Atomistic Modeling of Various Doped Mg2NiH4 as Conversion Electrode Materials for Lithium Storage
Abstract
1. Introduction
2. Methods
3. Results and Discussion
4. Summary and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Armand, M.; Tarascon, J.-M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef]
- Nitta, N.; Wu, F.X.; Lee, J.-T.; Yushin, G. Li-ion battery materials: Present and future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Yoon, G.; Kim, D.-H.; Park, I.; Chang, D.H.; Kim, B.; Lee, B.; Oh, K.; Kang, K. Using First-Principles Calculations for the Advancement of materials for Rechargeable Batteries. Adv. Funct. Mater. 2017, 27, 1702887. [Google Scholar] [CrossRef]
- Lu, K.; Hu, Z.Y.; Ma, J.Z.; Ma, H.Y.; Dai, L.M.; Zhang, J.T. A rechargeable iodine-carbon battery that exploits ion intercalation and iodine redox chemistry. Nat. Commun. 2017, 8, 527. [Google Scholar] [CrossRef]
- Urban, A.; Seo, D.-H.; Ceder, G. Computational understanding of Li-ion batteries. NPJ Comput. Mater. 2016, 2, 16002. [Google Scholar] [CrossRef]
- Oumellal, Y.; Rougier, A.; Nazri, G.A.; Tarascon, J.-M.; Aymard, L. Metal hydrides for lithium-ion batteries. Nat. Mater. 2008, 7, 916–921. [Google Scholar] [CrossRef]
- Qian, Z.; Sarkar, A.D.; Maark, T.A.; Jiang, X.; Deshpande, M.D.; Bououdina, M.; Ahuja, R. Pure and Li-doped NiTiH: Potential anode materials for Li-in rechargeable batteries. Appl. Phys. Lett. 2013, 103, 033902. [Google Scholar] [CrossRef]
- Qian, Z.; Jiang, X.; Sarkar, A.D.; Maark, T.A.; Deshpande, M.D.; Bououdina, M.; Johansson, B.; Ahuja, R. Screen study of light-metal and transition-metal-doped NiTiH hydrides as Li-ion battery anode materials. Solid State Ion. 2014, 258, 88–91. [Google Scholar] [CrossRef]
- Aymard, L.; Oumellal, Y.; Bonnet, J. Metal hydrides: An innovative and challenging conversion reaction anode for lithium-ion batteries. Beilstein J. Nanotechnol. 2015, 6, 1821–1839. [Google Scholar] [CrossRef]
