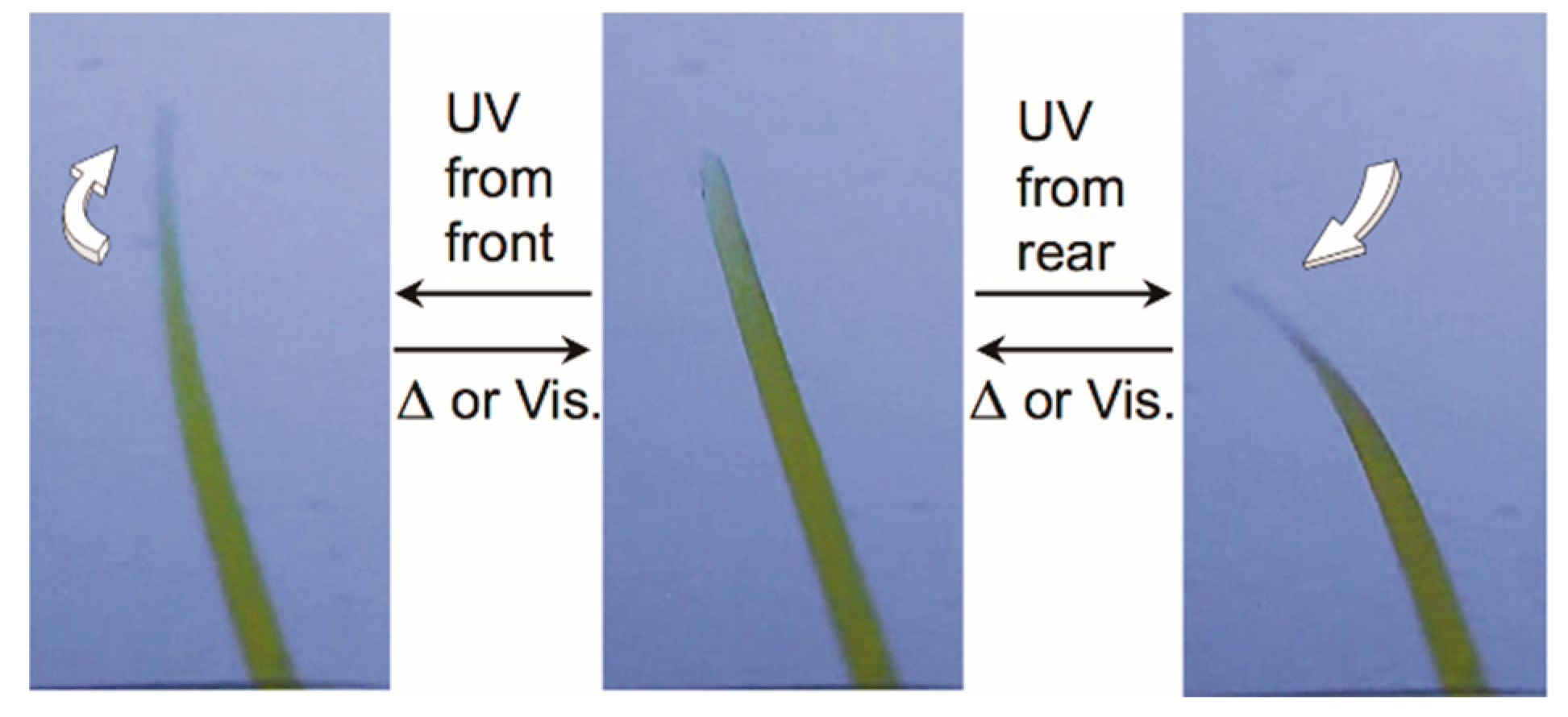
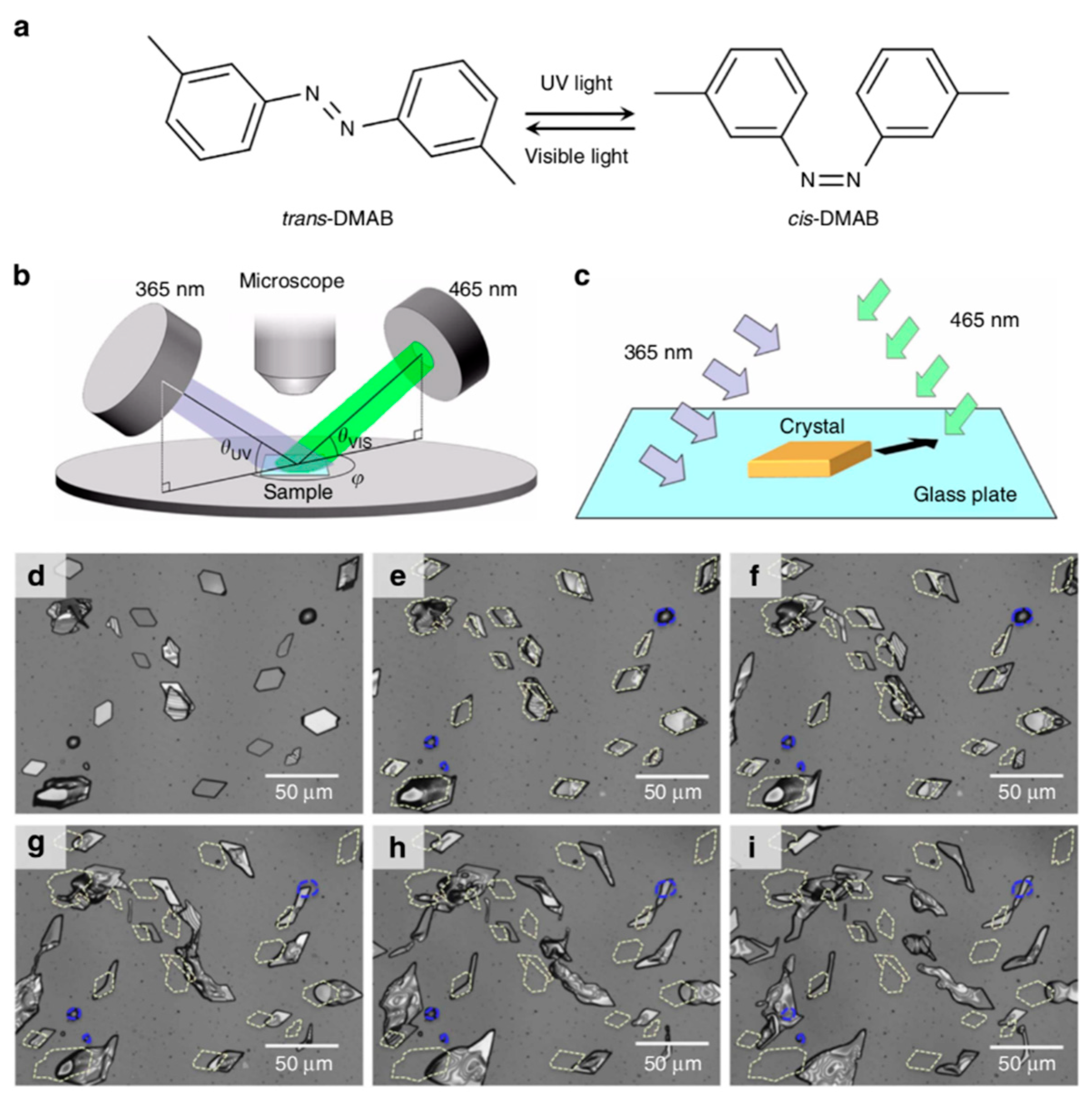
2.1. Early Stage of Photomechanical Azobenzene Crystals
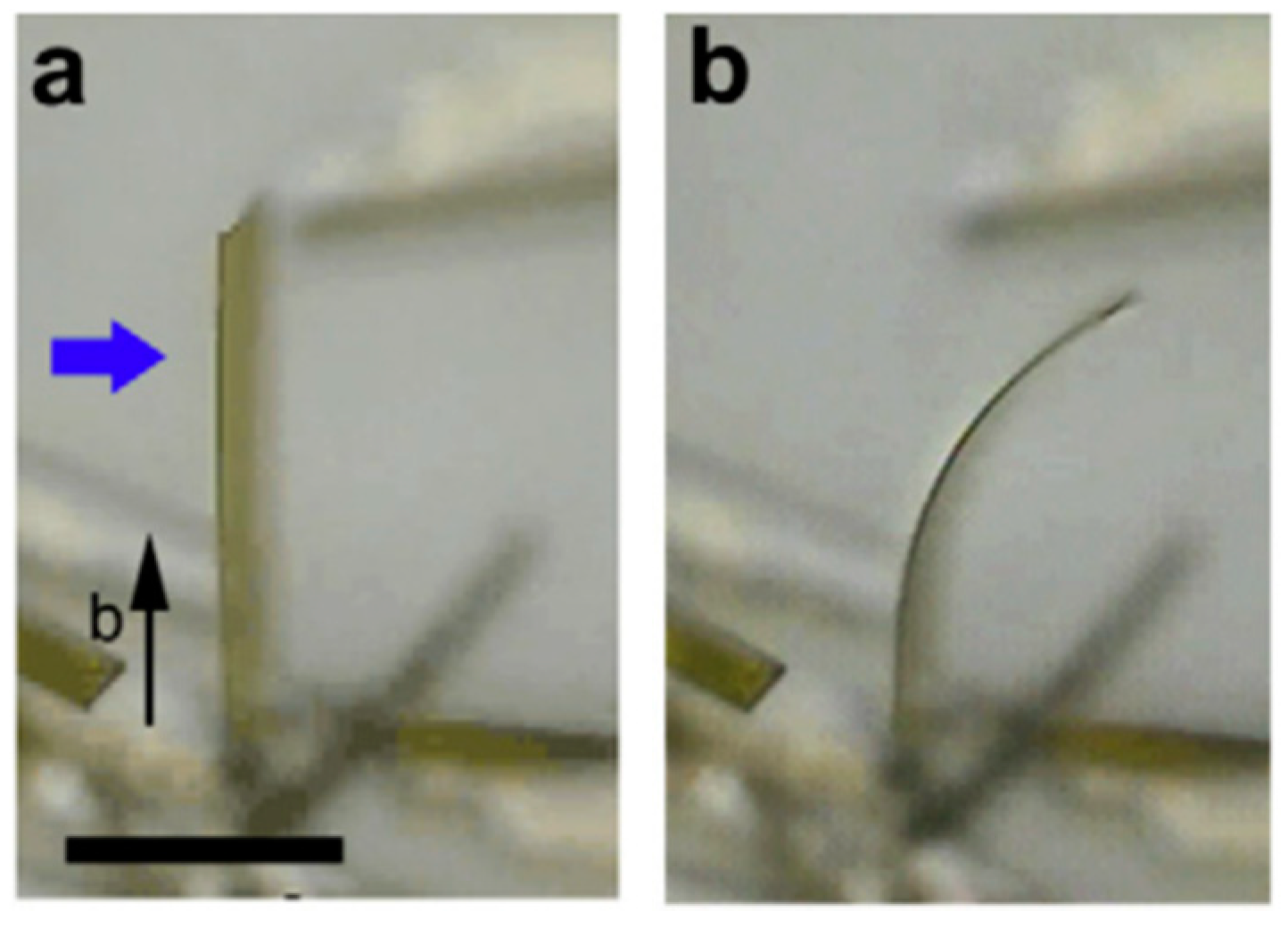
2.2. Fast Response
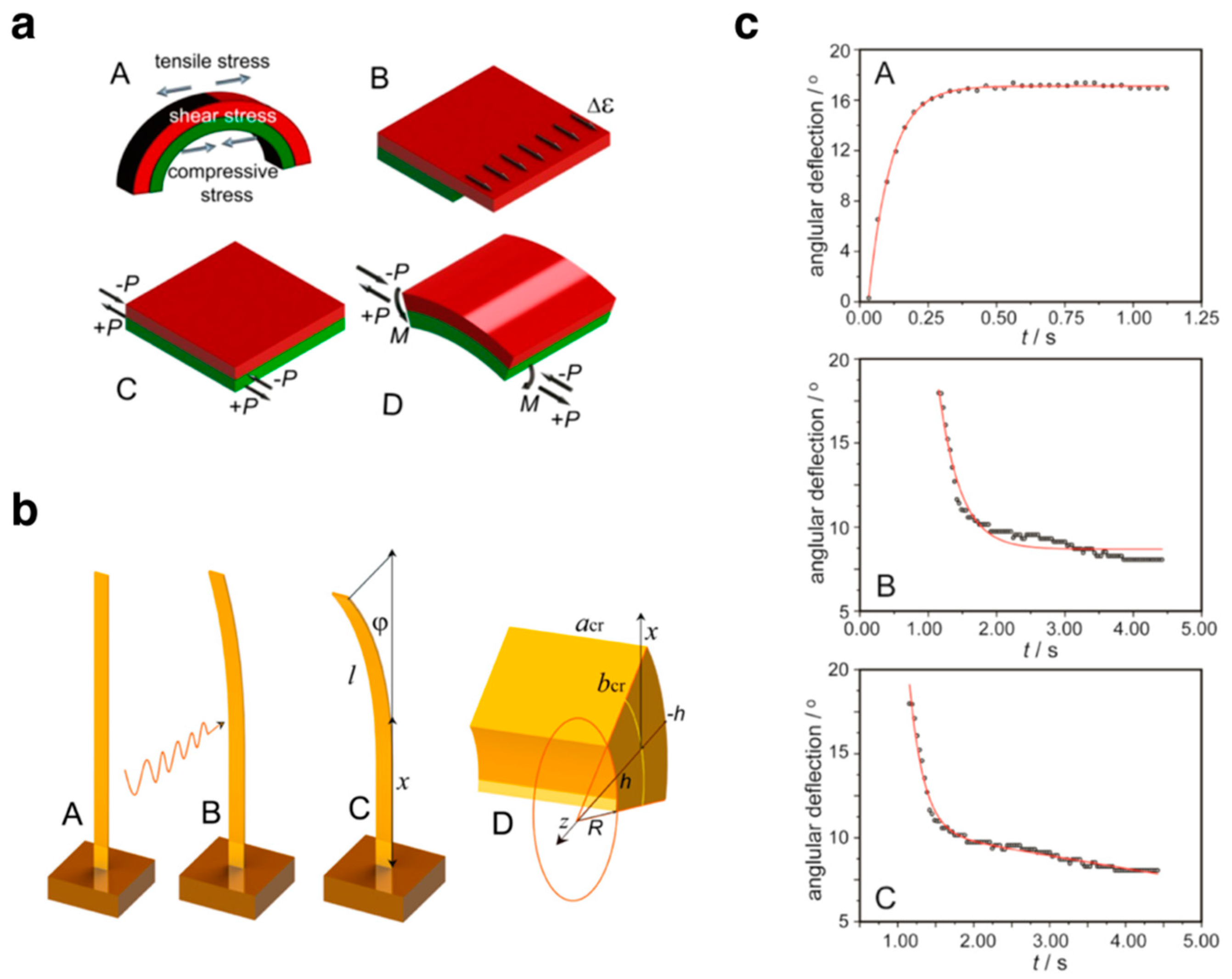
2.3. Model of Dynamical Photo-Bending
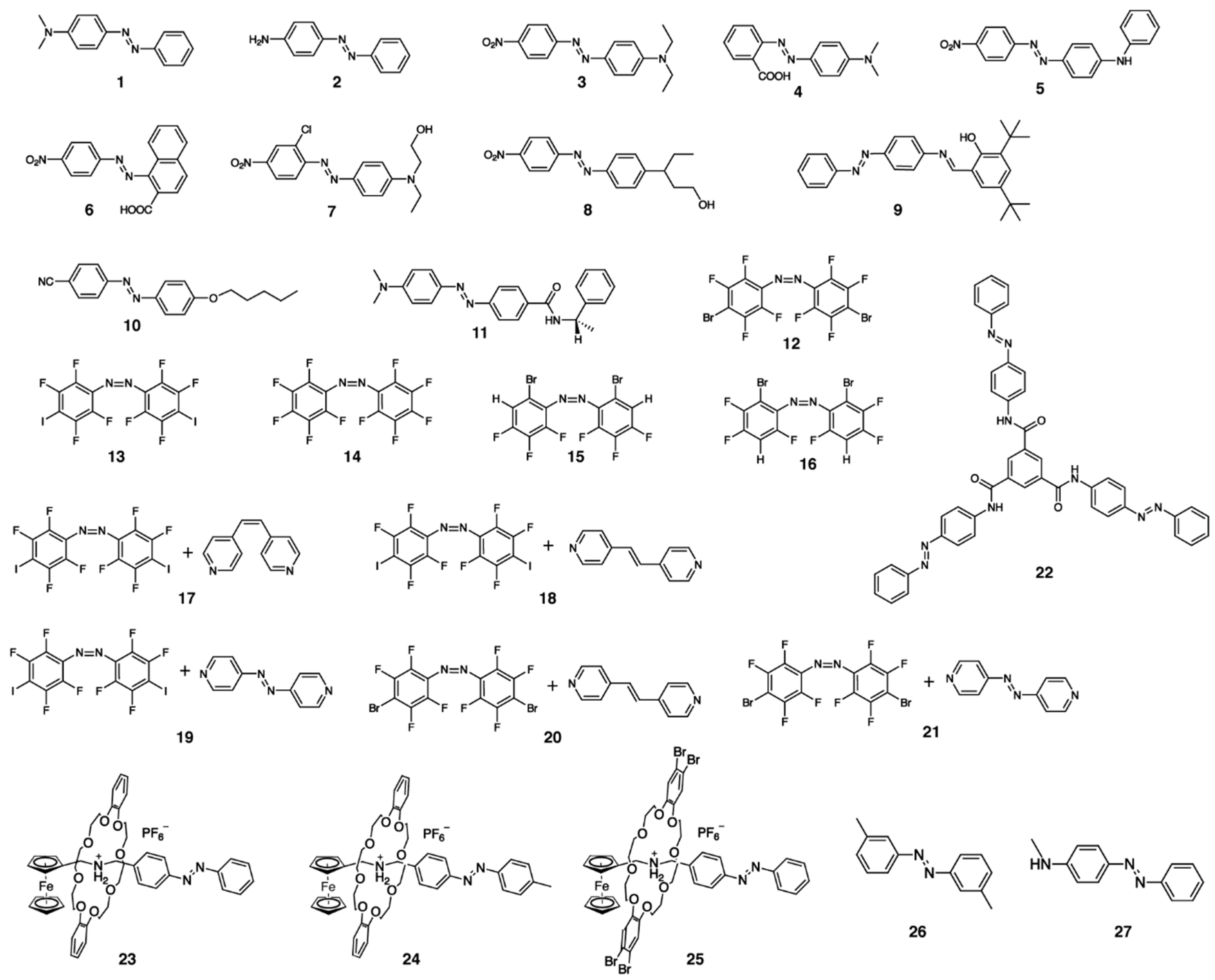
3. Design of New Azobenzene Crystals for Actuation
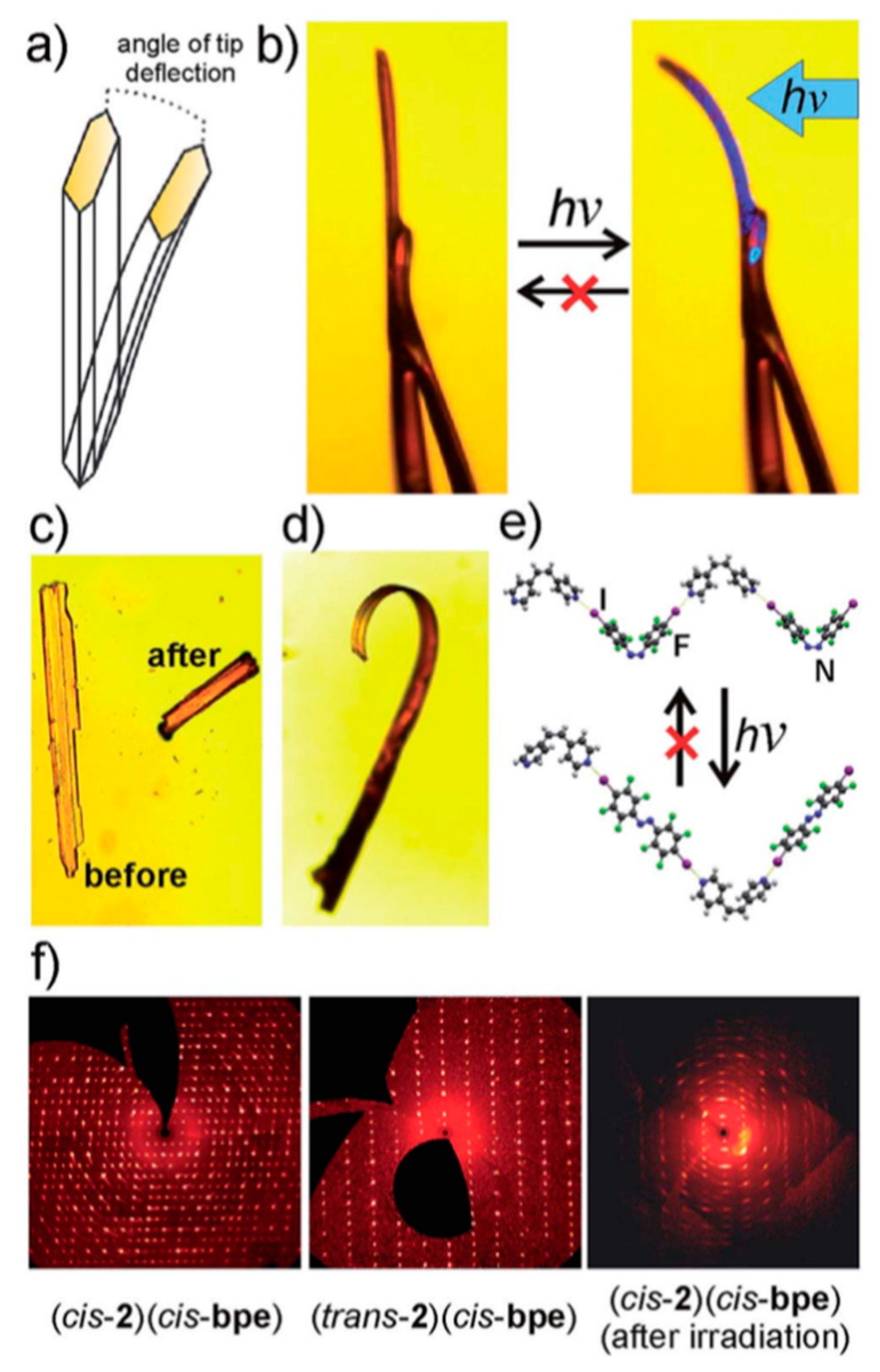
3.1. Chirality Induction
3.2. Crystallization with Multi-Molecular Components
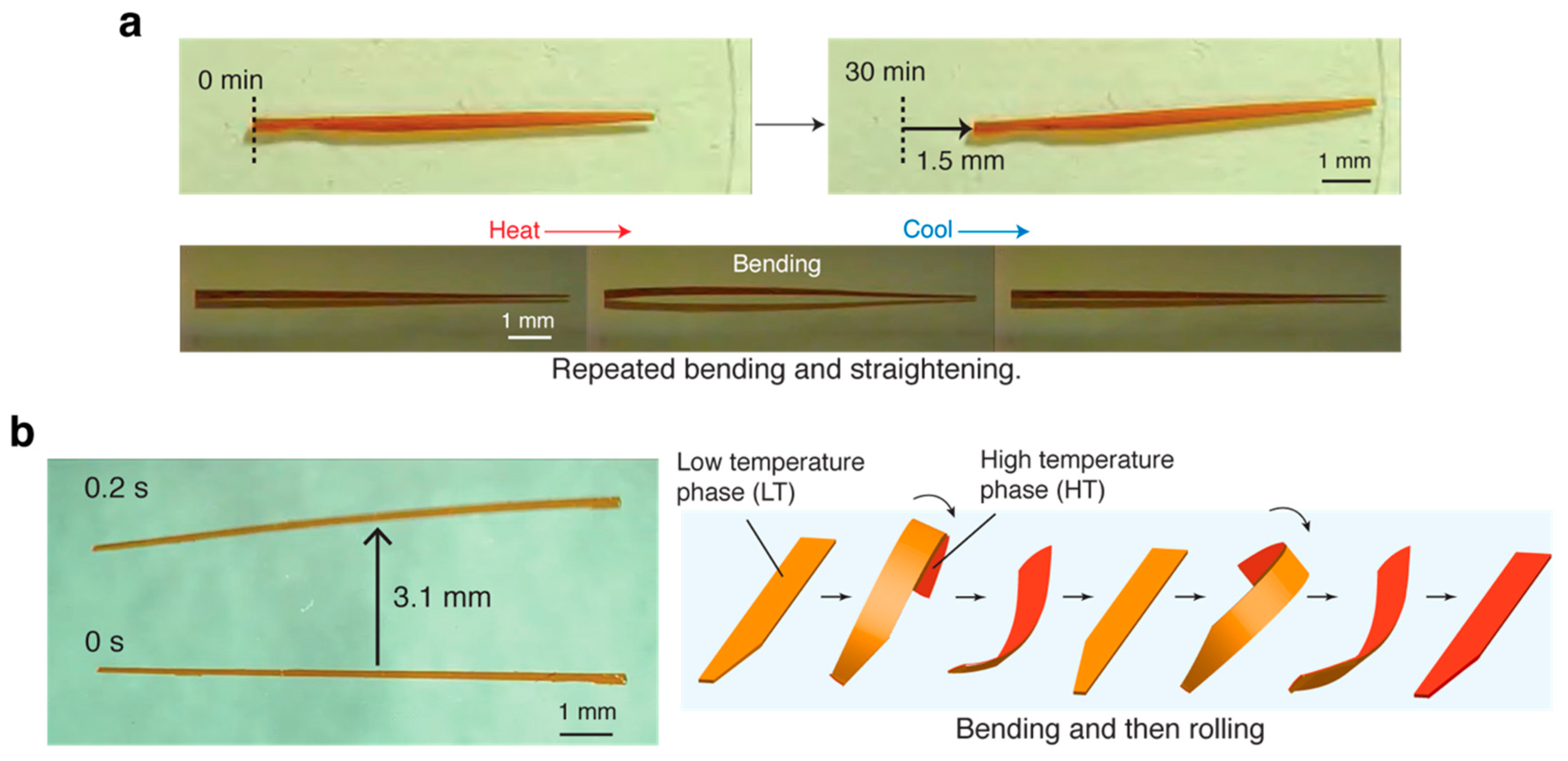
4. Other Mechanical Responses of Azobenzene Crystals
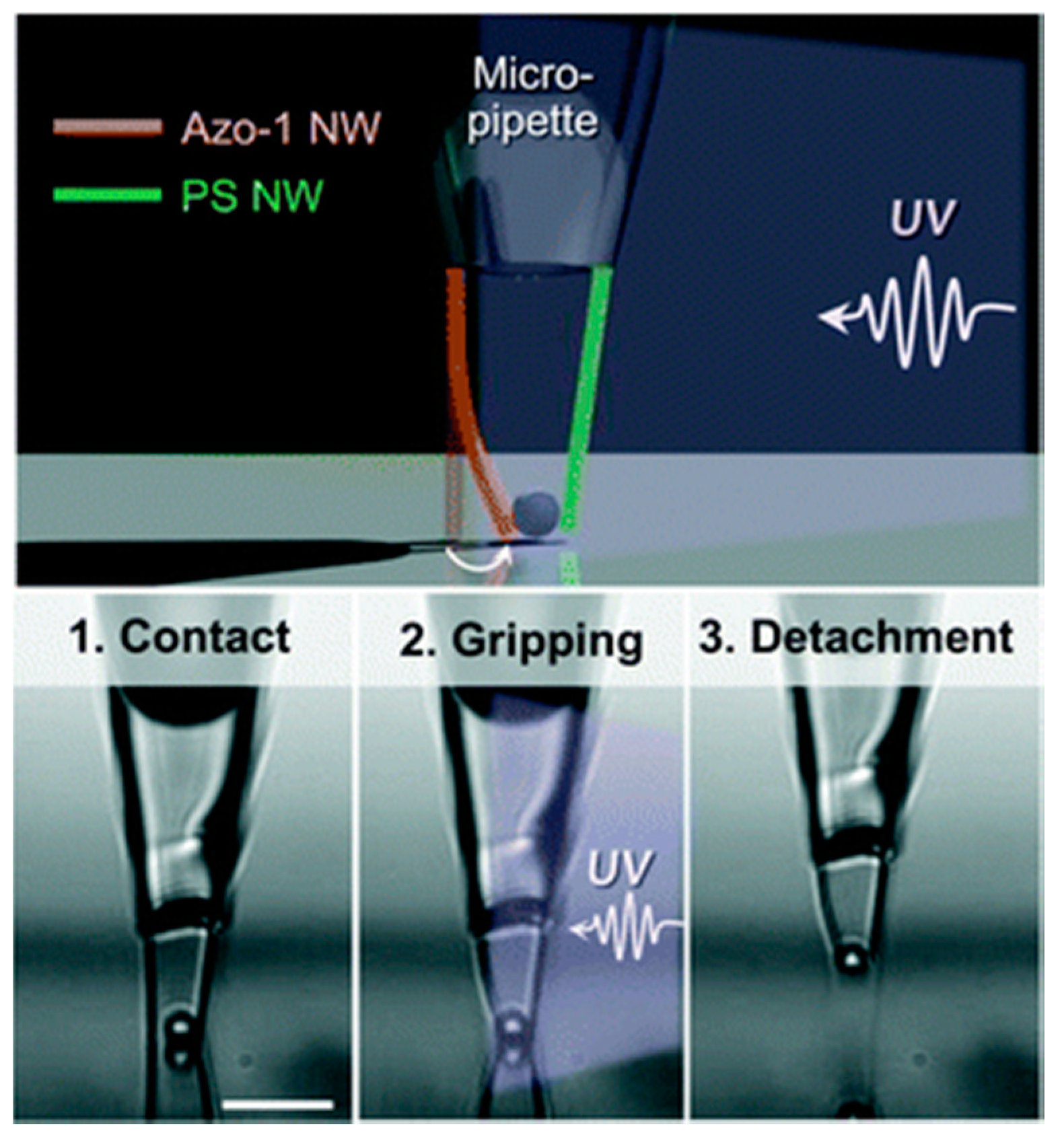
5. Possibilities for Future Applications
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, K.; Cheng, C.; Cheng, Z.; Wang, K.; Ramesh, R.; Wu, J. Giant-amplitude, high-work density microactuators with phase transition activated nanolayer bimorphs. Nano Lett. 2012, 12, 6302–6308. [Google Scholar] [CrossRef] [PubMed]
- Montero De Espinosa, L.; Meesorn, W.; Moatsou, D.; Weder, C. Bioinspired polymer systems with stimuli-responsive mechanical properties. Chem. Rev. 2017, 117, 12851–12892. [Google Scholar] [CrossRef] [PubMed]
- Eom, T.; Yoo, W.; Kim, S.; Khan, A. Biologically activatable azobenzene polymers targeted at drug delivery and imaging applications. Biomaterials 2018, 185, 333–347. [Google Scholar] [CrossRef] [PubMed]
- Stuart, M.A.C.; Huck, W.T.S.; Genzer, J.; Müller, M.; Ober, C.; Stamm, M.; Sukhorukov, G.B.; Szleifer, I.; Tsukruk, V.V.; Urban, M.; et al. Emerging applications of stimuli-responsive polymer materials. Nat. Mater. 