Crystal-Chemical Properties of Synthetic Almandine-Pyrope Solid Solution by X-Ray Single-Crystal Diffraction and Raman Spectroscopy
Abstract
1. Introduction
2. Experiments
2.1. Crystal Synthesis
2.2. Analysis Methods
3. Results and Discussion
3.1. Synthetic Garnet Crystal
3.2. Chemical Composition
3.3. Characteristics of Unit-Cell Parameters
3.4. Excess Mixing Volume in Solid Solutions
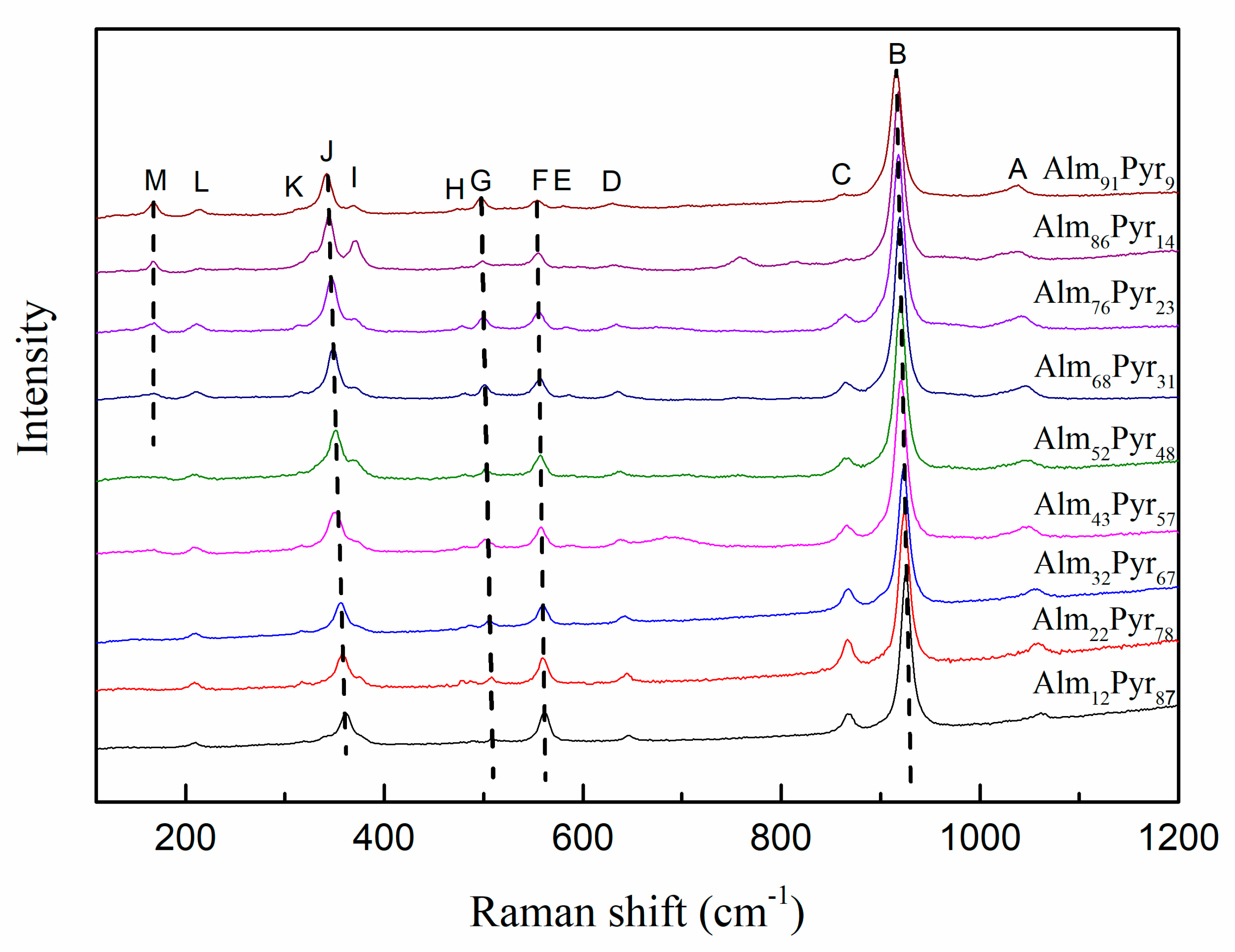
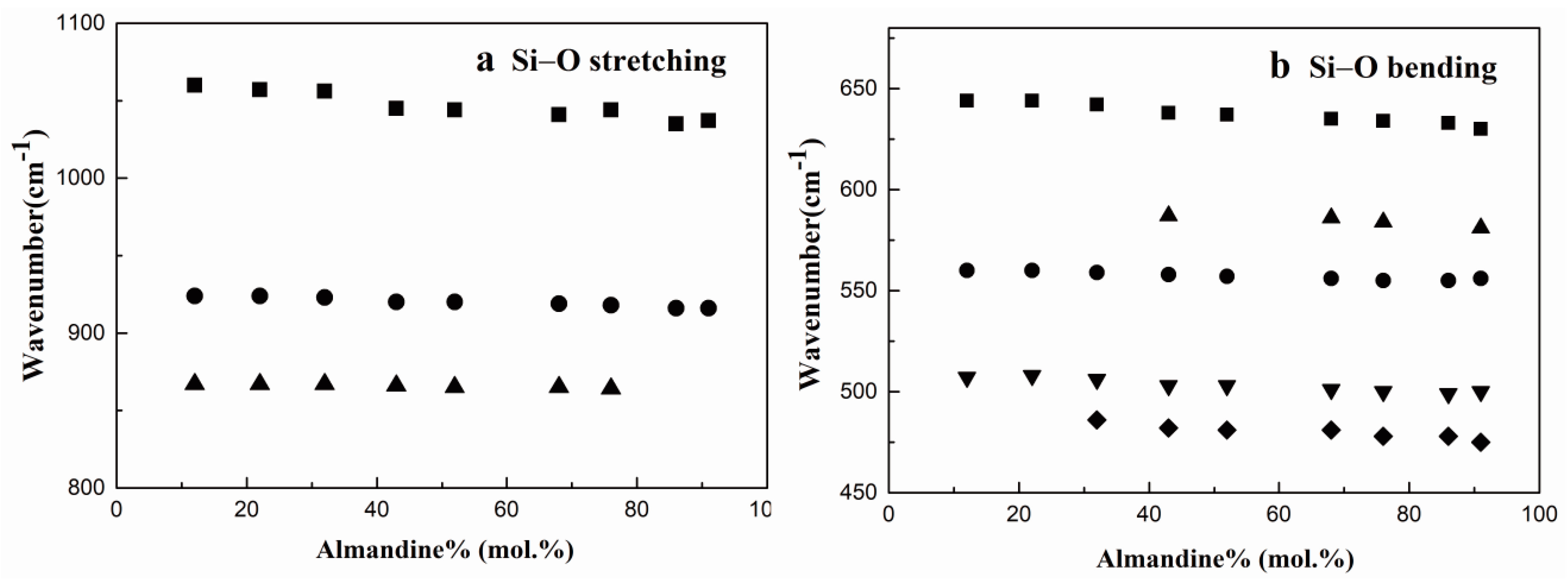
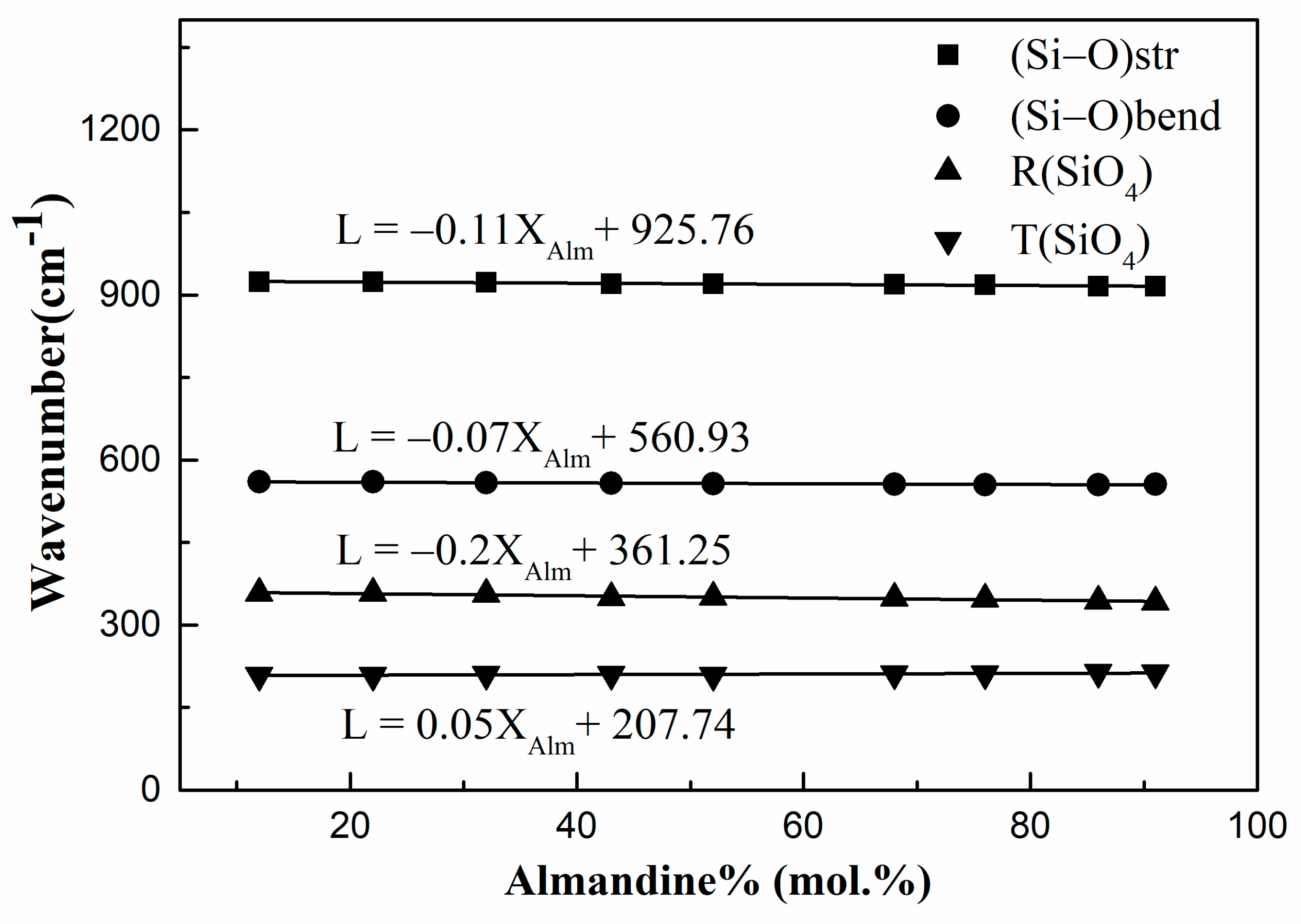
3.5. Characteristics of Raman Spectroscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ita, J.; Stixrude, L. Petrology, elasticity, and composition of the mantle transition zone. J. Geophys. Res. Solid Earth 1992, 97, 6849–6866. [Google Scholar] [CrossRef]
- Fan, D.; Li, B.; Chen, W.; Xu, J.; Kuang, Y.; Ye, Z.; Zhou, W.; Xie, H. Research Progress of the Equation of State for Garnet Minerals. Chin. J. High Press. Phys. 2018, 32, 1–13. [Google Scholar]
- Brey, G.P.; Kohler, T. Geothermobarometry in Four-phase Lherzolites II. New Thermobarometers, and Practical Assessment of Existing Thermobarometers. J. Petrol. 1990, 31, 1353–1378. [Google Scholar] [CrossRef]
- Newton, R.C.; Perkins, D. Thermodynamic calibration of geobarometers based on the assemblages garnet-plagioclase-orthopyroxene (clinopyroxene)-quartz1. Am. Mineral. 1982, 67, 203–222. [Google Scholar]
- Zhao, M. Introduction to Minearlogy, 2nd ed.; Geological Publishing House: Beijing, China, 2010; pp. 172–174. [Google Scholar]
- Nestola, F.; Milani, S.; Angel, R.J.; Pasqual, D.; Geiger, C.A. Pressure-volume equation of state for pyrope-almandine solid solutions. In Proceedings of the EGU General Assembly Conference, Vienna, Austria, 7–12 April 2013. [Google Scholar]
- Chopelas, A.; Savage, F. Single crystal raman spectroscopy and thermodynamics of garnet solid solutions I: Grossular-Andradite. In Proceedings of the American Geophysical Union Fall Meeting, San Francisco, CA, USA, 3–7 December 2012. [Google Scholar]
