Abstract
The development of drug-resistance and high morbidity rates due to life-threatening fungal infections account for a major global health problem. A new antifungal imidazole-based oximino ester 5 has been prepared and characterized with the aid of different spectroscopic tools. Single crystal X-ray analysis doubtlessly identified the (E)-configuration of the imine fragment of the title compound. Compound 5, C18H15N3O5, was crystallized in the monoclinic, P21/c, a = 10.4067 (5) Å, b = 6.8534 (3) Å, c = 23.2437 (12) Å, β = 94.627 (2)°, V = 1652.37 (14) Å3, Z = 4. Spectral and electronic features of compound 5 have been thoroughly explored with the aid of density function theory (DFT) simulations and the data were compared with the experimental results. In addition, Hirshfeld surface analysis and molecular docking simulations were executed on the target compound. Molecular docking results are fairly consistent with the experimental in vitro antifungal potential of the oximino ester 5.
1. Introduction
The development of drug-resistance and high morbidity rates due to life-threatening fungal infections increased dramatically during the last few decades and they account for a major global health problem [1,2]. Individuals with autoimmune diseases, organ transplants, acquired immunodeficiency syndrome (AIDS) or cancer are particularly more susceptible to life-threatening fungal infections than others [3,4]. The number of the available antifungal drugs is limited which could be attributed to the eukaryotic nature of the fungal’s cell which restricts the number of theantifungal drugs that can target fungal’s cell over human hosts [5]. Therefore, the search for new potent, safe, broad spectrum antifungal drug-like candidates is a mandatory medicinal chemistry challenge.
The azole (imidazole and triazole) antifungal agents are first-line drugs for the treatment of fungal infections, owing to their high potency, broad spectrum and low toxicity [6,7]. Competitive inhibition of the fungal lanosterol 14α-demethylase (CYP51) by the antifungal azoles results in inhibition of the oxidative removal of the 14α-methyl group in the sterol biosynthesis and hence the fungi cannot grow in a normal way [8]. Prior reports had documented the pharmacophore of antifungal imidazoles to contain two-carbon atoms joining the imidazole nucleus and an aromatic part. In addition, few studies reported the antifungal potential of compounds bearing three carbons linker between the azole scaffold and the aromatic residue [9,10,11].
Moreover, it has been reported that various bioactive molecules contain benzodioxole scaffold displaying a wide spectrum of biological activities including antimicrobial ones [12,13].
In view for the aforementioned premises and in our continued efforts to get new bioactive antifungal candidates, the title compound, namely ({[(1E)-1-(1,3-benzodioxol-5-yl) -3-(1H-imidazol -1-yl)propylidene] amino}oxy)(furan-2-yl)methanone (5) was designed and synthesized to examine its antifungal potential.. It combines the imidazole and benzodioxole pharmacophore fragments which are connected by a propyl carbon chain. Various spectroscopic tools were utilized to confirm the proposed chemical structure of the oximino ester 5 and single X-ray analysis assured the assumed (E)-configuration of its imine double bond.
2. Experimental
2.1. General
Gallenkamp melting point apparatus (LabAssets, Kent, UK) was utilized to measure the melting point of compound 5 and it is uncorrected. Deuterated chloroform (CDCl3) (Sigma-Aldrich Chemie GmbH, Taufkirchen, Germany) was used to dissolve compound 5 to measure its nuclear magnetic resonance (NMR) spectra with the aid of Bruker NMR spectrometer (Bruker, Reinstetten, Germany) at 500 MHz for 1H and 125.76 MHz for 13C at the Research Center, College of Pharmacy, King Saud University, Saudi Arabia. Chemical shifts are expressed in δ-values (ppm) relative to Tetramethylsilane (TMS) as an internal standard. Elemental analysis of the target compound 5 was executed at the Microanalysis Laboratory, Cairo University, Cairo, Egypt, and the results are consistent with the assumed chemical structure within ± 0.4% of the theoretical values.Agilent Quadrupole 6120 LC/MS (Agilent Technologies, Palo Alto, CA, USA) with ESI (Electrospray ionization) source recorded the mass spectrum of compound 5. Silica gel thin layer chromatography (TLC) plates from Merck (silica gel pre-coated aluminum cards with fluorescent indicator at 254 nm) were used for thin layer chromatography. Visualization was performed by illumination with UV light source (254 nm). All the commercially available chemicals were procured from Sigma-Aldrich Chemie GmbH (Taufkirchen, Germany).
2.2. Synthesis
2.2.1. Synthesis of (1E)-1-(2H-1,3-Benzodioxol-5-yl)-N-hydroxy-3-(1H-imidazol-1-yl)propan-1-imine (4)
The synthesis and spectroscopic characterization of the oxime 4 have been previously reported [14].
