Impact of Delay Time before Annealing MAI-PbI2-DMSO Intermediate Phase on Perovskite Film Quality and Photo-Physical Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Device Fabrication
2.3. Characterization
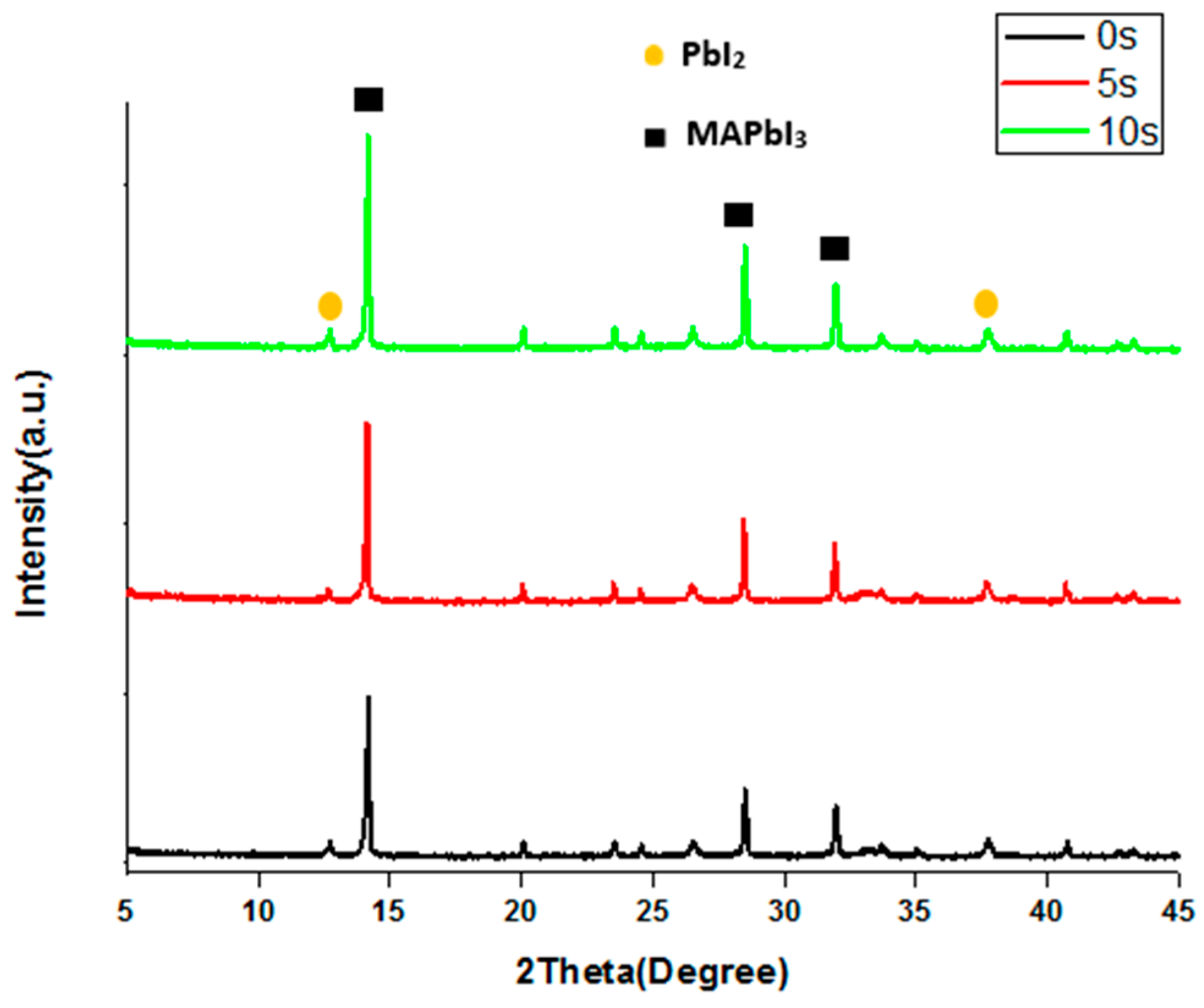
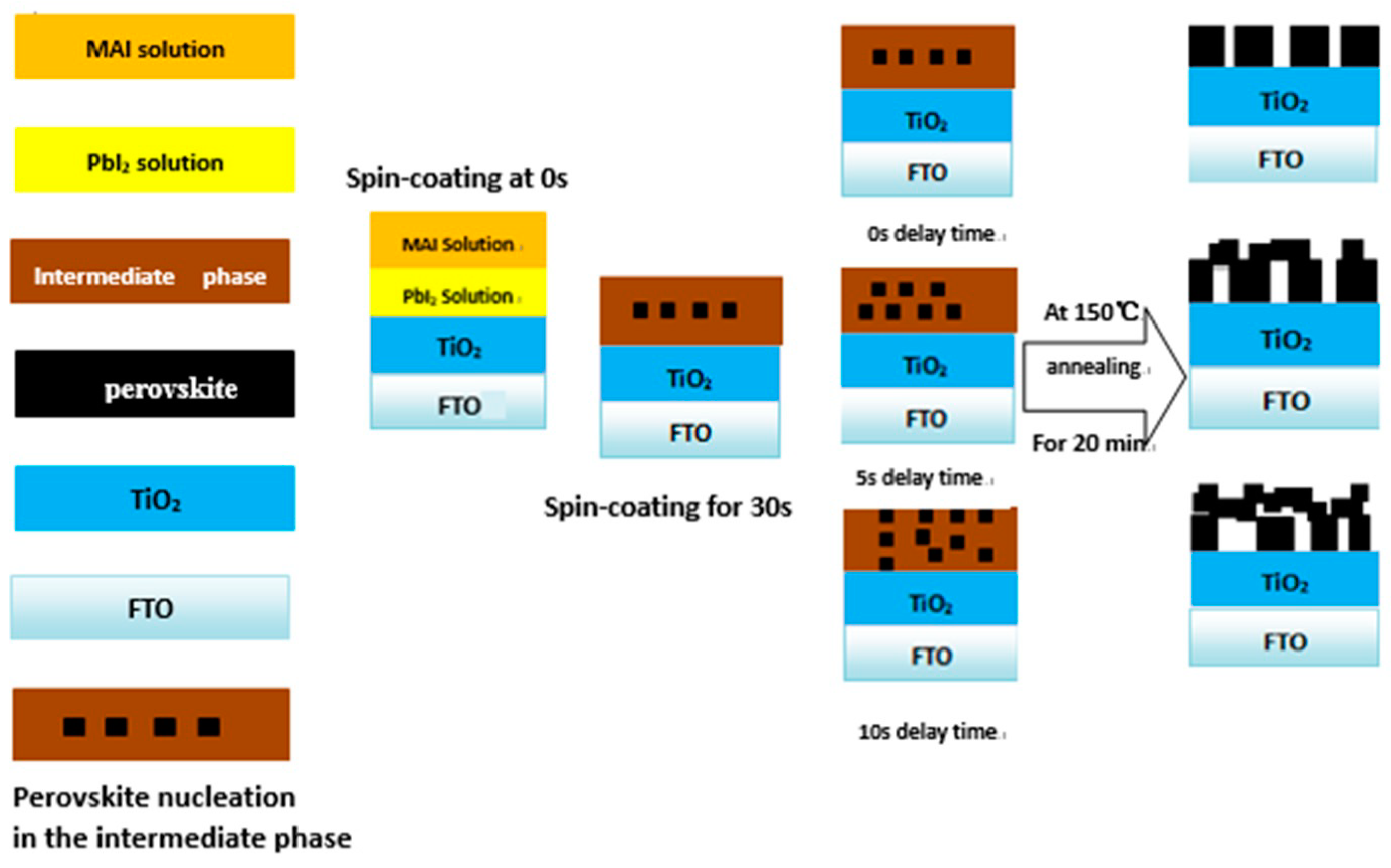
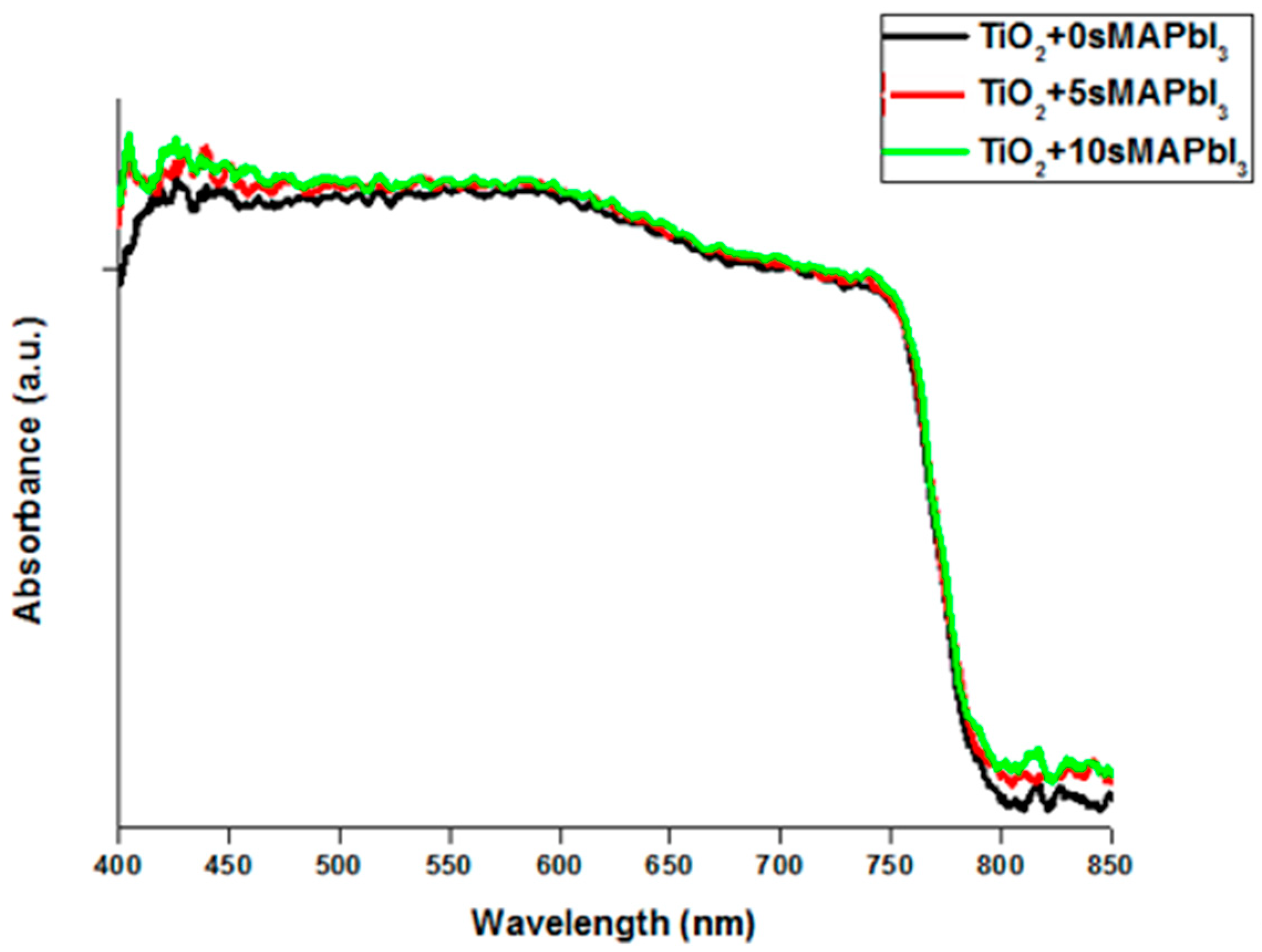
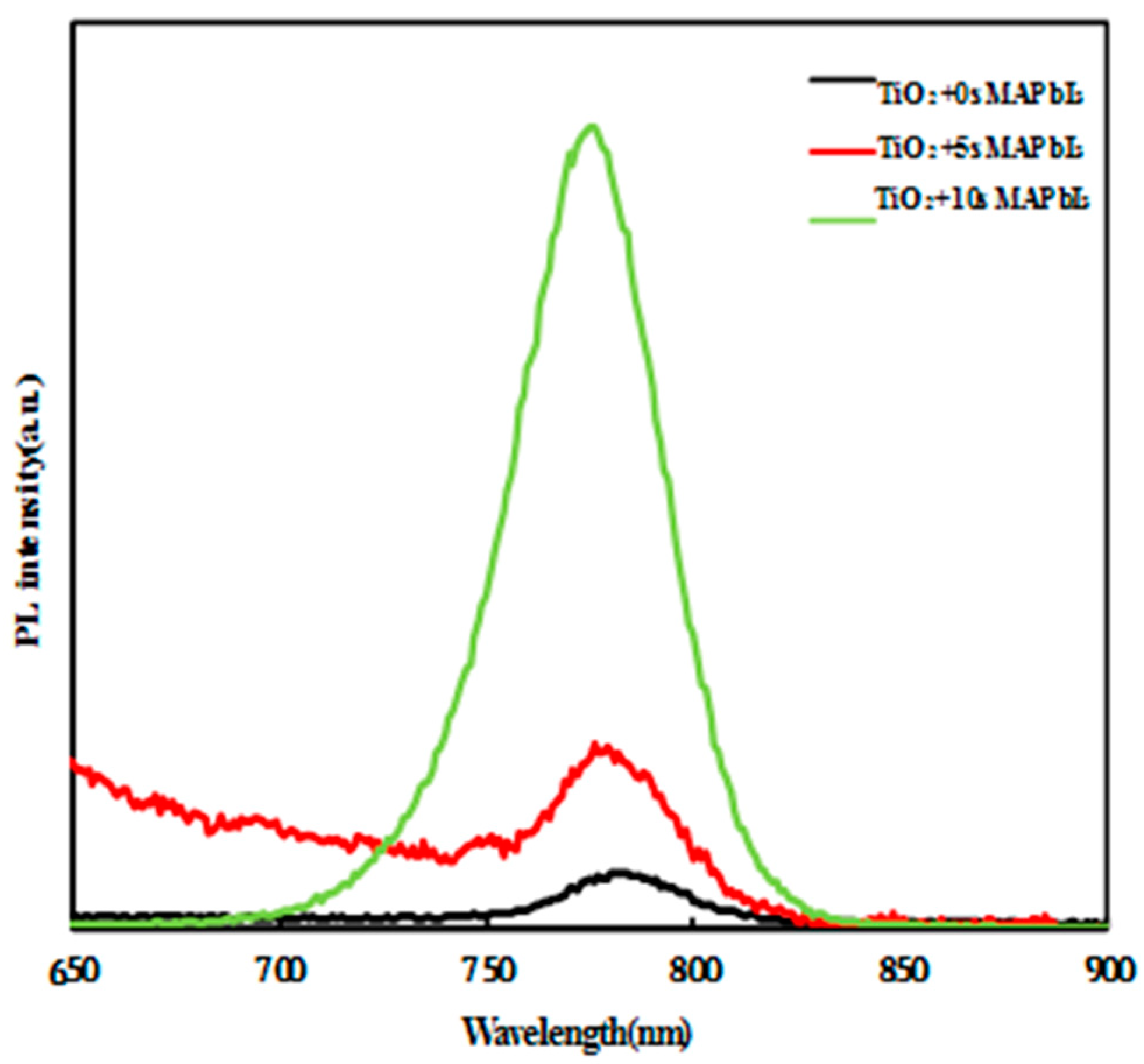
