1. Introduction
A large majority of active pharmaceutical ingredients (APIs) are manufactured into crystalline form by means of crystallization, the process modifies the physicochemical characteristics of manufactured APIs. Polymorphs, crystal morphology, particle size distribution and so on are known to have a significant impact on the manufacturability, dissolution rate, and bioavailability of APIs [
1,
2,
3]. Moreover, during the manufacturing process, the crystal morphology and particle size distribution of crystal powder have a huge impact on the dissolution rate and flowability of the final product [
4]. A poor flowability of APIs is an obviously huge limitation for the tablet-compression and capsule-filling during drug formulation process [
4,
5].
In 1982, Kawashima et al. applied a spherical crystallization technique and described the transformation process by which spherical agglomerates increased or decreased in size during their agglomeration [
6]. Moreover, by manufacturing spherical agglomerates with uniform PSD, it is possible to improve and overcome different problems such as crystal flowability and strong interfacial tension, encountered during the drug formulation process of microcrystalline [
7]. Accordingly, in the aim to improve APIs crystals characteristics and develop suitable APIs crystals for drug formulation, several studies regarding spherical crystallization have been carried out by many researchers, and spherical agglomerates could be produced using spherical crystallization methods.
In 1996, Ribardiere et al. presented two different spherical crystallization techniques to obtain spherical agglomerates. The first technique is spherical agglomeration (SA), carried out by the addition of bridging solvent during the drowning-crystallization process. The second one is the quasi-emulsion solvent diffusion (QESD) performed by forming quasi-emulsions during the drowning-out crystallization process [
8]. Kawashima et al. presented the ammonia diffusion method (ADM) which is carried out by adding ammonia water to the system in order to form spherical agglomerates [
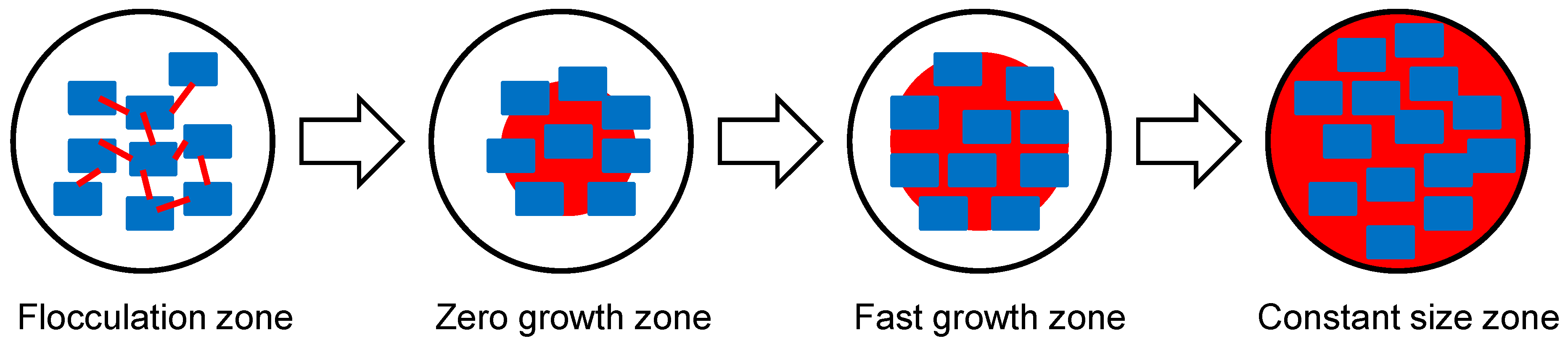
9]. When spherical agglomerates are induced during the spherical crystallization, the growth of the agglomeration is completed in 4 steps: 1) flocculation zone, 2) zero growth zone, 3) fast growth zone, and 4) constant size zone [
10]. These 4 steps are influenced by parameters, such as solubility, agitation rate, temperature, residence time, and so on [
10,
11]. Thati and Rasmuson described the spherical agglomeration mechanism in 2011 [
12], and Kovacic et al. presented a new spherical crystallization technique called crystallo-co-agglomeration (CCO) [
13]. Tahara et al. developed a solvent recycling system of the solvent used in the QESD spherical crystallization process [
14].