2.2.2. Synthesis of ({[(1E)-1-(1,3-Benzodioxol-5-yl)-3-(1H-imidazol-1-yl)propylidene]amino}oxy) (furan-2-yl)methanone (5)
N,N′-Carbonyldiimidazole (0.32 g, 2.0 mmol) was added to a stirred solution of furan-2-carboxylic acid (0.22 g, 2.0 mmol) in tetrahydrofuran (THF, 10 mL). The reaction mixture was allowed to stir for 18 h at ambient temperature after the addition of the oxime 4 (0.5 g, 2.0 mmol). THF was evaporated under vacuum, the residue was dissolved in ethyl acetate (30 mL) and the organic phase was washed successively with water (2 × 20 mL), 10% NaHCO3 solution (2 × 15 mL), and water (2 × 15 mL). The organic layer was dried (Na2SO4) and evaporated under reduced pressure. The corresponding oximino ester 5 was purified by re-crystallization from ethanol to furnish 0.3 g (30%)of the title compound 5 as colorless crystals suitable for X-ray analysis m.p. 149–151 °C. 1H-NMR (CDCl3): δ (ppm) 3.41 (t, J = 6.5 Hz, 2H, -CH2-CH2-N), 4.26 (t, J = 6.5 Hz, 2H, -CH2-CH2-N), 6.13 (s, 2H, -O-CH2-O-), 6.80 (s, 2H, -N-CH=CH-N=, Ar-H), 7.01 (d, J = 8.5 Hz, 1H, Ar-H), 7.16 (s, 1H, -N-CH=CH-N=), 7.29 (d, J = 6.0 Hz, 2H, Ar-H), 7.51 (d, J = 3.0 Hz, 1H, Ar-H), 7.56 (s, 1H, -N-CH=N-), 8.10 (s, 1H, Ar-H); 13C-NMR (CDCl3): δ (ppm) 30.4 (-CH2-CH2-N), 43.6 (-CH2-CH2-N), 102.3 (-O-CH2-O-), 107.3, 108.9, 113.0 (Ar-CH), 119.8 (-N-CH=CH-N=), 119.9, 122.9, 127.1(Ar-CH), 128.9 (-N–CH=CH-N=), 137.7 (-N-CH=N-), 142.5, 148.3, 148.9,150.2 (Ar-C), 155.5, 164.2 (C=N, C=O); MS m/z (ESI): 354.1 [M + H]+.
2.3. Crystal Structure Determination
Slow evaporation of the ethanolic solution of the oximino ester 5 at ambient temperature gave its single crystals. Bruker APEX-II D8 venture area diffractometer supported with graphite monochromatic Mo Kα radiation, λ = 0.71073 Å at 296 (2) K was used to collect data (Table 1). Bruker SAINT was utilized for cell refinement and data reduction [15]. Structure was solved with the aid of SHELXT [16,17]. The 2-furylring is disordered over two sites with an occupancy ratio of 0.68 (4):0.32 (4). SIMU, DELU and SAME restraints were used to model this disordered molecule. The H atoms of the whole molecule were located in difference-Fourier maps and refined freely. The final refinement was carried out by full-matrix least-squares techniques with anisotropic thermal data for non-hydrogen atoms on F. CCDC 1839849 contains the supplementary crystallographic data for this compound can be obtained free of charge from the Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.

Table 1.
X-ray experimental details of the oximino ester 5.
2.4. FT-IR and FT-Raman Measurements
Bruker RFS-27 FT-Raman spectrometer was utilized to record the FT-Raman spectrum of the oximino ester 5 in the spectral range of 3500–50 cm−1 and excitation was achieved via1064 nm line of Nd:YAG laser operating at 100 mW power. Perkin-Elmer spectrum FT-IR spectrometer was utilized to record the FT-IR spectrum with the spectral resolution of 1 cm−1 in the range of 4000–400 cm−1 for the KBr pellets of the title oximino ester 5.
2.5. Quantum Chemical Calculations
The optimized structure and vibrational wavenumbers of the title oximino ester 5 was obtained using the quantum mechanical methods boosted by the functional B3LYP/6-311+G [18]. The geometry of the molecule observed from the XRD values was compared with the calculated geometrical values from the quantum mechanical method. The Gaussian 09 program [19] embedded with the quantum mechanical methods with the advanced functional and basis sets were utilized for the theoretical study of the target oximino ester 5. The correlation and exchange functions included in the software with the advent of the density functional theory (DFT) play a vital role in obtaining results closer to the experimental data. Vibrational energy distribution analysis (VEDA) 4 program was used to evaluate the distributions of assignment of the calculated wavenumbers [20]. Pure Lorentizian band shapes with a bandwidth of full width half-maximum (FWHM) of 10 cm−1 were utilized to plot the simulated Raman and IR spectra of compound 5. Natural population, natural hybrid calculations and natural bonding orbital investigations were carried out with the aid of natural bond orbital (NBO) 3.1 program [21]. Crystal Explorer 3.1 was implemented to create fingerprint plots and Hirshfeld surface map of the oximino ester 5 [22].
2.6. Antifungal Activity
The minimum inhibitory concentration (MIC) values of the oximino ester 5 against different fungal strains were determined by adopting the previously reported protocols [14,23].
3. Results and Discussion
3.1. Chemistry
The title oximino ester 5 was prepared as depicted in Scheme 1. The ketone 3 was synthesized using the commercially available acetophenone derivative 1 by adopting Mannich reaction followed by substitution of the dimethyl amine moiety of compound 2 with imidazole. Subsequently, compound 3 was elaborated to the oxime 4 which was esterified under mild conditions to furnish the title oximino ester 5.
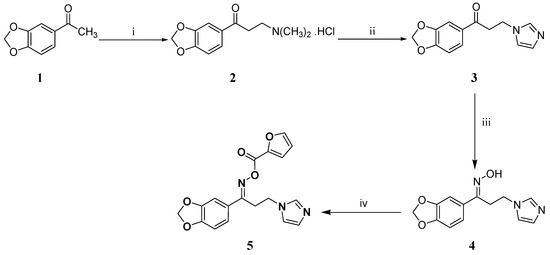
Scheme 1.