2010, 9, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Rus, D.; Tolley, M.T. Design, fabrication and control of origami robots. Nature 2015, 521, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Lahikainen, M.; Zeng, H.; Priimagi, A. Reconfigurable photoactuator through synergistic use of photochemical and photothermal effects. Nat. Commun. 2018, 9, 4148. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Nakano, M.; Ikeda, T. Directed bending of a polymer film by light. Nature 2003, 425, 145. [Google Scholar] [CrossRef] [PubMed]
- Bandara, H.M.D.; Burdette, S.C. Photoisomerization in different classes of azobenzene. Chem. Soc. Rev. 2012, 41, 1809–1825. [Google Scholar] [CrossRef] [PubMed]
- Aßhoff, S.J.; Lancia, F.; Iamsaard, S.; Matt, B.; Kudernac, T.; Fletcher, S.P.; Katsonis, N. High-power actuation from molecular photoswitches in enantiomerically paired soft springs. Angew. Chem. Int. Ed. 2017, 56, 3261–3265. [Google Scholar] [CrossRef]
- Gelebart, A.H.; Jan Mulder, D.; Varga, M.; Konya, A.; Vantomme, G.; Meijer, E.W.; Selinger, R.L.B.; Broer, D.J. Making waves in a photoactive polymer film. Nature 2017, 546, 632–636. [Google Scholar] [CrossRef]
- Wang, M.; Lin, B.P.; Yang, H. A plant tendril mimic soft actuator with phototunable bending and chiral twisting motion modes. Nat. Commun. 2016, 7, 13981. [Google Scholar] [CrossRef] [PubMed]
- Takashima, Y.; Hatanaka, S.; Otsubo, M.; Nakahata, M.; Kakuta, T.; Hashidzume, A.; Yamaguchi, H.; Harada, A. Expansion-contraction of photoresponsive artificial muscle regulated by host-guest interactions. Nat. Commun. 2012, 3, 1270–1278. [Google Scholar] [CrossRef] [PubMed]