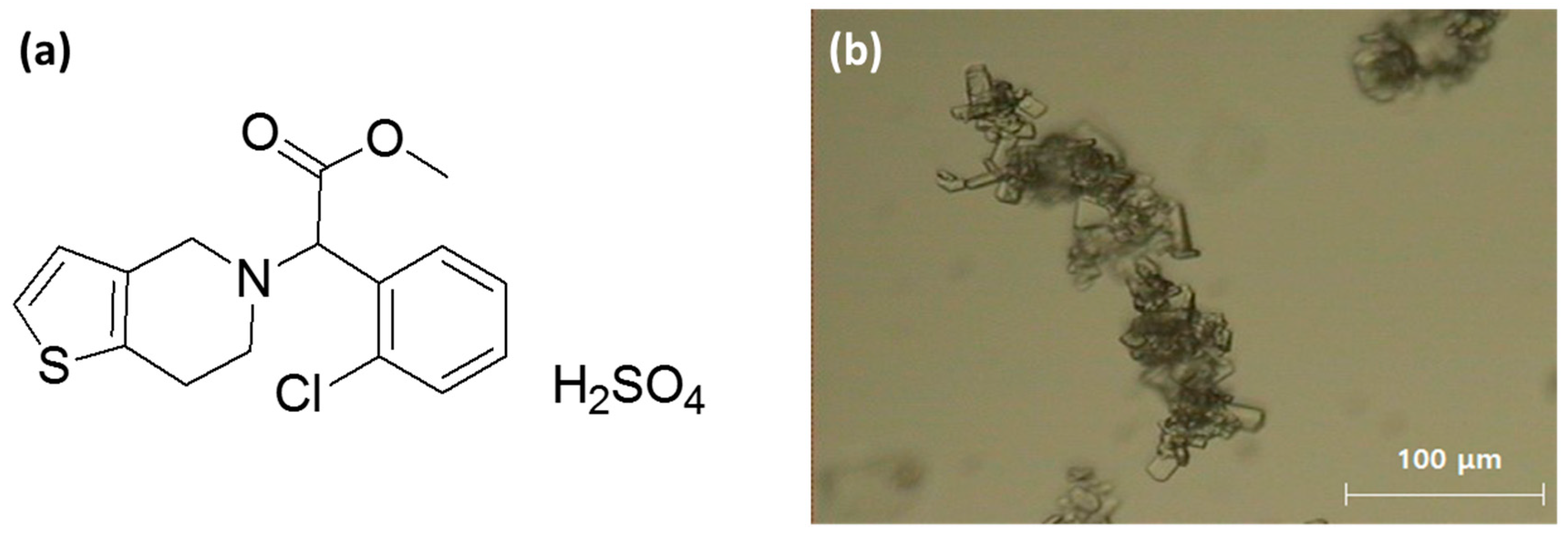
Clopidogrel bisulfate (CLP) is a drug with antiplatelet activity which is used for the treatment of several blood vessel diseases related to platelets such as angina, arrhythmia peripheral arterial occlusive disease, stroke, cerebral arteriosclerosis, and myocardial infarction. Its chemical name is (+)-methyl--5-[4,5,6,7-tetrahydro-[3,2-c] thienopyridyl]-(2-chlorophenyl)acetate hydrogen sulfate [
15] (
Figure 1a). At present, six types of CLP polymorphs have been reported, and among them form-I and form-II crystals are selectively used for the manufacturing of CLP drugs [
16,
17]. Form-I and form-II crystals are enantiotropic polymorphs. In addition, it is reported that form-II crystals are more stable than form-I crystals at room temperature because form-II crystals are thermodynamically stable at lower temperatures than their transition temperature and form-I crystals are thermodynamically stable at higher temperatures than their transition temperature [
18]. Besides, it is also reported that, monoclinic form-I crystals have higher density and solubility than orthorhombic form-II crystals [
19]. Therefore, pharmaceutical industries prefer the high soluble form-I crystals over form-II crystals. However, as the polymorphic transformation speed from form-I crystals to form-II crystals occurs within only 10 min, form-I crystals have been harvested via very complex techniques [
20,
21,
22]. Further, form-I crystals are irregular, rectangular shaped crystals and form microcrystalline powder (
Figure 1b).
This causes poor compressibility and flowability as well as a strong surficial tension which has a negative impact during the drug formulation process. In order to overcome these problems, CLP crystals are manufactured into agglomerated crystals and are commercially available as spherical agglomerates with uniform particle size distribution (PSD) [
19,
23]. Manufactures apply two different methods in order to obtain CLP spherical agglomerates. The first method is the harvest of CLP spherical agglomerates, three steps, such as CLP base and bisulfate synthesizing step, followed by the seeding and the crystallization steps, are carrying out simultaneously [
19]. The second method is as follows: While the synthesis of CLP base and bisulfate is occurring, an amount of acetic acid is added to the system, then the system is allowed to crystallize for more than 12 h [
23].
However, because of a poor yield and low reproducibility of CLP form-I spherical agglomerates when using the above mentioned two methods, developing a new crystallization technique for a high yield and high reproducibility of CLP form-I spherical agglomerates with uniform PSD is tremendously needed. Therefore, the present study was carried out utilizing a generally used crystallization method as well as varying the anti-solvent types, the temperature and the rotational speed, in order to develop an effective and reproducible crystallization technique for the manufacturing of CLP form-I spherical agglomerates with uniform PSD.
2. Materials and Methods
2.1. Materials
Clopidogrel bisulfate (purity higher than 99.9%, form-II) was supplied by J2H biotech. Co., Ltd. (Gyunggido, Korea). Ethanol (EtOH), isopropyl alcohol (IPA), n-hexane (Hex), and acetone (ACT) was purchased from Daejung Chemicals Co., Ltd. (Gyunggido, Korea).
2.2. Anti-Solvent Crystallization Method
The experimental conditions for spherical crystallization are summarized in
Table 1. A 25 mL mixture of IPA/Hex [40:60, v/v] in a double-jacketed crystallizer was constantly stirred at 10 °C. Then, 5 g of CLP Form-II was added to a round-bottom flask containing 25 mL of ethanol at 50 °C and stirred until completely dissolved. The ethanol solution containing CLP at 50 °C was added to the anti-solvent at 10 °C. The resulting solution was stirred for 10 min. The solution temperature was approximately 35 °C after the addition of the ethanol solution to the anti-solvent. The solution was continuously stirred for 20 min after the formation of precipitate, and then the precipitate was filtered using a vacuum filter (Exp. 1 in
Table 1). At first, the effect of solvent on spherical crystallization was studied by varying anti-solvents by using either an IPA/Hex mixture, pure Hex, or pure ACT while fixing the crystallization temperature at 35 °C and the rotation speed at 500 rpm (Exp. 1–3 in
Table 1). Additional experiments to investigate the effect of temperature on spherical crystallization were performed by varying crystallization temperatures from 20 to 40 °C (Exp. 1 and 4–7 in
Table 1). The effect of stirring rate on spherical crystallization was investigated by performing crystallization without stirring or varying the stirring speed from 500 to 800 rpm (Exp. 1 and 8–11 in
Table 1). Furthermore, the effect of the supersaturation level was also investigated (Exp. 1–3, 12 in
Table 1). Video-microscopy, field emission scanning electron microscopy (FE-SEM), powder X-ray diffraction (PXRD), particle size analysis (PSA), and Fourier Transform Infrared Spectroscopy (FT-IR) were carried out to determine the morphology, particle size distribution (PSD), and polymorphic forms of the resulting CLP agglomerates.