Synthesis of the target oximino ester 5. Reagents and conditions: (i) HN(CH3)2.HCl, (CH2O)n, conc. HCl, ethanol, reflux, 2 h; (ii) Imidazole, water, reflux, 5 h; (iii) H2NOH.HCl, KOH, ethanol, reflux, 18h; (iv) N,N′-Carbonyldiimidazole, furan-2-carboxylic acid, THF, rt, 18h.
3.2. Crystal Structure of the Target Oximino Ester 5
In the title compound, C18H15N3O5, the crystallographic information and refinement data are presented in Table 1. The selected bond angles and bond lengths are displayed in Table 2. The asymmetric unit is containing two independent molecules as illustrated in Figure 1. The 2-furyl ring in the molecule is disordered due to rotation through two orientations by approximately 180° about the single C–C bond; the ratio of site occupancy for the major and minor components is 0.68:0.32. The furan ring plane was nearly in the same plane of the 1,3-benzodioxol ring with small deviation equal to 8.38°. On the other hand, the imidazole ring occurred on another plane with dihedral angle 12.84° with 1,3-benzodioxol ring. All the bond angles and lengths are in normal ranges [24]. In the crystal packing, the molecules are connected through two hydrogen bonds between C13–H13A, C18–H18A and N3 as shown in Figure S1. The hydrogen-bond geometry of the title oximino ester 5 is presented in Table S1. The configuration of the title compound 5 was assured with the aid of X-ray crystallography approach as a doubtless analytical tool. Therefore, the assigned (E)-configuration of the imine group in the title compound was asserted via its single crystal X-ray molecular structure.

Table 2.
Selected geometric parameters (Å, °) of the oximino ester 5.
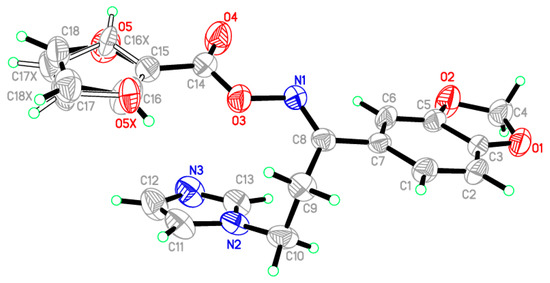
Figure 1.
ORTEP diagram of the oximino ester 5 drawn at 40% ellipsoids for non-hydrogen atoms.
3.3. Structural Geometry Analysis
The optimized molecular structure of the oximino ester 5 is calculated using DFT theory at B3LYP/6-311+G level of basis set by Gaussian 09 program package along with atom numbering scheme as illustrated in Figure 2. Most important optimized geometrical parameters for compound 5 along with experimental values are displayed in the Table 3. The target compound 5 bears a benzodioxole ring connected with a furan ring through an oximino ester functionality and the connection extended to an imidazole ring through two methylene carbons. Computational geometry optimization exposed that the non-planar structure is preferred by −1235.54997 Hartree as the compound undergoes steric repulsion to obtain the energetically preferred non-planar structure. The interesting geometrical properties of the title compound are the steric hindrance resulting in the twisting of the furan and imidazole rings due to the van der Waals repulsion between H36 and H27. Benzodioxole ring is almost planar with the imidazole ring. Also, the imidazole ring loses its planarity with the furan ring [C5-C4-C1-O8 = −7.57°].
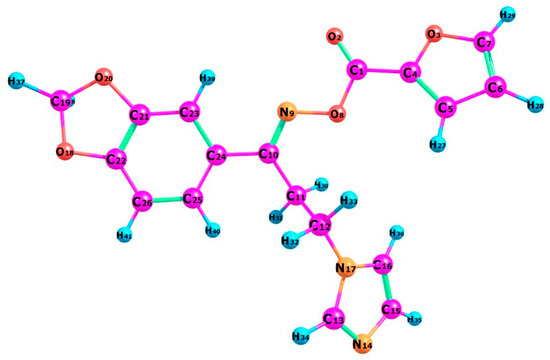
Figure 2.
The optimized molecular structure of the oximino ester 5.

Table 3.
The optimized structural geometry parameters for the oximino ester 5.
The calculated non-bonded hydrogen bond distance of C23-H39…N9 is 2.429 Å being less than the sum of the corresponding van der Waals radii (2.72 Å) and the angle (94.69°) is also within the limit which manifests the C-H…N intramolecular hydrogen bonding within the title oximino ester 5. The C-N bond lengths of the imidazole ring (C13-N14, C13-N17, C15-N14, C16-N17) are typical for the double bonds due to resonance interactions. The other interesting structural features of the title compound are the shortening of the endocyclic angle C23-C24-C25 and the increase in the exocyclic angle C10-C24-C25 which might be owing to the intramolecular charge transfer (ICT) interactions. DFT calculations indicate a raise in the C10-C24-C25 angle by 1.006° and a lowering in the endocyclic angle C23-C24-C25 by 3.21° as well as the C23-C24 bond length (1.418 Å) is also lengthened which are accompanied with a large charge transfer interactions.
