Annealing Control on the Anatase/Rutile Ratio of Nanostructured Titanium Dioxide Obtained by Sol-Gel
Abstract
:1. Introduction
2. Experimental Setup
2.1. Synthesis of TiO2
2.1.1. Sol-Gel Synthesis
2.1.2. Annealing
2.2. TiO2 Characterization
2.2.1. Raman Spectroscopy
2.2.2. Diffuse Reflectance Spectroscopy (DRS)
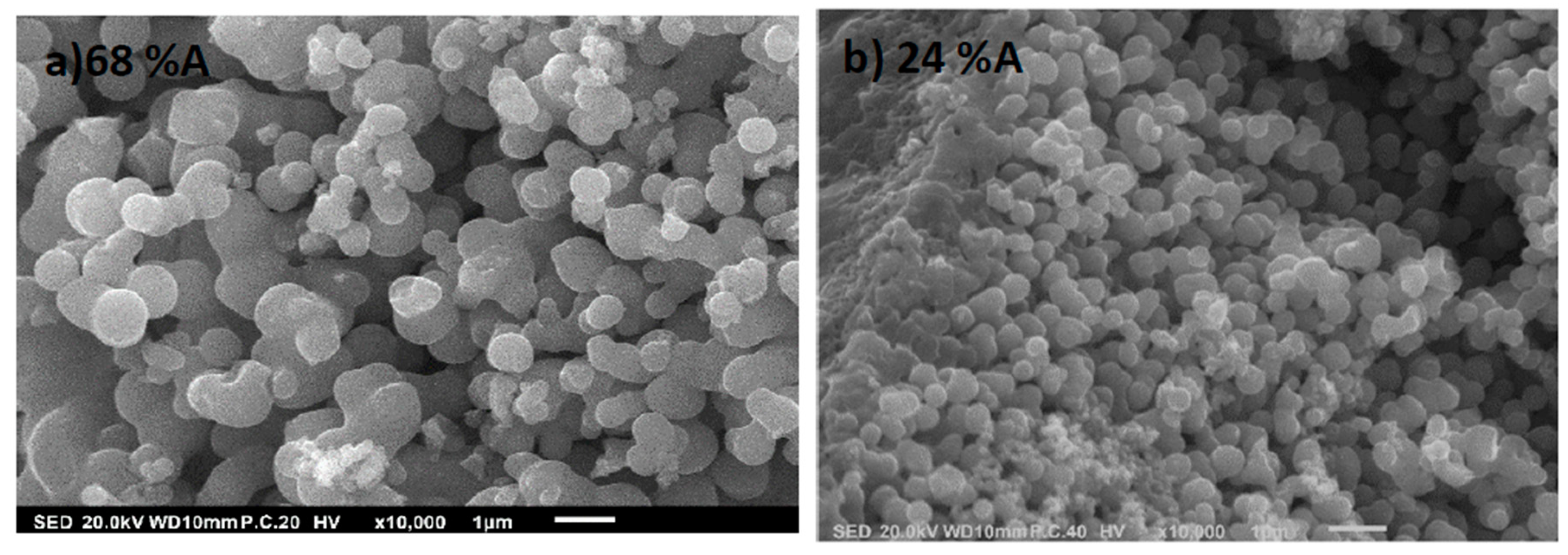
2.2.3. Scanning Electron Microscopy (SEM)
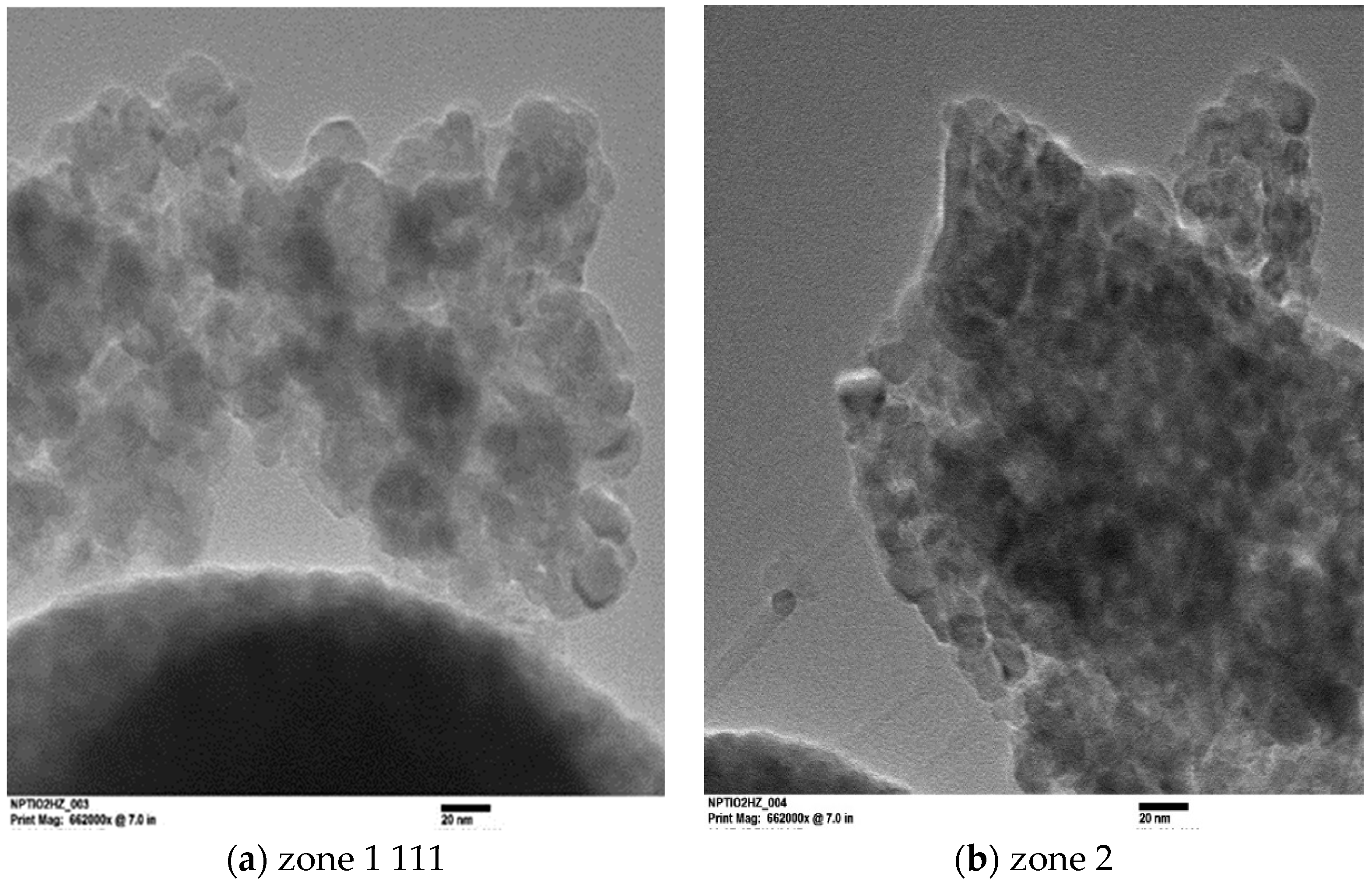
2.2.4. Transmission Electron Microscopy (TEM)
3. Results and Discussion
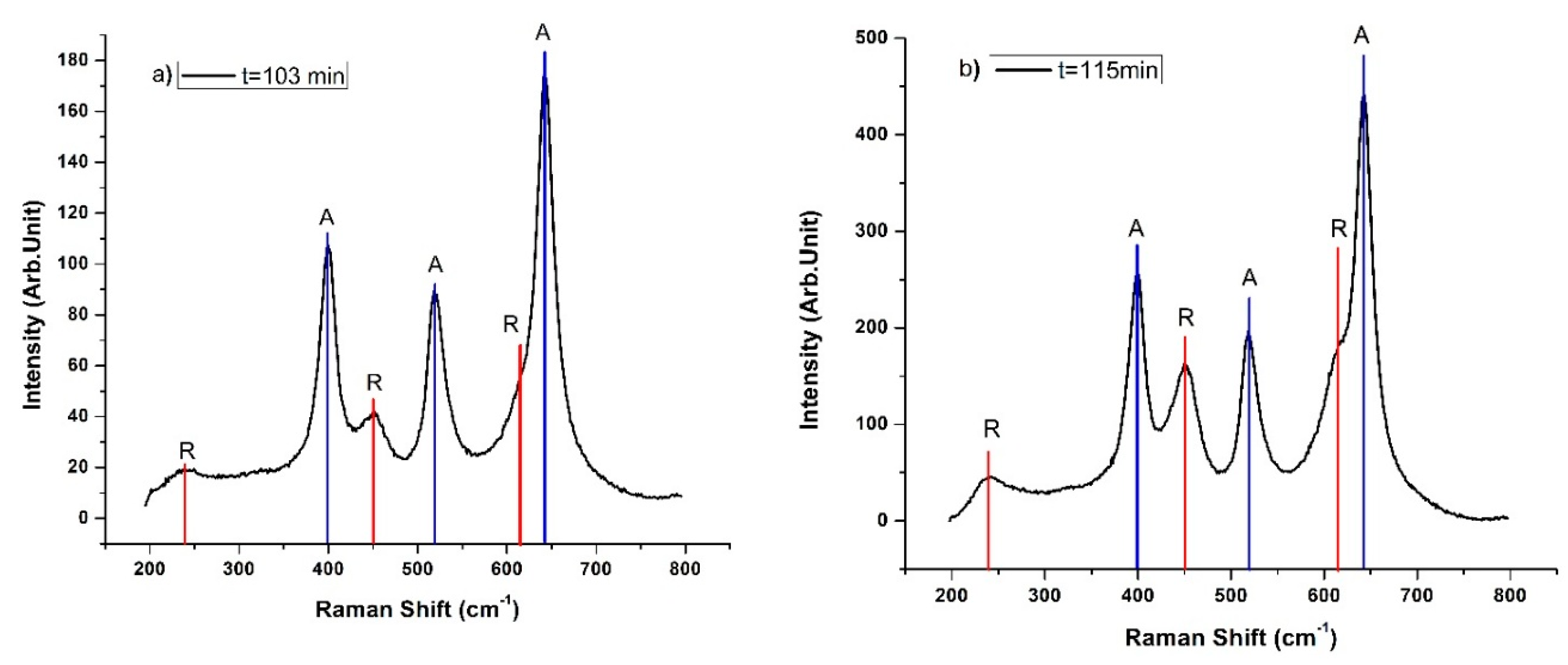
3.1. Raman Spectroscopy
3.2. Diffuse Reflectance Spectroscopy (DRS)
3.2.1. Optical Bandgap
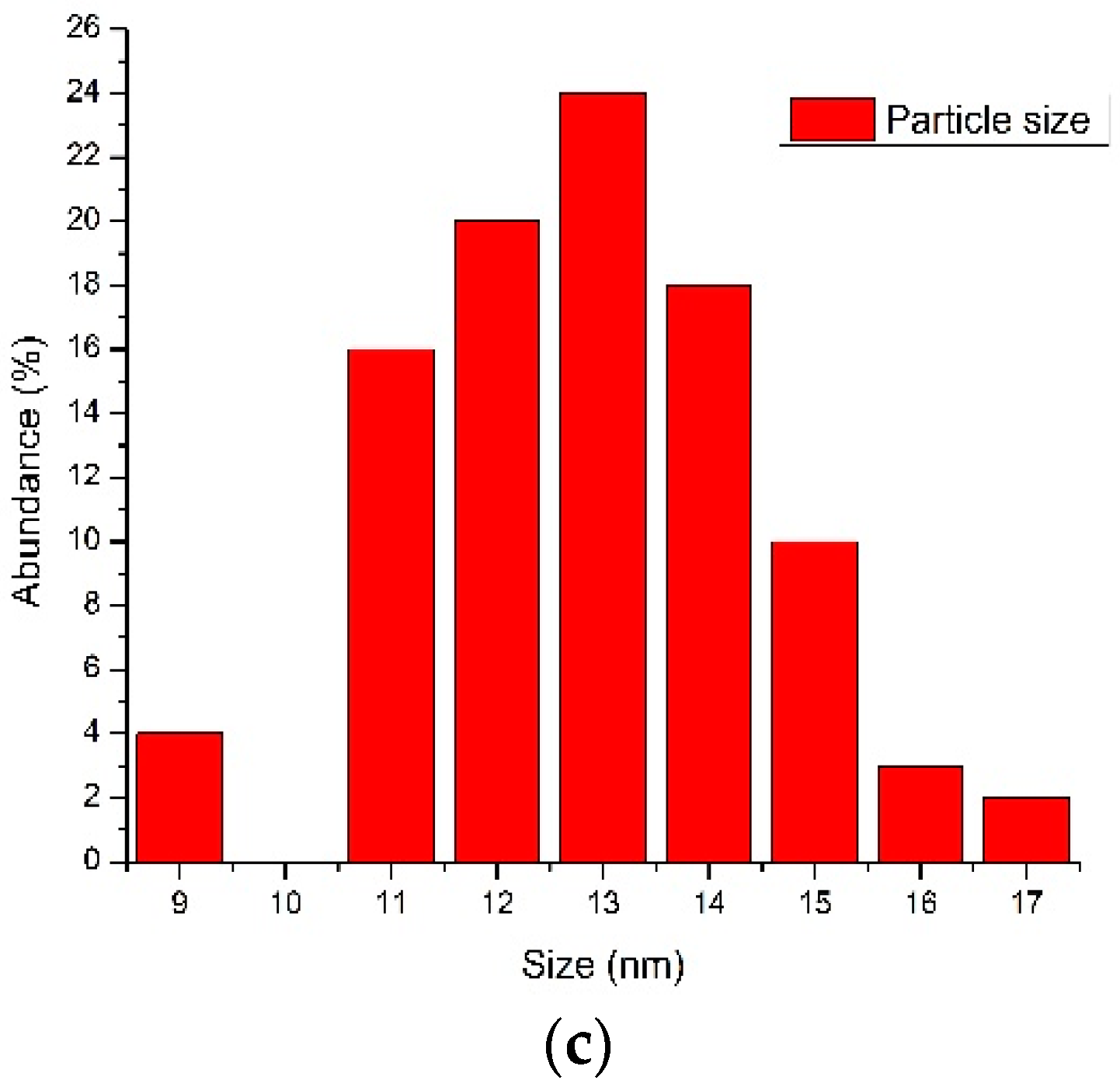
3.2.2. Particle Size Estimation
3.3. TiO2 Microstructure
3.3.1. Scanning Electron Microscopy (SEM)
3.3.2. Transmission Electron Microscopy (TEM)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Haider, A.J.; Al-Anabari, R.H.; Kadhim, G.R.; Salame, T.C. Exploring potential Environmental applications of TiO2 Nanoparticles. Energy Procedia 2017, 119, 332–345. [Google Scholar] [CrossRef]
- Fujishima, A.; Zhang, X.; Tryk, D.A. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Ouyang, F.; Zhu, R. A facile nonaqueous route for preparing mixed-phase TiO2 with high activity in photocatalytic hydrogen generation. Int. J. Hydrog. Energy 2017, 42, 11364–11371. [Google Scholar] [CrossRef]