2.3. Powder X-ray Diffraction (PXRD)
PXRD analyses were performed using a Powder X-ray diffractometer (Bruker, D8 Advance, Billerica, MA, USA) with Cu Kα radiation at 45 kV and 40 mA at a scan rate of 3°/min over a 2θ range from 5° to 35°. The divergence and scattering slits were 1mm while the receiving slit was 0.2 mm.
2.4. Video Microscope
Video photographs were taken using a video-microscope (SV-55, Sometech, Sungnam, Korea) at a total magnification of 600× in order to investigate the morphology of the CLP crystals.
2.5. Field Emission Scanning Electron Microscope (FE-SEM)
Agglomerates of CLP crystals were observed on FE-SEM (LEO SUPRA 55, GENESIS 200, Carl Zeiss, Philadelphia, PA, USA) at magnification of 100,000×.
2.6. Fourier Transform Infrared (FT-IR)
FT-IR (PerkinElmer Spectrum 100 FT-IR, Massachusetts, Boston, MA, USA) was employed to assay hydrogen-boding within the CLP polymorphs. An attenuated total reflectance (ATR) accessory was attached, and the crystals were analyzed in their powder form.
2.7. Differential Scanning Calorimetry (DSC)
DSC (Q20, TA Instruments, Philadelphia, PA, USA) was used for the thermal analysis of CLP crystals. The scan rate was 10 °C/min and the heat range was set from 20 to 250 °C.
2.8. Particle Size Analysis (PSA)
A Mastersizer 2000 particle size analyzer (Malvern Panalytical, Malvern, UK) equipped with Scirocco 2000 dry powder feeder (Malvern Panalytical, Malvern, UK) was used for measuring the particle size of CLP crystals. Samples were loaded at 1bar atmospheric pressure and the particle size distribution of CLP crystals was measured by determining the coefficient of variation for three characteristic diameters, 10% (d0.1), 50% (d0.5) and 90% (d0.9). In this study, values for 50% (d0.5) volume mean particle diameter are reported.
