Preparation of Mixed Matrix Membranes Containing ZIF-8 and UiO-66 for Multicomponent Light Gas Separation
Abstract
1. Introduction
2. Materials and Methods
2.1. Crystallization of ZIF-8 and UiO-66
2.2. Mixed Membrane Preparation
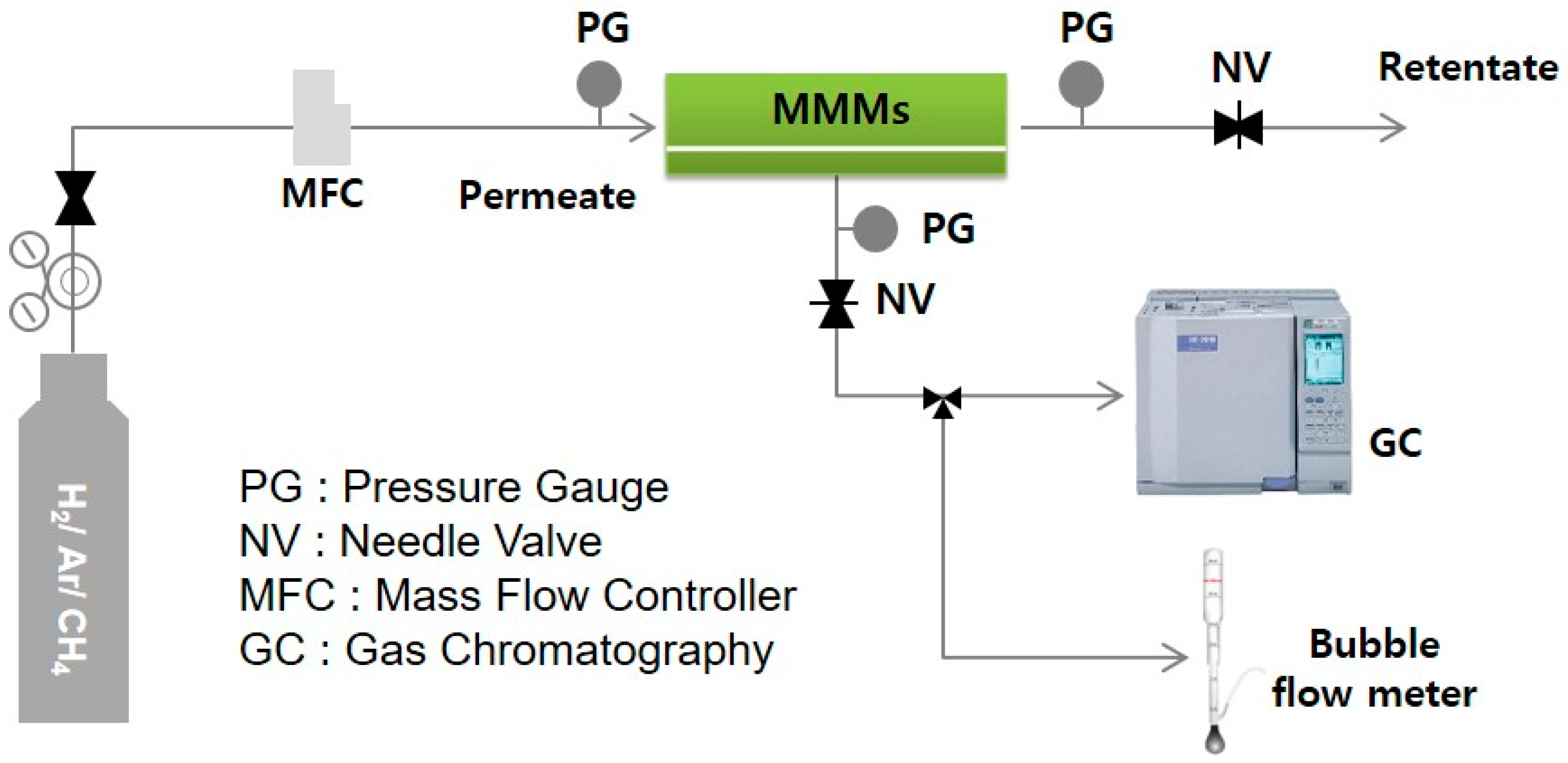
2.3. Characterizations