- Iwaso, K.; Takashima, Y.; Harada, A. Fast response dry-type artificial molecular muscles with [c2] daisy chains. Nat. Chem. 2016, 8, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Garibay, M.A. Molecular crystals on the move: From single-crystal-to-single-crystal photoreactions to molecular machinery. Angew. Chem. Int. Ed. 2007, 46, 8945–8947. [Google Scholar] [CrossRef] [PubMed]
- Kobatake, S.; Takami, S.; Muto, H.; Ishikawa, T.; Irie, M. Rapid and reversible shape changes of molecular crystals on photoirradiation. Nature 2007, 446, 778–781. [Google Scholar] [CrossRef] [PubMed]
- Irie, M.; Fukaminato, T.; Matsuda, K.; Kobatake, S. Photochromism of diarylethene molecules and crystals: Memories, switches, and actuators. Chem. Rev. 2014, 114, 12174–12277. [Google Scholar] [CrossRef]
- Kim, T.; Zhu, L.; Al-Kaysi, R.O.; Bardeen, C.J. Organic photomechanical materials. Chem. Phys. Chem. 2014, 15, 400–414. [Google Scholar] [CrossRef]
- Abendroth, J.M.; Bushuyev, O.S.; Weiss, P.S.; Barrett, C.J. Controlling motion at the nanoscale: Rise of the molecular machines. ACS Nano 2015, 9, 7746–7768. [Google Scholar] [CrossRef]
- Naumov, P.; Chizhik, S.; Panda, M.K.; Nath, N.K.; Boldyreva, E. Mechanically responsive molecular crystals. Chem. Rev. 2015, 115, 12440–12490. [Google Scholar] [CrossRef]
- Commins, P.; Desta, I.T.; Karothu, D.P.; Panda, M.K.; Naumov, P. Crystals on the move: Mechanical effects in dynamic solids. Chem. Commun. 2016, 52, 13941–13954. [Google Scholar] [CrossRef]
- Bushuyev, O.S.; Friščić, T.; Barrett, C.J. Photo-induced motion of azo dyes in organized media: From single and liquid crystals, to MOFs and machines. Cryst. Eng. Comm. 2016, 18, 7204–7211. [Google Scholar] [CrossRef]