2.9. Solubility Measurements
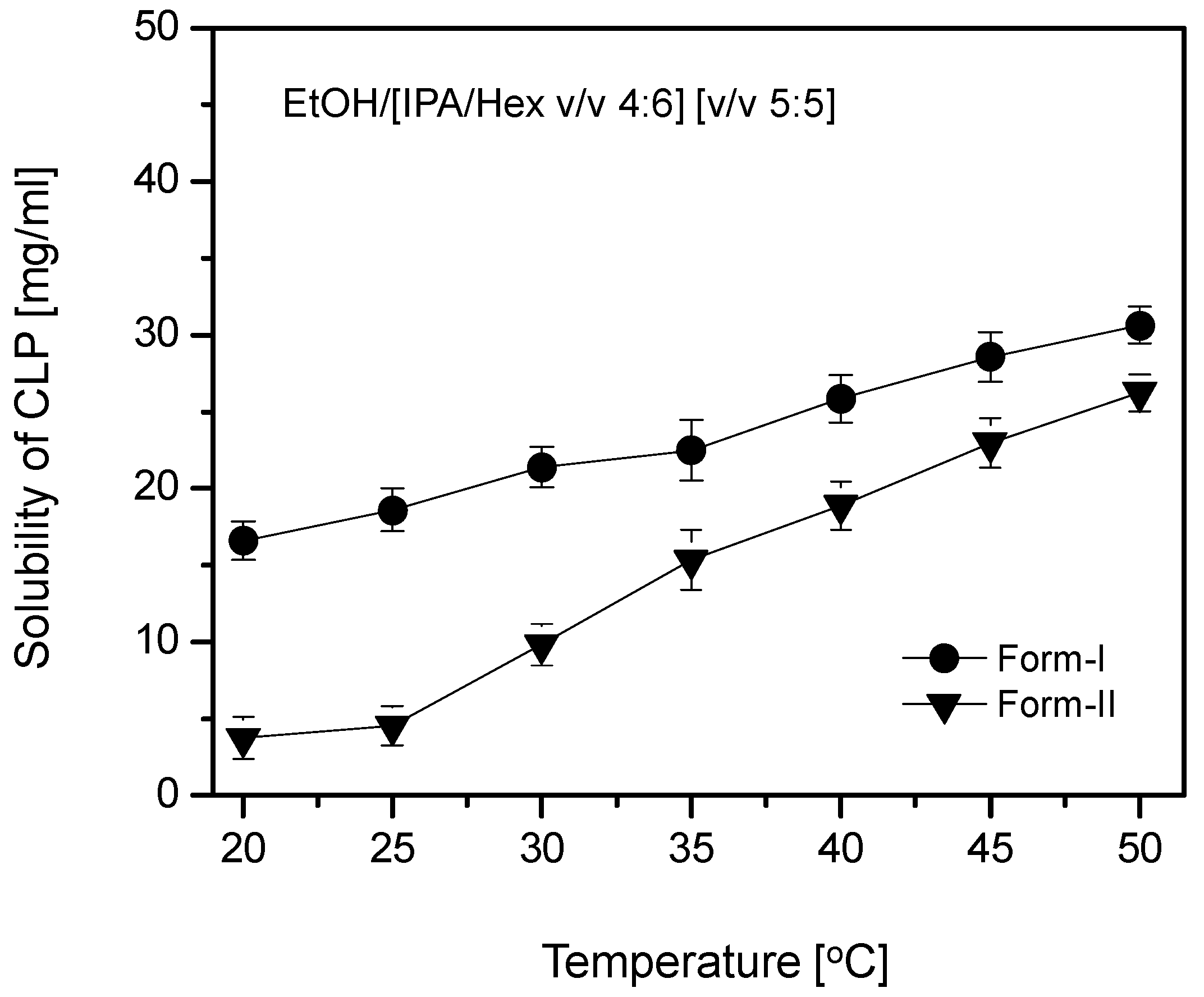
High-performance liquid chromatography (HPLC-Agilent 1260, Agilent Technology, Santa Clara, CA, USA) was used to investigate the solubility of CLP form-I and form-II crystals at 35 °C in solvent [ethanol]/Anti-solvent [IPA/Hex, 4:6, v/v] [5:5, v/v]. It was also employed to study how the solubility of CLP form-I and form-II crystals in the same solvent changed from 20 to 50 °C. The analysis was performed using a C18 column (4.6 × 50 mm) (3.5 μm) (YMC Co., Ltd., Tokyo, Japan). The mobile phase was 0.1% triethylamine water/acetonitrile [25:75, v/v] (pH adjusted to 4 using dilute ortho-phosphoric acid) at a flow rate of 1mL/min and the UV detection wavelength was set to 225 nm. CLP samples, from 1 mg to 5 mg were dissolved in 1mL of ethanol/[IPA/Hex [4:6, v/v]] [5:5, v/v] and then diluted ten-fold in methanol. The solubility was measured from the calibration curve determined after each sample was run in triplicate.
3. Results
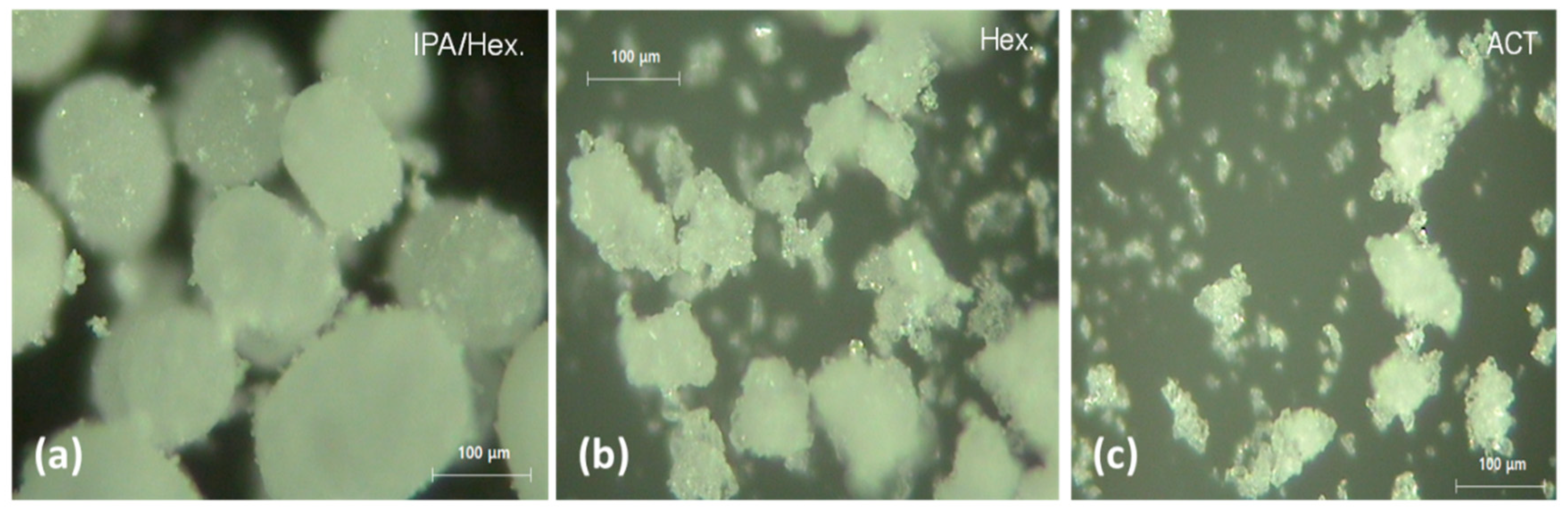
Figure 2 presents Video-microscopic images of CLP agglomerates crystallized at 35 °C at a stirring speed of 500 rpm when either isopropyl alcohol/
n-hexane (IPA/Hex),
n-hexane (Hex), or acetone (ACT) was used as the anti-solvent. Spherical CLP crystals were obtained when the IPA/Hex mixture was used as an anti-solvent (
Figure 2a). However, non-spherical agglomerates were obtained when Hex or ACT was used as an anti-solvent (
Figure 2b,c).