- Xiong, J.; Das, S.N.; Kim, S.; Lim, J.; Choi, H.; Myoung, J.M. Photo-induced hydrophilic properties of reactive RF magnetron sputtered TiO2 thin films. Surf. Coat. Technol. 2010, 204, 3436–3442. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Li, X. Preparation and Photocatalytic Performance of Anatase/Rutile Mixed-Phase TiO2 Nanotubes. Catal. Lett. 2010, 139, 129–133. [Google Scholar] [CrossRef]
- Hashimoto, K.; Irie, H.; Fujishima, A. TiO2 Photocatalysis: A Historical Overview and Future Prospects. Jpn. J. Appl. Phys. 2005, 44, 8269–8285. [Google Scholar] [CrossRef]
- Hanaor, D.A.; Sorrel, C.C. Review of the anatase to rutile phase transformation. J. Mater. Sci. 2011, 46, 855–874. [Google Scholar] [CrossRef]
- Boehme, M.; Ensinger, W. Mixed Phase Anatase/rutile Titanium Dioxide Nanotubes for Enhanced Photocatalytic Degradation of Methylene-blue. Nano Micro Lett. 2011, 3, 236–241. [Google Scholar] [CrossRef]
- Bernardini, C.; Cappelletti, G.; Dozzi, M.V.; Selli, E.J. Photocatalytic degradation of organic molecules in water: photoactivity and reaction paths in relation to TiO2 particles features. Photochem. Photobiol. A Chem. 2010, 211, 185–192. [Google Scholar] [CrossRef]
- Hussain, M.; Ceccarelli, R.; Marchisio, D.; Fino, N.R.D.; Geobaldo, F. Synthesis, characterization, and photocatalytic application of novel TiO2 nanoparticles. Chem. Eng. J. 2010, 157, 45–51. [Google Scholar] [CrossRef]