- Koshima, H.; Taniguchi, T.; Asahi, T. Chapter 3: Photomechanical and thermomechanical crystals. In Mechanically Responsive Materials for Soft Robotics; Koshima, H., Ed.; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2019; pp. 57–82. [Google Scholar]
- Brown, C.J. A refinement of the crystal structure of azobenzene. Acta Crystallogr. 1966, 21, 146–152. [Google Scholar] [CrossRef]
- Mostad, A.; Rømming, C. A refinement of the crystal structure of cis-azobenzene. Acta Chem. Scand. 1971, 25, 3561–3568. [Google Scholar] [CrossRef]
- Tsuda, M.; Kuratani, K. Isomerization of cis-azobenzene in the solid phase. Bull. Chem. Soc. Jpn. 1964, 37, 1284–1288. [Google Scholar] [CrossRef]
- Koshima, H.; Ojima, N.; Uchimoto, H. Mechanical motion of azobenzene crystals upon photoirradiation. J. Am. Chem. Soc. 2009, 131, 6890–6891. [Google Scholar] [CrossRef] [PubMed]
- Koshima, H.; Ojima, N. Photomechanical bending of 4-aminoazobenzene crystals. Dyes Pigment. 2012, 92, 798–801. [Google Scholar] [CrossRef]
- Beharry, A.A.; Woolley, G.A. Azobenzene photoswitches for biomolecules. Chem. Soc. Rev. 2011, 40, 4422–4437. [Google Scholar] [CrossRef]
- Bushuyev, O.S.; Singleton, T.A.; Barrett, C.J. Fast, reversible, and general photomechanical motion in single crystals of various Azo compounds using visible light. Adv. Mater. 2013, 25, 1796–1800. [Google Scholar] [CrossRef]
- Timoshenko, S. Analysis of Bi-Metal Thermostats. J. Opt. Soc. Am. Rev. Sci. Instrum. 1925, 11, 233–255. [Google Scholar] [CrossRef]
- Kitagawa, D.; Iwaihara, C.; Nishi, H.; Kobatake, S. Quantitative evaluation of photoinduced bending speed of diarylethene crystals. Crystals 2015, 5, 551–561. [Google Scholar] [CrossRef]