3.4. Natural Bond Orbital (NBO) Analysis
It is proved that the NBO analysis is an efficient approach to interpret chemical hyperconjugative interactions and electron density transfer from the filled lone pair electrons. DFT level computations are used to investigate the different second-order interactions between the filled orbitals of one subsystem and vacant orbitals of another vacant orbitals of another subsystem, which is a measure of the delocalization or hyperconjugation. The NBO 3.1 program was used to analyze the fundamental natural orbital interactions of the target compound. Table S2 presents certain remarkable donor-acceptor interactions and their second-order perturbation energies E(2). The NBO analysis of the title molecule 5 exposed the significance of the charge transfer between the lone pair of the proton acceptor and the anti-bonds of the proton donor. It also clearly explains the proof of the formation of intramolecular hydrogen bond interaction between n1(N9) and σ*(C23-H39) anti-bonding orbital with a stabilization energy of 0.65 kcal/mol. The hyperconjugative interactions of π(C4-C5) → π*(C1-O2), π(C4-C5) → π*(C6-C7), π(C6-C7) → π*(C4-C5), π(C13-N14) →π*(C15-C16), π(C15-C16) → π*(C13-N14), π(C21-C23) → π*(C22-C26), π(C21-C23) → π*(C24-C25), π(C22-C26) → π*(C21-C23), π(C22-C26) → π*(C24-C25),π(C24-C25) → π*(N9-C10), π(C24-C25) → π*(C21-C23) andπ(C24-C25) →π *(C22-C26)are 19.86, 15.18, 17.66, 21.73, 14.92, 20.89, 17.76, 18.21, 19.66, 18.10, 17.04 and 17.20 kcal/mol, respectively. This might be due to the charge delocalization and hence stabilization of the system.
3.5. Natural Population Analysis (NPA)
NPA is known as a mathematical way to partition electron density or wave function. It portrays the distribution of electrons and gives an improved numerical stability in a better way [25]. The atomic charge distribution of the target oximino ester 5 is presented in Figure 3. C2 is the most positive atom owing to its connection to two electronegative oxygen atoms, while O2, O18 and O20 are the most negative atoms in the oximino ester 5.
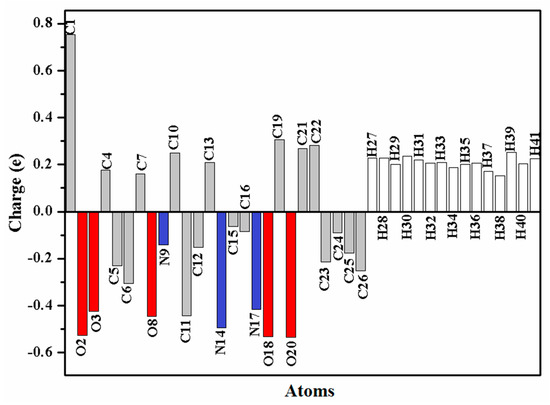
Figure 3.
The atomic charge distribution of the oximino ester 5.
3.6. Hirshfeld Surface Analysis
The space occupied by a molecule in a crystal was defined via its Hirshfeld surface and it is utilized to partition the crystal electron density into molecular fragments [26,27]. The fingerprint plot summarizes the 3D picture of close contacts in the crystal [28]. The occurrence of different types of intermolecular interactions is recognized through plotting di versus de in a 2D fingerprint plot. The Hirshfeld surfaces of the target oximino ester 5 are presented in Figure 4 explaining the surfaces that have been mapped over dnorm, de, di and curvedness (3D plots) where dnorm is the normalized distance of chemical contacts and de and di are the distances from the Hrishfeld surface to the nearest atoms outside and inside the surface, respectively.
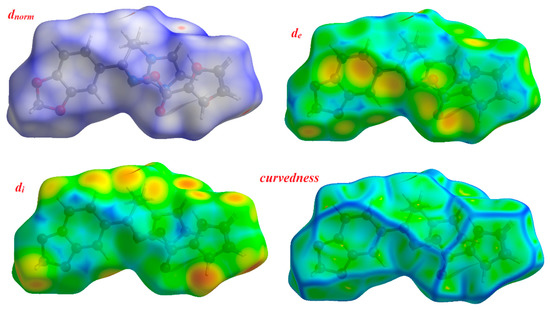
Figure 4.
Hirshfeld surfaces for dnorm, di, de and curvedness for the oximino ester 5.
The distance from the Hirshfeld surface to the nearest atoms outside and inside the surface are characterized by the quantities de and di, respectively. The function dnorm (normalized chemical contacts) is a ratio encompassing the distances of any surface point to the nearest di and de atom and the van der Waals radii of the atoms. The negative value of dnorm indicates the sum of di and de is shorter than the sum of the relevant van der Waals radii, which is considered to be a closest contact and is visualized as red color in the Hirshfeld surfaces. The white color denotes intermolecular distances close to van der Waals contacts with dnorm equal to zero whereas contacts longer than the sum of van der Waals radii with positive dnorm values are colored with blue. The intermolecular interactions outwards the O...H/C…C/C…H/N…H bonds as well as the overall fingerprint region of the title molecule are depicted in Figure S2. With these analyses, the division of contributions is possible for different interactions including N…H, O…H, C…H and C…C, which commonly overlap in the full fingerprint plots. Figure S2 shows the dnorm surface of compound 5, highlighting only O…H/N…H intermolecular contacts. These interactions comprise 26.1% and 10.5% of the total Hirshfeld surface area for this molecule, respectively. O…H/N…H intermolecular interactions are represented by two sharp spikes in the 2D fingerprint plot (Figure S2). The relative contributions of the C…H and C…C contacts are 14.2% and 3.6%, respectively.