- Sabeen, H.M.; Ramanujam, N.; Morton, A.C. The provenance of garnet: Constraints provided by studies of coastal sediments from southern India. Sediment. Geol. 2002, 152, 279–287. [Google Scholar] [CrossRef]
- Du, W.; Han, B.; Clark, S.M.; Wang, Y.; Liu, X. Raman spectroscopic study of synthetic pyrope–grossular garnets: Structural implications. Phys. Chem. Miner. 2018, 45, 197–209. [Google Scholar] [CrossRef]
- Savage, F.B.; Chopelas, A. Single Crystal Raman Spectroscopy and Thermodynamics of Garnet Solid Solutions II: Pyrope-Almandine Binary. In Proceedings of the American Geophysical Union Fall Meeting, San Francisco, CA, USA, 8–12 December 2003. [Google Scholar]
- Milani, S.; Nestola, F.; Alvaro, M.; Pasqual, D.; Mazzucchelli, M.L.; Domeneghetti, M.C.; Geiger, C.A. Diamond–garnet geobarometry: The role of garnet compressibility and expansivity. Lithos 2015, 227, 140–147. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, D.; Fan, D.; Zhang, J.S.; Hu, Y.; Guo, X.; Dera, P.; Zhou, W. Phase Transitions in Orthoenstatite and Subduction Zone Dynamics: Effects of Water and Transition Metal Ions. J. Geophys. Res. Solid Earth 2018, 123, 2723–2737. [Google Scholar] [CrossRef]
- Fan, D.; Chang, L.; Xu, J.; Yan, B.; Yang, B.; Chen, J. Effects of water on P-V-T equation of state of pyrope. Phys. Earth Planet. Inter. 2017, 267, 9–18. [Google Scholar] [CrossRef]
- Zhang, D.; Dera, P.K.; Eng, P.J.; Stubbs, J.E.; Zhang, J.S.; Prakapenka, V.B.; Rivers, M.L. High Pressure Single Crystal Diffraction at PX^2. J. Vis. Exp. 2017, 119, e54660. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2010, 42, 339–341. [Google Scholar] [CrossRef]
- Cherepanova, T.A.; Bennema, P.; Yanson, Y.A.; Vogels, L.J.P.; Cherepanova, T.A.; Bennema, P.; Yanson, Y.A.; Vogels, L.J.P. Morphology of synthetic and natural garnets: Theory and observations. J. Cryst. Growth 1992, 121, 17–32. [Google Scholar] [CrossRef]
- Grew, E.S.; Locock, A.J.; Mills, S.J.; Galuskina, I.O.; Galuskin, E.V.; Hålenius, U. Nomenclature of the garnet supergroup. Am. Mineral. 2013, 98, 785–811. [Google Scholar] [CrossRef]