- Zhao, T.; Wang, Q.; Jena, P. Cluster-Inspired Design of Hige-Capacity Anode for Li-Ion Batteries. ACS Energy Lett. 2016, 1, 202–208. [Google Scholar] [CrossRef]
- Liu, J.; Pang, W.K.; Zhou, T.; Chen, L.; Wang, Y.; Peterson, V.K.; Yang, Z.; Guo, Z.; Xia, Y. Li2TiSiO5: A low potential and large capacity Ti-based anode material for Li-ion batteries. Energy Environ. Sci. 2017, 10, 1456–1464. [Google Scholar] [CrossRef]
- Oumellal, Y.; Rougier, A.; Tarascon, J.M.; Aymard, L. 2LiH + M (M = Mg, Ti): New concept of negative electrode for rechargeable lithium-ion batteries. J. Power Sources 2009, 192, 698–702. [Google Scholar] [CrossRef]
- Mahata, A.; Bhauriyal, P.; Rawat, K.S.; Pathak, B. Pt3Ti (Ti19@Pt60)-Based Cuboctahedral Core–Shell Nanocluster Favors a Direct over Indirect Oxygen Reduction Reaction. ACS Energy Lett. 2016, 1, 797–805. [Google Scholar] [CrossRef]
- Jasen, P.V.; González, E.A.; Brizuela, G.; Nagel, O.A.; González, G.A.; Juan, A. A theoretical study of the electronic structure and bonding of the monoclinic phase of Mg2NiH4. Int. J. Hydrog. Energy 2007, 32, 4943–4948. [Google Scholar] [CrossRef]
- Häussermann, U.; Blomqvist, H.; Noréus, D. Bonding and stability of the hydrogen storage material Mg2NiH4. Inorg. Chem. 2002, 41, 3684–3692. [Google Scholar] [CrossRef]
- Wu, Z.; Zhu, L.; Yang, F.; Jiang, Z.; Zhang, Z. Influences of interstitial nitrogen with high electronegativity on structure and hydrogen storage properties of Mg-based metal hydride: A theoretical study. Int. J. Hydrog. Energy 2016, 41, 18550–18561. [Google Scholar] [CrossRef]
- Martínez-Coronado, R.; Retuerto, M.; Torres, B.; Martínez-Lope, M.J.; Fernández-Díaz, M.T.; Alonso, J.A. High-pressure synthesis, crystal structure and cyclability of the Mg2NiH4 hydride. Int. J. Hydrog. Energy 2013, 38, 5738–5745. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, D.W.; He, L.P.; Peng, P.; Liu, J.S. First-principles investigation of Mg2Ni phase and high/low temperature Mg2NiH4 complex hydrides. J. Phys. Chem. Solids 2008, 70, 32–39. [Google Scholar] [CrossRef]