3.7. Vibrational Spectral Analysis
The vibrational spectral assignments have been performed on the basis of characteristic vibrations of imidazole ring, benzodioxole ring, furan ring, side chain C=N-O-C=O and methylene groups. Vibrational assignment were performed at DFT level with B3LYP/6-311++G (d,p) level of basis set with possible assignments and potential energy distribution (PED) are summarized in Table 4. The computed wavenumbers were compared with the experimental FT-Raman and FT-IR spectra of the oximino ester 5. Both the experimental and the calculated FT-Raman and FT-IR spectra of the target compound 5 are displayed in Figures S3 and S4, respectively. The experimental FT-IR spectrum of the title oximino ester 5 was recorded in the solid form, whereas its simulated spectrum was computed in the monomer gas phase.

Table 4.
Vibrational assignments of compound 5 based on potential energy distribution (PED) analysis.
3.7.1. Imidazole Ring Vibrations
Calculations on the molecule provide a reasonable model to reach an acceptable agreement between the observed and the calculated spectral profiles. Nevertheless, when the calculations ignore intermolecular H-bonding between successive molecules, the wavenumber agreement is rather pleasing. Imidazole C-H stretching vibrations usually appear in the region 3145–3115 cm−1 [29]. These bands occurred at 3141 and 3106 cm−1 in the Raman and IR spectra of the target molecule, respectively. The computational wavenumber of the imidazole C-H stretching vibration coincides exactly with the experimental IR observation. Imidazole C=N and C=C stretching vibrations usually occur in the region 1660–1450 cm−1. The C=C and C=N stretching modes are the most characteristic vibrations of the heterocyclic imidazole ring which were observed as weak intensity bands at 1553 and 1477 in the IR spectrum of the target molecule, respectively, while its C=N Raman band was observed at 1471 cm−1. The noticed IR band at 751 cm−1 could be attributed to the out-of-plane C-H wagging modes, as advocated by the literature data [30].
3.7.2. Methylene Group Vibrations
The neighbouring rings π system and nitrogen lone pair affects the spectral behavior of SP3 hybridized methylene moiety. Methylene asymmetric and symmetric C-H stretching vibrations usually occur in the region 2960–3100 cm−1 [31]. The asymmetric Raman band appeared at 3090 cm−1, while the symmetric IR bands occurred at 2993 and 3036 and their respective Raman bands appeared at 2951 and 2998 cm−1 medium and very weak intensity bands in the spectra of the target molecule. Lowering of symmetric stretching wavenumbers might be related to the hyperconjugative interactions between the nitrogen lone pair and σ*(C–H) bond. The twisting, wagging, and rocking vibrational modes of the title compound were noted in the region of 1400–900 cm−1.
3.7.3. Benzodioxole Ring Vibrations
Aromatic C-H stretching mode appears in the region of 3000–3100 cm−1 [31]. Medium and very strong Raman bands were identified at 2801 and 3141 cm−1 and have been assigned to the H-C-H symmetric and asymmetric stretching modes, respectively. Also, a medium IR band was identified at 2930 cm−1 and has been assigned to the H-C-H symmetric stretching mode. Ring C-C stretching mode has been noted in the Raman spectrum of the title compound at 1176 cm−1. The bands related to the ring C-H out-of-plane and in-plane bending vibrations are usually occurred in the region of 750–1000 and 1000–1300 cm−1, respectively [29]. In the title compound, the C-H in-plane bending vibration has been observed as a very weak intensity IR band at 1161cm−1. The very weak intensity IR bands observed at 894 and 805 cm−1 have been assigned to C-H out-of-plane bending mode. The ring asymmetric deformation has been identified at 918 cm−1in the IR spectrum of the target compound.
3.7.4. Side Chain C=N-O-C=O Vibrations
The C=N, C=O and C-O stretching vibrations usually occur in the regions of 1610–1680, 1680–1820 and 970–1250 cm−1, respectively [31]. In the target molecule, the C=N stretching vibration has been noted as a very weak intensity IR band at 1611 cm−1. The strong intensity bands observed at 1772 (IR) and 1814 (Raman) cm−1 have been assigned to C=O stretching vibrations.
3.7.5. Furan Ring Vibrations
Bands related to the C-H stretching vibration for furans usually exist in the region of 3180–3000 cm−1 [31]. The observed medium Raman band at 3269 cm−1 was assigned to furan C-H stretching. A very strong C-O band was observed at 1009 cm−1 in the Raman spectrum of the target compound.
3.7.6. Skeletal Vibrations
C-N and C-C stretching vibrations usually happen in 1150–850 cm−1 region [32]. The observed IR bands at 1343 (C-C) and 1220 (C-N) cm−1 as well as the Raman bands at 1349 (C-C) are attributed to the skeletal vibrations of the title oximino ester 5.
3.8. Frontier Molecular Orbital (FMO) Analysis
The capability of electron giving is expressed by the highest occupied molecular orbital (HOMO), while the ability of electron accepting is expressed by the lowest unoccupied molecular orbital (LUMO). However, energy gap describes the energy difference between HOMO and LUMO orbitals and it constitutes a substantial stability factor for the chemical structures indicating electron transport within molecular systems [33]. Figure 5 indicates the graphical representation of the frontier molecular orbitals of the target molecule 5 in which the FMO energy gap was calculated at the B3LYP/6-311++G(d,p) level.