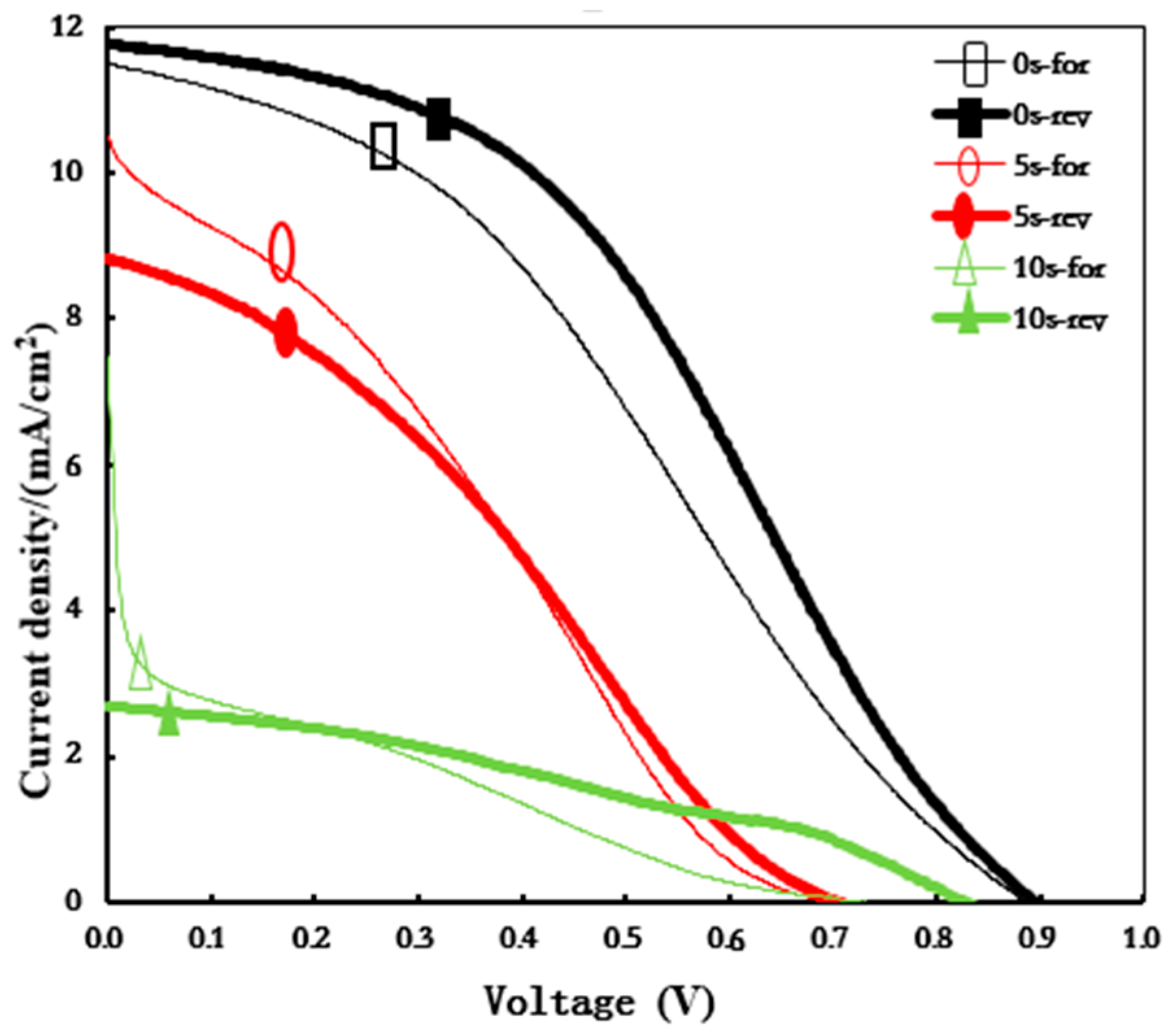
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mokhtar, M.Z.; Chen, M.; Whittaker, E.; Hamilton, B.; Aristidou, N.; Ramadan, S.; Gholinia, A.; Haque, S.A.; Saunders, B.R. CH3NH3PbI3 films prepared by combining 1- and 2-step deposition: How crystal growth conditions affect properties. Phys. Chem. Chem. Phys. 2017, 19, 7204–7214. [Google Scholar] [CrossRef] [PubMed]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef]
- Available online: https://www.nrel.gov/pv/assets/pdfs/pv-efficiency-chart.20190103.pdf (accessed on 3 January 2019).