- Nath, N.K.; Pejov, L.; Nichols, S.M.; Hu, C.; Saleh, N.; Kahr, B.; Naumov, P. Model for photoinduced bending of slender molecular crystals. J. Am. Chem. Soc. 2014, 136, 2757–2766. [Google Scholar] [CrossRef] [PubMed]
- Panda, M.K.; El Azhary, N.; Alzaabi, M.A.; Wahba, B.M.; Jacob, J.; Naumov, P. Photomechanical and photochromic behavior of a molecule containing multiple photoactive groups. Croat. Chem. Acta 2014, 87, 475–479. [Google Scholar] [CrossRef]
- Zhu, L.; Al-Kaysi, R.O.; Bardeen, C.J. Reversible photoinduced twisting of molecular crystal microribbons. J. Am. Chem. Soc. 2011, 133, 12569–12575. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, D.; Nishi, H.; Kobatake, S. Photoinduced twisting of a photochromic diarylethene crystal. Angew. Chem. Int. Ed. 2013, 52, 9320–9322. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Zhu, L.; Mueller, L.J.; Bardeen, C.J. Mechanism of photoinduced bending and twisting in crystalline microneedles and microribbons composed of 9-methylanthracene. J. Am. Chem. Soc. 2014, 136, 6617–6625. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, D.; Tsujioka, H.; Tong, F.; Dong, X.; Bardeen, C.J.; Kobatake, S. Control of photomechanical crystal twisting by illumination direction. J. Am. Chem. Soc. 2018, 140, 4208–4212. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, T.; Fujisawa, J.; Shiro, M.; Koshima, H.; Asahi, T. Mechanical motion of chiral azobenzene crystals with twisting upon photoirradiation. Chem. Eur. J. 2016, 22, 7950–7958. [Google Scholar] [CrossRef] [PubMed]