3. Results and Discussion
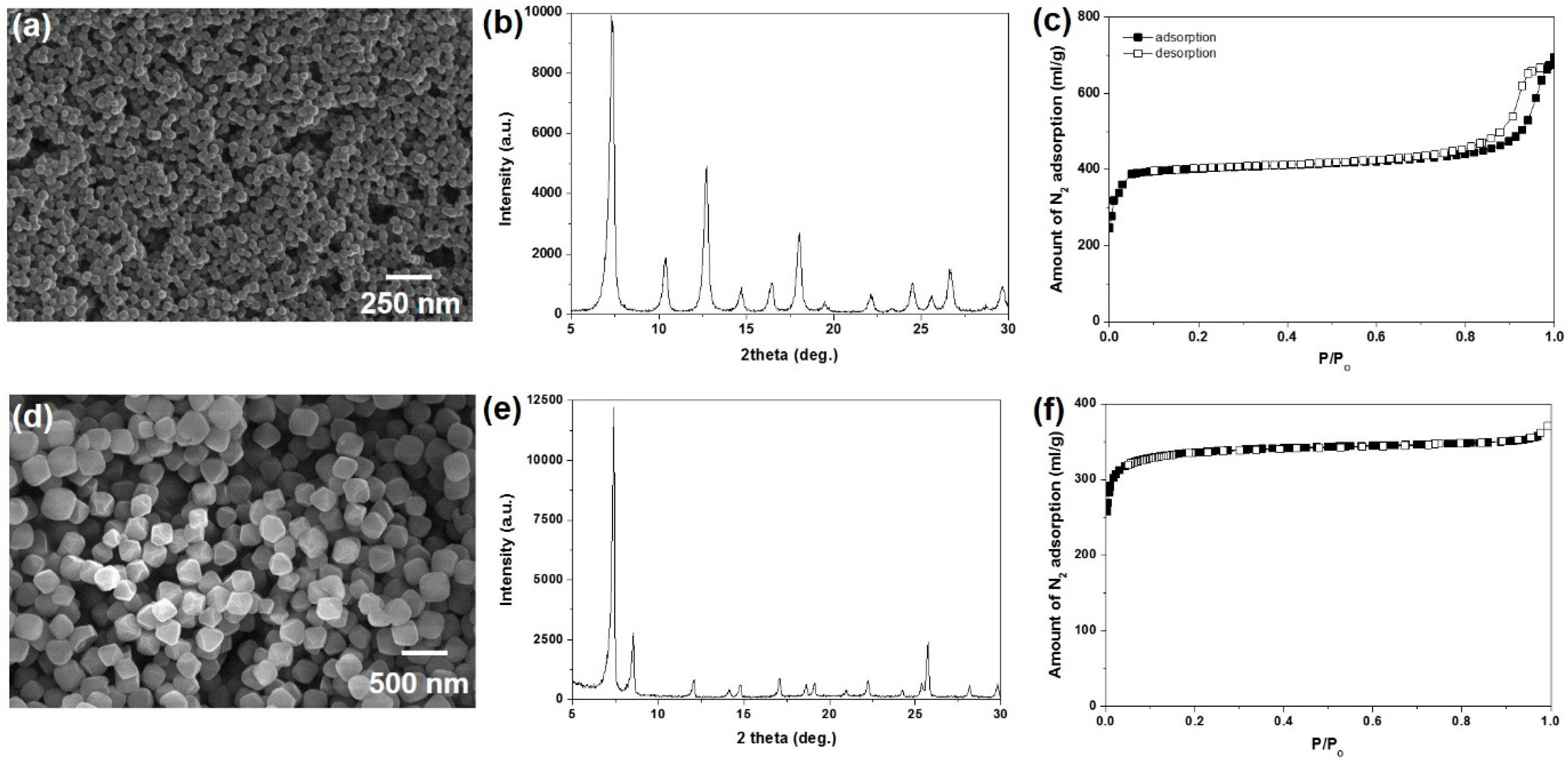
3.1. Characterization of ZIF-8 and UiO-66
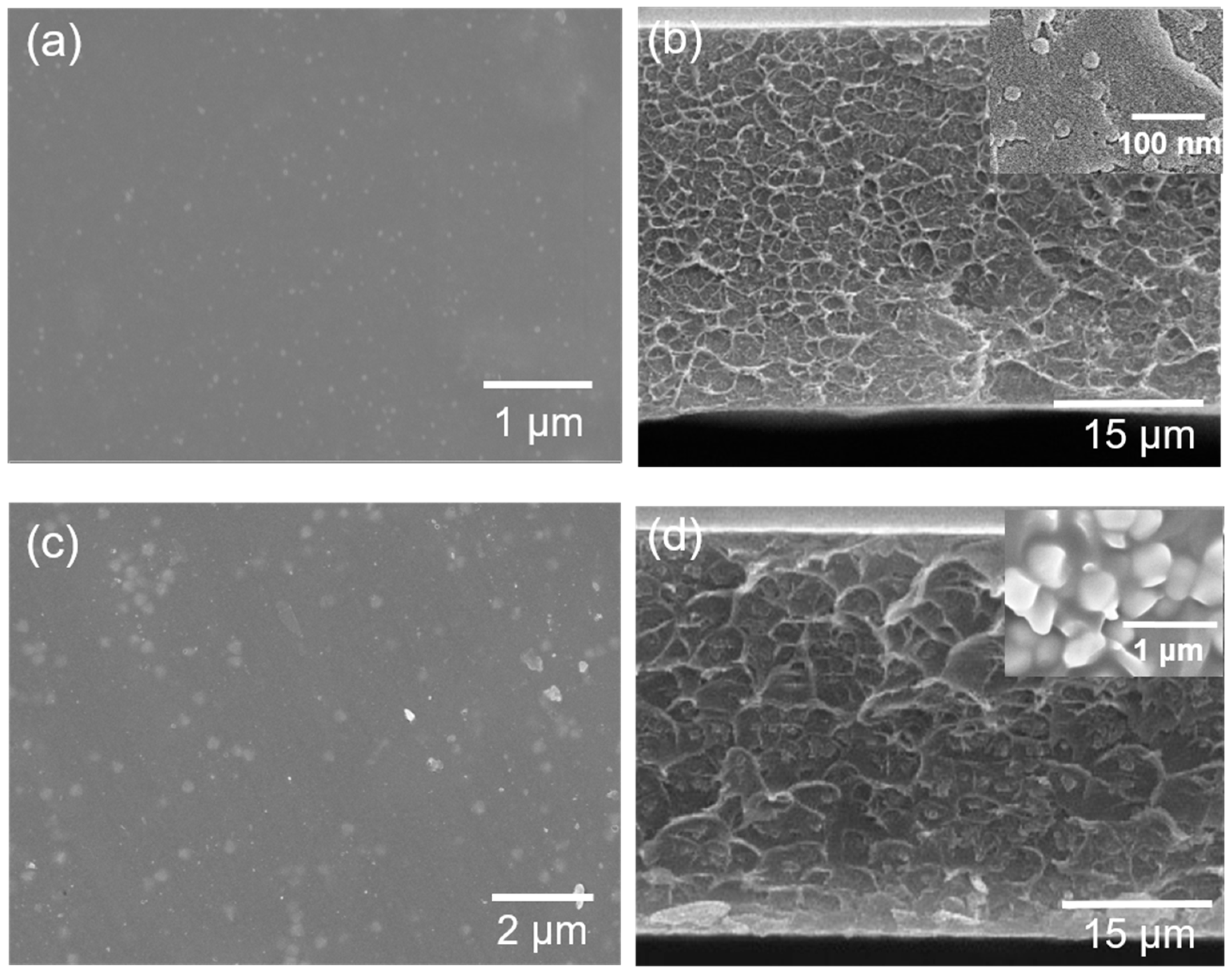