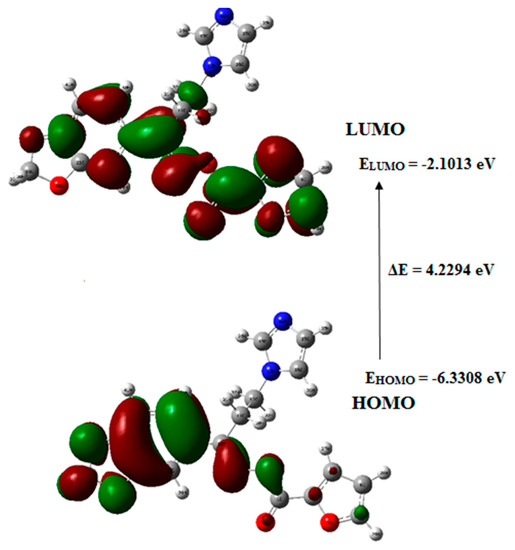
Figure 5.
Graphical representation of frontier molecular orbital (FMOs) of the oximino ester 5; HOMO: highest occupied molecular orbital; LOMO: lowest unoccupied molecular orbital.
The HOMO of the target compound 5 existed on the furan ring, while its LUMO is laying on methylenedioxy phenyl moiety indicating the charge transfer between the furan ring to the methylenedioxy phenyl ring system. The Eigenvalues of LUMO (−2.1013 eV) and HOMO (−6.3308 eV) and their energy gap (4.2294 eV), explain the eventual charge transfer interactions exist within the target compound 5.
3.9. Molecular Docking Simulations
The structure of the oximino ester 5 was optimized depending on DFT using Gaussian 09 program. The molecular docking was accomplished using AutoDock Tools-1.5.4 interfaced with the MGL Tools-1.5.4 package [34]. The antifungal target protein lanosterol 14α-demethylase (CYP51) crystal structure in complex with ketoconazole (PDB ID:3I3K), was selected for the present docking analysis [35]. The three-dimensional (3D) coordinates of the protein file was downloaded from the RCSB (Research Collaboratory for Structural Bioinformatics) protein data bank [36] with a resolution of 2.8 Å. The protein preparation has been executed via adopting the following steps (i) all water molecules were removed (ii) the protein crystal structure was provided with hydrogen atoms (iii) add Coulomb charges (iv) previous docked inhibitor (ketoconazole) was removed from the protein. An affinity grid 126 × 126 × 126 with a spacing of 0.42 Å was created in the center of the active site with the aid of AutoGrid 4.2 [37]. Flexible ligand and rigid protein dockings were executed using AutoDock 4.2 implemented with Lamarckian genetic algorithm according to the following schedule: population size of 200, trials of 100 dockings, a mutation rate of 0.02, energy evaluations of 25,000,000, an elitism value of 1, and a crossover rate of 0.8. The docking results were evaluated by sorting the binding free energy predicted by the docked confirmations of the title compound. The title molecule 5 was docked with the binding pocket of 3I3K protein forming three hydrogen bonds with VAL138 and SER60. These hydrophobic interactions revealed the substantial value of oxygen atoms in the title molecule for binding and hence its inhibitory ability. The best binding score of the target oximino ester 5 was obtained as −2.27 kcal/mol and its best binding pose is illustrated in Figure 6. The results clearly predicted the preclinical antifungal potential of the target molecule 5.
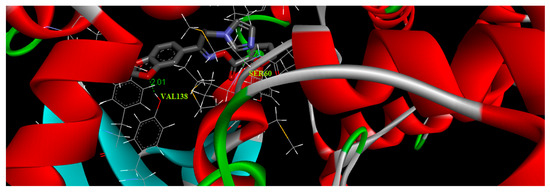
Figure 6.
Binding pose diagram of the oximino ester 5 with its target protein.
3.10. Antifungal Activity of the Target Oximino Ester 5
Table 5 illustrates the minimum inhibitory concentrations (MICs) values of the antifungal activity of the target oximino ester 5 and the reference standard antifungal drugs, fluconazole and ketoconazole. Compound 5 showed an equipotent activity against C. albicans and C. parapsilosis with MIC value of 0.181 µmol/mL. It also manifests an equipotent activity against C. tropicalis and Aspergillus niger with MIC value of 0.724 µmol/mL in the broth microdilution assay.

Table 5.
The minimum inhibitory concentrations (MICs) values of the antifungal activity of the target compound 5, fluconazole and ketoconazole against different Candida species and Aspergillus niger.
4. Conclusions
({[(1E)-1-(1,3-Benzodioxol-5-yl)-3-(1H-imidazol-1-yl)propylidene]amino}oxy)(furan-2-yl)methanone (5) has been synthesized and comprehensively verified with various spectroscopic tools. Single crystal X-ray analysis of the target compound assured its assigned structure and proved doubtlessly the (E)-configuration of its imine double bond.
The molecular structural features of the target molecule 5 have been explored using DFT method at B3LYP/6-311+G level. The investigated conformation of the target molecule revealed its non-planarity. Its optimized geometry showed the occurrence of C23-H39…N9 intramolecular hydrogen bonding which was confirmed by NBO and NPA analyses. NBO, FMO analyses and vibrational analysis also confirmed the existence of intramolecular charge transfer within the title molecule, which results in its stability and in turn its bioactivity. Hirshfeld surface analysis revealed that strong and weak intermolecular interactions like O...H and N...H bonding are present in the crystalline state of the target molecule. The molecular docking results predicted the antifungal activity of the title molecule which is consistent with its experimental in vitro antifungal examination. Our ongoing laboratory research efforts are continued aiming to synthesize new imidazole-bearing candidates with an improved antifungal activity to be harnessed in clinic.