- Shu, H.; Chen, J. Equation of state of pyrope–almandine solid solution measured using a diamond anvil cell and in situ synchrotron X-ray diffraction. Phys. Earth Planet. Inter. 2014, 228, 88–91. [Google Scholar]
- Takahashi, T.; Liu, L.G. Compression of Ferromagnesian Garnets and the Effect of solid solutions on the Bulk Modulus. J. Geophys. Res. 1970, 75, 5757–5766. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Geiger, C.A.; Newton, R.C.; Kleppa, O.J. Enthalpy of mixing of synthetic almandine-grossular and almandine-pyrope garnets from high-temperature solution calorimetry. Geochimica Et Cosmochimica Acta 1987, 51, 1755–1763. [Google Scholar] [CrossRef]
- Geiger, C.A.; Feenstra, A. Molar volumes of mixing of almandine-pyrope and almandine-spessartine garnets and the crystal chemistry and thermodynamic-mixing properties of the aluminosilicate garnets. Am. Mineral. 1997, 82, 571–581. [Google Scholar] [CrossRef]
- Antao, S.; Zaman, M.; Suarez Nieto, N.; Gontijo, V.; Marr, R. Structural variations in pyrope-almandine solid solutions. Adv. X-Ray Anal. 2014, 58, 90–107. [Google Scholar]
- Du, W.; Clark, S.M.; Walker, D. Excess mixing volume, microstrain, and stability of pyrope-grossular garnets. Am. Mineral. 2016, 101, 193–204. [Google Scholar] [CrossRef]
- Koningstein, J.A.; Mortensen, O.S. Electronic Raman Spectra. III. Absolute Cross Sections for Electronic Raman and Rayleigh Scattering. Phys. Rev. 1968, 168, 75–77. [Google Scholar] [CrossRef]
- Moore, R.K.; White, W.B.; Long, T.V. Vibrational spectra of the common silicates: I. The garnets. Am. Mineral. 1971, 56, 54–71. [Google Scholar]
- Fateley, W.G.; Mcdevitt, N.T.; Bentley, F.F. Infrared and Raman Selection Rules for Lattice Vibrations: The Correlation Method. Appl. Spectrosc. 1971, 25, 155–173. [Google Scholar] [CrossRef]
- Kolesov, B.A.; Geiger, C.A. Raman spectra of silicate garnets. Phys. Chem. Miner. 1998, 25, 142–151. [Google Scholar] [CrossRef]