- Desiraju, G.R. Supramolecular synthons in crystal engineering—A new organic synthesis. Angew. Chem. Int. Ed. Engl. 1995, 34, 2311–2327. [Google Scholar] [CrossRef]
- Saha, S.; Desiraju, G.R. Using structural modularity in cocrystals to engineer properties: Elasticity. Chem. Commun. 2016, 52, 7676–7679. [Google Scholar] [CrossRef]
- Christopherson, J.C.; Topić, F.; Barrett, C.J.; Friščić, T. Halogen-bonded cocrystals as optical materials: Next-generation control over light-matter interactions. Cryst. Growth Des. 2018, 18, 1245–1259. [Google Scholar] [CrossRef]
- Bushuyev, O.S.; Tomberg, A.; Friščić, T.; Barrett, C.J. Shaping crystals with light: Crystal-to-crystal isomerization and photomechanical effect in fluorinated azobenzenes. J. Am. Chem. Soc. 2013, 135, 12556–12559. [Google Scholar] [CrossRef] [PubMed]
- Bushuyev, O.S.; Tomberg, A.; Vinden, J.R.; Moitessier, N.; Barrett, C.J.; Friščić, T. Azo⋯phenyl stacking: A persistent self-assembly motif guides the assembly of fluorinated cis-azobenzenes into photo-mechanical needle crystals. Chem. Commun. 2016, 52, 2103–2106. [Google Scholar] [CrossRef] [PubMed]
- Bushuyev, O.S.; Corkery, T.C.; Barrett, C.J.; Friščić, T. Photo-mechanical azobenzene cocrystals and in situ X-ray diffraction monitoring of their optically-induced crystal-to-crystal isomerisation. Chem. Sci. 2014, 5, 3158–3164. [Google Scholar] [CrossRef]
- Cheng, S.C.; Chen, K.J.; Suzaki, Y.; Tsuchido, Y.; Kuo, T.S.; Osakada, K.; Horie, M. Reversible laser-induced bending of pseudorotaxane crystals. J. Am. Chem. Soc. 2018, 140, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Uchida, E.; Azumi, R.; Norikane, Y. Light-induced crawling of crystals on a glass surface. Nat. Commun. 2015, 6, 7310. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Ohnuma, M.; Norikane, Y. Negative phototactic behaviour of crystals on a glass surface. Chem. Commun. 2019, 55, 9303–9306. [Google Scholar] [CrossRef] [PubMed]