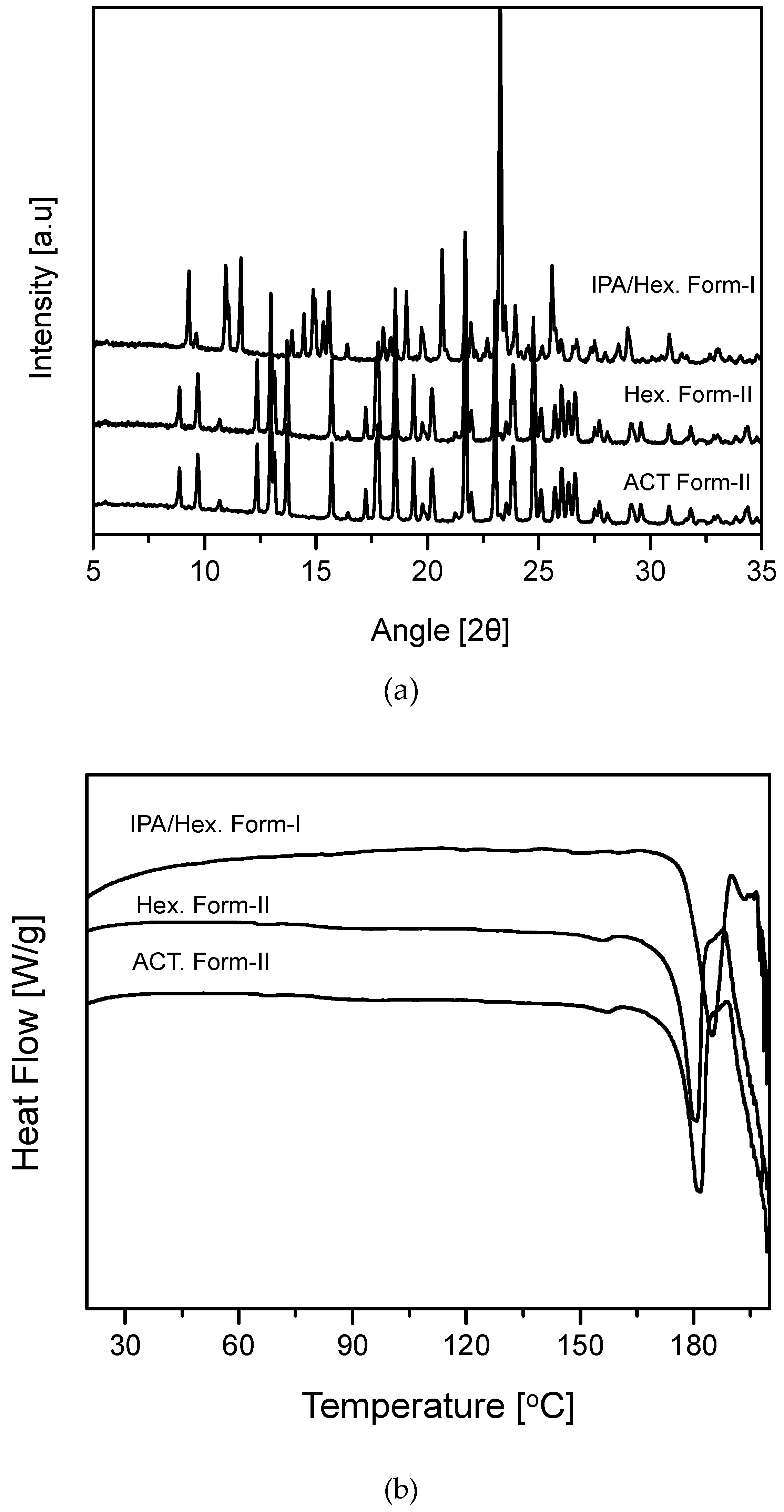
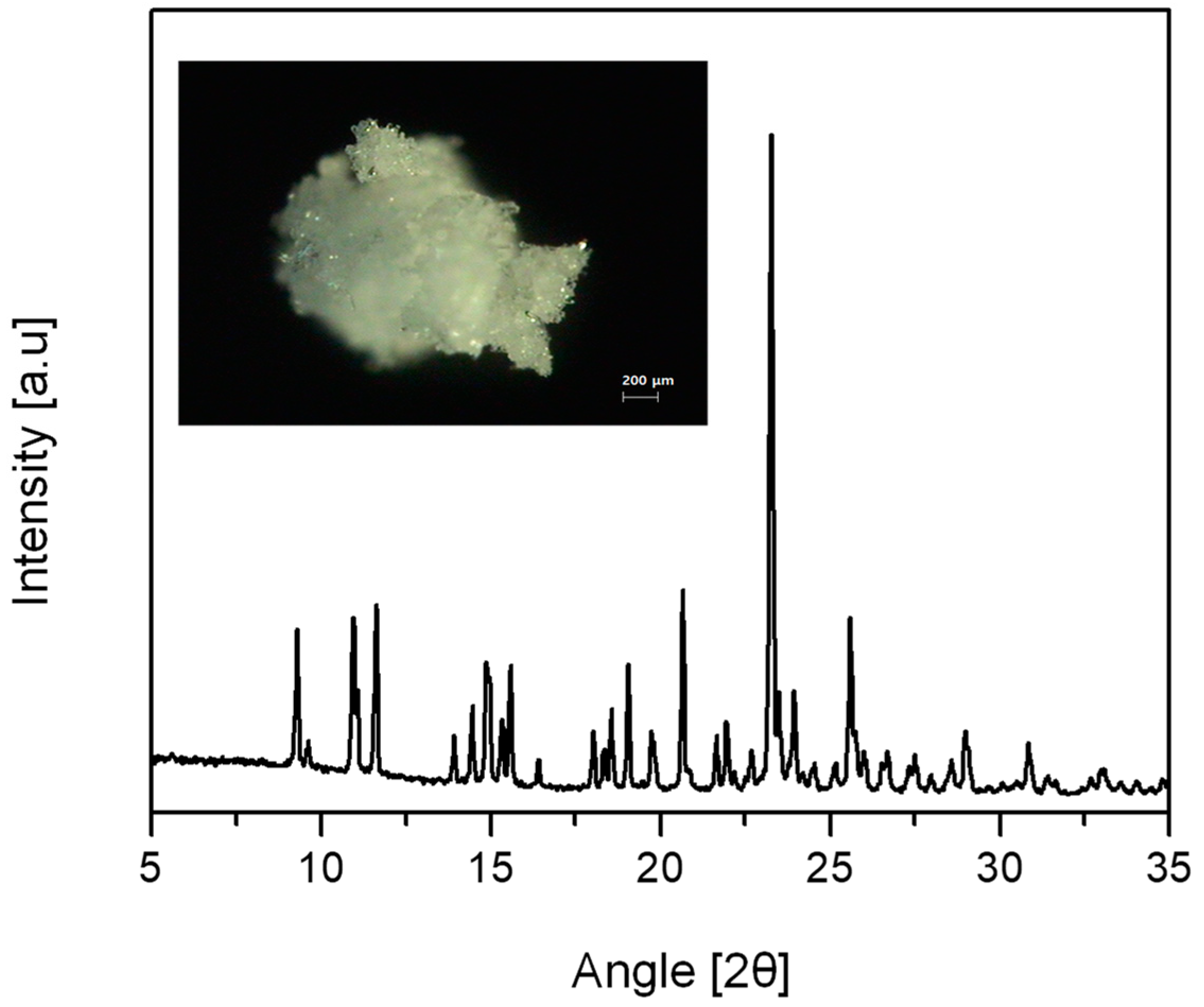
The PXRD patterns suggest that the spherical CLP crystals obtained with the IPA/Hex mixture were Form-I but that the non-spherical CLP agglomerates obtained with Hex or ACT were Form-II (
Figure 3a). Moreover, the results revealed that the DSC data (
Figure 3b) were in accordance with the PXRD data illustrate in
Figure 3a. The
Figure 3 results obtained in this study were in good agreement with the previously reported PXRD and DSC data for Form-I and Form-II [
18].
Figure 4 illustrates the PSD D50 and morphology of the CLP crystals formed at stirring speeds from 500 to 800 rpm under the same conditions (35 °C and IPA/Hex [40:60, v/v] as an anti-solvent). When the stirring speed was 500 rpm, the PSD D50 was 105.4 μm with a uniform particle size distribution (
Figure 4a). As the stirring speed increases to 600, 700, and then 800 rpm, the PSD D50 decreased to 90, 74.5 and then 62.4 μm (
Figure 4b–d). It was also noted that, according to the PXRD analysis, the spherical CLP agglomerates with rough surfaces generated under 800 rpm were a mixture of Forms I and II (
Figure S1). PXRD data confirmed that the spherical agglomerates with smooth surfaces obtained at stirring speeds of 500, 600, and 700 rpm were Form-I (
Figure S1) [
18].
Figure 5 shows the FE-SEM images of CLP spherical agglomerates magnified 100,000×, precipitated at stirring speeds of 500 and 800 rpm. The size of the particles on the surface of the agglomerates obtained at a stirring speed of 500 rpm were much smaller and more uniform than those obtained at a stirring speed of 800 rpm (