- Sibi, N.; Subodh, G. Structural and Microstructural Correlations of Physical Properties in Natural Almandine-Pyrope Solid Solution: Al70Py29. J. Electron. Mater. 2017, 46, 1–10. [Google Scholar] [CrossRef]
- Mingsheng, P.; Mao, H.K.; Dien, L.; Chao, E.C.T. Raman spectroscopy of garnet-group minerals. Chin. J. Geochem. 1994, 13, 176–183. [Google Scholar] [CrossRef]
- Ganetsos, T.; Katsaros, T.; Vandenabeele, P.; Greiff, S.; Hartmann, S. Raman spectroscopy as a tool for garnet analysis and investigation on samples from different sources. Int. J. Mater. Chem. 2013, 3, 5–9. [Google Scholar]
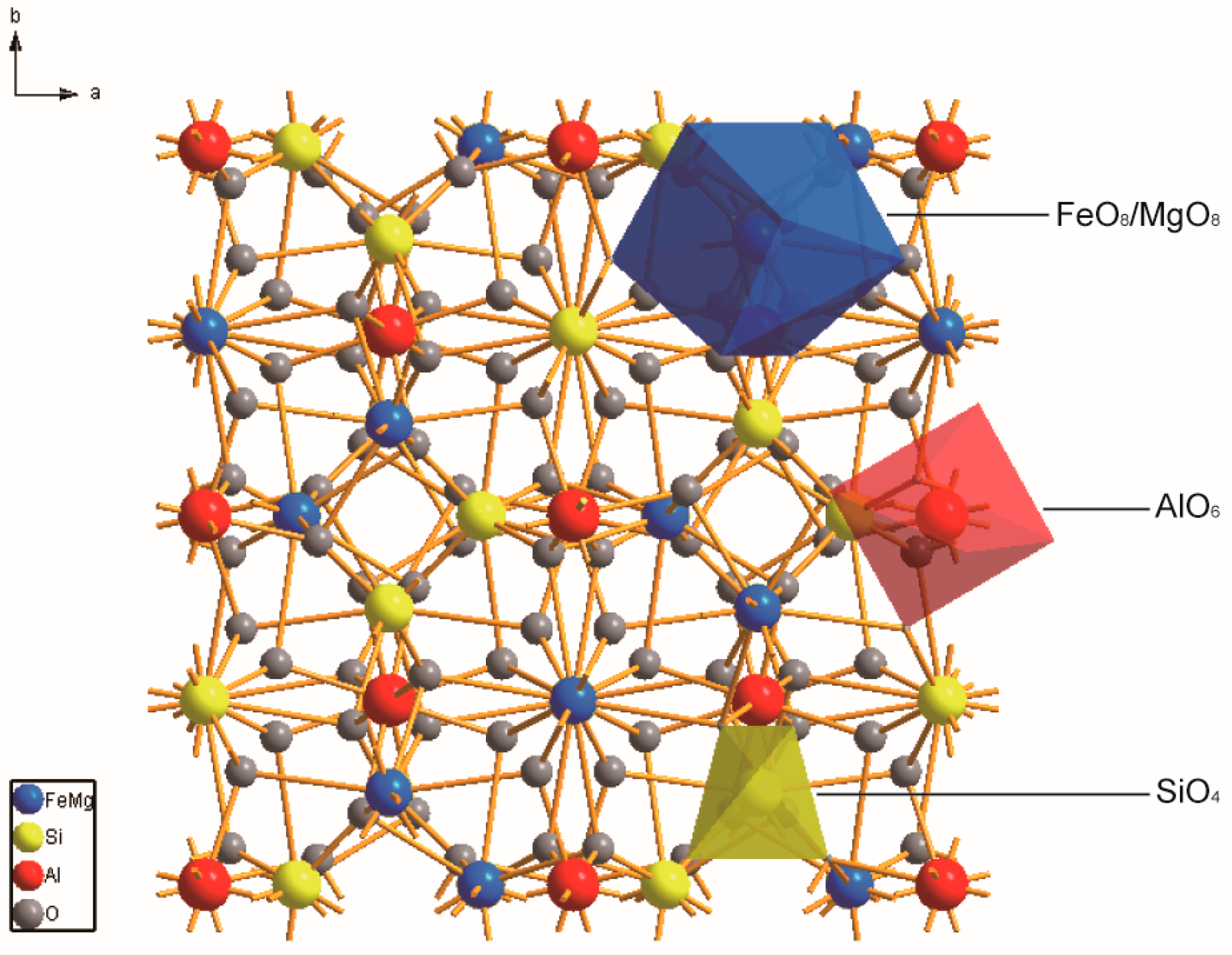
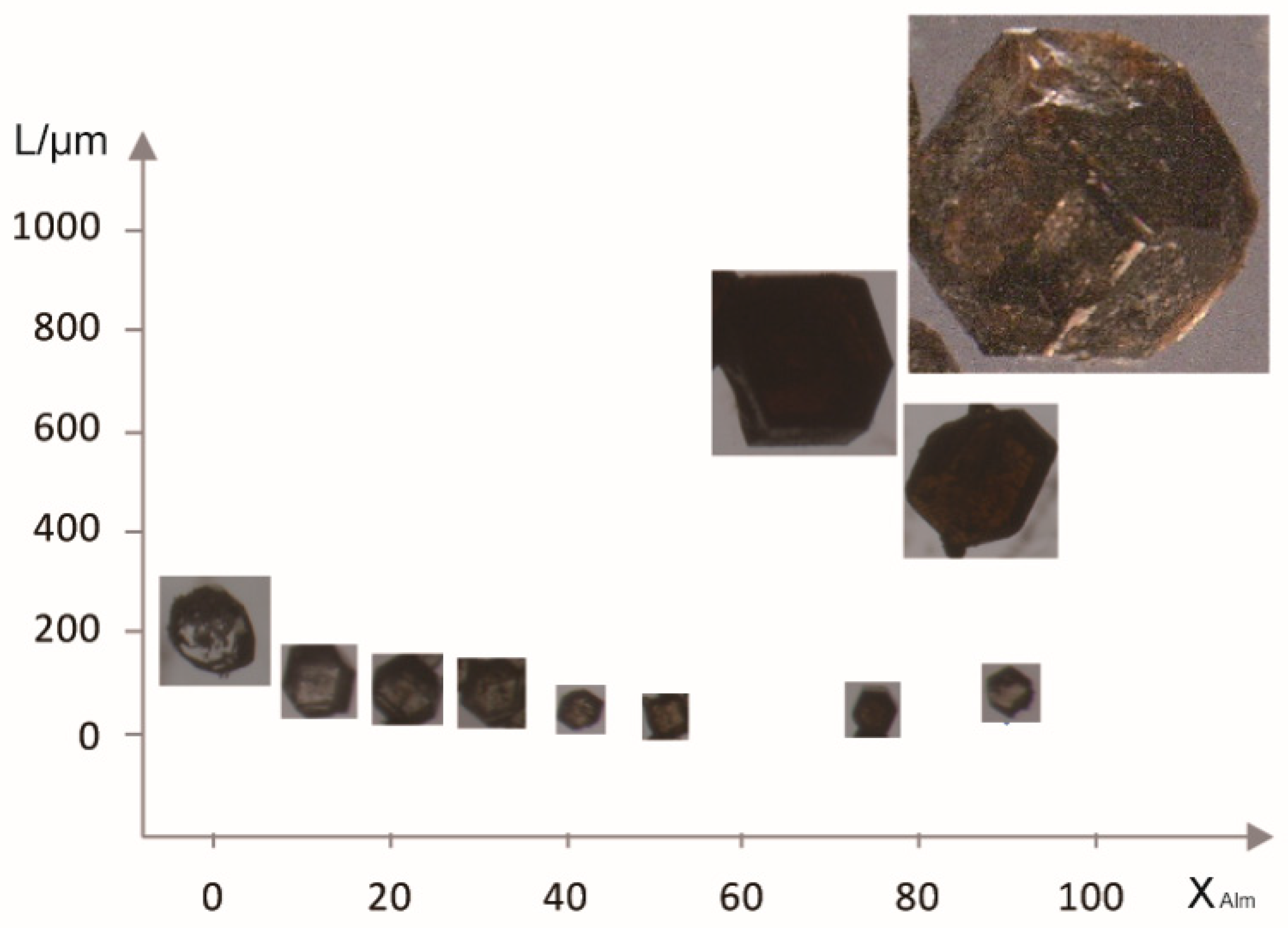
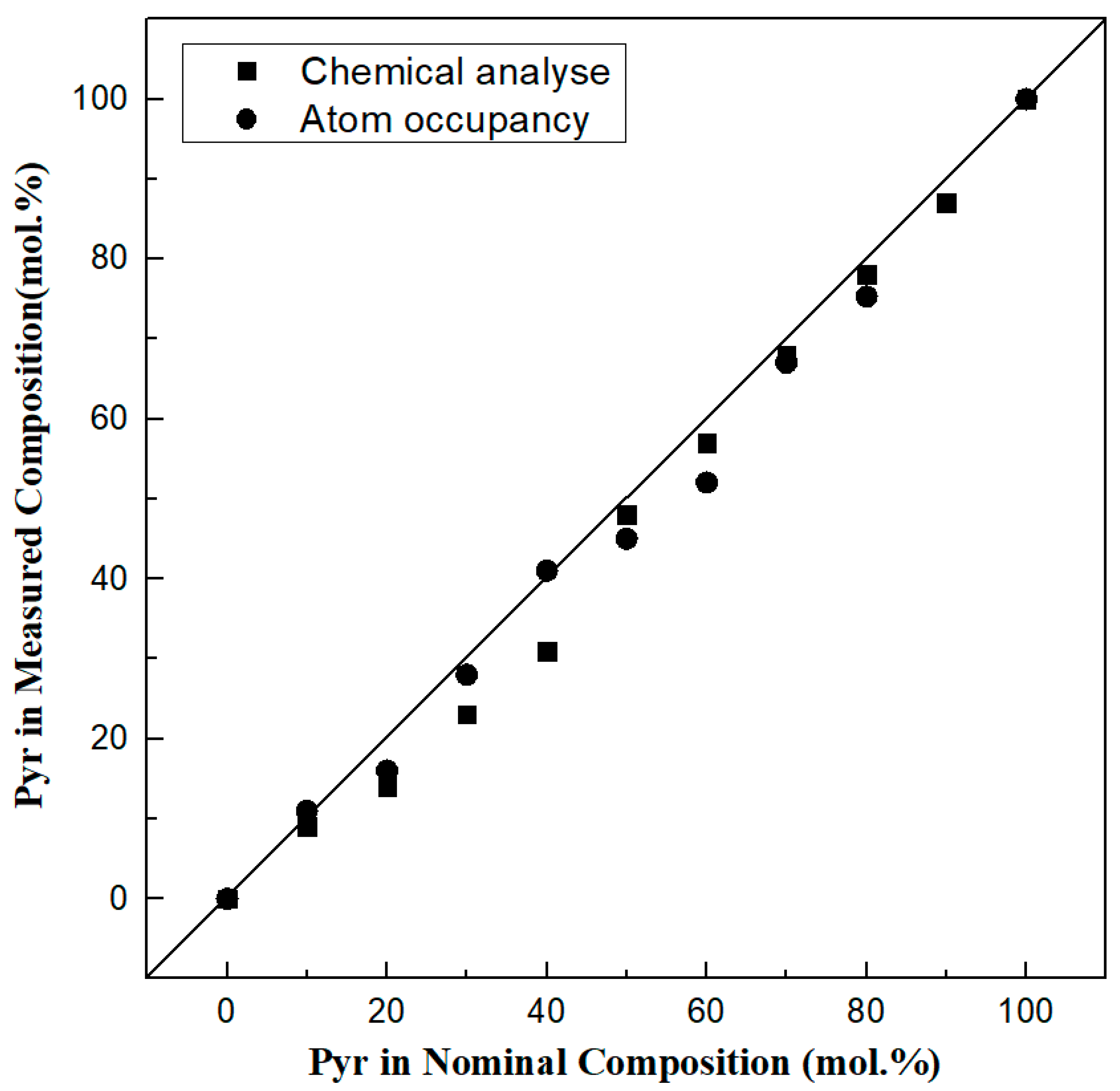
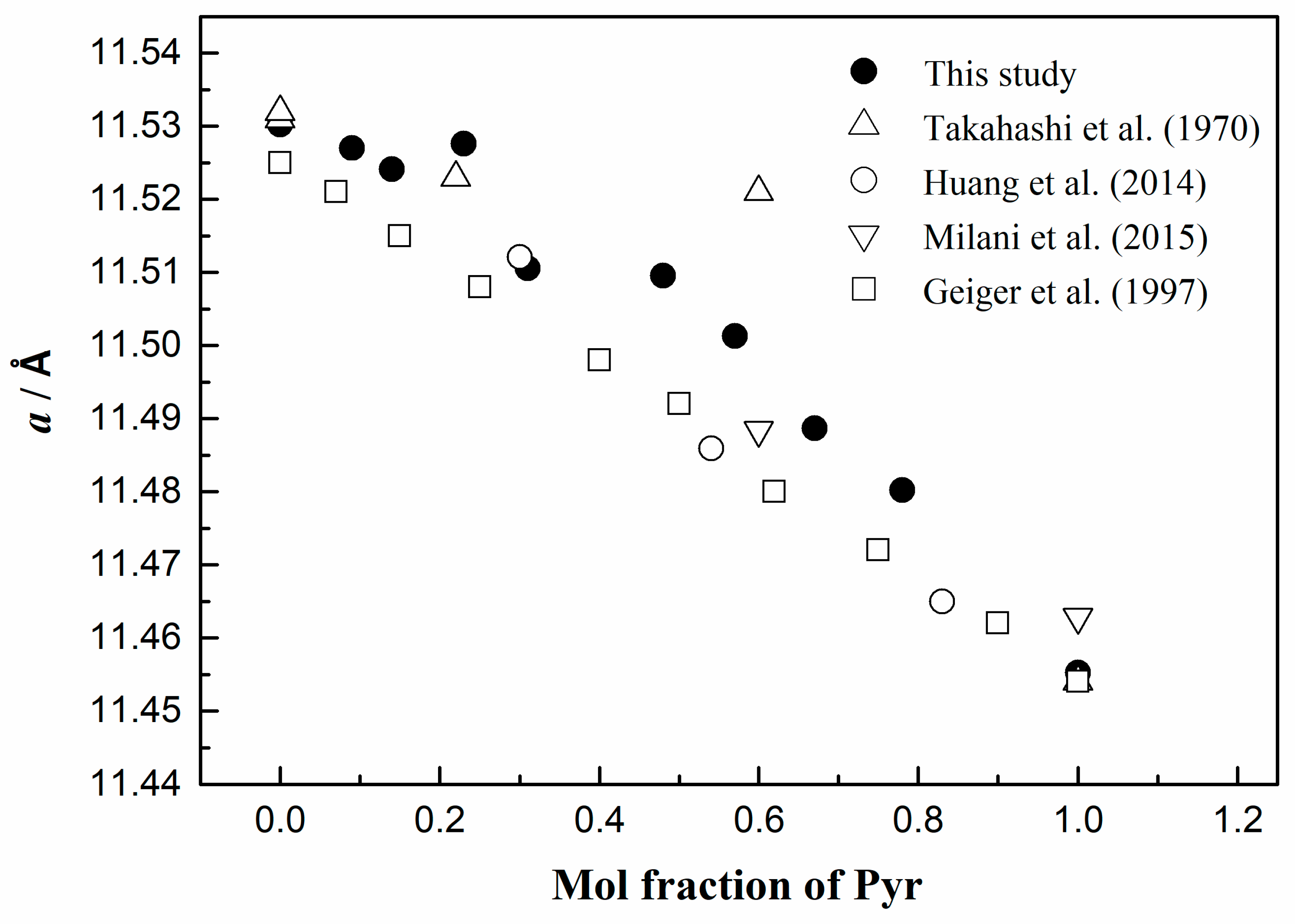

| Nominal Compositions | Alm100 | Pyr10Alm90 | Pyr20Alm80 | Pyr30Alm70 | Pyr40Alm60 | Pyr50Alm50 | Pyr60Alm40 | Pyr70Alm30 | Pyr80Alm20 | Pyr90Alm10 | Pyr100 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| EMPA Compositions | Alm100 | Pyr9Alm91 | Pyr14Alm86 | Pyr23Alm76 | Pyr31Alm68 | Pyr48Alm52 | Pyr57Alm43 | Pyr67Alm32 | Pyr78Alm22 | Pyr87Alm12 | Pyr100 |
| wt.% of oxides | |||||||||||
| SiO2 | 35.76 (27) | 36.83 (102) | 36.37 (175) | 36.26 (177) | 38.33 (26) | 38.88 (174) | 40.11 (57) | 41.21 (28) | 42.68 (59) | 43.28 (33) | 43.34 (101) |
| TiO2 | 1.58 (147) | 0.90 (75) | 1.20 (112) | 0.87 (67) | 1.34 (4) | 0.05 (5) | 0.18 (22) | 0.55 (55) | 0.01 (1) | 0.20 (4) | 0.11 (12) |
| Al2O3 | 19.81 (53) | 20.10 (44) | 20.25 (123) | 19.96 (31) | 21.15 (28) | 21.57 (275) | 22.23 (16) | 22.72 (16) | 22.20 (167) | 24.13 (24) | 24.36 (52) |
| FeO | 46.04 (72) | 43.09 (15) | 40.32 (95) | 38.33 (234) | 33.94 (38) | 23.37 (147) | 22.17 (70) | 17.13 (22) | 11.98 (37) | 6.83 (200) | 0.05 (5) |
| MnO | 0.01 (2) | 0.01 (1) | 0.01 (3) | 0.01 (1) | 0.02 (2) | 0.01 (2) | 0.02 (1) | 0.01 (1) | 0.01 (2) | 0.00 | 0.01 (1) |
| MgO | 0.01 (1) | 2.30 (21) | 3.56 (94) | 6.59 (158) | 8.74 (22) | 12.11 (74) | 16.65 (69) | 19.95 (48) | 23.68 (72) | 26.91 (162) | 30.66 (37) |