- Orimo, S.; Züttel, A.; Ikeda, K.; Saruki, S.; Fukunaga, T.; Fujii, H.; Schlapbach, L. Hydriding properties of the MgNi-based systems. J. Alloy. Compd. 1999, 293, 437–442. [Google Scholar] [CrossRef]
- Yvon, K.; Schefer, J.; Stucki, F. Structural studies of the hydrogen storage material Mg2NiH4. 1. Cubic high-temperature structure. Inorg. Chem. 1980, 20, 2776–2778. [Google Scholar] [CrossRef]
- Mitov, M.; Chorbadzhiyska, E.; Nalbandian, L.; Hubenova, Y. Nickel-based electrodeposits as potential cathode catalysts for hydrogen production by microbial electrolysis. J. Power Sources 2017, 356, 467–472. [Google Scholar] [CrossRef]
- Hohenberg, P.; Kohn, W. Inhomogeneous electron gas. Phys. Rev. 1964, 11, B864. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Nisar, J.; Pathak, B.; Ahuja, R. Screened hybrid density functional study on Sr2Nb2O7 for visible light photocatalysis. Appl. Phys. Lett. 2012, 100, 181903. [Google Scholar] [CrossRef]
- Cui, X.Y.; Medvedeva, J.E.; Delley, B.; Freeman, A.J.; Newman, N.; Stampfl, C. Role of embedded clustering in dilute magnetic semiconductors: Cr doped GaN. Phys. Rev. Lett. 2005, 95, 256404. [Google Scholar] [CrossRef]
- Jiang, G.; Qian, Z.; Bououdina, M.; Ahuja, R.; Liu, X. Exploring pristine and Li-doped Mg2NiH4 compounds with potential lithium-storage properties: Ab initio insight. J. Alloy. Compd. 2018, 746, 140–146. [Google Scholar] [CrossRef]
- Ceder, G.; Aydinol, M.K.; Kohan, A.F. Application of first-principles calculations to the design of rechargeable Li-batteries. Comput. Mater. Sci. 1997, 8, 161–169. [Google Scholar] [CrossRef]
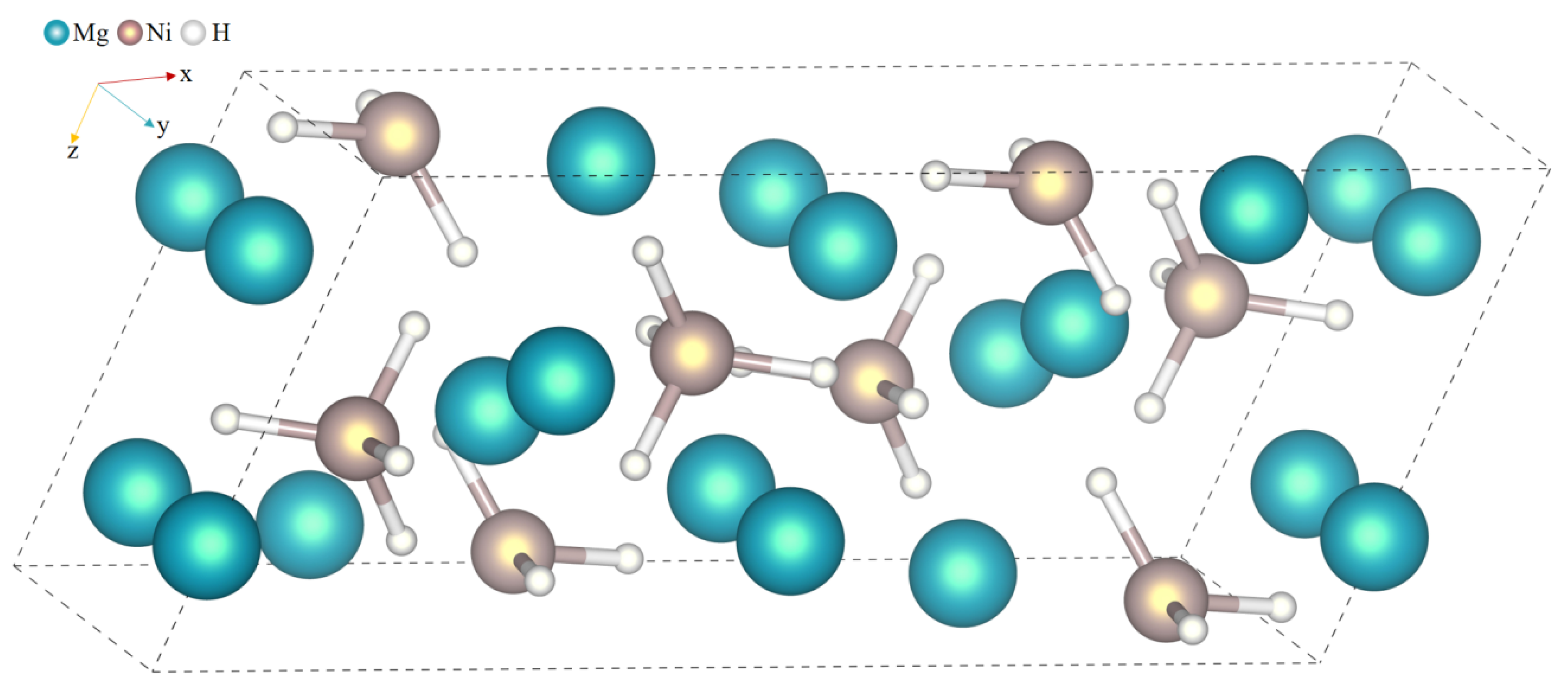

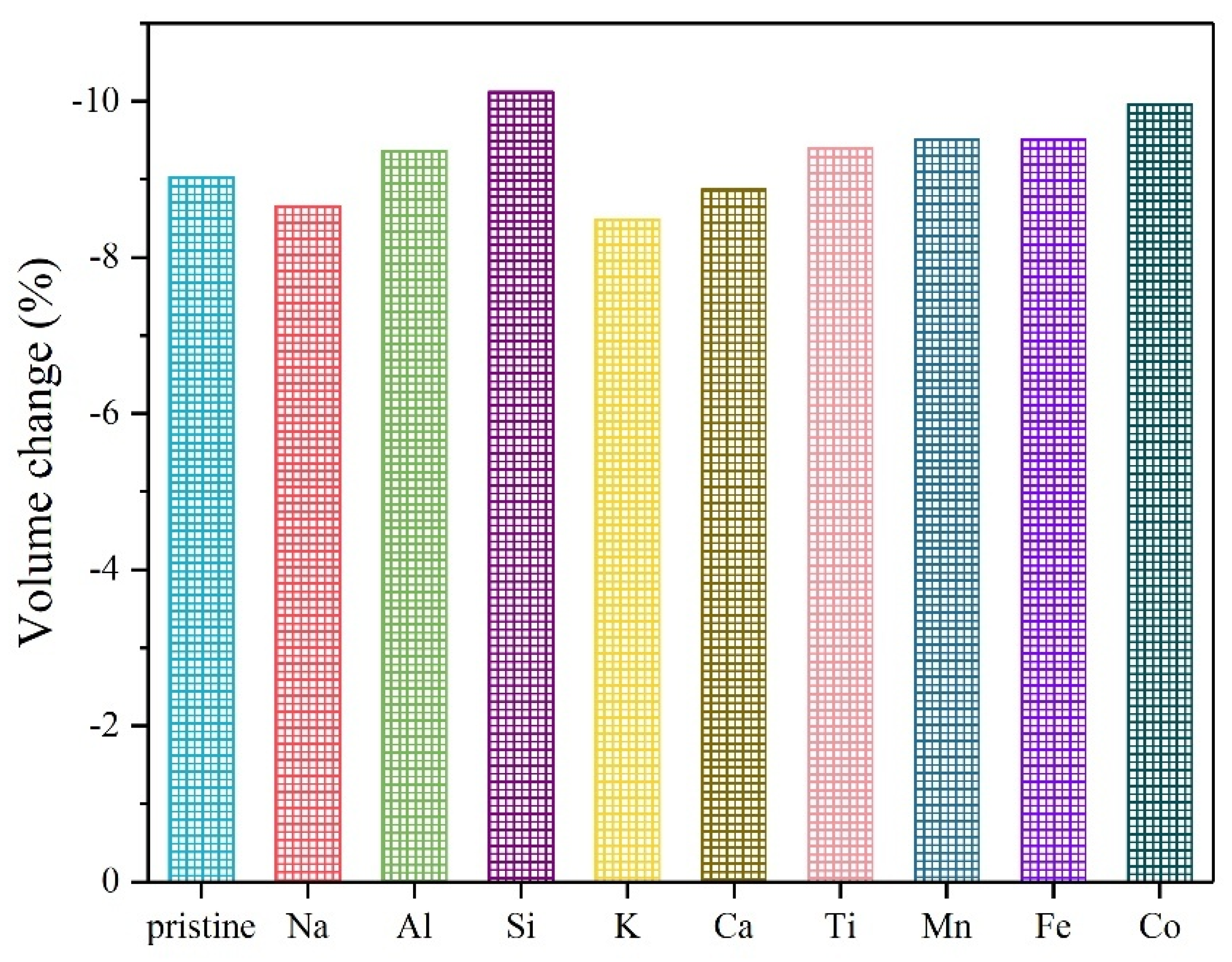
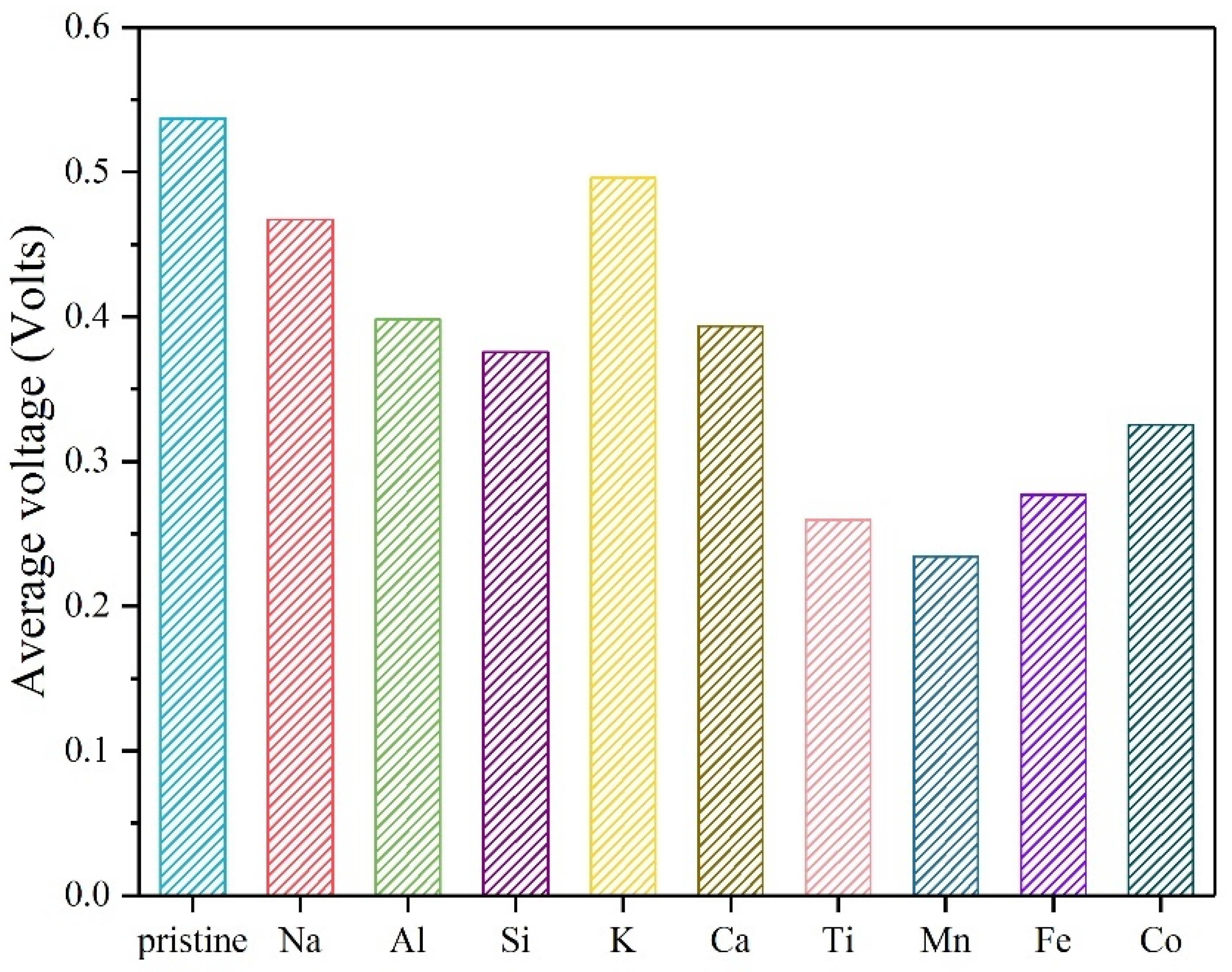
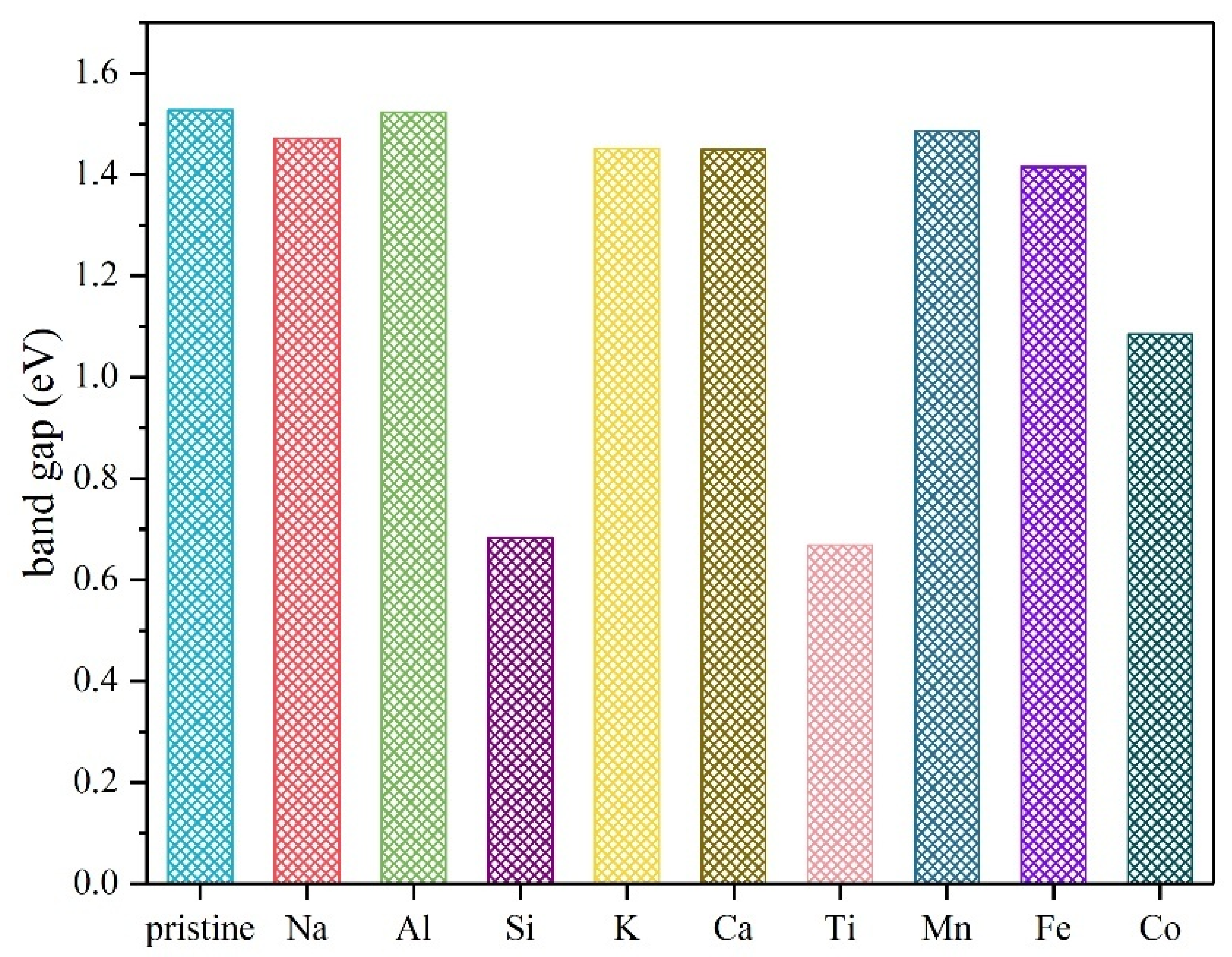
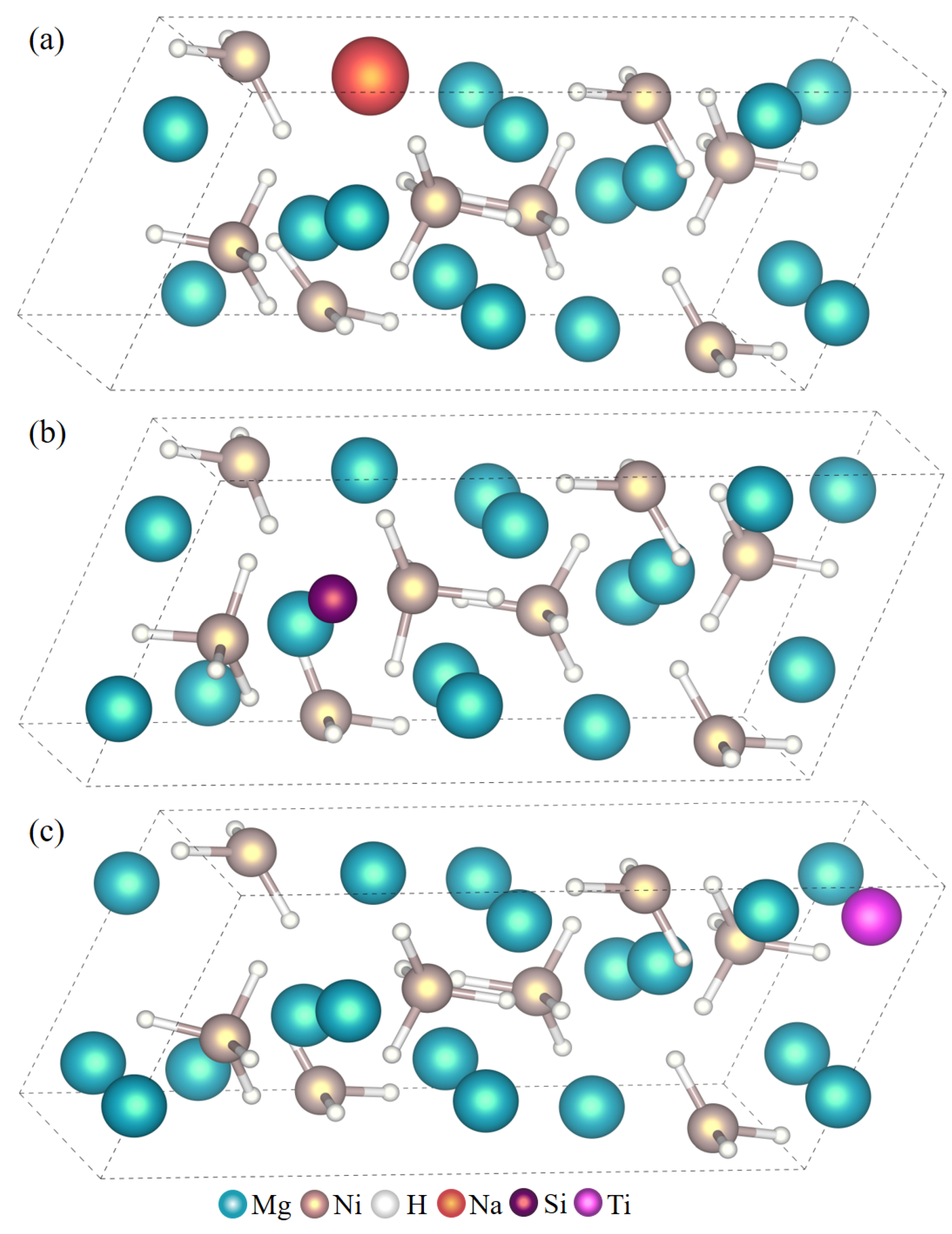
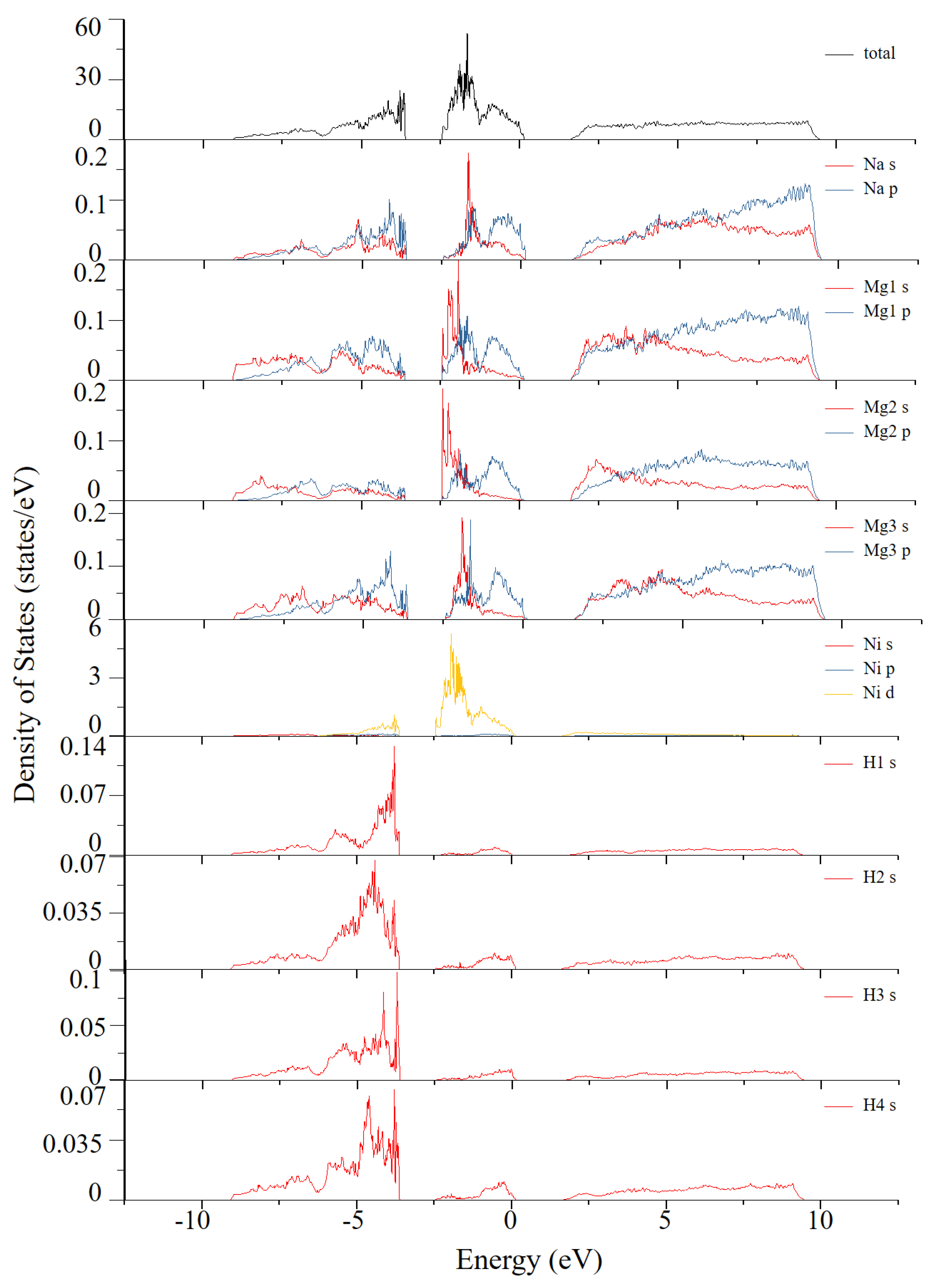
