The Lyotropic Analog of the Polar SmC* Phase
Abstract
1. Introduction
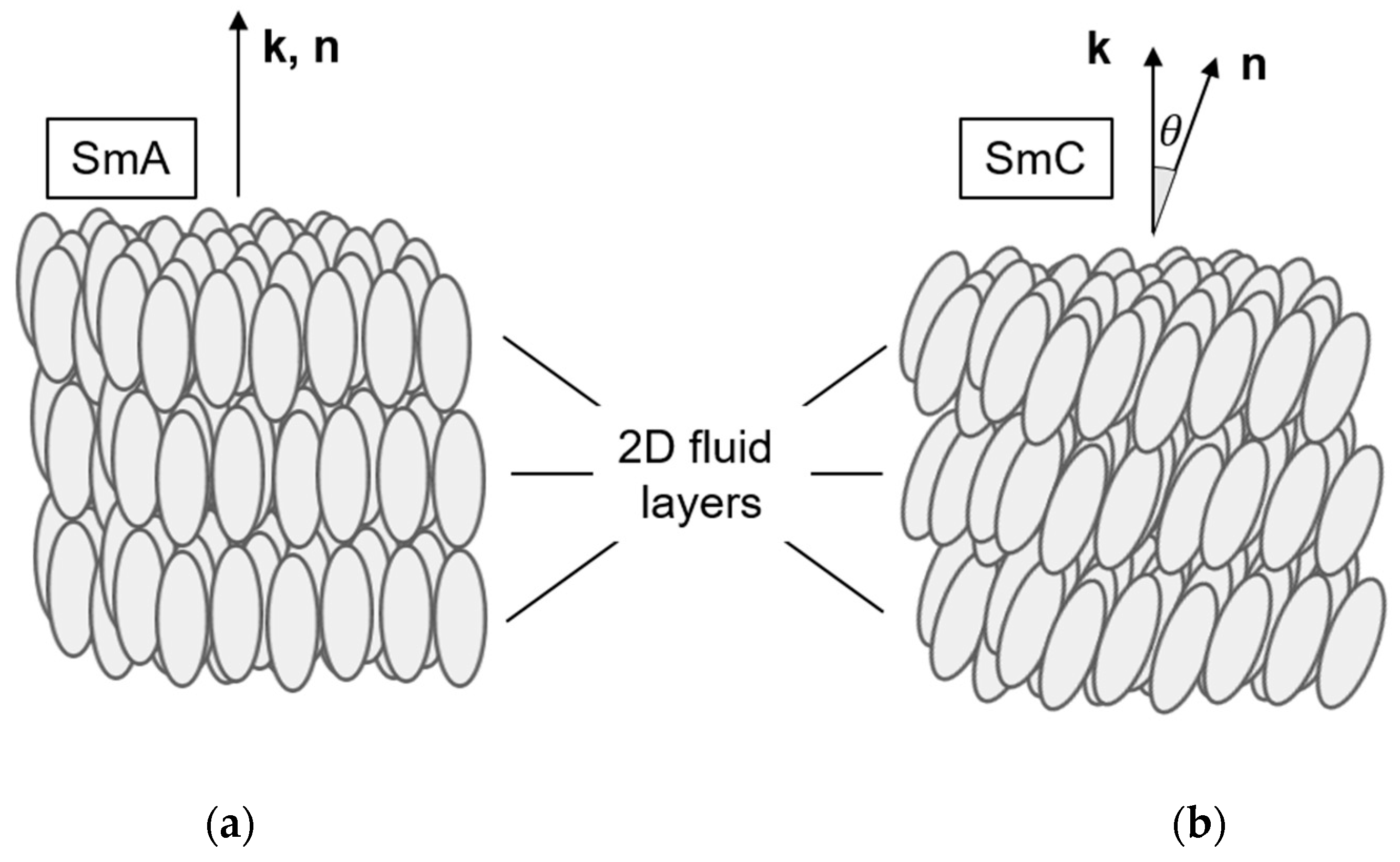
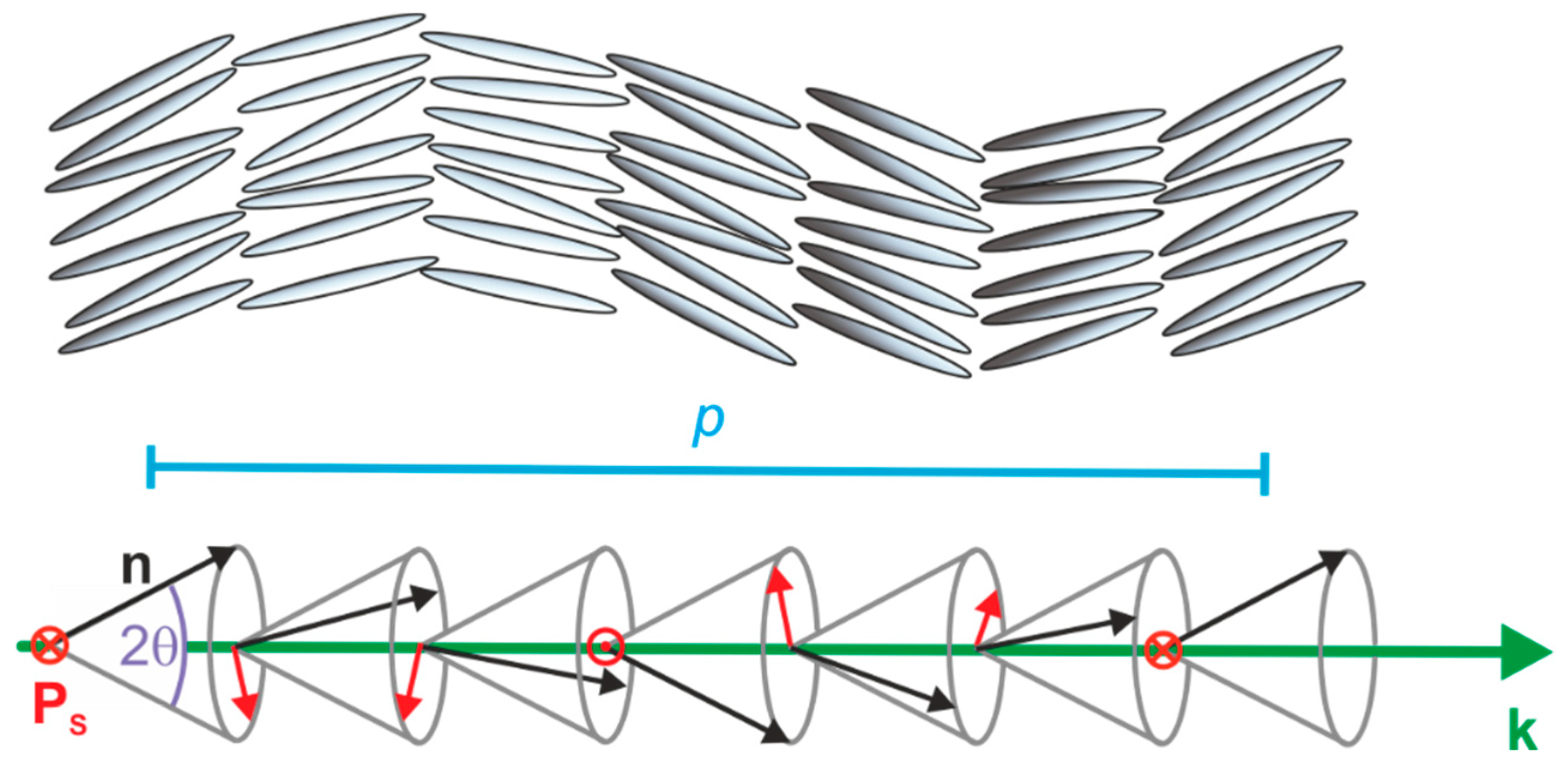
1.1. Ferroelectricity in Liquid Crystals
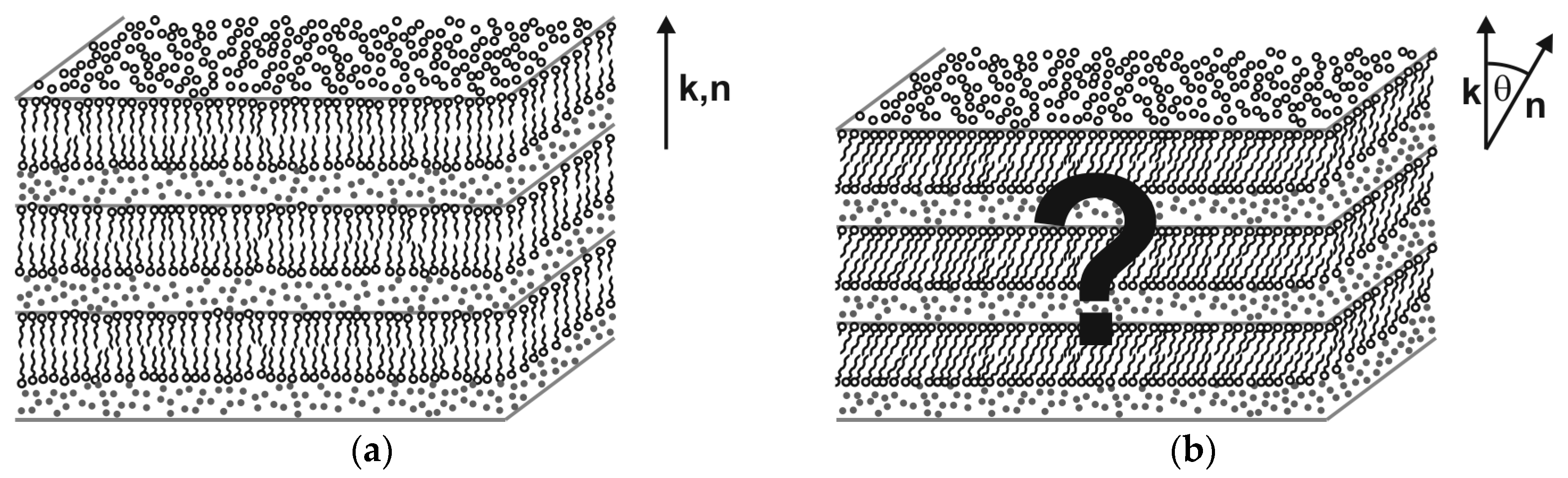
1.2. The Challenge of a Lyotropic SmC* Phase
- Intra-layer tilt correlation: In order to form a lyotropic C-phase the surfactant bilayers must be in a 2D-fluid state, the hydrophobic tails in each single bilayer must be tilted and the direction of tilt must be more or less the same throughout the bilayer. A uniform tilt direction in each single fluid bilayer might be hardly achieved with the flexible alkyl-tails of classic surfactants. The incorporation of rigid core segments (mesogenic cores) into the tails might, however, promote a collective tilt direction, e.g., by steric interactions between the rigid segments, and might lead to uniformly tilted amphiphiles in the bilayer.
- Inter-layer tilt correlation: The formation of a lyo-SmC structure further requires that in a 1D stack of bi-layers, each of which has a uniform tilt direction, the tilt directions become correlated between the bi-layers such that they all point into the same direction (synclinic correlation). In thermotropic SmC, where the smectic layers are in direct contact with each other, the synclinic tilt correlation is explained by short-range interactions, such as steric interactions or out-of-layer fluctuations, which align the tilt directions of molecules in adjacent layers [18]. In the lyotropic case, however, where the by-layers are separated from each other by solvent layers, these short-range interaction mechanisms are less relevant. Probably, the inter-layer correlation of tilt directions is the most critical step in the formation of lyo-SmC phases.
- Helical correlation: In addition to the first two points, the formation of a chiral lyo-SmC* structure requires that also subtle chiral perturbations of the synclinic tilt correlation, namely the helical precession of the tilt direction from bilayer to bilayer, are transmitted through the solvent layers.