| CaO | 0.02 (2) | 0.03 (1) | 0.05 (3) | 0.07 (4) | 0.10 (3) | 0.07 (10) | 0.09 (6) | 0.14 (2) | 0.15 (11) | 0.11 (9) | 0.01 (1) |
| Total | 103.23 (20) | 103.26 (124) | 101.76 (440) | 102.08 (12) | 103.62 (57) | 96.10 (328) | 101.44 (80) | 101.72 (63) | 100.72 (77) | 101.46 (64) | 98.54 (191) |
| mol.% of garnets | |||||||||||
| Pyrope | 0.05 (4) | 8.68 (69) | 13.56 (334) | 23.41 (545) | 31.37 (76) | 47.83 (260) | 57.09 (187) | 67.25 (42) | 77.59 (108) | 87.30 (406) | 99.88 (11) |
| Spessartine | 0.03 (2) | 0.03 (3) | 0.03 (6) | 0.01 (1) | 0.04 (4) | 0.03 (5) | 0.03 (3) | 0.02 (1) | 0.03 (3) | 0.00 | 0.01 (3) |
| Grossular | 0.04 (4) | 0.08 (3) | 0.13 (8) | 0.18 (11) | 0.26 (6) | 0.20 (28) | 0.23 (14) | 0.34 (4) | 0.36 (27) | 0.26 (21) | 0.02 (3) |
| Almandine | 99.88 (8) | 91.20 (69) | 86.28 (333) | 76.40 (532) | 68.33 (83) | 51.86 (286) | 42.65 (172) | 32.39 (45) | 22.02 (104) | 12.45 (384) | 0.08 (9) |
| Total | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00.00 | 100.00.00 | 100.00 | 100.00 | 100.00 | 100.00 |
| Chemical formula | Fe3.13Al1.90Ti0.1Si2.91O12 | (Fe2.88Mg0.27) Al1.89Ti0.05Si2.94O12 | (Fe2.71Mg0.43) Al1.92Ti0.07Si2.92O12 | (Fe2.55Mg0.78) Al1.87Ca0.01 Ti0.05Si2.88O12 | (Fe2.16Mg0.99) Al1.9Ca0.01Ti0.08Si2.92O12 | (Fe1.52Mg1.41) Al1.99Ca0.01Si3.04O12 | (Fe1.36Mg1.82) Al1.93Ca0.01 Ti0.01Si2.95O12 | (Fe1.03Mg2.13) Al1.92Ca0.01Ti0.03Si2.95O12 | (Fe0.71Mg2.49) Al1.85Ca0.01 Si3.01O12 | (Fe0.39Mg2.74) Al1.95Ca0.01Ti0.01Si2.96O12 | Mg3.12Al1.96 Ti0.01Si2.96O12 |
| Atom occupancy composition | Alm100 | Pyr11Alm90 | Pyr16Alm84 | Pyr28Alm73 | Pyr41Alm60 | Pyr45Alm55 | Pyr52Alm48 | Pyr67Alm33 | Pyr75Alm24 | Pyr100 | |
| Composition | Pry% | a (Å) | V (Å3) | Reference |
|---|---|---|---|---|
| Alm100 | 0 | 11.5320 (5) | 1533.52 | Takahashi et al. 1970 [20] |
| Alm100 | 0 | 11.5330 (5) | 1533.61 | Takahashi et al. 1970 [20] |
| Alm100 | 0 | 11.5303 (1) | 1532.91 (4) | This study |
| Alm100 | 0 | 11.530 | 1533.52 (10) | Milani et al. 2015 [11] |