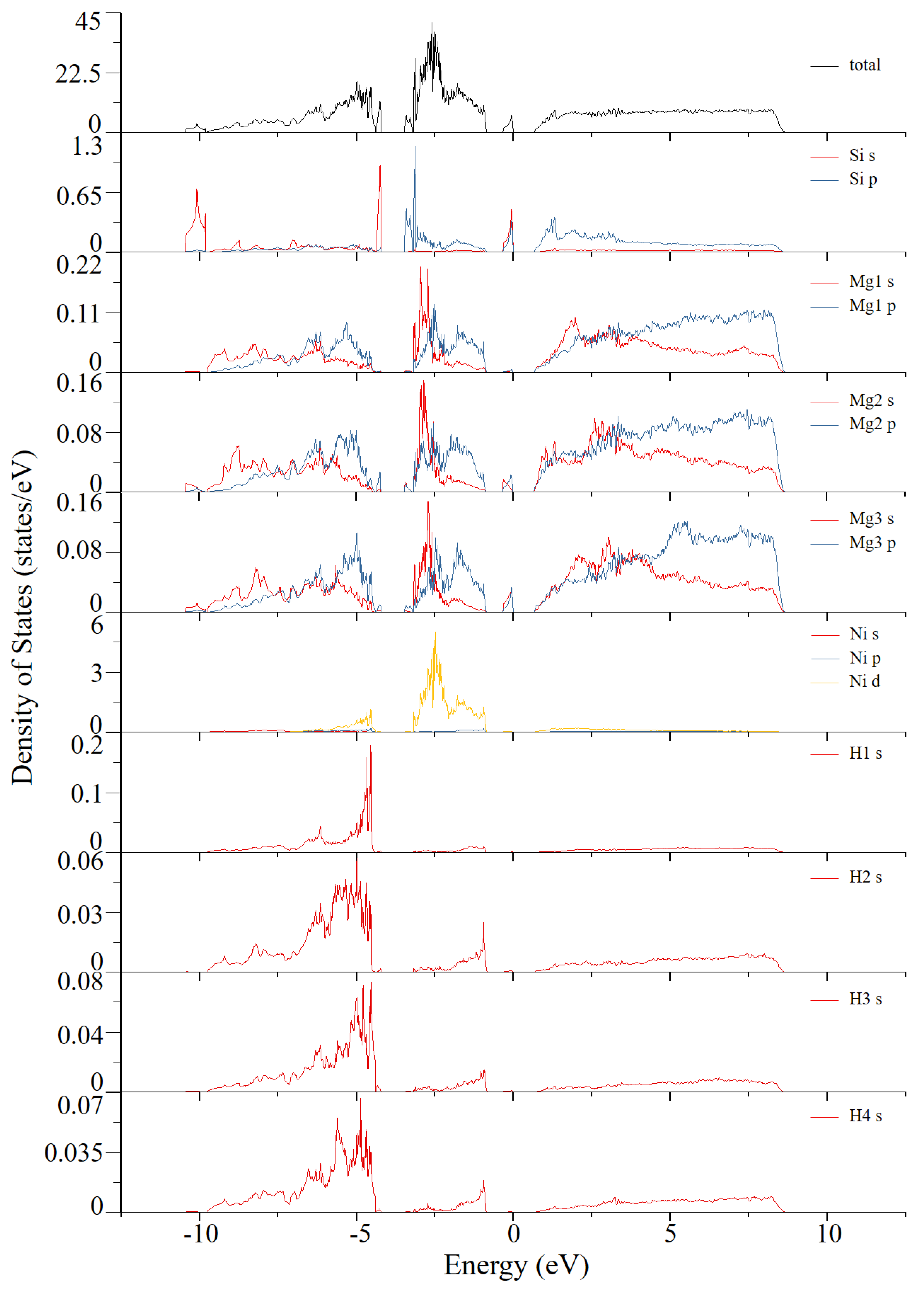
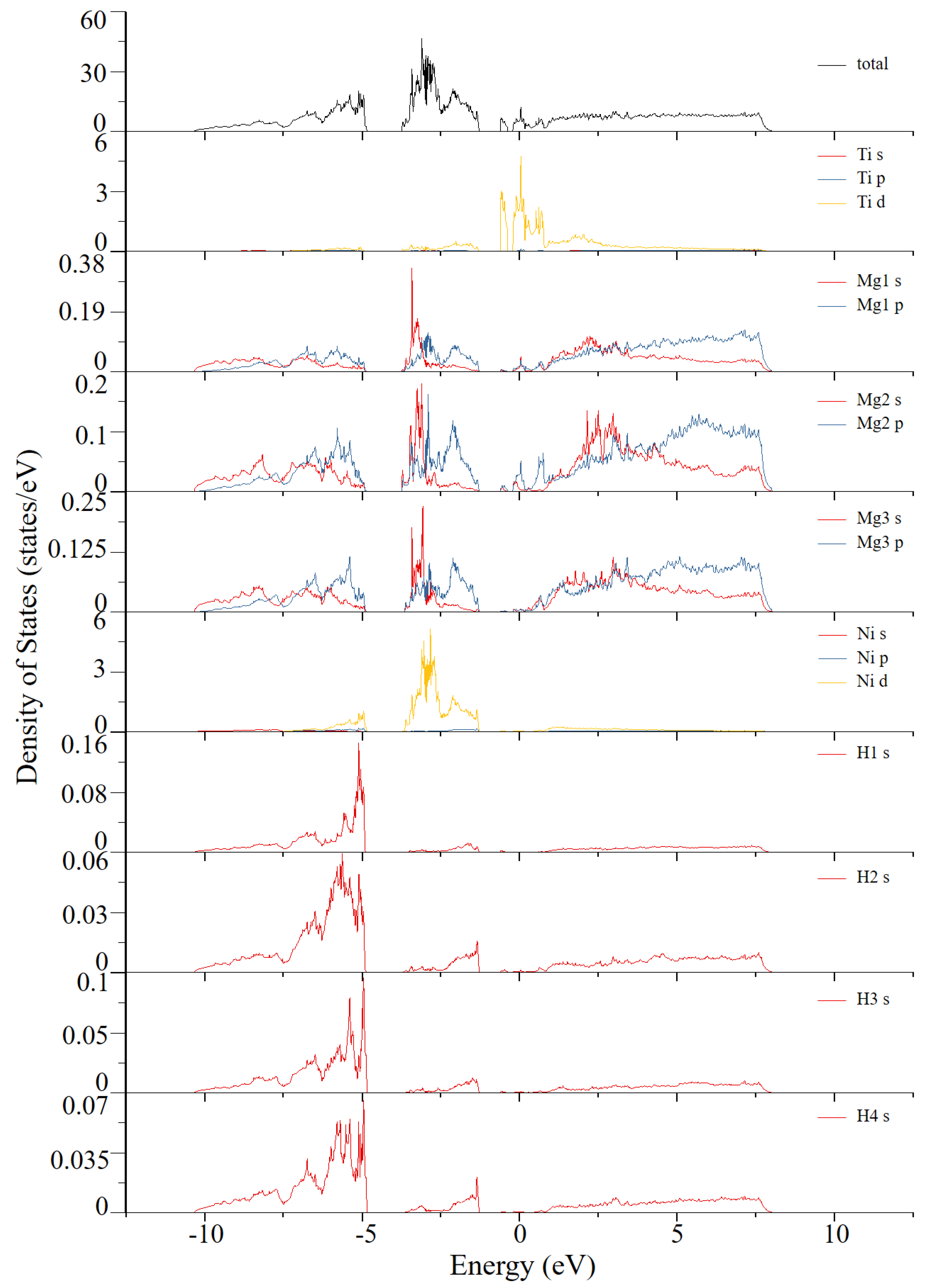
| Atom Type | Site | x | y | z |
|---|---|---|---|---|
| Mg1 | 8f | 0.26389 | 0.48813 | 0.08155 |
| Mg2 | 4e | 0.00000 | 0.02287 | 0.25000 |
| Mg3 | 4e | 0.00000 | 0.52380 | 0.25000 |
| Ni | 8f | 0.11991 | 0.23067 | 0.07987 |
| H1 | 8f | 0.21074 | 0.30420 | 0.30318 |
| H2 | 8f | 0.13847 | 0.31959 | 0.87295 |
| H3 | 8f | 0.00890 | 0.28864 | 0.05115 |
| H4 | 8f | 0.12497 | 0.98652 | 0.07292 |
| Element | Mg1 Site | Mg2 Site | Mg3 Site | Ni Site |
|---|---|---|---|---|
| Na | 0.18689 | 0.19680 | 0.20369 | 0.88411 |
| Al | −0.05240 | −0.08164 | −0.04217 | 0.59143 |
| Si | −0.09707 | −0.12752 | −0.04054 | 0.43428 |
| K | 0.29441 | 0.37751 | 7.66576 | 0.97687 |
| Ca | −0.12695 | −0.11233 | −0.12014 | 0.72187 |
| Ti | −0.33983 | −0.32535 | −0.34227 | 0.30145 |
| Mn | −0.12189 | −0.11608 | −0.12628 | 0.39103 |
| Fe | −0.17242 | −0.15257 | −0.19421 | 0.14697 |