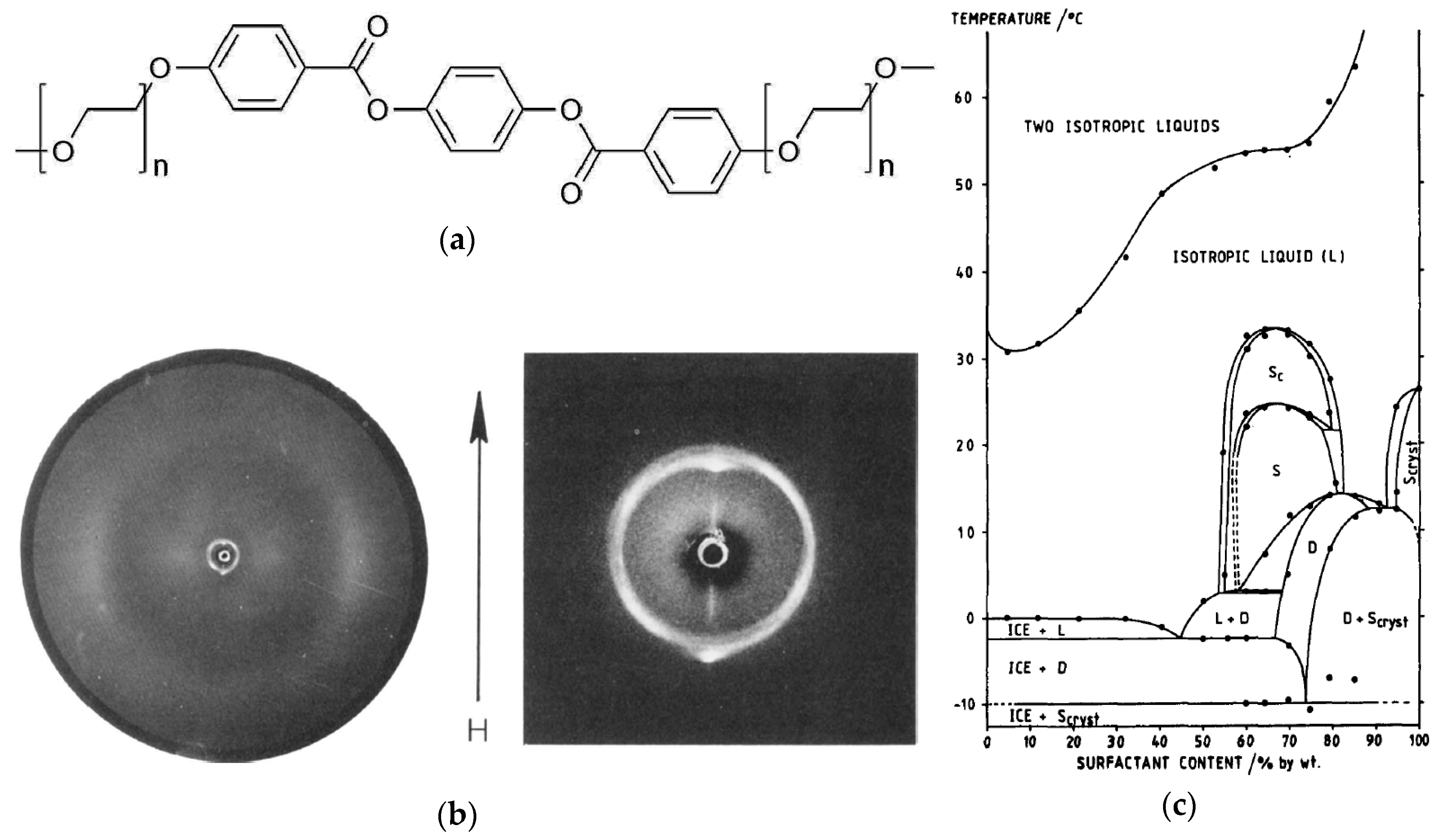
2. Examples of Swollen Thermotropic and Lyotropic SmC Phases
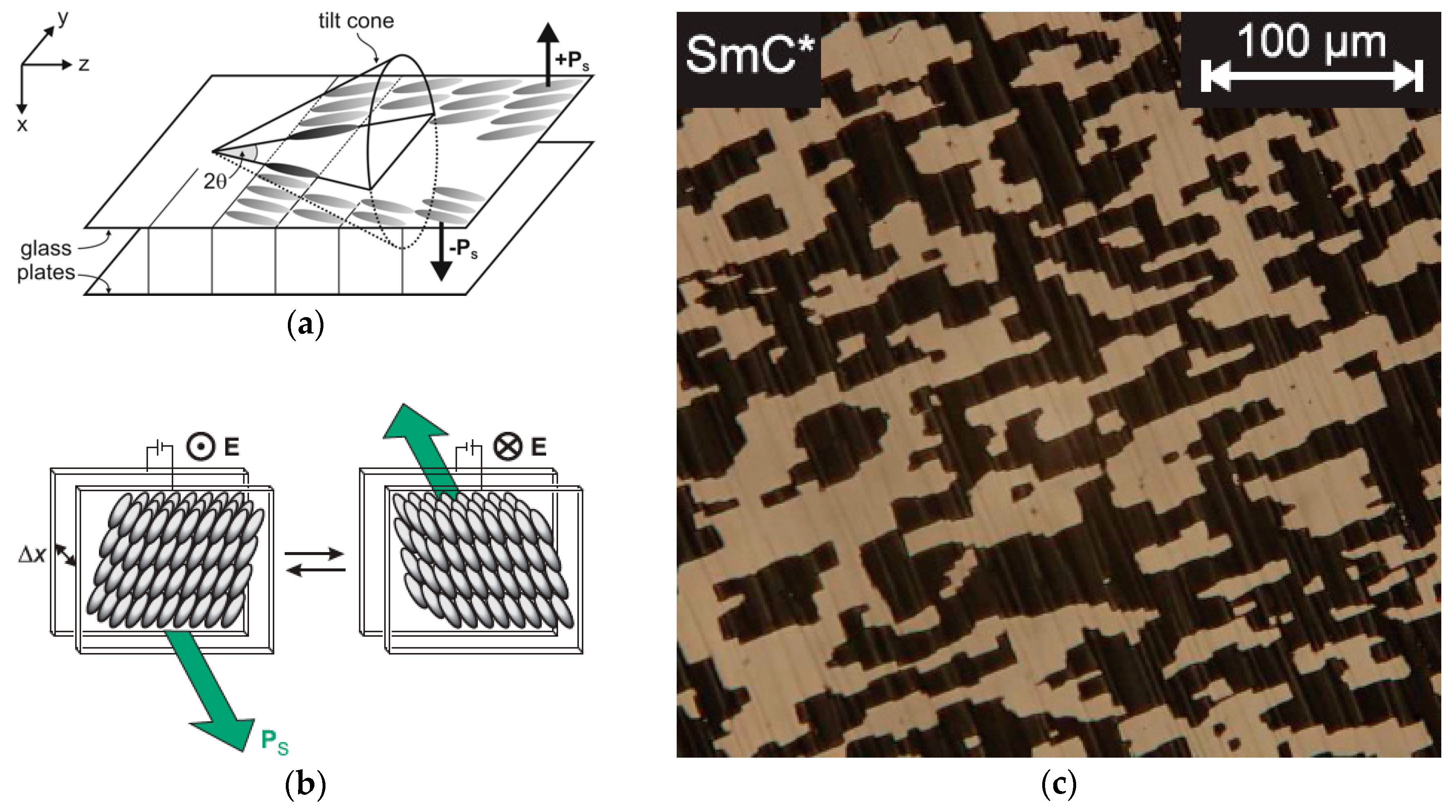
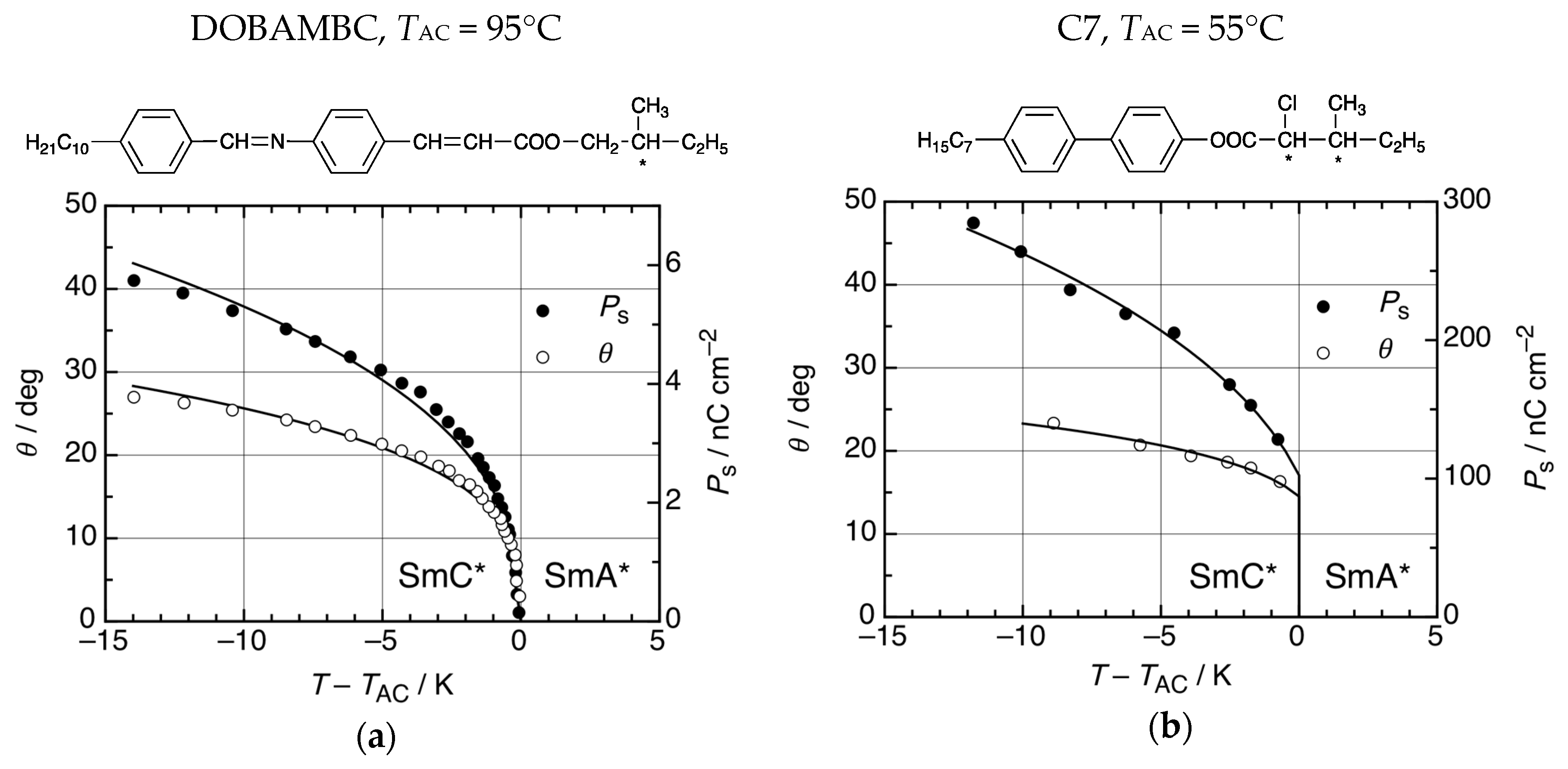
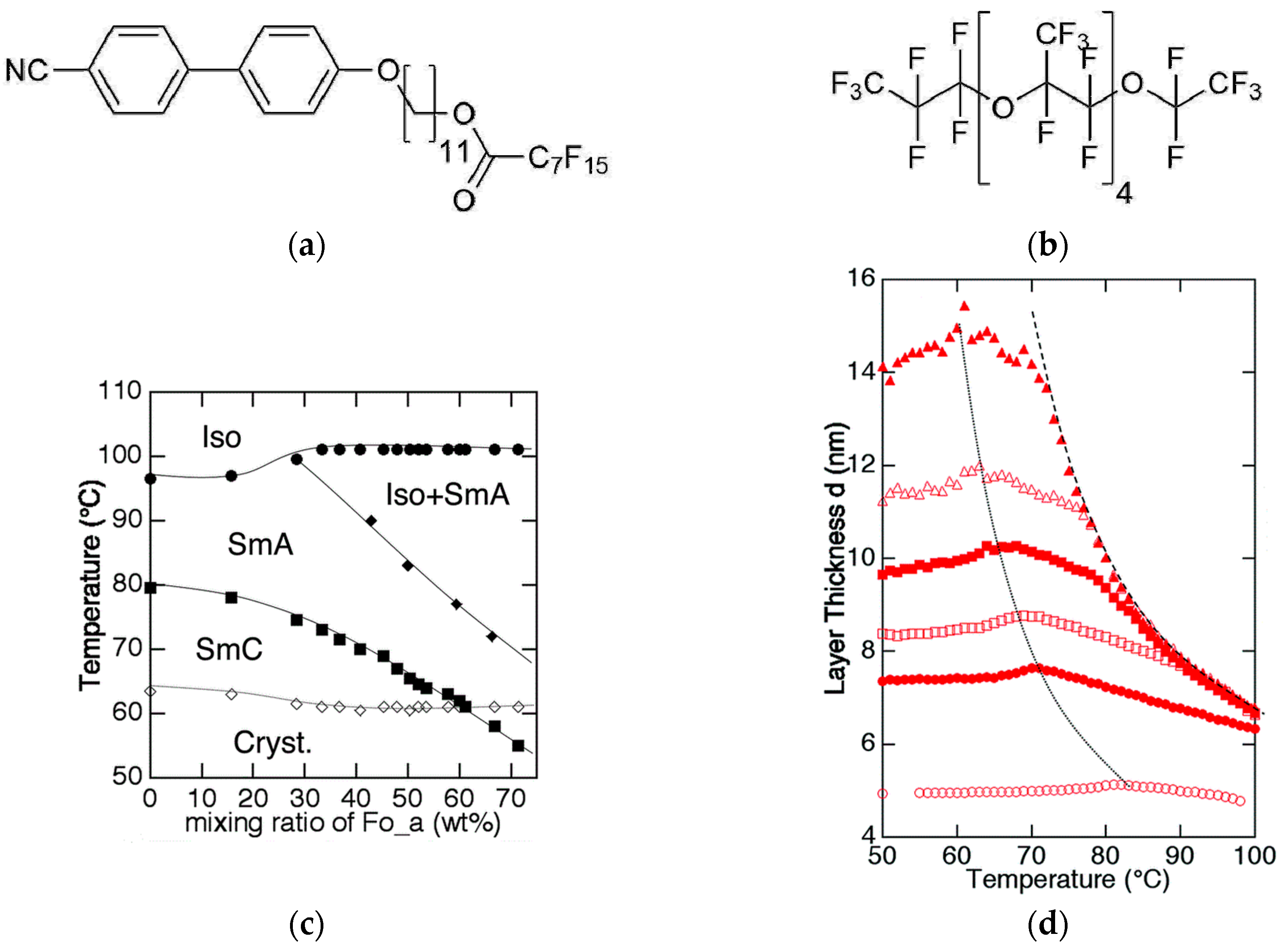
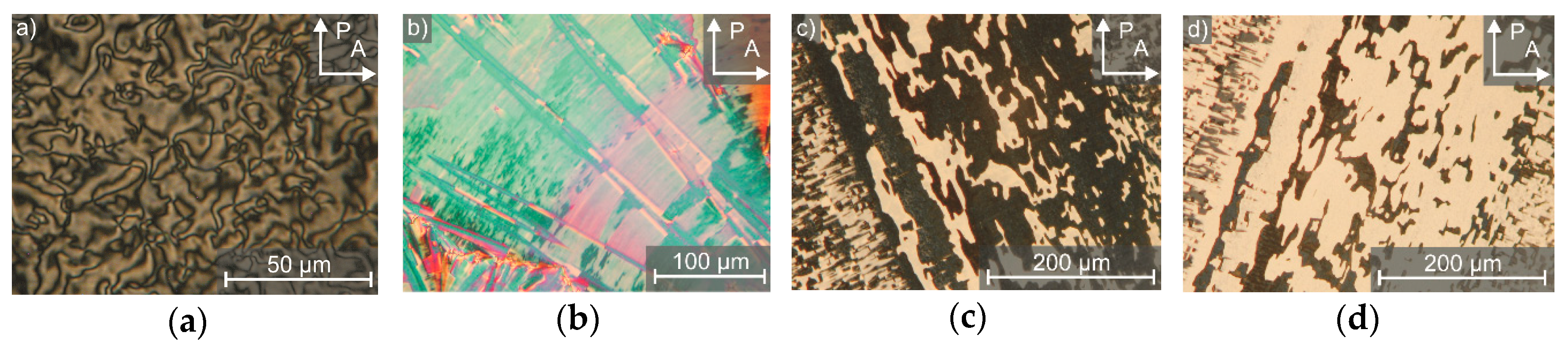
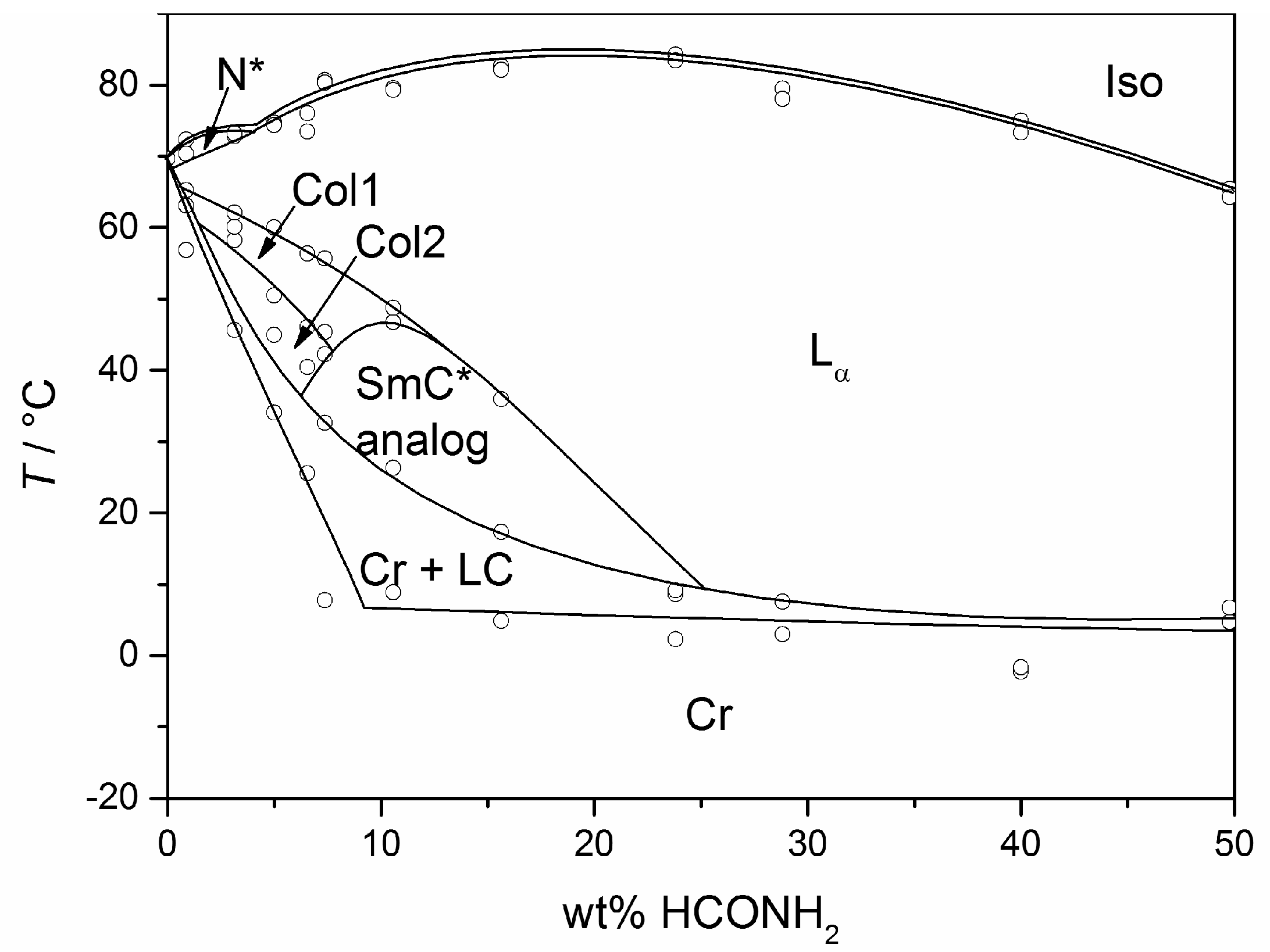