- Ohno, T.; Sarukawa, K.; Tokieda, K.; Matsumura, M.J. Morphology of a TiO2 Photocatalyst (Degussa, P-25) Consisting of Anatase and Rutile Crystalline Phases. J. Catal. 2001, 203, 82–86. [Google Scholar] [CrossRef]
- Ohtani, B.; Prieto-Mahaney, O.O.; Li, D.; Abe, R.J. What is Degussa (Evonik) P25? Crystalline composition analysis, reconstruction from isolated pure particles and photocatalytic activity test. Photochem. Photobiol. A Chem. 2010, 216, 179–182. [Google Scholar] [CrossRef]
- Jiang, X.; Manawan, M.; Feng, T.; Qian, R.; Zhao, T.; Zhou, G.; Kong, F.; Wange, Q.; Dai, S.; Pan, J.H. Anatase and rutile in evonik aeroxide P25: Heterojunctioned or individual nanoparticles? Catal. Today 2018, 300, 12–17. [Google Scholar] [CrossRef]
- Markowska-Szczupak, A.; Wang, K.; Rokicka, P.; Endo, M.; Wei, Z.; Ohtani, B.; Morawski, A.W.; Kowalska, E. J. The effect of anatase and rutile crystallites isolated from titania P25 photocatalyst on growth of selected mould fungi. Photochem. Photobiol. B Biol. 2015, 151, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.-Y.; Lin, H.-C.; Yen, C.-C. Influence of metal plasma ion implantation on photo-sensitivity of anatase TiO2 thin films. Thin Solid Films 2006, 515, 1047–1052. [Google Scholar] [CrossRef]