Supplementary Materials
The following are available online at http://www.mdpi.com/2073-4352/9/1/25/s1, Figure S1: Molecular packing of the oximino ester 5 manifesting hydrogen bonds which are drawn as dashed lines, Table S1: Hydrogen-bond geometry (Å, °) of the oximino ester 5, Table S2: Second-order perturbation theory analysis of Fock matrix in NBO basis for the oximino ester 5, Figure S2: 2D fingerprint plots showing various intermolecular contacts in the oximino ester 5, Figure S3: Comparison of (a) calculated and (b) observed FT-IR spectra of the oximino ester 5, Figure S4: Comparison of (a) calculated and (b) observed FT-Raman spectra of the oximino ester 5.
Author Contributions
R.I.A.-W. and A.R.A.-G. synthesized and characterized the title molecule. H.A.G. carried out X-ray analysis. M.H.A.-A. performed the antifungal screening. S.V.A. and I.H.J. conducted the computational work. M.I.A. proposed the work and prepared the manuscript for publication. All authors discussed the contents of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for its funding of this research through the Research Group Project No. RGP-196.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Vandeputte, P.; Ferrari, S.; Coste, A.T. Antifungal resistance and new strategies to control fungal infections. Int. J. Microbiol. 2011, 2012, 713687. [Google Scholar] [CrossRef] [PubMed]
- Groll, A.H.; Lumb, J. New developments in invasive fungal disease. Future Microbiol. 2012, 7, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Cuenca-Estrella, M.; Bernal-Martinez, L.; Buitrago, M.J.; Castelli, M.V.; Gomez-Lopez, A.; Zaragoza, O.; Rodriguez-Tudela, J.L. Update on the epidemiology and diagnosis of invasive fungal infection. Int. J. Antimicrob. Agents 2008, 32, S143–S147. [Google Scholar] [CrossRef]
- Crunkhorn, S. Fungal infection: Protecting from Candida albicans. Nat. Rev. Drug Discov. 2016, 15, 604. [Google Scholar] [CrossRef] [PubMed]
- Lino, C.I.; de Souza, I.G.; Borelli, B.M.; Matos, T.T.S.; Teixeira, I.N.S.; Ramos, J.P.; de Souza Fagundes, E.M.; de Oliveira Fernandes, P.; Maltarollo, V.G.; Johann, S. Synthesis, molecular modeling studies and evaluation of antifungal activity of a novel series of thiazole derivatives. Eur. J. Med. Chem. 2018, 151, 248–260. [Google Scholar] [CrossRef] [PubMed]
- Aperis, G.; Mylonakis, E. Newer triazole antifungal agents: Pharmacology, spectrum, clinical efficacy and limitations. Expert Opin. Investig. Drugs 2006, 15, 579–602. [Google Scholar] [CrossRef] [PubMed]
- Pappas, P.G.; Kauffman, C.A.; Andes, D.R.; Clancy, C.J.; Marr, K.A.; Ostrosky-Zeichner, L.; Reboli, A.C.; Schuster, M.G.; Vazquez, J.A.; Walsh, T.J. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2015, 62, 1–50. [Google Scholar] [CrossRef]
- Aoyama, Y.; Yoshida, Y.; Sato, R. Yeast cytochrome P-450 catalyzing lanosterol 14 alpha-demethylation. II. Lanosterol metabolism by purified P-450 (14) DM and by intact microsomes. J. Biol. Chem. 1984, 259, 1661–1666. [Google Scholar]
- Aboul-Enein, M.N.; El-Azzouny, A.A.; Attia, M.I.; Saleh, O.A.; Kansoh, A.L. Synthesis and anti-Candida potential of certain novel 1-[(3-substituted-3-phenyl)propyl]-1H-imidazoles. Arch. Pharm. 2011, 344, 794–801. [Google Scholar] [CrossRef]
- Roman, G.; Mares, M.; Nastasa, V. A novel antifungal agent with broad spectrum: 1-(4-biphenylyl)-3-(1H-imidazol-1-yl)-1-propanone. Arch. Pharm. 2013, 346, 110–118. [Google Scholar] [CrossRef]
- Attia, M.I.; Radwan, A.A.; Zakaria, A.S.; Almutairi, M.S.; Ghoneim, S.W. 1-Aryl-3-(1H-imidazol-1-yl)propan-1-ol esters: Synthesis, anti-Candida potential and molecular modeling studies. Chem. Cent. J. 2013, 7, 168. [Google Scholar] [CrossRef] [PubMed]
- Leite, A.C.L.; da Silva, K.P.; de Souza, I.A.; de Araújo, J.M.; Brondani, D.J. Synthesis, antitumour and antimicrobial activities of new peptidyl derivatives containing the 1,3-benzodioxole system. Eur. J. Med. Chem. 2004, 39, 1059–1065. [Google Scholar] [CrossRef] [PubMed]
- Cotinguiba, F.; Regasini, L.O.; da Silva Bolzani, V.; Debonsi, H.M.; Passerini, G.D.; Cicarelli, R.M.B.; Kato, M.J.; Furlan, M. Piperamides and their derivatives as potential anti-trypanosomal agents. Med. Chem. Res. 2009, 18, 703–711. [Google Scholar] [CrossRef]