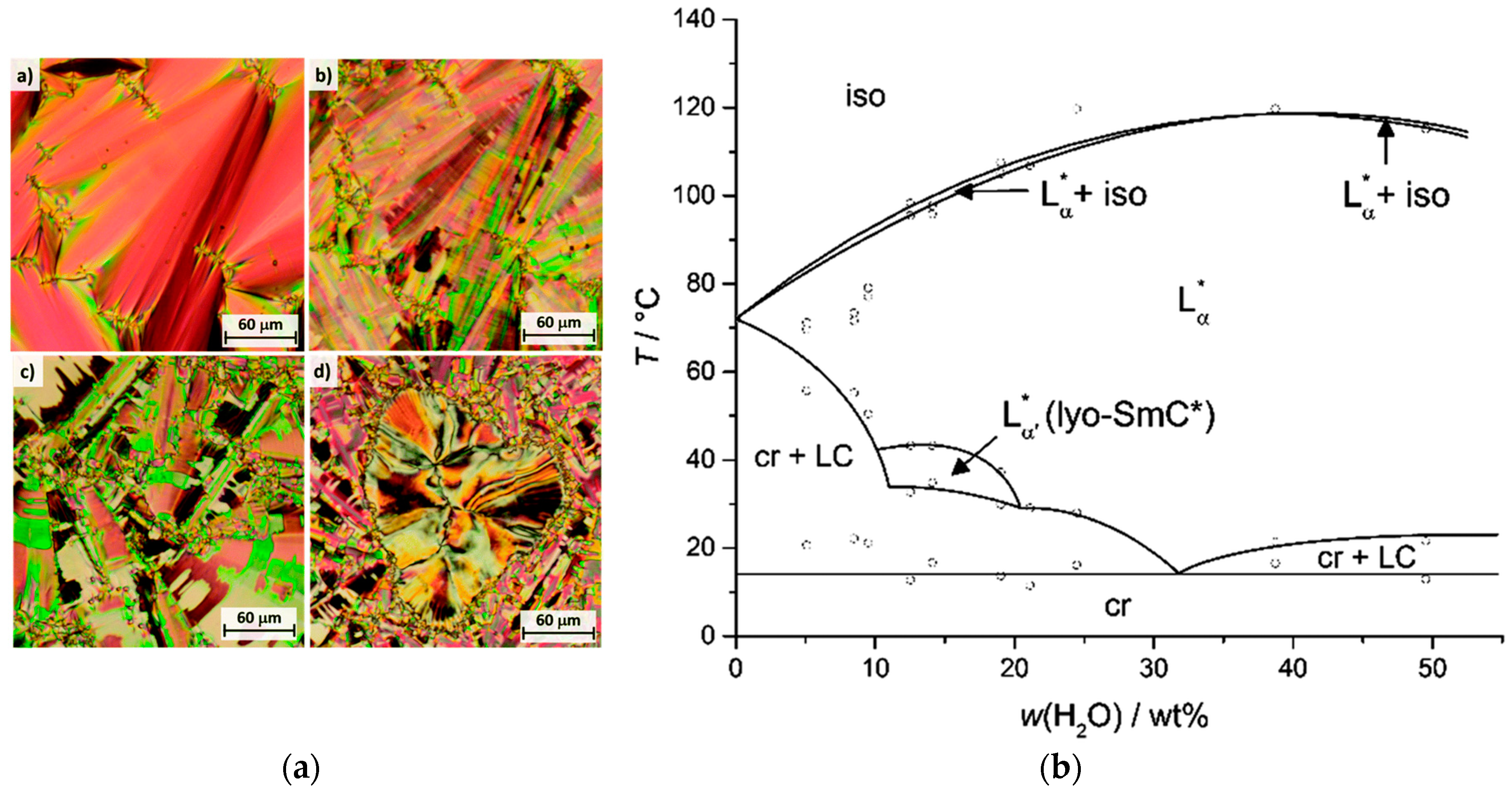
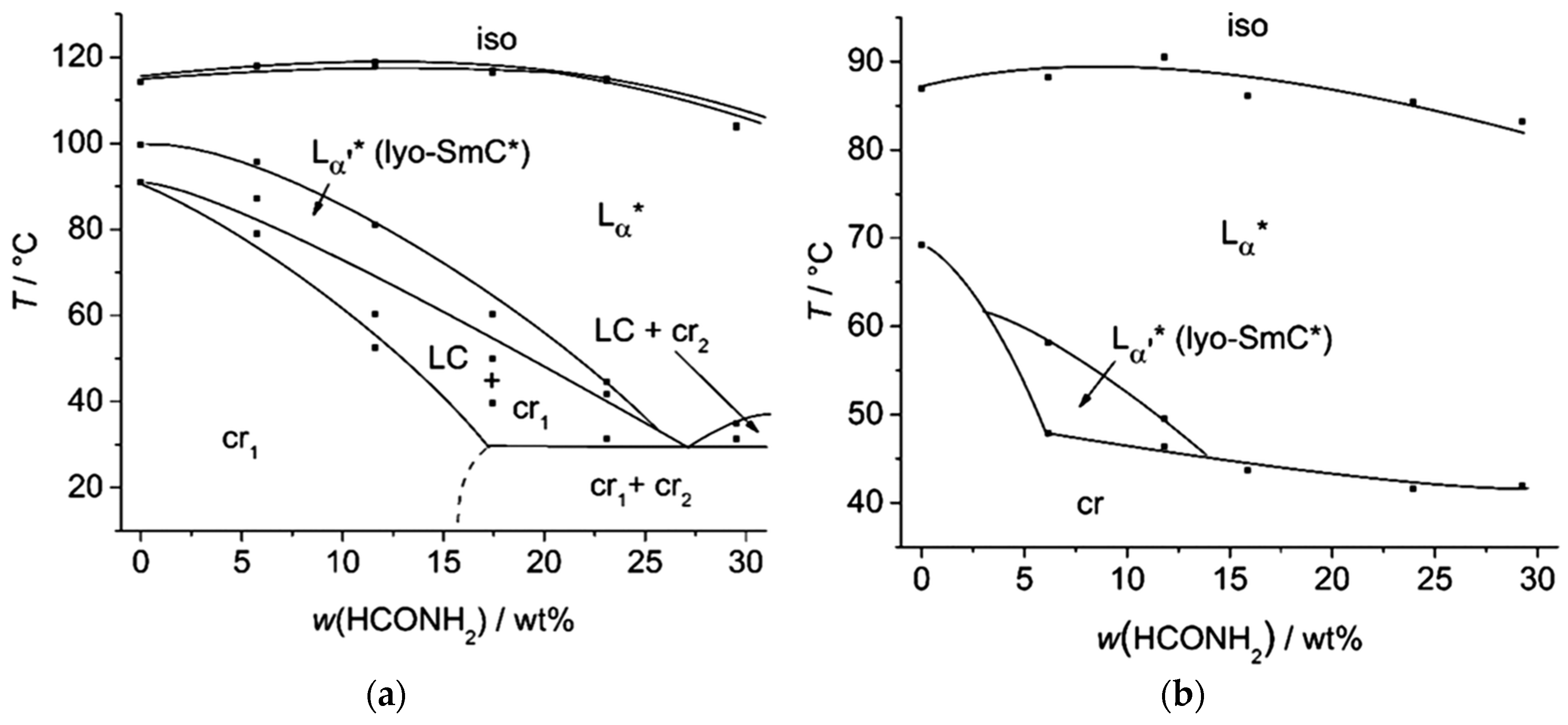
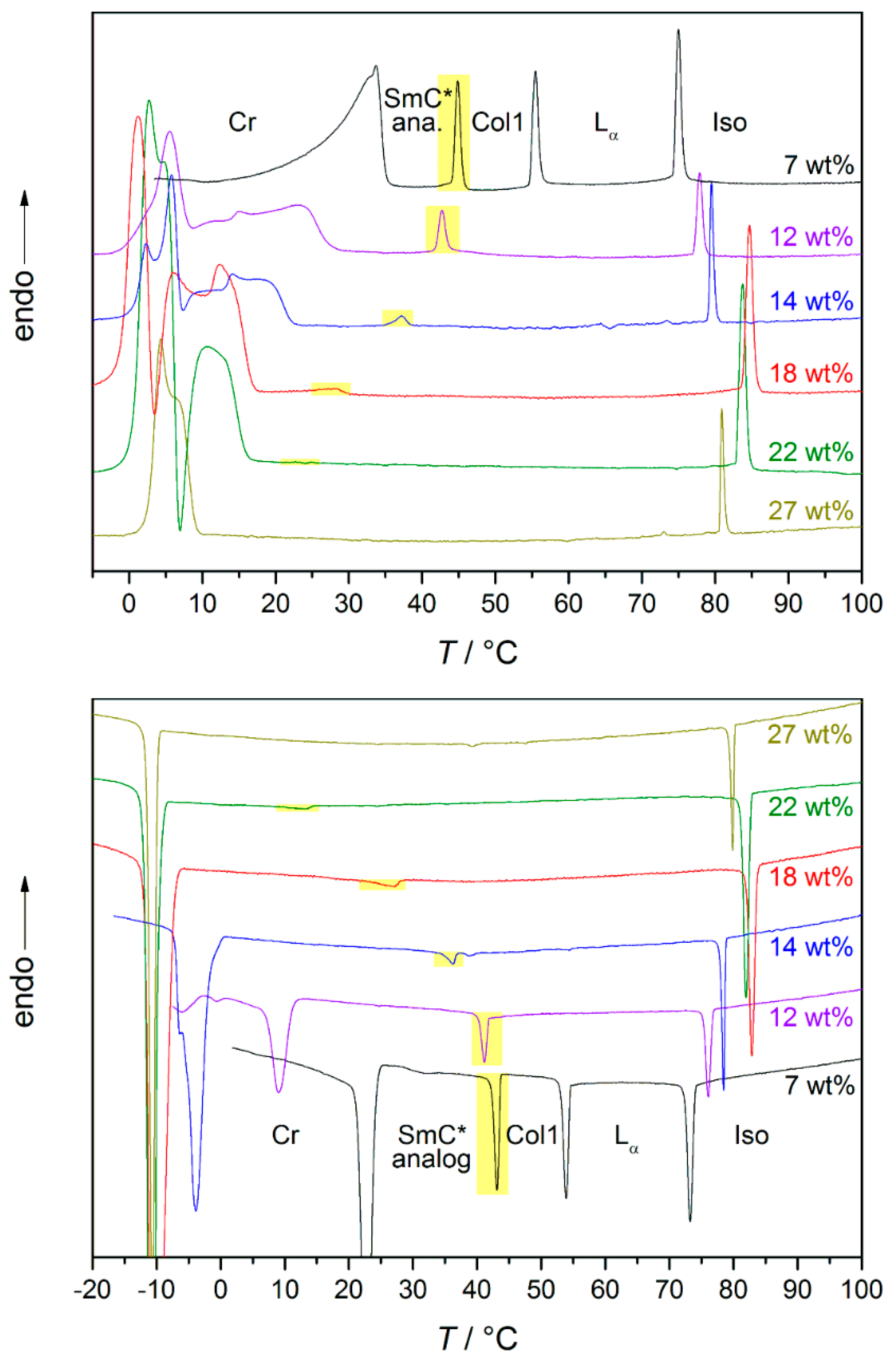
3. The Recognition of a First Lyotropic SmC* Phase
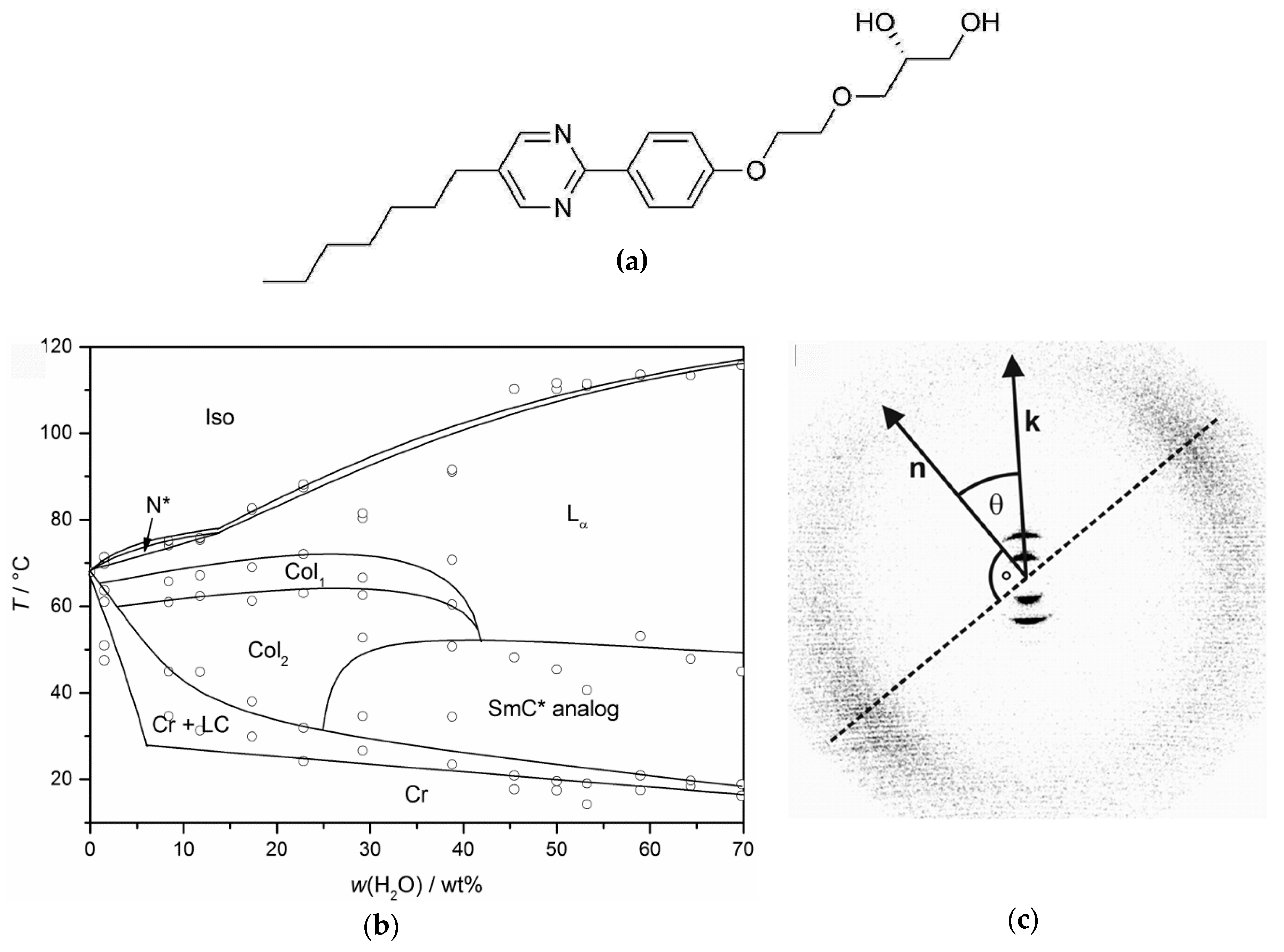
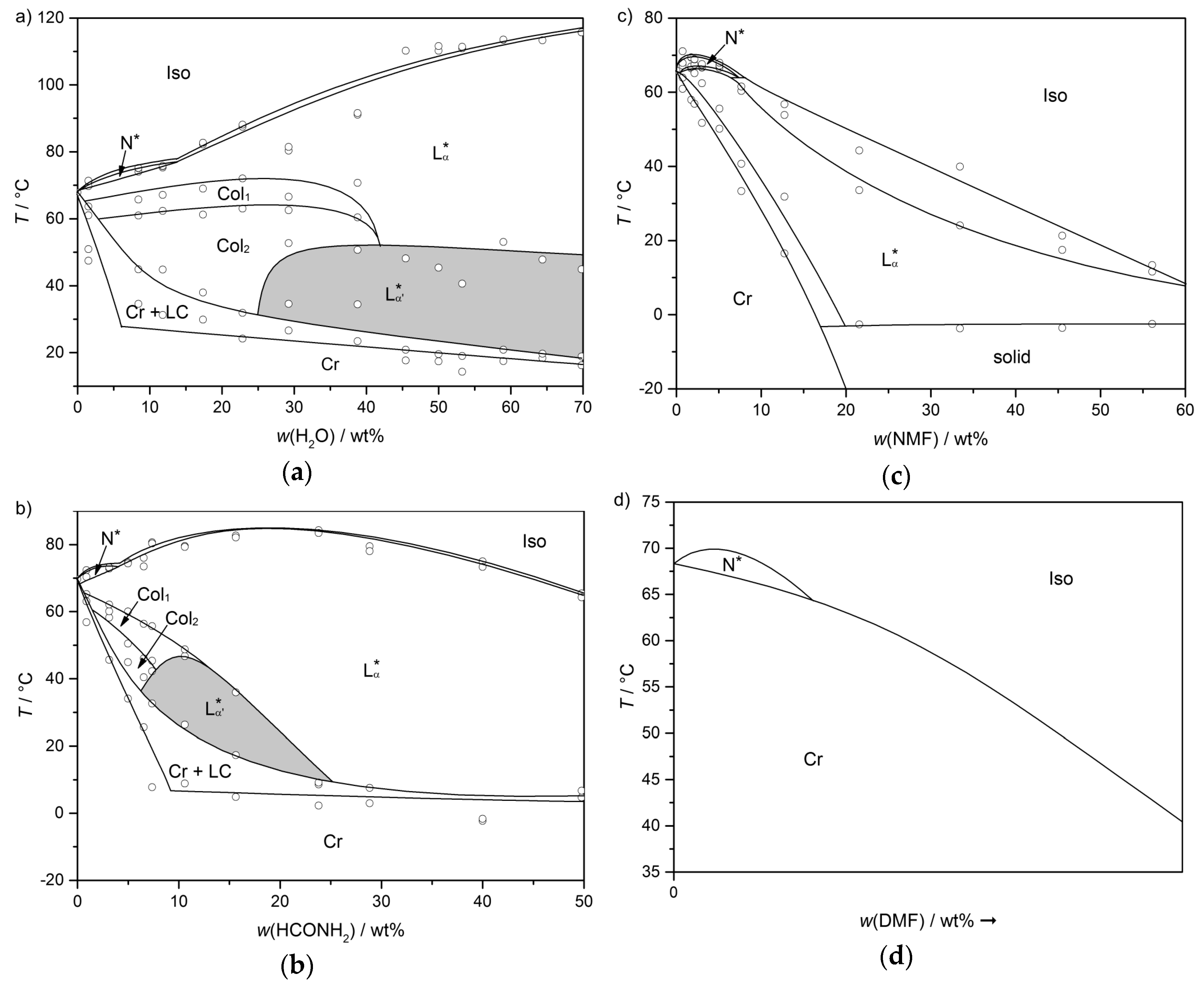
4. Prerequisites and Properties of Lyotropic SmC* Phases
4.1. Lyotropic SmC* Systems—A Delicate Balance
- A rigid aromatic core;
- A polar head group which is attached to the core by a slightly hydrophilic linker (e.g., ethylene glycol units);
- Another flexible chain attached to the other side of the core;
- Water as solvent.