- Choi, Y.C.; Lee, S.W.; Kim, D.H. Antisolvent-assisted powder engineering for controlled growth of hybrid CH3NH3PbI3 perovskite thin films. APL Mater. 2017, 5, 026101. [Google Scholar] [CrossRef]
- Khadka, D.B.; Shirai, Y.; Yanagida, M.; Masuda, T.; Miyano, K. Enhancement in efficiency and optoelectronic quality of perovskite thin films annealed in MACl vapor. Sustain. Energy Fuels 2017, 1, 755–766. [Google Scholar] [CrossRef]
- Zhou, Z.M.; Wang, Z.W.; Zhou, Y.Y.; Pang, S.P.; Wang, D.; Xu, H.X.; Liu, Z.H.; Padture, N.P.; Cui, G.L. Methylamine-gas-induced defect-healing behavior of CH3NH3PbI3 thin films for perovskite solar cells. Angew. Chem. Int. Ed. 2015, 54, 9705–9709. [Google Scholar] [CrossRef] [PubMed]
- Rahaq, Y.; Moussa, M.; Mohammad, A.; Wang, H.M.; Hassan, A. Highly reproducible perovskite solar cells via controlling the morphologies of the perovskite thin films by the solution-processed two-step method. J. Mater. Sci. Mater. Electron. 2018, 29, 16426–16436. [Google Scholar] [CrossRef]
- Mo, J.J.; Zhang, C.F.; Chang, J.J.; Yang, H.F.; Xi, H.; Chen, D.Z.; Lin, Z.H.; Lu, G.; Zhang, J.H.; Hao, Y. Enhanced efficiency of planar perovskite solar cells via a two-step deposition using DMF as an additive to optimize the crystal growth behavior. J. Mater. Chem. A 2017, 5, 13032–13038. [Google Scholar] [CrossRef]
- Liu, D.; Li, Y.; Shi, B.; Yao, X.; Fan, L.; Zhao, S.Z.; Liang, J.H.; Ding, Y.; Wei, C.C.; Zhang, D.K.; et al. Tailoring morphology and thickness of perovskite layer for flexible perovskite solar cells on plastics: The role of CH3NH3I concentration. Sol. Energy 2017, 147, 222–227. [Google Scholar] [CrossRef]
- Zhou, Y.Y.; Yang, M.J.; Vasiliev, A.L.; Garces, H.F.; Zhao, Y.X.; Wang, D.; Pang, S.P.; Zhu, K.; Padture, N.P. Growth control of compact CH3NH3PbI3 thin films via enhanced solid-state precursor reaction for efficient planar perovskite solar cells. J. Mater. Chem. A 2015, 3, 9249–9256. [Google Scholar] [CrossRef]
- Chang, J.; Zhu, H.; Li, B.; Isikgor, F.H.; Hao, Y.; Xu, Q.H.; Jian, Y. Boosting the performance of planar heterojunction perovskite solar cells by controlling the precursor purity of perovskite materials. J. Mater. Chem. A 2016, 4, 887–893. [Google Scholar] [CrossRef]
- Sun, C.Y.; Guo, Y.P.; Fang, B.J.; Guan, L.; Duan, H.A.; Chen, Y.J.; Li, H.; Liu, H.Z. Facile preparation of high-quality perovskites for efficient solar cells via a fast conversion of wet PbI2 precursor films. RSC Adv. 2017, 7, 22492–22500. [Google Scholar] [CrossRef]
- Acuna, D.; Krishnan, B.; Shaji, S.; Avellaneda, D. On the structure and physical properties of methyl ammonium lead iodide perovskite thin films by the two-step deposition method. Mater. Chem. Phys. 2018, 215, 137–147. [Google Scholar]
- Ling, T.; Zou, X.P.; Cheng, J.; Bai, X.; Ren, H.Y.; Chen, D. Modified sequential deposition route through localized-liquid-liquid-diffusion for improved perovskite multi-crystalline thin films with micrometer-scaled grains for solar cells. Nanomaterials 2018, 8, 416. [Google Scholar] [CrossRef] [PubMed]
- Jeon, N.J.; Noh, J.H.; Kim, Y.C.; Yang, W.S.; Ryu, S.C.; Seok, S.L. Solvent engineering for high-performance inorganic-organic hybrid perovskite solar cells. Nat. Mater. 2014, 13, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.Y.; Zou, X.P.; Cheng, J.; Ling, T.; Bai, X.; Chen, D. Facile solution spin-coating SnO2 thin film covering cracks of TiO2 hole blocking layer for perovskite solar cells. Coatings 2018, 8, 314. [Google Scholar] [CrossRef]
- Yang, Y.; Zou, X.; Pei, Y.X.; Bai, X.; Jin, W.B.; Chen, D. Effect of doping of NaI monovalent cation halide on the structural, morphological, optical and optoelectronic properties of MAPbI3 perovskite. J. Mater. Sci.: Mater. Electron. 2017, 29, 205–210. [Google Scholar] [CrossRef]