- Norikane, Y.; Tanaka, S.; Uchida, E. Azobenzene crystals swim on water surface triggered by light. Cryst. Eng. Comm. 2016, 18, 7225–7228. [Google Scholar] [CrossRef]
- Hao, Y.; Huang, S.; Guo, Y.; Hao, H.; Zhou, L.; Barrett, C.J.; Yu, H. Photoinduced multi-directional deformation of azobenzene molecular crystals. J. Mater. Chem. C 2019, 7, 503–508. [Google Scholar] [CrossRef]
- Taniguchi, T.; Sugiyama, H.; Uekusa, H.; Shiro, M.; Asahi, T.; Koshima, H. Walking and rolling of crystals induced thermally by phase transition. Nat. Commun. 2018, 9, 538. [Google Scholar] [CrossRef]
- Kitagawa, D.; Kawasaki, K.; Tanaka, R.; Kobatake, S. Mechanical behavior of molecular crystals induced by combination of photochromic reaction and reversible single-crystal-to-single-crystal phase transition. Chem. Mater. 2017, 29, 7524–7532. [Google Scholar] [CrossRef]
- Taniguchi, T.; Sato, H.; Hagiwara, Y.; Asahi, T.; Koshima, H. Photo-triggered phase transition of a crystal. Commun. Chem. 2019, 2, 19. [Google Scholar] [CrossRef]
- Lee, J.; Oh, S.; Pyo, J.; Kim, J.M.; Je, J.H. A light-driven supramolecular nanowire actuator. Nanoscale 2015, 7, 6457–6461. [Google Scholar] [CrossRef] [PubMed]
- Momeni, F.; Hassani, N.S.M.M.; Liu, X.; Ni, J. A review of 4D printing. Mater. Des. 2017, 122, 42–79. [Google Scholar] [CrossRef]