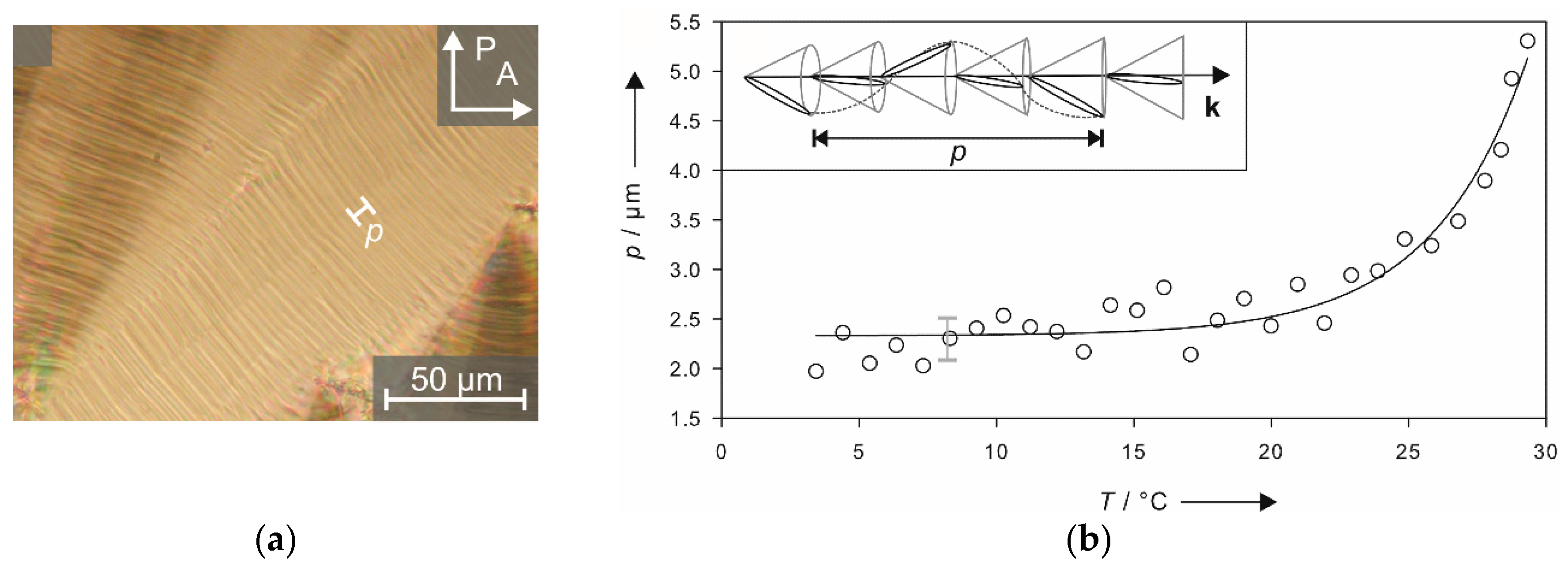
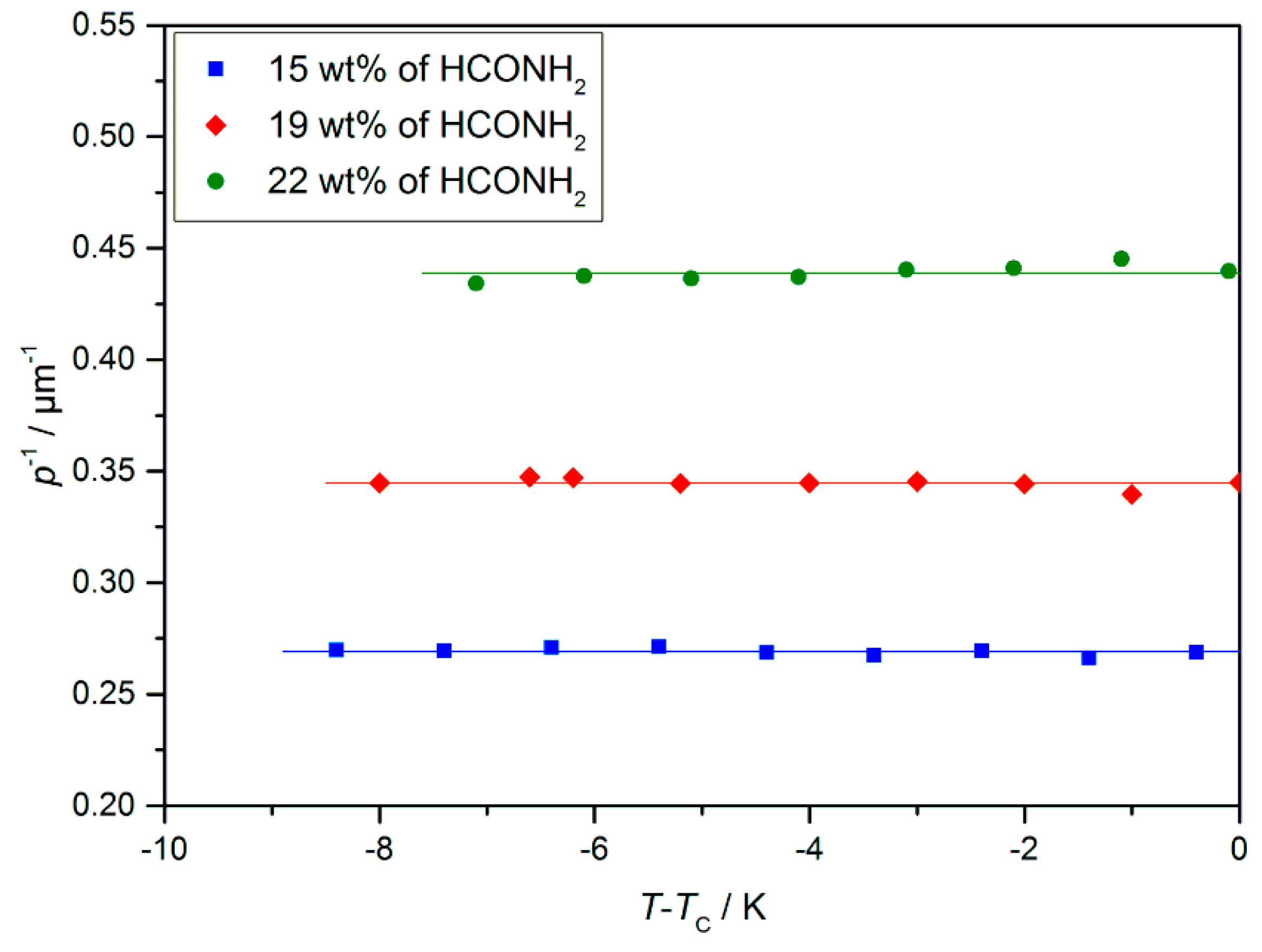
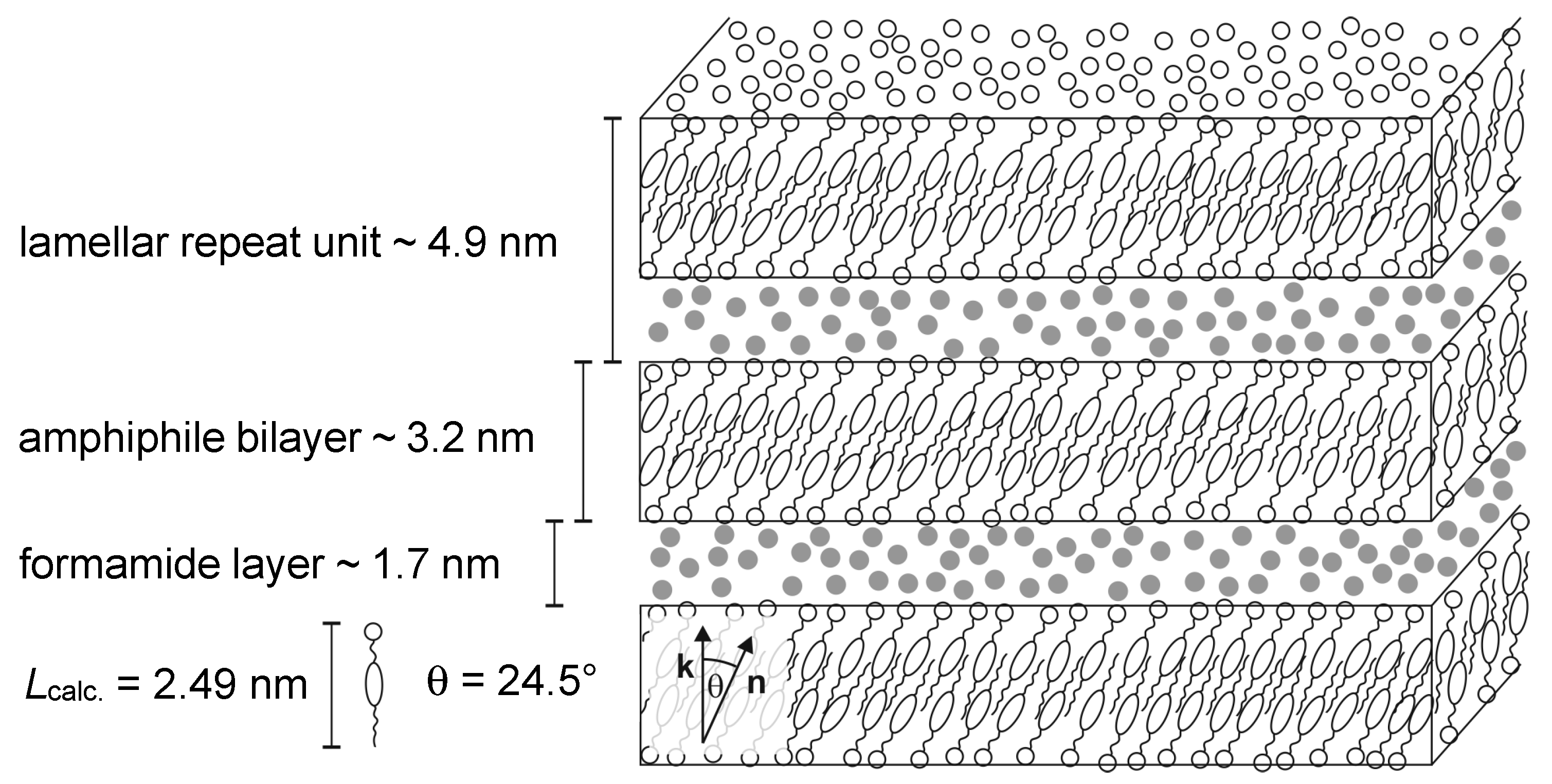
4.2. Structure and Phase Transitions of Lyotropic SmC* Phases
- The thickness of the solvent layer ds, which mainly depends on the mass fraction w of solvent;
- The thickness of the amphiphile bilayer dbl, which changes with the temperature T.