| Alm100 | 0 | 11.5291 (3) | 1532.45 | Geiger et al. 1997 [23] |
| Pyr7Alm93 | 7 | 11.5227 (2) | 1529.90 | Geiger et al. 1997 [23] |
| Pyr9Alm91 | 9 | 11.5270 (1) | 1531.62 (4) | This study |
| Pyr14Alm86 | 14 | 11.5241 (3) | 1530.46 (12) | This study |
| Pyr15Alm85 | 15 | 11.51570 (2) | 1527.63 | Geiger et al. 1997 [23] |
| Pyr22Alm72 | 22 | 11.5230 (5) | 1530.02 | Takahashi et al. 1970 [20] |
| Pyr23Alm76 | 23 | 11.5276 (2) | 1531.85 (6) | This study |
| Pyr25Alm75 | 25 | 11.5105 (2) | 1525.045 | Geiger et al. 1997 [23] |
| Pyr30Alm70 | 30 | 11.5121 (3) | 1526 (1) | Huang et al. 2014 [19] |
| Pyr31Alm68 | 31 | 11.5105 (1) | 1525.06 (2) | This study |
| Pyr40Alm60 | 40 | 11.4995 (2) | 1520.677 | Geiger et al. 1997 [23] |
| Pyr48Alm52 | 48 | 11.5096 (1) | 1524.67 (2) | This study |
| Pyr50Alm50 | 50 | 11.4925 (3) | 1517.90 | Geiger et al. 1997 [23] |
| Pyr54Alm46 | 54 | 11.4859 (1) | 1515 (2) | Huang et al. 2014 [19] |
| Pyr57Alm43 | 57 | 11.5013 (4) | 1521.39 (16) | This study |
| Pyr60Alm40 | 60 | 11.488 | 1516.32 (13) | Milani et al. 2015 [11] |
| Pyr60Alm31 | 60 | 11.521 (1) | 1529.22 | Takahashi et al. 1970 [20] |
| Pyr62Alm38 | 62 | 11.4830 (2) | 1514.14 | Geiger et al. 1997 [23] |
| Pyr67Alm32 | 67 | 11.4887 (3) | 1516.40 (12) | This study |
| Pyr75Alm25 | 75 | 11.4737 (2) | 1510.46 | Geiger et al. 1997 [23] |
| Pyr78Alm22 | 78 | 11.4802 (1) | 1513.04 (3) | This study |
| Pyr83Alm17 | 83 | 11.4650 (3) | 1511 (1) | Huang et al. 2014 [19] |
| Pyr90Alm10 | 90 | 11.4612 (2) | 1505.53 | Geiger et al. 1997 [23] |
| Pyr100 | 100 | 11.4552 (1) | 1503.18 (4) | This study |
| Pyr100 | 100 | 11.4555 (3) | 1503.29 | Geiger et al. 1997 [23] |
| Pyr100 | 100 | 11.4540 (5) | 1502.70 | Takahashi et al. 1970 [20] |
| Pyr100 | 100 | 11.463 | 1506.15 (16) | Milani et al. 2015 [11] |
| Bond Distances | Alm100 | Pyr9Alm91 | Pyr14Alm86 | Pyr23Alm76 | Pyr31Alm69 | Pyr48Alm52 | Pyr57Alm43 | Pyr67Alm32 | Pyr78Alm22 | Pyr100 |
|---|---|---|---|---|---|---|---|---|---|---|