- Sydney Gladman, A.; Matsumoto, E.A.; Nuzzo, R.G.; Mahadevan, L.; Lewis, J.A. Biomimetic 4D printing. Nat. Mater. 2016, 15, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, M.; Irie, M. A diarylethene cocrystal that converts light into mechanical work. J. Am. Chem. Soc. 2010, 132, 14172–14178. [Google Scholar] [CrossRef] [PubMed]
- Koshima, H.; Matsuo, R.; Matsudomi, M.; Uemura, Y.; Shiro, M. Light-driven bending crystals of salicylidenephenylethylamines in enantiomeric and racemate forms. Cryst. Growth Des. 2013, 13, 4330–4337. [Google Scholar] [CrossRef]
- Thorpe, S.K.; Li, Y.; Crompton, R.H.; Alexander, R.M. Stresses in human leg muscles in running and jumping determined by force plate analysis and from published magnetic resonance images. J. Exp. Biol. 1998, 201, 63–70. [Google Scholar]
- Yu, Q.; Yang, X.; Chen, Y.; Yu, K.; Gao, J.; Liu, Z.; Cheng, P.; Zhang, Z.; Aguila, B.; Ma, S. Fabrication of light-triggered soft artificial muscles via a mixed-matrix membrane strategy. Angew. Chem. Int. Ed. 2018, 57, 10192–10196. [Google Scholar] [CrossRef]
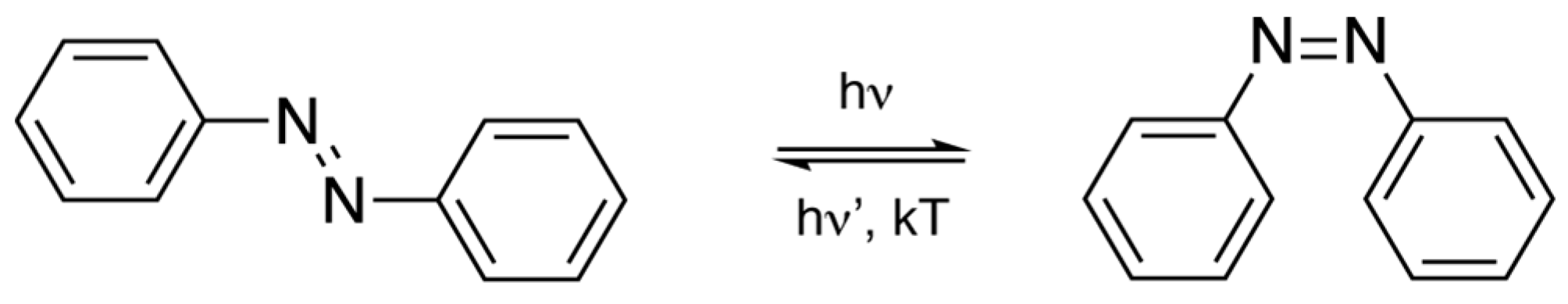
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taniguchi, T.; Asahi, T.; Koshima, H. Photomechanical Azobenzene Crystals. Crystals 2019, 9, 437. https://doi.org/10.3390/cryst9090437
Taniguchi T, Asahi T, Koshima H. Photomechanical Azobenzene Crystals. Crystals. 2019; 9(9):437. https://doi.org/10.3390/cryst9090437
Chicago/Turabian StyleTaniguchi, Takuya, Toru Asahi, and Hideko Koshima. 2019. "Photomechanical Azobenzene Crystals" Crystals 9, no. 9: 437. https://doi.org/10.3390/cryst9090437
APA StyleTaniguchi, T., Asahi, T., & Koshima, H. (2019). Photomechanical Azobenzene Crystals. Crystals, 9(9), 437. https://doi.org/10.3390/cryst9090437