- Al-Wabli, R.I.; Al-Ghamdi, A.R.; Ghabbour, H.A.; Al-Agamy, M.H.; Monicka, J.C.; Joe, I.H.; Attia, M.I. Synthesis, X-ray single crystal structure, molecular docking and DFT computations on N-[(1E)-1-(2H-1,3-benzodioxol-5-yl)-3-(1H-imidazol-1-yl)propylidene]-hydroxylamine: A new potential antifungal agent precursor. Molecules 2017, 22, 373. [Google Scholar] [CrossRef] [PubMed]
- Brucker. APEX2, SAINT and SADABS; Brucker AXS: Madison, WI, USA, 2009. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SHELXTL-PC (Version 7); Siemens Analytical Instruments, Inc.: Madison, WI, USA, 2014. [Google Scholar]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian-09; Revision A.02; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Jamróz, M.H. Vibrational energy distribution analysis (VEDA): Scopes and limitations. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 114, 220–230. [Google Scholar] [CrossRef]
- Reed, A.E.; Curtiss, L.A.; Weinhold, F. Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem. Rev. 1988, 88, 899–926. [Google Scholar] [CrossRef]
- Wolff, S.; Grimwood, D.; McKinnon, J.; Jayatilaka, D.; Spackman, M. Crystal Explorer 2.0; University of Western Australia: Perth, Australia, 2007. [Google Scholar]
- Al-Wabli, R.I.; Al-Ghamdi, A.R.; Primsa, I.; Ghabbour, H.A.; Al-Agamy, M.H.; Joe, I.H.; Attia, M.I. (2E)-2-[1-(1,3-benzodioxol-5-yl)-3-(1H-imidazol-1-yl)propylidene]-N-(4-methoxy phenyl)hydrazinecarboxamide: Synthesis, crystal structure, vibrational analysis, DFT computations, molecular docking and antifungal activity. J. Mol. Struct. 2018, 1166, 121–130. [Google Scholar] [CrossRef]
- Allen, F.H.; Kennard, O.; Watson, D.G.; Brammer, L.; Orpen, A.G.; Taylor, R. Tables of bond lengths determined by X-ray and neutron diffraction. Part 1. Bond lengths in organic compounds. J. Chem. Soc. Perkin Trans. 1987, 2, S1–S19. [Google Scholar] [CrossRef]
- Reed, A.E.; Weinstock, R.B.; Weinhold, F. Natural population analysis. J. Chem. Phys. 1985, 83, 735–746. [Google Scholar] [CrossRef]
- Spackman, M.A.; Byrom, P.G. A novel definition of a molecule in a crystal. Chem. Phys. Lett. 1997, 267, 215–220. [Google Scholar] [CrossRef]
- McKinnon, J.J.; Jayatilaka, D.; Spackman, M.A. Towards quantitative analysis of intermolecular interactions with Hirshfeld surfaces. Chem. Commun. 2007, 37, 3814–3816. [Google Scholar] [CrossRef]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld surface analysis. CrystEngComm 2009, 11, 19–32. [Google Scholar] [CrossRef]
- Govindarajan, M.; Abdelhameed, A.S.; Al-Saadi, A.A.; Attia, M.I. Experimental and theoretical studies of the vibrational and electronic properties of (2E)-2-[3-(1H-imidazol-1-yl)-1-phenyl-propylidene]-N-phenylhydrazinecarboxamide: An anticonvulsant agent. Appl. Sci. 2015, 5, 955–972. [Google Scholar] [CrossRef]
- Naumov, P.; Ristova, M.; Šoptrajanov, B.; Zugik, M. Vibrational spectra of bis (acetato) tetrakis (imidazole) copper (II). J. Mol. Struct. 2001, 598, 235–243. [Google Scholar] [CrossRef]
- Smith, B.C. Infrared Spectral Interpretation: A Systematic Approach; CRC Press: Boca Raton, FL, USA, 1998. [Google Scholar]
- Bellamy, L. The Infrared Spectra of Complex Molecules; Chapman and Hill: London, UK, 1975. [Google Scholar]
- Fleming, I. Frontier Orbitals and Organic Chemical Reactions; Wiley: Hoboken, NJ, USA, 1977. [Google Scholar]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comp. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- Strushkevich, N.; Usanov, S.; Park, H. Crystal structure of human lanosteroal 14alpha-demethylase (CYP51) in complex with ketoconazole. In Proceedings of the 16th International Conference on Cytochrome P, Graz, Austria, 21–26 June 2009; pp. 21–25. [Google Scholar]
- Bernstein, F.C.; Koetzle, T.F.; Williams, G.J.; Meyer, E.F., Jr.; Brice, M.D.; Rodgers, J.R.; Kennard, O.; Shimanouchi, T.; Tasumi, M. The protein data bank: A computer-based archival file for macromolecular structures. Arch. Biochem. Biophys. 1978, 185, 584–591. [Google Scholar] [CrossRef]
- Morris, G.M.; Goodsell, D.S.; Halliday, R.S.; Huey, R.; Hart, W.E.; Belew, R.K.; Olson, A.J. Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J. Comp. Chem. 1998, 19, 1639–1662. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).