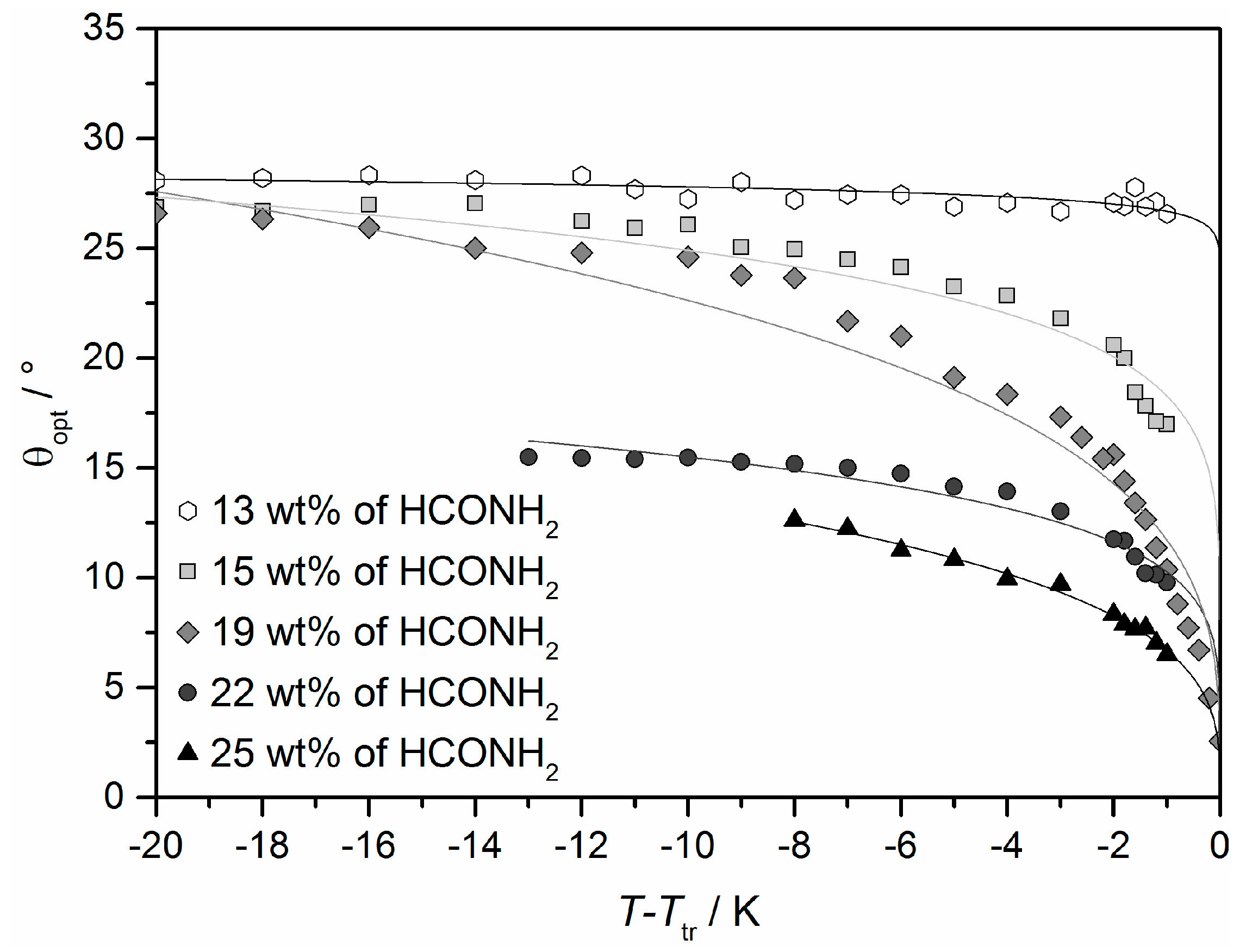
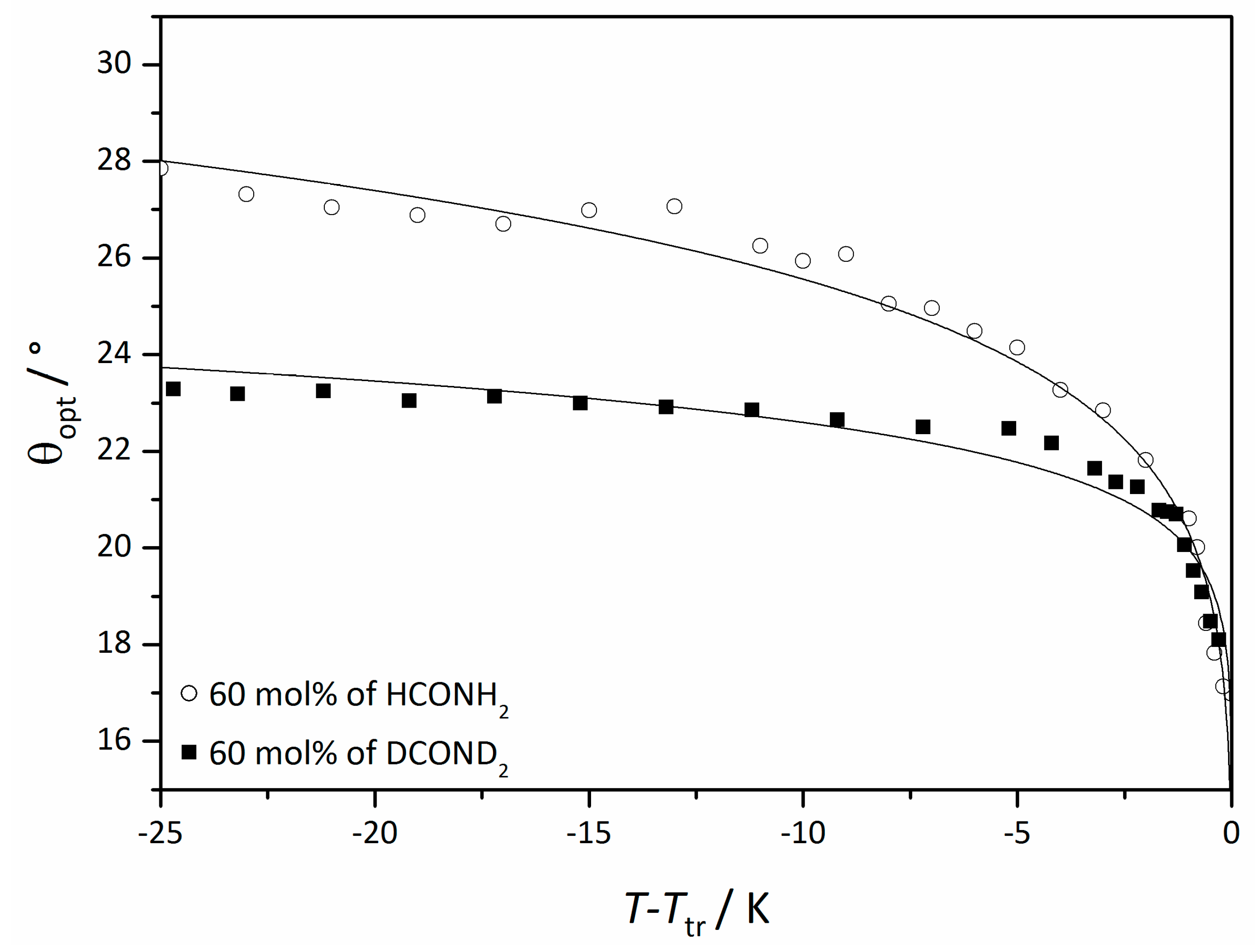
4.3. Origin of the Director Tilt
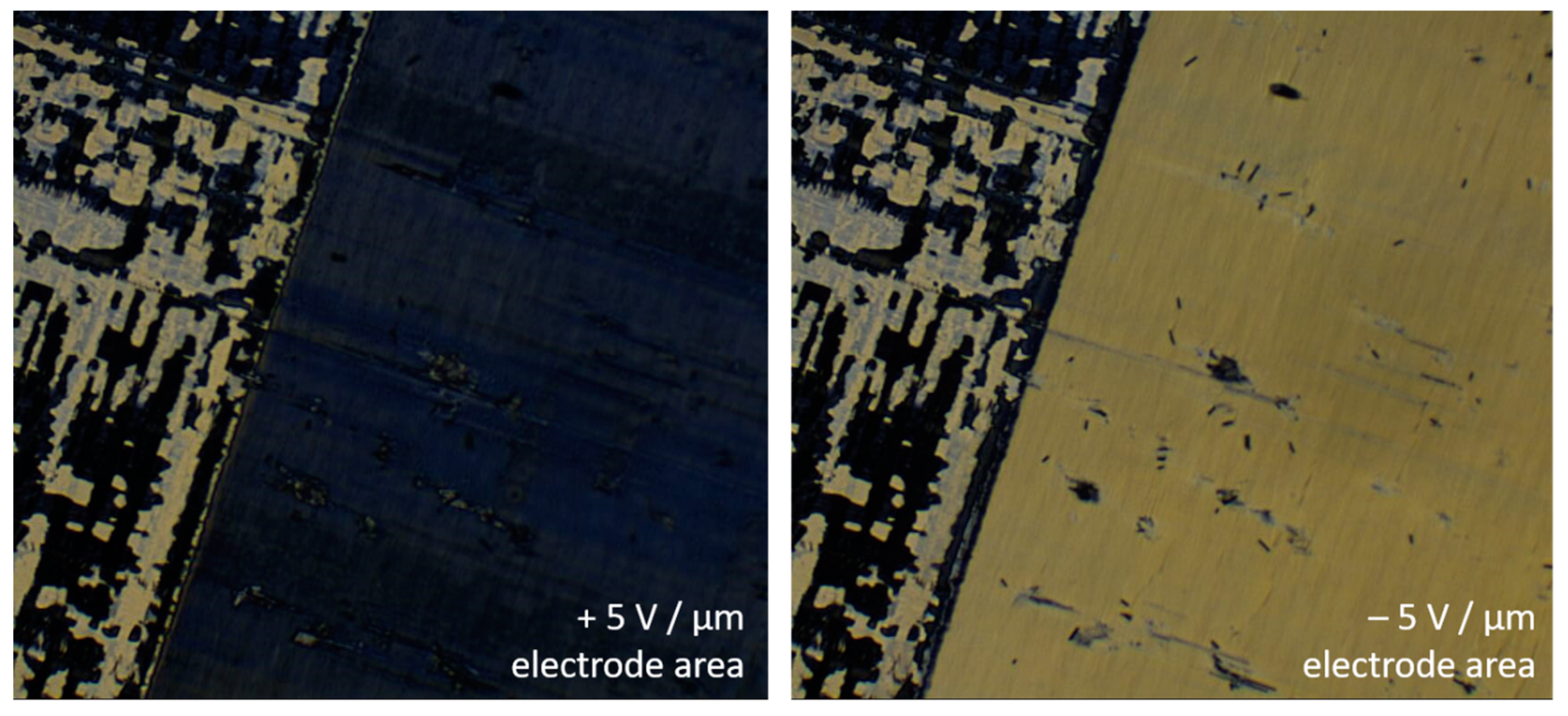
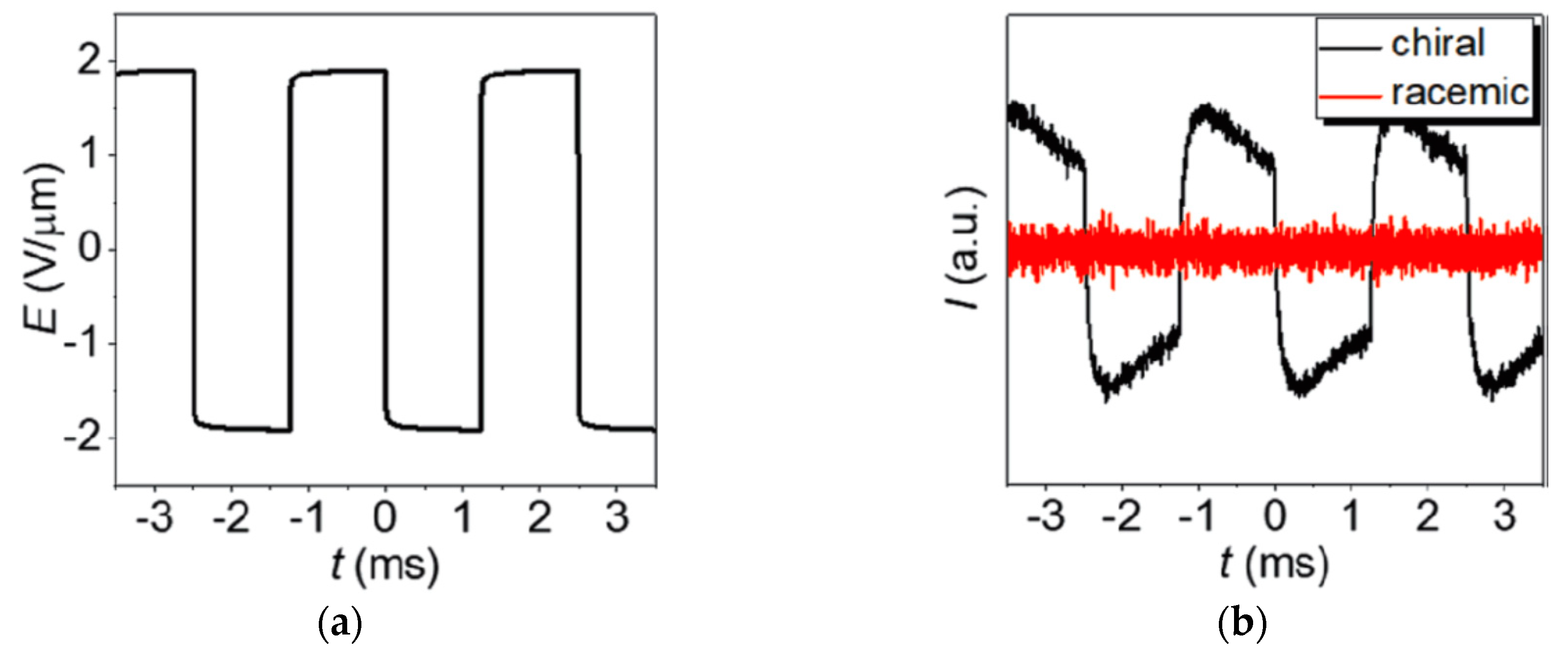
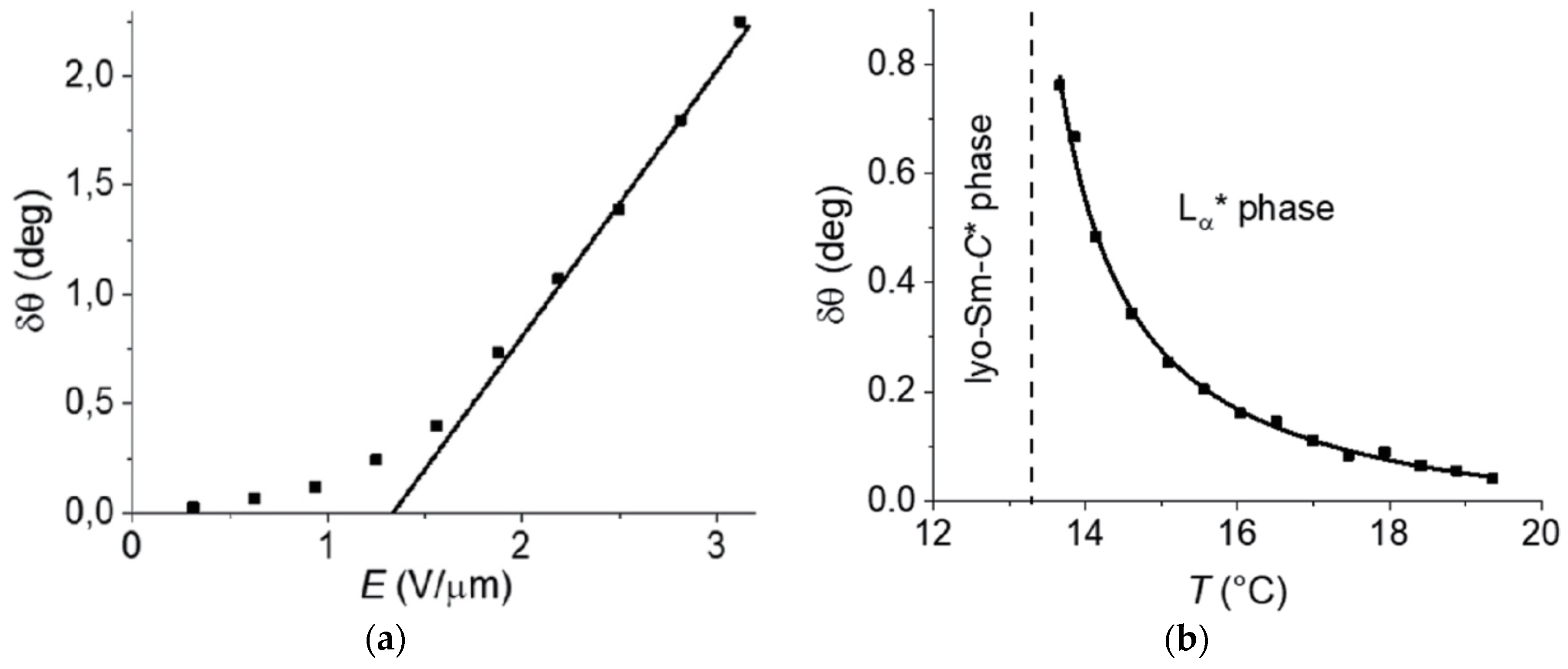
4.4. Electroclinic Effect
5. Conclusions and Outlook
- It has the same fundamental SmC structure; namlely, a 1D periodic layer structure of 2D-fluid bilayers with the liquid crystal director macroscopically tilted from the layer normal by typically 10–30 degrees.