- Fassier, M.; Peyratout, C.; Smith, D.; Ducroquetz, C.; Voland, T. Photocatalytic activity of titanium dioxide coatings: Influence of the firing temperature of the chemical gel. J. Eur. Ceram. Soc. 2010, 30, 2757–2762. [Google Scholar] [CrossRef]
- Terabe, K.; Kato, K.; Miyazaki, H.; Yamaguchi, S.; Imai, A.; Iguchi, Y. Microstructure and crystallization behaviour of TiO2 precursor prepared by the sol-gel method using metal alkoxide. J. Mater. Sci. 1994, 29, 1617–1622. [Google Scholar] [CrossRef]
- Ohno, T.; Haga, D.; Fujihara, K.; Kaizaki, K.; Matsumara, M.J. Unique Effects of Iron(III) Ions on Photocatalytic and Photoelectrochemical Properties of Titanium Dioxide. Phys. Chem. B 1997, 101, 6415–6419. [Google Scholar] [CrossRef]
- Okada, K.; Yamamoto, N.; Kameshima, Y.; Yasumori, A. Effect of Silica Aditive on the Anatase-to-Rutile transition. J. Am. Ceram. Soc. 2001, 84, 1591–1596. [Google Scholar] [CrossRef]
- Shin, H.; Jung, H.S.; Hong, K.S.; Lee, J.K. Crystal phase evolution of TiO2 nanoparticles with reaction time in acidic solutions studied via freeze-drying method. J. Solid State Chem. 2005, 178, 15–21. [Google Scholar] [CrossRef]
- Ghosh, T.B.; Dhabal, S.J.; Datta, A.K. On crystallite size dependence of phase stability of nanocrystalline TiO2. Appl. Phys. 2003, 94, 4577–4582. [Google Scholar] [CrossRef]