Figure 5). In the case of agglomerates obtained at 800 rpm, most of the particles on the surface were large well-shaped particles and the agglomerates were relatively rough-surfaced.
Temperature during crystallization is a crucial factor since it can modify the supersaturation level, the driving force of polymorphic transformation, by changing the ratio of the solubilities of the metastable form to the stable form, thus yielding different crystallization outcomes. As a result, if the polymorphic form can be controlled by temperature, it is important to find an optimal crystallization temperature and sometimes maintain a high temperature.
Controlling the polymorphic form during the crystallization process is very important since it is well known that the polymorphic form can affect the solubility, dissolution rate, and even bioavailability [
1,
2,
3]. CLP is known to have six polymorphic forms, of which Forms I and II are presently used in commercially available products [
16,
17,
18]. Since Forms I and II are enantiotropically related, the temperature during crystallization can play a major role in determining the final polymorphic form [
18].
Table 1 shows the polymorphic forms crystallizing at various temperatures (Exp. 1, 8–11). The FTIR spectra suggest that Form-II was obtained below 25 °C, while Form-I was obtained above 35 °C. At 30 °C, a mixture of Forms I and II was obtained (
Figure S2) [
18,
22]. However, it should be noted that Form-I eventually transformed to Form-II at all temperatures studied, with the rate of polymorphic transformation being faster at 35 °C than at 40 °C (
Figure S3).