- The lyo-SmC* phase has a helical ground state with a pitch typically in the micron range and textures showing pitch lines familiar from thermotropic SmC* phase.
- In thin samples the lyo-SmC* phase can be surface stabilized, showing ferroelectric tilt domains, sometimes prevaded by zig-zag defects, and it can be electrically switched between the two stable states just like a thermotropic ferroelectric SmC* sample.
- At higher temperatures and/or solvent concentrations, the lyo-SmC* phase often transforms into the chiral Lα* phase, the lyotropic equivalent of thermotropic SmA*. As in thermotropic SmC*–SmA* materials, the transition can be second order and an electroclinic effect is observed in the Lα* phase.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lines, M.E.; Glass, A.M. Principles and Applications of Ferroelectrics and Related Materials; Oxford University Press: Oxford, UK, 2001; ISBN 978-0-19850778-9. [Google Scholar]
- Valasek, J. Piezo-Electric and Allied Phenomena in Rochelle Salt. Phys. Rev. 1921, 17, 475–481. [Google Scholar] [CrossRef]
- Meyer, R.B.; Liebert, L.; Strzelecki, L.; Keller, P. Ferroelectric Liquid Crystals. J. Phyique Lett. 1975, 36, 69–71. [Google Scholar] [CrossRef]
- Meyer, R.B. Ferroelectric Liquid Crystals; a Review. Mol. Cryst. Liq. Cryst. 1977, 40, 33–48. [Google Scholar] [CrossRef]
- Lagerwall, S.T. Ferroelectric Liquid Crystals. In Handbook of Liquid Crystals; Goodby, J.W., Collings, P.J., Kato, T., Tschierske, C., Gleeson, H.F., Raynes, P., Eds.; Wiley-VCH: Weinheim, Germany, 2014; Volume 4, ISBN 978-3-527-32773-7. [Google Scholar]
- Takezoe, H.; Cepic, M. Antiferroelectric Liquid Crystals. In Handbook of Liquid Crystals; Goodby, J.W., Collings, P.J., Kato, T., Tschierske, C., Gleeson, H.F., Raynes, P., Eds.; Wiley-VCH: Weinheim, Germany, 2014; Volume 4, ISBN 978-3-527-32773-7. [Google Scholar]
- Takezoe, H.; Eremin, A. Bent-Shaped Liquid Crystals; CRC Press: Boca Raton, FL, USA, 2017; ISBN 978-1-4822-4759-6. [Google Scholar]
- Lagerwall, S.T. Ferroelectric and Antiferroelectric Liquid Crystals; Wiley-VCH: Weinheim, Germany, 1999; p. 129. ISBN 3-527-29831-2. [Google Scholar]
- Garoff, S.; Meyer, R.B. Electroclinic Effect at the A—C Phase Change in a Chiral Smectic Liquid Crystal. Phys. Rev. Lett 1977, 38, 848–851. [Google Scholar] [CrossRef]
- Garoff, S.; Meyer, R.B. Electroclinic Effect at the A—C Phase Change in a Chiral Smectic Liquid Crystal. Phys. Rev. A 1979, 19, 338–347. [Google Scholar] [CrossRef]
- Bahr, C.; Heppke, G. Optical and Dielectric Investigations on the Electroclinic Effect Exhibited by a Ferroelectric Liquid Crystal with High Spontaneous Polarization. Liquid Cryst. 1987, 2, 825–831. [Google Scholar] [CrossRef]
- Clark, N.A.; Lagerwall, S.T. Submicrosecond bistable electro-optic switching in liquid crystals. Appl. Phys. Lett. 1980, 36, 899–901. [Google Scholar] [CrossRef]
- Bruckner, J.R. A First Example of a Lyotropic Smectic C* Analog Phase—Design, Properties and Chirality Effects; Springer: Cham, Switzerland, 2015; ISBN 978-3-319-27202-3. [Google Scholar]
- Bogner, A. Maßgeschneiderte smektische Flüssigkristalle vom ‚de Vries‘-Typ: Struktur-Eigenschaftsvariationen in nanosegregierenden Organosiloxanen und Organocarbosilanen. Ph.D. Thesis, University of Stuttgart, Stutgart, Germany, 2015. [Google Scholar]
- Dumrongrattana, S.; Huang, C.C. Polarization and Tilt-Angle Measurements Near the Smectic-a-Chiral-Smectic-C Transition of P-(N-Decyloxybenzylidene)-P-Amino-(2-Methyl-Butyl)Cinnamate (DOBAMBC). Phys. Rev. Lett 1986, 56, 464–467. [Google Scholar] [CrossRef]
- Bahr, C.; Heppke, G. Ferroelectric Liquid-Crystals: Properties of Binary Mixtures and Pure Compounds with High Spontaneous Polarization. Mol. Cryst. Liq. Cryst. 1987, 148, 29–43. [Google Scholar] [CrossRef]
- Bahr, C.; Heppke, G. Influence of Electric Field on a First-Order Smectic-A–Ferroelectric-Smectic-C Liquid-Crystal Phase Transition: A Field-Induced Critical Point. Phys. Rev. A 1990, 41, 4335–4342. [Google Scholar] [CrossRef]
- Glaser, M.A.; Clark, N.A. Fluctuations and Clinicity in Tilted Smectic Liquid Crystals. Phys. Rev. E 2002, 66, 021711–021714. [Google Scholar] [CrossRef] [PubMed]
- Tschierske, C.; Schroeter, J.A.; Lindner, N.; Sauer, C.; Diele, S. Formation of columnar mesophases by rodlike molecules. SPIE 1998, 3319, 8–13. [Google Scholar] [CrossRef]
- Lindner, N.; Kölbel, M.; Sauer, C.; Diele, S.; Jokiranta, J.; Tschierske, C. Formation of Columnar and Cubic Mesophases by Calamitic Molecules: Novel Amphotropic Biphenyl Derivatives. J. Phys. Chem. B 1998, 102, 5261–5273. [Google Scholar] [CrossRef]
- Neumann, B.; Sauer, C.; Diele, S.; Tschierske, C. Molecular design of amphotropic materials: Influence of oligooxyethylene groups on the mesogenic properties of calamitic liquid crystals. J. Mater. Chem. 1996, 6, 1087–1098. [Google Scholar] [CrossRef]
- Kanie, K.; Sekiguchi, J.; Zeng, X.; Ungar, G.; Atsushi, M. Phospholipids with a stimuli-responsive thermotropic liquid-crystalline moiety. Chem. Commun. 2011, 47, 6885–6887. [Google Scholar] [CrossRef]
- Tan, X.; Zhang, R.; Guo, C.; Cheng, X.; Gao, H.; Liu, F.; Bruckner, J.R.; Giesselmann, F.; Prehm, M.; Tschierske, C. Amphotropic azobenzene derivatives with oligooxyethylene and glycerol based polar groups. J. Mater. Chem. C 2015, 3, 11202–11211. [Google Scholar] [CrossRef]
- Murase, M.; Takanishi, Y.; Nishiyama, I.; Yoshizawa, A.; Yamamoto, J. Hyper swollen perfluorinated smectic liquid crystal by perfluorinated oils. RSC Adv. 2015, 5, 215–220. [Google Scholar] [CrossRef]
- Schafheutle, M.A.; Finkelmann, H. Shapes of Micelles and Molecular Geometry Synthesis and Studies on the Phase Behaviour, Surface Tension and Rheology of Rigid Rod-Like Surfactants in Aqueous Solutions. Liq. Cryst. 1988, 3, 1369–1386. [Google Scholar] [CrossRef]
- Ujiie, S.; Yano, Y. Thermotropic and lyotropic behavior of novel amphiphilic liquid crystals having hydrophilic poly(ethyleneimine) units. Chem. Commun. 2000, 79–80. [Google Scholar] [CrossRef]
- Pietschmann, N.; Lunow, A.; Brezesinski, G.; Tschierske, C.; Kuschel, F.; Zaschke, H. The first liquid crystalline diol compound which exhibits nematic, smectic A+, and smectic C+ mesophases in the presence of water. Colloid Polym. Sci. 1991, 269, 636–639. [Google Scholar] [CrossRef]
- Bruckner, J.R.; Krueerke, D.; Porada, J.H.; Jagiella, S.; Blunk, D.; Giesselmann, F. The 2D-correlated structures of a lyotropic liquid crystalline diol with a phenylpyrimidine core. J. Mater. Chem. 2012, 22, 18198–18203. [Google Scholar] [CrossRef]
- Bruckner, J.R.; Porada, J.H.; Dietrich, C.F.; Dierking, I.; Giesselmann, F. A Lyotropic Chiral Smectic C Liquid Crystal with Polar Electrooptic Switching. Angew. Chem. Int. Ed. 2013, 52, 8934–8937. [Google Scholar] [CrossRef] [PubMed]
- Clark, N.A.; Rieker, T.P.; Maclennan, J.E. Director and layer structure of SSFLC cells. Ferroelectrics 1988, 85, 79–97. [Google Scholar] [CrossRef]
- Kuczyński, W. Behavior of the helix in some chiral smectic-C* liquid crystals. Phys. Rev. E 2010, 2, 021708. [Google Scholar] [CrossRef]
- Martinot-Lagarde, P. Flexo and ferro-electricity observation of ferroelectrical monodomains in the chiral smectic liquid crystal. J. Phys. Colloques 1976, 37, 129–132. [Google Scholar] [CrossRef]
- Brunet, M.; Isaert, N. Periodic structures in smectic C*—Pitch and unwinding lines. Ferroelectrics 1988, 84, 25–52. [Google Scholar] [CrossRef]
- Osipov, M.A.; Bruckner, J.R.; Giesselmann, F. On the Theory of Helical Twisting in Ferroelectric Liquid Crystals, 2. In Proceedings of the Joint German British Liquid Crystal Conference, Würzburg, Germany, 3–5 April 2017. [Google Scholar]
- Gorb, L.; Asensio, A.; Tuñón, I.; Ruiz-López, M.F. The Mechanism of Formamide Hydrolysis in Water from Ab Initio Calculations and Simulations. Chem. Eur. J. 2005, 11, 6743. [Google Scholar] [CrossRef]
- Notley, J.M.; Spiro, M. The Purification of Formamide, and the Rate of its Reaction with Dissolved Water. J. Chem. Soc. B 1966, 362. [Google Scholar] [CrossRef]
- Harjung, M.D.; Schubert, C.P.J.; Knecht, F.; Porada, J.H.; Lemieux, R.P.; Giesselmann, F. New amphiphilic materials showing the lyotropic analogue to the thermotropic smectic C* liquid crystal phase. J. Mater. Chem. C 2017, 5, 7452–7457. [Google Scholar] [CrossRef]