- Zhang, H.; Banfield, J.F. Thermodynamic analysis of phase stability of nanocrystalline titania. J. Mater. Chem. 1998, 8, 2073–2076. [Google Scholar] [CrossRef]
- Jamieson, J.C.; Olinger, B. Pressure-Temperature studies of anatase, brookite rutile, and TiO2(ii): A discussion. Am. Mineral. 1969, 54, 1447–1481. [Google Scholar]
- Kim, J.; Song, K.C.; Fondacillas, S.; Pratsinis, S.E. Dopants for synthesis of stable bimodally porous titania. J. Eur. Ceram. Soc. 2001, 21, 2863–2872. [Google Scholar] [CrossRef]
- Shannon, R.D.; Pask, J.A. Kinetics of the anatase-rutile transformation. J. Am. Ceram. Soc. 1969, 48, 391–398. [Google Scholar] [CrossRef]
- Zhang, H.; Banfield, J.F. Phase transformation of nanocrystalline anatase-to-rutile via combined interface and surface nucleation. J. Mater. Res. 2000, 15, 437–448. [Google Scholar] [CrossRef]
- Mahshid, S.; Askari, M.; Ghamsari, M.S.; Afshr, N.; Lahuti, S. Mixed-phase TiO2 nanoparticles preparation using sol–gel method. J. Alloys Compd. 2009, 478, 586–589. [Google Scholar] [CrossRef]
- Barringer, E.A.; Bowen, H.K. Formation, Packing, and Sintering of Monodisperse TiO2 Powders. J. Am. Ceram. Soc. 1982, 65, C-199–C-201. [Google Scholar] [CrossRef]
- Castrejón-Sánchez, V.; Camps, E.; Camacho-López, M. Quantification of phase content in TiO2 thin films by Raman spectroscopy. Superf. Vacío 2014, 27, 88–92. [Google Scholar]