| Co | −0.15074 | −0.13761 | −0.16507 | 0.05557 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qian, Z.; Jiang, G.; Ren, Y.; Nie, X.; Ahuja, R. Atomistic Modeling of Various Doped Mg2NiH4 as Conversion Electrode Materials for Lithium Storage. Crystals 2019, 9, 254. https://doi.org/10.3390/cryst9050254
Qian Z, Jiang G, Ren Y, Nie X, Ahuja R. Atomistic Modeling of Various Doped Mg2NiH4 as Conversion Electrode Materials for Lithium Storage. Crystals. 2019; 9(5):254. https://doi.org/10.3390/cryst9050254
Chicago/Turabian StyleQian, Zhao, Guanzhong Jiang, Yingying Ren, Xi Nie, and Rajeev Ahuja. 2019. "Atomistic Modeling of Various Doped Mg2NiH4 as Conversion Electrode Materials for Lithium Storage" Crystals 9, no. 5: 254. https://doi.org/10.3390/cryst9050254
APA StyleQian, Z., Jiang, G., Ren, Y., Nie, X., & Ahuja, R. (2019). Atomistic Modeling of Various Doped Mg2NiH4 as Conversion Electrode Materials for Lithium Storage. Crystals, 9(5), 254. https://doi.org/10.3390/cryst9050254