- Knecht, F. Neue Einblicke in die Lyotrope Smektische C* Phase: Untersuchungen zur Phasenstabilität und zum Mechanismus Interlamellarer Direktorkorrelation. Ph.D. Thesis, University of Stuttgart, Stutgart, Germany, 2019. [Google Scholar]
- Harjung, M.D. Chirale Lyotrop-Lamellare Flüssigkristalle: Phasenverhalten Neuer Chiraler Amphiphile und Nachweis des Elektroklinen Effektes. Ph.D. Thesis, University of Stuttgart, Stutgart, Germany, 2019. [Google Scholar]
- Li, L.; Jones, C.D.; Magolan, J.; Lemieux, R.P. Siloxane-terminated phenylpyrimidine liquid crystal hosts. J. Mater. Chem. 2007, 17, 2313–2318. [Google Scholar] [CrossRef]
- Roberts, J.C.; Kapernaum, N.; Giesselmann, F.; Lemieux, R.P. Design of liquid crystals with “de Vries-like” properties: Organosiloxane mesogen with a 5-phenylpyrimidine core. J. Am. Chem. Soc. 2008, 130, 13842–13843. [Google Scholar] [CrossRef] [PubMed]
- McCubbin, J.A.; Tong, X.; Wang, R.; Zhao, Y.; Snieckus, V.; Lemieux, R.P. Directed metalation route to ferroelectric liquid crystals with a chiral fluorenol core: The effect of restricted rotation on polar order. J. Am. Chem. Soc. 2004, 126, 1161–1167. [Google Scholar] [CrossRef] [PubMed]
- Takatoh, K.; Sunohara, K.; Sakamoto, M. Mesophase Transition of Series Materials Containing Fluorene, Fluorenone and Biphenyl Structures with Chiral End Groups. Mol. Cryst. Liq. Cryst. 1988, 164, 167–178. [Google Scholar] [CrossRef]
- Roberts, J.C.; Kapernaum, N.; Song, Q.; Nonnenmacher, D.; Ayub, K.; Giesselmann, F.; Lemieux, R.P. Design of liquid crystals with “de Vries-like” properties: Frustration between SmA- and SmC-promoting elements. J. Am. Chem. Soc. 2010, 132, 364–370. [Google Scholar] [CrossRef]
- Bruckner, J.R.; Knecht, F.; Giesselmann, F. Origin of the Director Tilt in the Lyotropic Smectic C* Analog Phase: Hydration Interactions and Solvent Variations. ChemPhysChem 2016, 17, 86–92. [Google Scholar] [CrossRef]
- Rumble, J.R. (Ed.) CRC Handbook of Chemistry and Physics, 99th ed.; CRC Press: Boca Raton, FL, USA, 2018; ISBN 1138561630. [Google Scholar]
- Sengwa, R.J.; Kaur, K.; Chaudhary, R. Dielectric properties of low molecular weight poly(ethylene glycol)s. Polym. Int. 2000, 49, 599–608. [Google Scholar] [CrossRef]
- LeNeveu, D.M.; Rand, R.P.; Parsegian, V.A. Measurement of forces between lecithin bilayers. Nature 1976, 259, 601–603. [Google Scholar] [CrossRef]
- Lis, L.J.; McAlister, D.; Fuller, N.; Rand, R.P.; Parsegian, V.A. Interactions between neutral phospholipid bilayer membranes. Biophys. J. 1982, 37, 657–666. [Google Scholar]
- Leikin, S.; Parsegian, V.A.; Rau, D.C.; Rand, R.P. Hydration Forces. Ann. Rev. Phys. Chem. 1993, 44, 369–395. [Google Scholar] [CrossRef]
- McIntosh, T.J. Short-range interactions between lipid bilayers measured by X-ray diffraction. Curr. Opin. Struct. Biol. 2000, 10, 481–485. [Google Scholar] [CrossRef]
- Milhaud, J. New insights into water-phospholipid model membrane interactions. Biochim. Biophys. Acta 2004, 1663, 19–51. [Google Scholar] [CrossRef] [PubMed]
- Berkowitz, M.L.; Vácha, R. Aqueous solutions at the interface with phospholipid bilayers. Acc. Chem. Res. 2012, 45, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Bergenstaahl, B.A.; Stenius, P. Phase diagrams of dioleoylphosphatidylcholine with formamide, methylformamide and dimethylformamide. J. Phys. Chem. 1987, 91, 5944–5948. [Google Scholar] [CrossRef]
- Cheng, J.-X.; Pautot, S.; Weitz, D.A.; Xie, X.S. Ordering of water molecules between phospholipid bilayers visualized by coherent anti-Stokes Raman scattering microscopy. Proc. Natl. Acad. Sci. USA 2003, 100, 9826–9830. [Google Scholar] [CrossRef]
- Tayebi, L.; Ma, Y.; Vashaee, D.; Chen, G.; Sinha, S.K.; Parikh, A.N. Long-range interlayer alignment of intralayer domains in stacked lipid bilayers. Nat. Mater. 2012, 11, 1074–1080. [Google Scholar] [CrossRef]
- Marrink, S.J.; Berkowitz, M.; Berendsen, H. Molecular dynamics simulation of a membrane/water interface: The ordering of water and its relation to the hydration force. Langmuir 1993, 9, 3122–3131. [Google Scholar] [CrossRef]
- Kjellander, R.; Marčelja, S. Perturbation of hydrogen bonding in water near polar surfaces. Chem. Phys. Lett. 1985, 393–396. [Google Scholar] [CrossRef]
- Marčelja, S.; Radić, N. Repulsion of interfaces due to boundary water. Chem. Phys. Lett. 1976, 42, 129–130. [Google Scholar] [CrossRef]
- Attard, P.; Batchelor, M.T. A mechanism for the hydration force demonstrated in a model system. Chem. Phys. Lett. 1988, 149, 206–211. [Google Scholar] [CrossRef]
- Tokarev, A.V.; Bondarenko, G.N.; Yampol’skii, Y.P. Chain structure and stiffness of Teflon AF glassy amorphous fluoropolymers. Polym. Sci. Ser. A 2007, 49, 909–920. [Google Scholar] [CrossRef]
- Parra, R.D.; Zeng, X.C. Staggered and eclipsed conformations of C2F6: A systematic ab initio study. J. Fluor. Chem. 1997, 83, 51–60. [Google Scholar] [CrossRef]
- Salerno, K.M.; Bernstein, N. Persistence Length, End-to-End Distance, and Structure of Coarse-Grained Polymers. J. Chem. Theory Comput. 2018, 14, 2219–2229. [Google Scholar] [CrossRef] [PubMed]
- Teoh, M.M.; Chung, T.-S.; Pramoda, K.P. Thin-film polymerization and Metropolis Monte Carlo simulation of thermotropic liquid crystalline poly(ester-amide)s. Synth. Met. 2004, 147, 191–197. [Google Scholar] [CrossRef]
- Koestner, R.; Roiter, Y.; Kozhinova, I.; Minko, S. AFM imaging of adsorbed Nafion polymer on mica and graphite at molecular level. Langmuir 2011, 27, 10157–10166. [Google Scholar] [CrossRef] [PubMed]
- Giesselmann, F.; Zugenmaier, P. Mean-Field Coefficients and the Electroclinic Effect of a Ferroelectric Liquid-Crystal. Phys. Rev. E 1995, 52, 1762–1772. [Google Scholar] [CrossRef]
- Jakli, A.; Harden, J.; Notz, C.; Bailey, C. Piezoelectricity of Phospholipids: A Possible Mechanism for Mechanoreception and Magnetoreception in Biology. Liquid Cryst. 2008, 35, 395–400. [Google Scholar] [CrossRef]
- Harden, J.; Diorio, N.; Petrov, A.G.; Jakli, A. Chirality of Lipids Makes Fluid Lamellar Phases Piezoelectric. Phys. Rev. E 2009, 79. [Google Scholar] [CrossRef]
- Harjung, M.D.; Giesselmann, F. Electroclinic effect in the chiral lamellar α phase of a lyotropic liquid crystal. Phys. Re. E 2018, 97, 032705. [Google Scholar] [CrossRef]
- Clark, N.A.; Lagerwall, S.T. Applications of Ferroelectric Liquid Crystals in Ferroelectric Liquid Crystals—Principles, Properties and Applications; Taylor, G.W., Shuvalov, L.A., Eds.; Gordon and Breach Science Publishers: Philadelphia, PA, USA, 1991; ISBN 2-88124-282-0. [Google Scholar]



| No° | Structure | TLC 1 | Lyo-SmC* 1 | Ref. | |
|---|---|---|---|---|---|
| 1 | 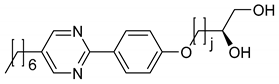 | j = 1 | - | - | [13] |
| 2 | j = 3 | - | - | [28] | |
| 3 | j = 4 | - | - | [13] | |
| 4 | 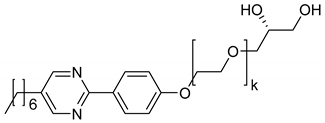 | k = 1 | (N*) | H2O, formamide | [13,29] |
| 5 | k = 2 | (SmA*) | H2O | [37] | |
| 6 | k = 3 | SmA* | - | [37] | |
| 7 | 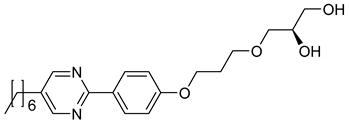 | SmA* | - | [13] | |
| 8 | 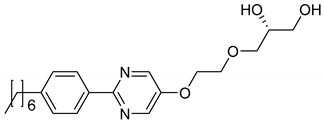 | SmA*, SmC* | H2O, formamide | [37] | |
| 9 | 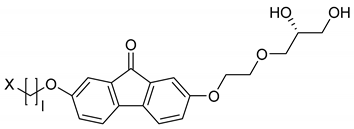 | l = 4 X = H | - | - | [37,39] |
| 10 | l = 5 X = H | SmA* | - | [37,39] | |
| 11 | l = 6 X = H | SmA*, SmC* | H2O, formamide | [37] | |
| 12 | l = 6 X = Cl | SmA* | (H2O, formamide) | [37,39] | |
| Solvent | Dielectric Permittivity εr [46] | Dipole Moment µ/D [46] | Number of H-bond Donor Atoms | Number Density 1 nρ/1022·cm−3 |
|---|---|---|---|---|
| Water | 80.1 | 1.85 | 2 | 3.27 |
| Formamide | 111.0 | 3.73 | 2 | 1.52 |
| Ethylene glycol | 41.4 | 2.36 | 2 | 1.08 |
| NMF | 189.0 | 3.83 | 1 | 1.03 |
| DMF | 38.3 | 3.82 | 0 | 0.78 |
| PEG 200 | 22.1 [47] | 3.28 [47] | 2 | 0.34 |
| PEG 300 | 19.2 [47] | 3.91 [47] | 2 | 0.23 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bruckner, J.R.; Giesselmann, F. The Lyotropic Analog of the Polar SmC* Phase. Crystals 2019, 9, 568. https://doi.org/10.3390/cryst9110568
Bruckner JR, Giesselmann F. The Lyotropic Analog of the Polar SmC* Phase. Crystals. 2019; 9(11):568. https://doi.org/10.3390/cryst9110568
Chicago/Turabian StyleBruckner, Johanna R., and Frank Giesselmann. 2019. "The Lyotropic Analog of the Polar SmC* Phase" Crystals 9, no. 11: 568. https://doi.org/10.3390/cryst9110568
APA StyleBruckner, J. R., & Giesselmann, F. (2019). The Lyotropic Analog of the Polar SmC* Phase. Crystals, 9(11), 568. https://doi.org/10.3390/cryst9110568