- Spurr, R.A.; Myers, H. Quantitative Analysis of Anatase-Rutile Mixtures with an X-Ray Diffractometer. Anal. Chem. 1957, 29, 760–762. [Google Scholar] [CrossRef]
- Boukrouh, S.; Bensaha, R.; Bourgeois, S.; Finot, E.; Marcos de Lucas, M.C. Reactive direct current magnetron sputtered TiO2 thin films with amorphous to crystalline structures. Thin Solid Films 2008, 516, 6353–6358. [Google Scholar] [CrossRef]
- Schulte, K.L.; DeSario, P.A.; Gray, K.A. Effect of crystal phase composition on the reductive and oxidative abilities of TiO2 nanotubes under UV and visible light. Appl. Catal. B Environ. 2010, 97, 354–360. [Google Scholar] [CrossRef]
- Hurum, D.; Agrios, A.; Crist, S.; Gray, K.; Rajh, T.; Thurnauer, M.J. Probing reaction mechanisms in mixed phase TiO2 by EPR. Electron. Spectros. Relat. Phenom. 2006, 150, 155–163. [Google Scholar] [CrossRef]
- Luis, A.M.; Neves, M.C.; Mendoça, M.H.; Monteiro, O.C. Influence of calcination parameters on the TiO2 photocatalytic properties. Mater. Chem. Phys. 2011, 125, 20–25. [Google Scholar] [CrossRef]
- Paul, S.; Choudhury, A.A. Investigation of the optical property and photocatalytic activity of mixed phase nanocrystalline titania. Appl. Nanosci. 2014, 4, 839–847. [Google Scholar] [CrossRef]