| M-O bond I length | 2.2241 (9) | 2.2218 (10) | 2.2193 (12) | 2.2203 (9) | 2.2159 (9) | 2.2153 (6) | 2.2140 (9) | 2.2088 (8) | 2.2057 (7) | 2.1993 (8) |
| M-O bond I length | 2.3701 (10) | 2.3704 (10) | 2.3680 (12) | 2.3662 (9) | 2.3606 (9) | 2.3598 (7) | 2.3599 (9) | 2.3532 (8) | 2.3501 (7) | 2.3400 (7) |
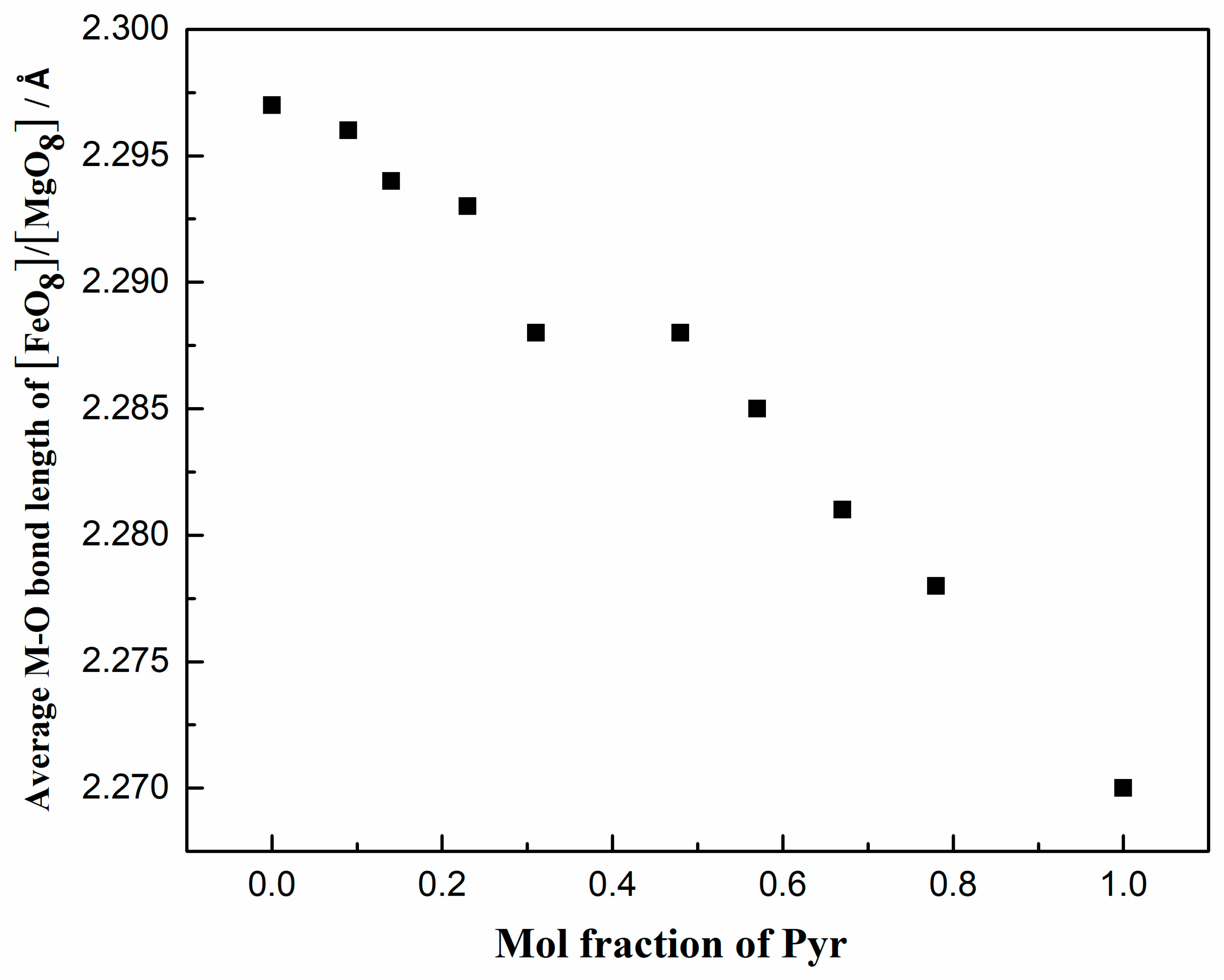
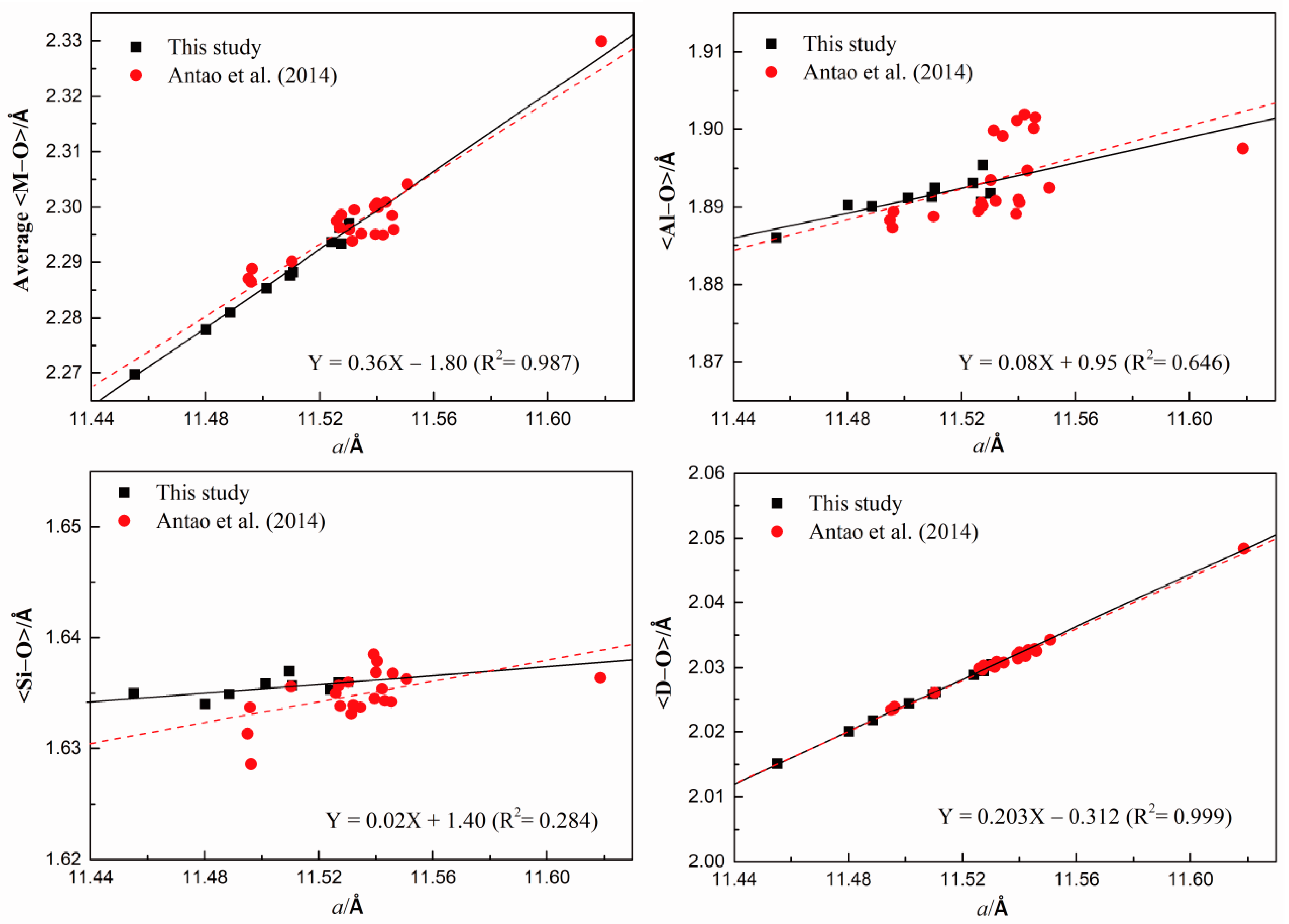
| Average <M-O> | 2.2971 (10) | 2.2962 (10) | 2.2936 (12) | 2.2933 (9) | 2.2882 (9) | 2.2876 (7) | 2.2853 (9) | 2.2810 (8) | 2.2779 (7) | 2.2779 (7) |
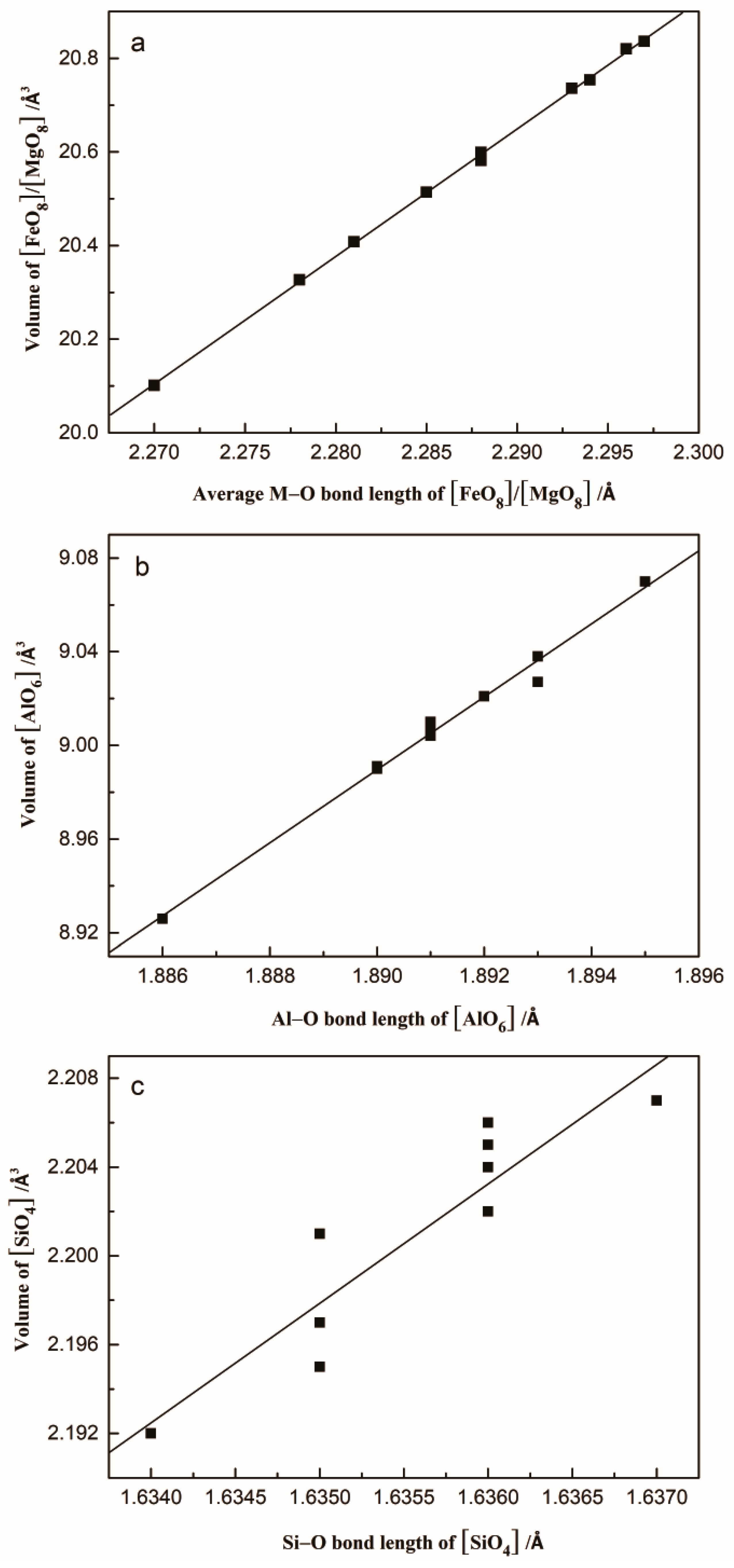
| [FeO8]/[MgO8] volume | 20.836 | 20.82 | 20.754 | 20.736 | 20.599 | 20.582 | 20.514 | 20.408 | 20.327 | 20.101 |
| <Al-O> | 1.8918 (10) | 1.8907 (11) | 1.8931 (13) | 1.8954 (10) | 1.8925 (10) | 1.8913 (7) | 1.8912 (10) | 1.8901 (8) | 1.8903 (7) | 1.8860 (8) |
| [AlO6] volume | 9.021 | 9.004 | 9.038 | 9.07 | 9.027 | 9.01 | 9.007 | 8.99 | 8.991 | 8.926 |
| <Si-O> | 1.6360 (10) | 1.6360 (10) | 1.6353 (13) | 1.6360 (10) | 1.6357 (10) | 1.6370 (7) | 1.6359 (10) | 1.6349 (8) | 1.6340 (7) | 1.6350 (8) |
| [SiO4] volume | 2.206 | 2.205 | 2.201 | 2.204 | 2.202 | 2.207 | 2.202 | 2.197 | 2.192 | 2.195 |
| a <D-O> | 2.029 | 2.02977 | 2.0289 | 2.0295 | 2.02615 | 2.02588 | 2.02442 | 2.02175 | 2.02003 | 2.0151 |
| a (Å) | 11.53025 (9) | 11.52701 (11) | 11.5241 (3) | 11.52759 (16) | 11.51055 (6) | 11.50955 (6) | 11.5013 (4) | 11.4887 (3) | 11.48023 (8) | 11.45522 (10) |
| Alm12Pyr87 | Alm22Pyr78 | Alm32Pyr67 | Alm43Pyr57 | Alm52Pyr48 | Alm68Pyr31 | Alm76Pyr23 | Alm86Pyr14 | Alm91Pyr9 | Assignment | Symmetry Species | Site Motion |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1060 | 1057 | 1056 | 1045 | 1044 | 1041 | 1044 | 1035 | 1037 | (Si-O)str | F2g | v1 and v3 |
| 924 | 924 | 923 | 920 | 920 | 919 | 918 | 916 | 916 | (Si-O)str | A1g | |
| 867 | 867 | 867 | 866 | 865 | 865 | 864 | (Si-O)str | F2g | |||
| 759 b | F2g | ||||||||||
| 688 a | (Si-O)bend | F2g | v2 and v4 | ||||||||