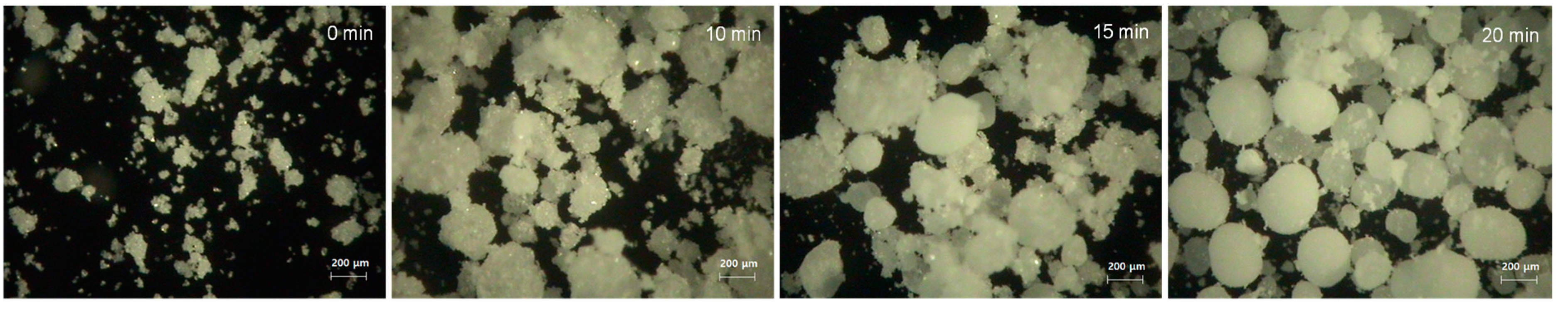
In-situ monitoring of agglomerate formation during crystallization (35 °C, 500 rpm, and IPA/Hex [v/v 40:60] as an anti-solvent) was conducted using a video-microscope at 600× magnification. At the beginning of the crystallization, small non-agglomerated particles were formed (
Figure 6). The microscopic image taken at 10 min showed that the agglomerates had begun to form but were not yet spherical and did not have a smooth surface. Spherical agglomerates with a smooth surface started forming at 15 min and had finished by approximately 20 min. The schematic process of forming spherical agglomerates with smooth surfaces is illustrated in
Figure 7.
On the basis of the in-situ monitoring, it can be hypothesized that the formation of spherical CLP agglomerations follows the four stages proposed by Bermer and Zuider Wag, the flocculation zone, zero growth zone, fast growth zone, and constant size zone [
10]. In the flocculation zone, a bridging liquid is often considered necessary, but in our experiment floccules were formed without it. The floccules started to form into agglomerates at 10 min. In the fast growth zone, the rough agglomerates began to be filled in with small particles to make densely packed agglomerates. Finally, the surface smoothing process began and the formation of spherical particles with smooth surfaces was completed in the constant size zone (
Figure 6).
We applied the four steps mechanism in order to explain the growth of the agglomerations. However, we did not use bridging liquid so were able to remove the complex bridging liquid addition step.
4. Discussion
According to classical nucleation theory (CNT), the crystallization process is mostly governed by the supersaturation level and temperature. When polymorphic control is important, we consider the “Ostwald Rule of Stages” and the driving force of crystallization. The “Ostwald Rule of Stages” states that the system seeks a more stable condition, that is, not the most stable one but a more stable one close to the current condition. In practice, a solution with a high supersaturation level is more likely to produce a metastable polymorph than a solution with a low supersaturation level. In order to be formed by crystallization, the desired polymorphic form has to be kinetically stable. The important factor determining the kinetic stability of metastable Form-I is known to be the thermodynamic driving force of polymorphic transformation. The thermodynamic driving force of polymorphic transformation can be estimated by measuring the ratio of the solubilities of the metastable and stable forms. As the driving force of polymorphic transformation increases, the rate of polymorphic transformation tends to increase [
22,
24,
25].
Table 2 includes the solubilities of Forms I and II, the supersaturation level S (where S = the solution concentration/the equilibrium solubility at a given temperature), and the ratio of the solubilities of Form-I and Form-II, C
Form-I/C
Form-II (where C
Form-I/C
Form-II = the solubility of Form-I/the solubility of Form-II at a given temperature) from the three different solvent and anti-solvent systems at 35 °C. The solubility data shows that over the temperature range we studied Form-II was the stable form. However, above 30 °C Form-I could be obtained in the experiment. As mentioned previously, the supersaturation level plays an important role in determining the resulting polymorphic form following the “Ostwald Rule of Stages” [
21]. In addition, the ratio of the solubilities of Form-I/Form-II, the driving force for polymorphic transformation, is a critical factor controlling the resulting polymorphic form because it can kinetically stabilize the metastable form [
22,
24,
25].
In the ethanol/IPA/Hex system, the level of supersaturation for Form-I was the highest (S
Form-I = 4.44) and the driving force for polymorphic transformation is the lowest (C
Form-I/C
Form-II = 1.47). In the other two solvent systems, the level of supersaturation was small but the driving force for crystallization was large, and therefore, the formation of the stable form, Form-II, was favored. In order to investigate the effect of the supersaturation level on the polymorphic form crystallizing out, a similar level of supersaturation (S = 2.7) was applied to crystallization in the ethanol/IPA/Hex system. Since the driving force for polymorphic transformation in this system is low (C
Form-I/C
Form-II = 1.47), it can be assumed that the form initially generated by crystallization would be maintained. The generation of Form-II in this case confirmed that the level of supersaturation is an important factor for producing the metastable form of CLP, Form-I, during crystallization (
Table 2).