- Escobedo-Morales, A.; Sánchez-Mora, E.; Pal, U. Use of diffuse reflectance spectroscopy for optical characterization of un-supported nanostructures. Rev. Mex. Física 2007, 53, 18–22. [Google Scholar]
- Brus, L. Electronic wave functions in semiconductor clusters: experiment and theory. J. Phys. Chem. 1986, 90, 2555–2560. [Google Scholar] [CrossRef]
- Lee, H.S.; Woo, C.S.; Youn, B.K.; Kim, S.; Oh, S.; Sung, Y.; Lee, H. Bandgap modulation of TiO2 and its effect on the activity in photocatalytic oxidation of 2-isopropyl-6-methyl-4-pyrimidinol. Top. Catal. 2005, 35, 255–260. [Google Scholar] [CrossRef]
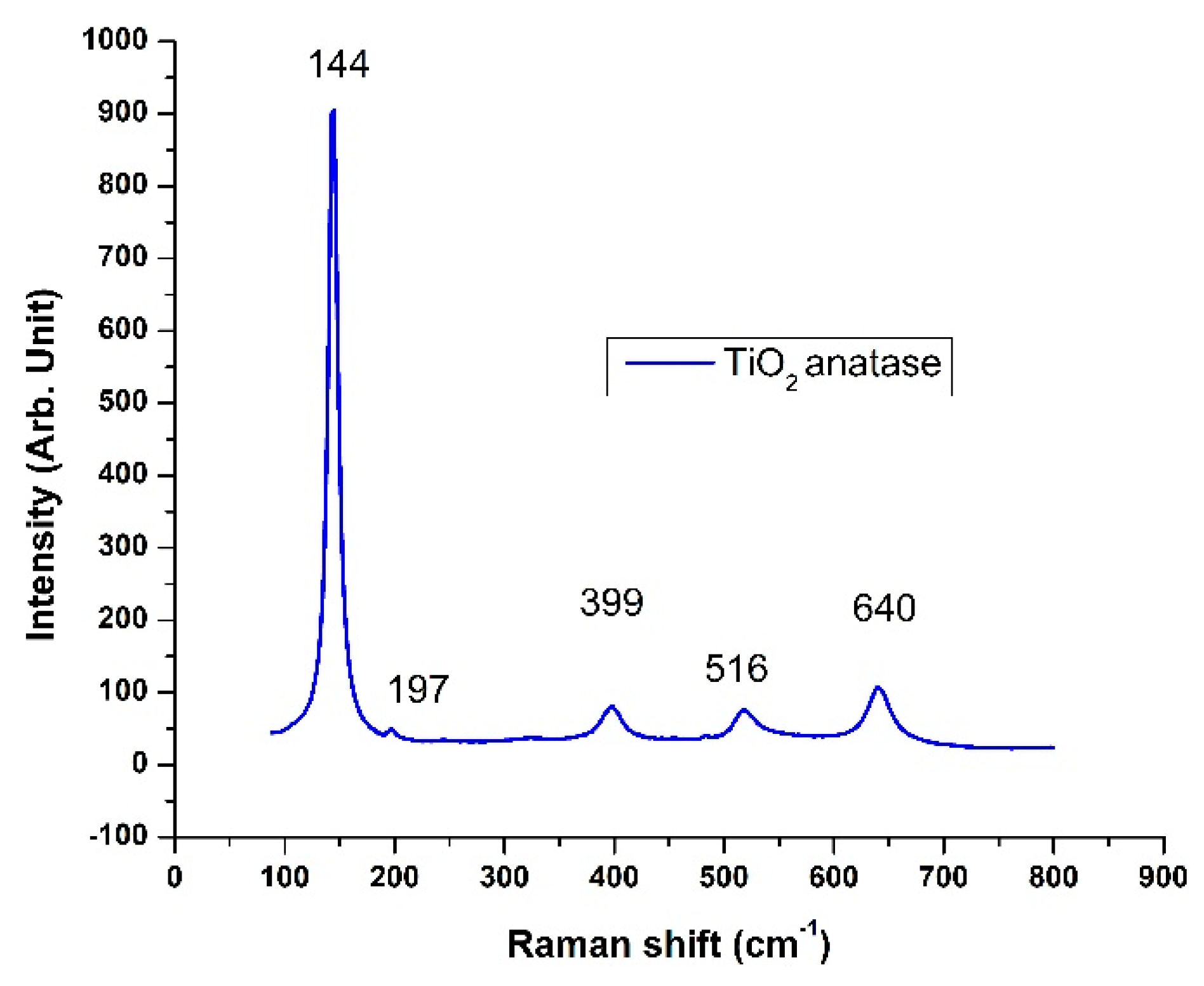
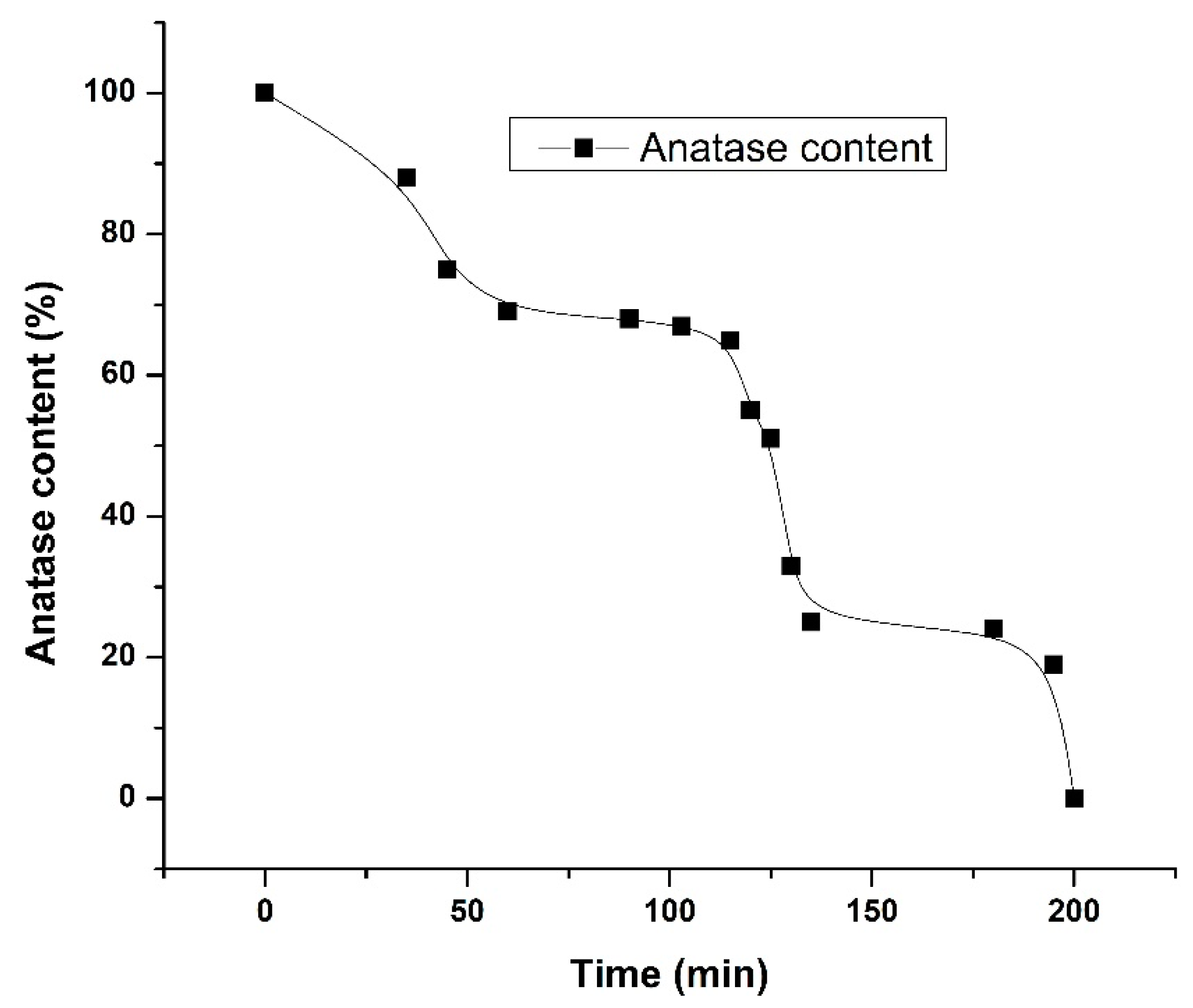
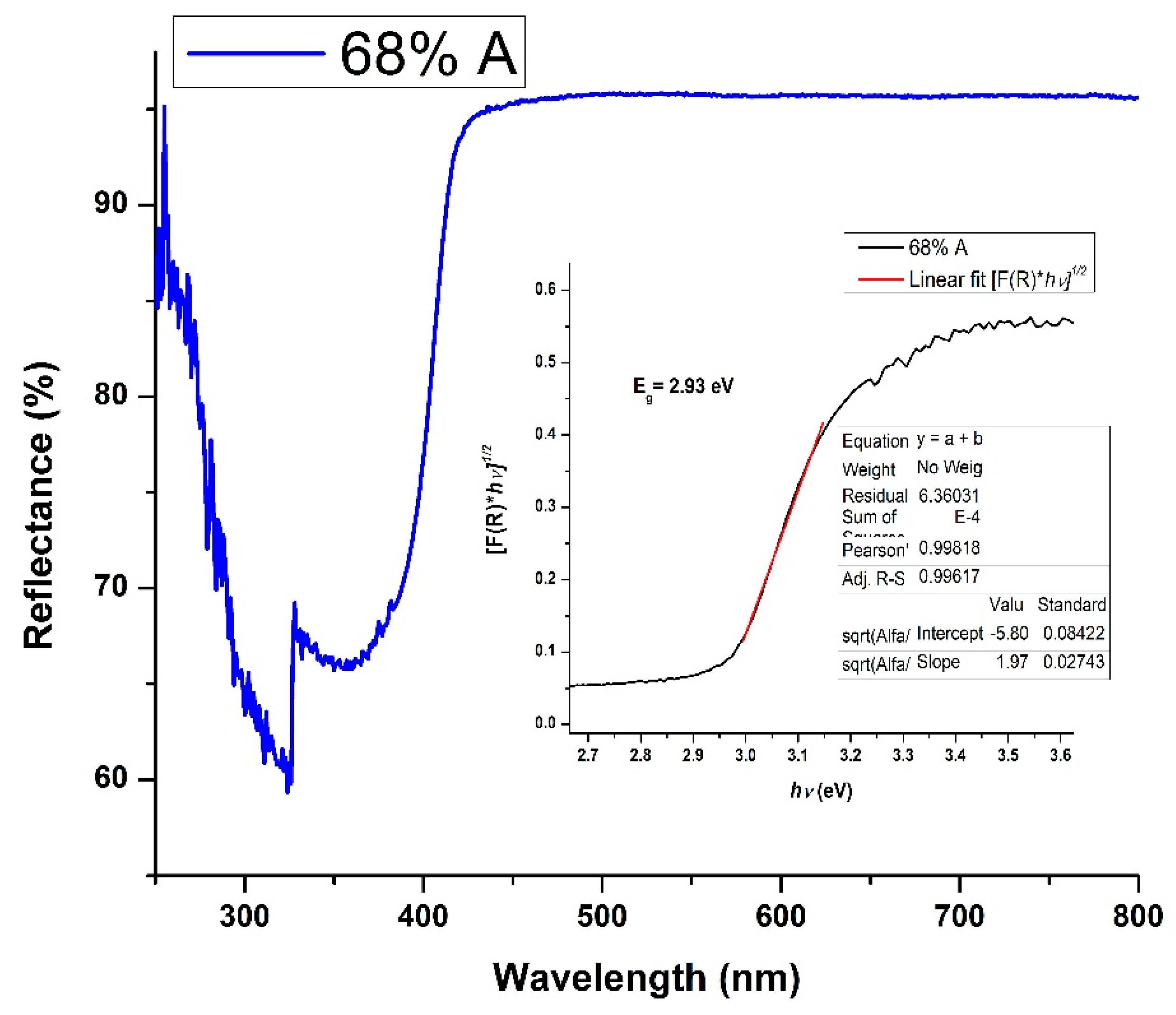
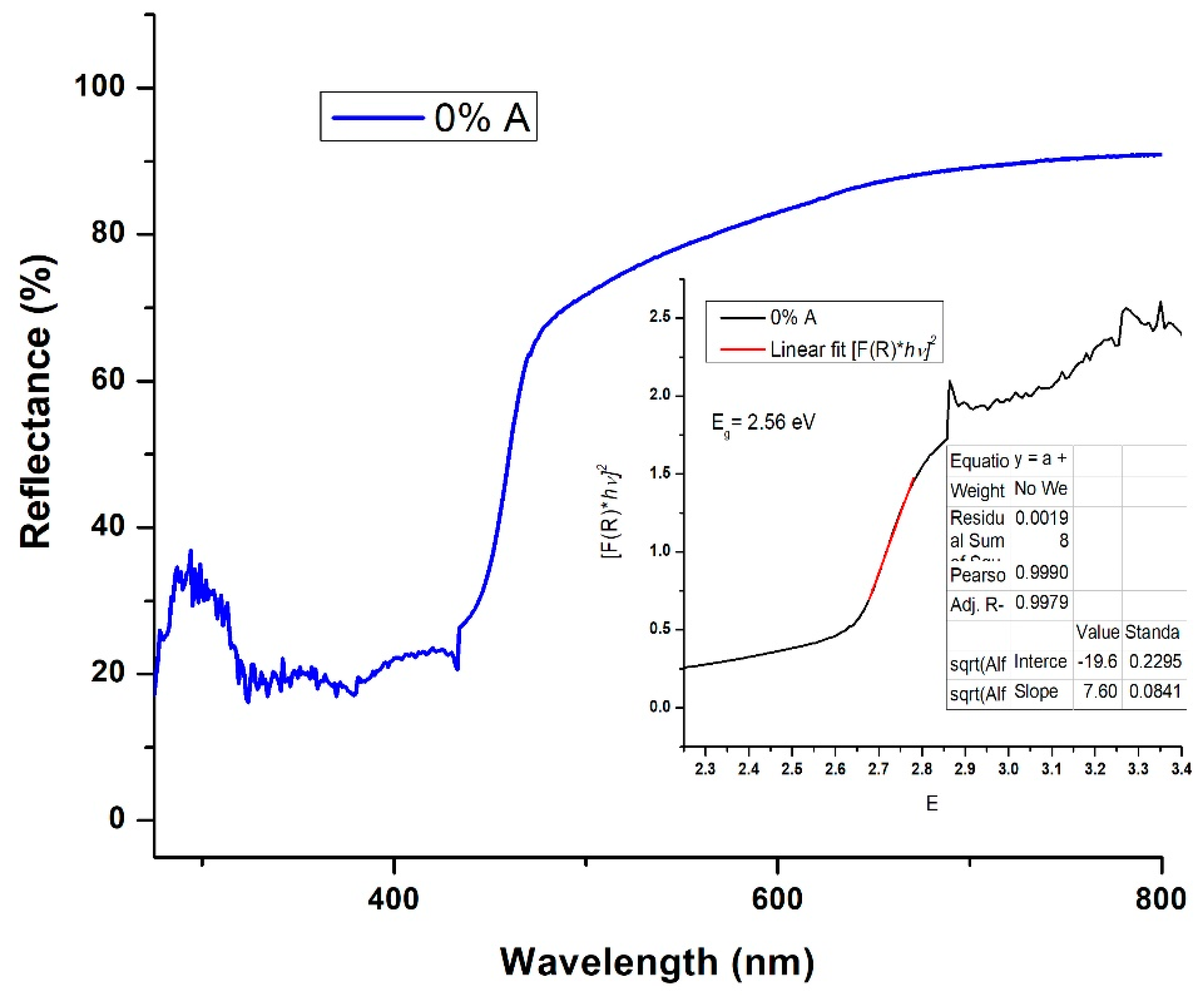


| Time (min) | IA399/IR447 | WA/WR | % Anatase | % Rutile | ΔEg (eV) | 2R (nm) |
|---|---|---|---|---|---|---|
| 0 | - | - | 100 | 0 | 2.90 | 20.35 |
| 35 | 7.48 | 7.22 | 88 | 12 | 2.78 | 15.02 |
| 45 | 3.35 | 3.05 | 75 | 25 | 2.88 | 15.37 |
| 60 | 2.54 | 2.24 | 69 | 31 | 2.86 | 14.26 |
| 90 | 2.41 | 2.10 | 68 | 32 | 2.93 | 15.89 |
| 103 | 2.38 | 2.07 | 67 | 33 | 2.78 | 17.20 |
| 115 | 2.14 | 1.83 | 65 | 35 | 2.77 | 14.82 |
| 120 | 1.53 | 1.21 | 55 | 45 | 2.80 | 12.02 |
| 125 | 1.38 | 1.06 | 51 | 49 | 2.83 | 12.21 |
| 130 | 0.81 | 0.48 | 33 | 67 | 2.85 | 11.46 |
| 135 | 0.67 | 0.34 | 25 | 75 | 2.81 | 10.48 |