| 644 | 644 | 642 | 638 | 637 | 635 | 634 | 633 | 630 | (Si-O)bend | F2g | |
| 587 | 586 | 584 | 581 | (Si-O)bend | Eg | ||||||
| 560 | 560 | 559 | 558 | 557 | 556 | 555 | 555 | 556 | (Si-O)bend | A1g | |
| 507 | 508 | 506 | 503 | 503 | 501 | 500 | 499 | 500 | (Si-O)bend | F2g | |
| 486 | 482 | 481 | 481 | 478 | 478 | 475 | (Si-O)bend | F2g | |||
| 375 | 375 | 374 | 370 | 372 | 372 | 367 | 370 | 365 | R(SiO4) | F2g | Rotation of SiO4 |
| 358 | 358 | 356 | 350 | 351 | 349 | 347 | 344 | 342 | R(SiO4) | A1g | |
| 350 | 351 | 353 | 333 | 332 | 323 | 329 | R(SiO4) | F2g | |||
| 318 | 318 | 318 | 317 | 315 | 314 | 314 | 316 | R(SiO4) | F2g | ||
| 209 | 209 | 210 | 210 | 209 | 211 | 211 | 214 | 213 | T(SiO4) | Eg | Translation of SiO4 |
| 167 | 168 | 168 | T(X2+) | F2g | Translation of X cation |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuang, Y.; Xu, J.; Li, B.; Ye, Z.; Huang, S.; Chen, W.; Zhang, D.; Zhou, W.; Ma, M. Crystal-Chemical Properties of Synthetic Almandine-Pyrope Solid Solution by X-Ray Single-Crystal Diffraction and Raman Spectroscopy. Crystals 2019, 9, 541. https://doi.org/10.3390/cryst9100541
Kuang Y, Xu J, Li B, Ye Z, Huang S, Chen W, Zhang D, Zhou W, Ma M. Crystal-Chemical Properties of Synthetic Almandine-Pyrope Solid Solution by X-Ray Single-Crystal Diffraction and Raman Spectroscopy. Crystals. 2019; 9(10):541. https://doi.org/10.3390/cryst9100541
Chicago/Turabian StyleKuang, Yunqian, Jingui Xu, Bo Li, Zhilin Ye, Shijie Huang, Wei Chen, Dongzhou Zhang, Wenge Zhou, and Maining Ma. 2019. "Crystal-Chemical Properties of Synthetic Almandine-Pyrope Solid Solution by X-Ray Single-Crystal Diffraction and Raman Spectroscopy" Crystals 9, no. 10: 541. https://doi.org/10.3390/cryst9100541
APA StyleKuang, Y., Xu, J., Li, B., Ye, Z., Huang, S., Chen, W., Zhang, D., Zhou, W., & Ma, M. (2019). Crystal-Chemical Properties of Synthetic Almandine-Pyrope Solid Solution by X-Ray Single-Crystal Diffraction and Raman Spectroscopy. Crystals, 9(10), 541. https://doi.org/10.3390/cryst9100541