The same approach can be applied to explain the crystallization outcome across other temperatures. As the temperature increased from 20 to 40 °C, the supersaturation level decreased (
Table 3). It was also notable that the driving force for polymorphic transformation, the solubility ratio of Form-I/Form-II, decreased as the temperature increased. As shown in
Figure 8, Form-II was the stable form over the temperature range of the study. However, the crystallization outcome does not only rely on thermodynamic stability in this case. Over the temperature ranges studied, the level of supersaturation (S
Form-I ≥ 3.87) appears to be high enough to produce the metastable form following the ‘Ostwald Rule of Stages’. However, the driving force for polymorphic transformation, the ratio of the solubilities of Form-I and Form-II was observed to be low enough (C
Form-I/C
Form-II ≤ 1.47) to delay polymorphic transformation above 35 °C. As a result, the final polymorphic was Form-I. Below 25 °C, Form-II was produced, and at 30 °C a mixture of Form-I and Form-II was produced. This observation was also confirmed by monitoring the rate of polymorphic transformation during crystallization (
Figure S3). The rate of polymorphic transformation is faster at 35 °C than 40 °C, confirming that the ratio of the solubilities of Form-I and Form-II plays an important role in determining the rate of polymorphic transformation and thus, the final polymorphic form [
18,
22,
24,
25].
The purpose of this study was to propose a simple and efficient method of producing spherical CLP particles of the desired polymorphic form by varying the level of supersaturation.
In this study, the type of anti-solvent, the stirring speed, and temperature were shown to significantly affect the size, polymorphic form, and formation of the spherical agglomerates. According to this study, the type of anti-solvent and crystallization temperature determines the level of supersaturation during crystallization and the driving force for polymorphic transformation, and thus, the final polymorphic form. Stirring is also important for producing spherical CLP particles. Without stirring, agglomerates of the desired polymorphic form could be obtained but the particles were not spherical (
Figure 9).
In addition, spherical particles of Form-I could not be obtained when a certain supersaturation level was not met – i.e., the cases where either Hex or ACT were used as anti-solvents. (
Figure 2 and
Figure 3,
Table 2).
Stirring speed affects the hydrodynamics of the crystallization solution and thus can modify crystallization conditions by varying mass transfer of not only solute molecules but even crystals, both in the bulk solution and near the growing crystal surfaces, varying heat transfer between the wall of crystallizer and the bulk solution, thus varying the frequency and intensity of collisions between solute molecules or crystals. As a result, stirring speed can significantly impact nucleation, crystal growth, breakage, and crystal agglomeration (
Figure 9). In our case, the stirring speed modified the size and shape of the agglomerates, and even their polymorphic form. Increasing the stirring speeds from 500 to 700 rpm, decreased the size of the spherical agglomerates and by 800 rpm not only was their size further decreased but their polymorphic form changed (
Figure 5,
Figure S1).
Spherical crystallization processes involve the transport and the attachment of small crystals to the surface of the agglomerates/core crystals. After forming small core crystals, the pores within agglomerates were filled with small crystals. Hydrodynamics begin to play an important role at this stage. Stirring speed can impact the frequency and intensity of collisions between small crystals and core agglomerates. Intense turbulence will separate each crystal apart so that agglomerates will not form. However, appropriate turbulence can allow agglomerates to form and help to maintain highly concentrated solution inside of them by creating external turbulence that separates the highly concentrated solution within the agglomerates from the bulk solution. After forming the core agglomerates, the solution in the pores within the agglomerates is confined, and crystallization can occur within the pores. Without stirring, the agglomerates of Form I were formed, however, the small crystals filling the void spaces within the agglomerates were not (
Figure S4). This further indicates that concentrated solutions were confined within the agglomerates and generated small crystals. Therefore, appropriate stirring is essential for the spherical crystallization of CLP. Needless to say, a certain level of supersaturation is also required for spontaneous crystallization within pores [
6,
10,
11].
It is also notable that the sizes of the individual crystals forming the agglomerates prepared at a stirring speed of 800 rpm are larger than those formed at a stirring speed of 500 rpm (
Figure 5). The larger individual crystal sizes may indicate that the high stirring speed of 800 rpm facilitates polymorphic transformation, and thus, produces the larger crystals. Interestingly, small crystals are also observed in the agglomerate prepared at 800 rpm. In fact, the small crystals within the large crystals looked similar to those of the spherical particles shown in
Figure 5a and may be crystallized from a confined solution within agglomerates. PXRD data showed that the agglomerates formed at a stirring speed of 800 rpm were a mixture of Forms I and II, and it is speculated that the large crystals were Form II and the small crystals were Form I (
Figure S1).
5. Conclusions
This study was carried out with the aim of developing a simple and effective process for the production of CLP form-I spherical agglomerates in order to overcome difficulties due to the rapid phase transformation of form-I crystals and form-II crystals. Our purpose was also to provide an alternative approach to enhance and resolve problems regarding the complexity of the crystallization process raised during the manufacturing of CLP form-I spherical agglomerates.
This study showed that temperature, solvents, supersaturation level, and stirring speeds are equally important for producing spherical CLP agglomerates of optimal morphology, size, size distribution, and in a polymorphic form, that is, Form-I. It was concluded that the supersaturation level and the driving force of polymorphic transformation play critical roles in determining the polymorphic form and that the supersaturation level and stirring play a major role in forming spherical agglomerates with a smooth surface. Furthermore, the conventional need for a bridging solvent or large volumes of anti-solvent by spherical crystallization techniques proved unnecessary for the spherical crystallization of CLP Form-I.