- Zhao, Y.C.; Zhou, W.K.; Zhou, X.; Liu, K.H.; Yu, D.P.; Zhao, Q. Quantification of light-enhanced ionic transport in lead iodide perovskite thin films and its solar cells applications. Light Sci. Appl. 2017, 6, e16243. [Google Scholar] [CrossRef]
- Zhao, J.; Cai, B.; Luo, Z.L.; Dong, Y.Q.; Zhang, Y.; Xu, H.; Hong, B.; Yang, Y.J.; Li, L.B.; Zhang, W.H. Investigation of the hydrolysis of perovskite organometallic halide CH3NH3PbI3 in humidity environment. Sci. Rep. 2016, 6, 21976. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.L.; Chu, Q.Q.; Dong, H.; Yao, J.T.; Zhou, Y.; Yang, G. Tuning nucleation sites to enable monolayer perovskite films for highly efficient perovskite solar cells. Coatings 2018, 8, 408. [Google Scholar] [CrossRef]
- Huang, Y.C.; Tsao, C.S.; Cho, Y.J.; Chen, K.C.; Chiang, K.M.; Hsiao, S.Y.; Chen, C.W.; Su, C.J.; Jeng, U.S. Insight into evolution, processing and performance of multi-length-scale structures in planar heterojunction perovskite solar cells. Sci. Rep. 2015, 5, 13657. [Google Scholar] [CrossRef]
- Khadka, D.B.; Shirai, Y.; Yanagida, M.; Ryan, J.W.; Miyano, K. Exploring the effects of interfacial carrier transport layers on device performance and optoelectronic properties of planar perovskite solar cells. J. Mater. Chem. C 2017, 5, 8819–8827. [Google Scholar] [CrossRef]
- Mojtaba, A.J.; Ibrahim, D.M.; Aditya, S.; Senanayak, S.P.; Fabrizio, G.; Mohammed, Z.S.; Michael, G. Impact of a mesoporous titania-perovskite interface on the performance of hybrid organic-inorganic perovskite solar cells. J. Phys. Chem. Lett. 2016, 7, 3264–3269. [Google Scholar]
- Tan, H.; Che, F.; Wei, M.; Zhao, Y.C.; Saidaminov, M.I.; Todorović, P.; Broberg, D.; Walters, G.; Tan, F.; Zhuang, T.T.; et al. Dipolar cations confer defect tolerance in wide-bandgap metal halide perovskites. Nat. Commun. 2018, 9, 3100. [Google Scholar] [CrossRef]
- Zhang, W.; Pathak, S.; Sakai, N.; Stergiopoulos, T.; Nayak, P.K.; Noel, N.K.; Haghighirad, A.A.; deQuilettes, D.W.; Sadhanala, A.; Li, W.; et al. Enhanced optoelectronic quality of perovskite thin films with hypophosphorous acid for planar heterojunction solar cells. Nat. Commun. 2015, 6, 10030. [Google Scholar] [CrossRef]
- Gedamu, D.; Asuo, I.M.; Benetti, D.; Basti, M.; Ka, I.; Cloutier, S.G.; Rose, F. Solvent-antisolvent ambient processed large grain size perovskite thin films for high-performance solar cells. Sci. Rep. 2018, 8, 12885. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, Y.; Zou, X.; Cheng, J.; Chen, D.; Chang, C.; Ling, T.; Ren, H. Impact of Delay Time before Annealing MAI-PbI2-DMSO Intermediate Phase on Perovskite Film Quality and Photo-Physical Properties. Crystals 2019, 9, 151. https://doi.org/10.3390/cryst9030151
Yao Y, Zou X, Cheng J, Chen D, Chang C, Ling T, Ren H. Impact of Delay Time before Annealing MAI-PbI2-DMSO Intermediate Phase on Perovskite Film Quality and Photo-Physical Properties. Crystals. 2019; 9(3):151. https://doi.org/10.3390/cryst9030151
Chicago/Turabian StyleYao, Yujun, Xiaoping Zou, Jin Cheng, Dan Chen, Chuangchuang Chang, Tao Ling, and Haiyan Ren. 2019. "Impact of Delay Time before Annealing MAI-PbI2-DMSO Intermediate Phase on Perovskite Film Quality and Photo-Physical Properties" Crystals 9, no. 3: 151. https://doi.org/10.3390/cryst9030151
APA StyleYao, Y., Zou, X., Cheng, J., Chen, D., Chang, C., Ling, T., & Ren, H. (2019). Impact of Delay Time before Annealing MAI-PbI2-DMSO Intermediate Phase on Perovskite Film Quality and Photo-Physical Properties. Crystals, 9(3), 151. https://doi.org/10.3390/cryst9030151