| 180 | 0.64 | 0.31 | 24 | 76 | 2.90 | 11.90 |
| 195 | 0.56 | 0.24 | 19 | 81 | 2.86 | 14.91 |
| 200 | - | - | 0 | 100 | 2.56 | 7.43 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castrejón-Sánchez, V.H.; López, R.; Ramón-González, M.; Enríquez-Pérez, Á.; Camacho-López, M.; Villa-Sánchez, G. Annealing Control on the Anatase/Rutile Ratio of Nanostructured Titanium Dioxide Obtained by Sol-Gel. Crystals 2019, 9, 22. https://doi.org/10.3390/cryst9010022
Castrejón-Sánchez VH, López R, Ramón-González M, Enríquez-Pérez Á, Camacho-López M, Villa-Sánchez G. Annealing Control on the Anatase/Rutile Ratio of Nanostructured Titanium Dioxide Obtained by Sol-Gel. Crystals. 2019; 9(1):22. https://doi.org/10.3390/cryst9010022
Chicago/Turabian StyleCastrejón-Sánchez, V. H., Roberto López, M. Ramón-González, Ángeles Enríquez-Pérez, M. Camacho-López, and G. Villa-Sánchez. 2019. "Annealing Control on the Anatase/Rutile Ratio of Nanostructured Titanium Dioxide Obtained by Sol-Gel" Crystals 9, no. 1: 22. https://doi.org/10.3390/cryst9010022
APA StyleCastrejón-Sánchez, V. H., López, R., Ramón-González, M., Enríquez-Pérez, Á., Camacho-López, M., & Villa-Sánchez, G. (2019). Annealing Control on the Anatase/Rutile Ratio of Nanostructured Titanium Dioxide Obtained by Sol-Gel. Crystals, 9(1), 22. https://doi.org/10.3390/cryst9010022