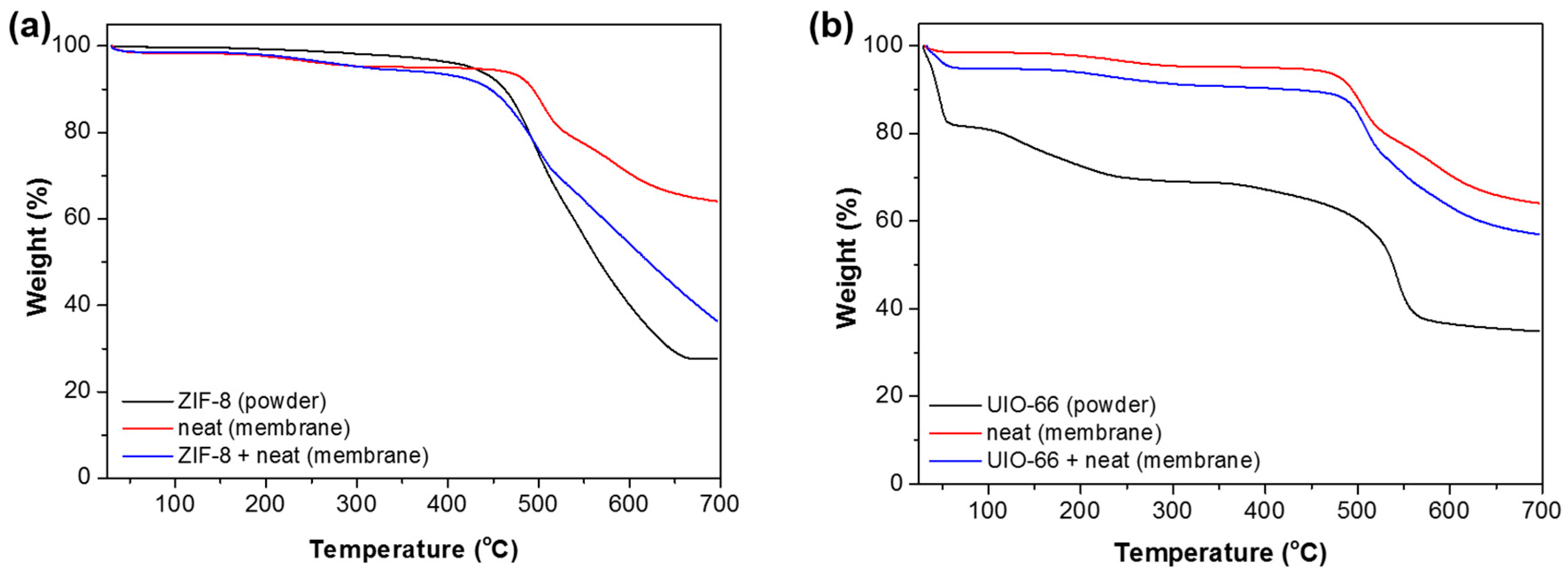
3.2. Characterization of Membranes
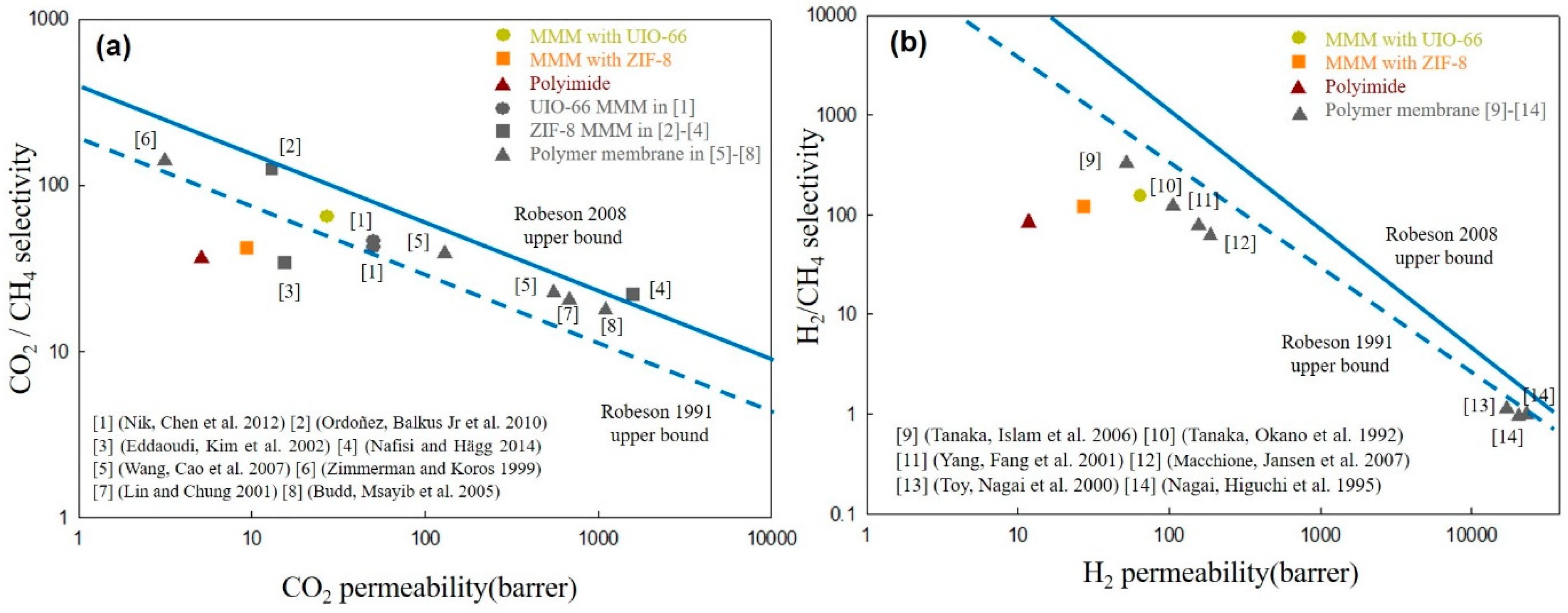
3.3. Single Gas Permeation Through Membranes
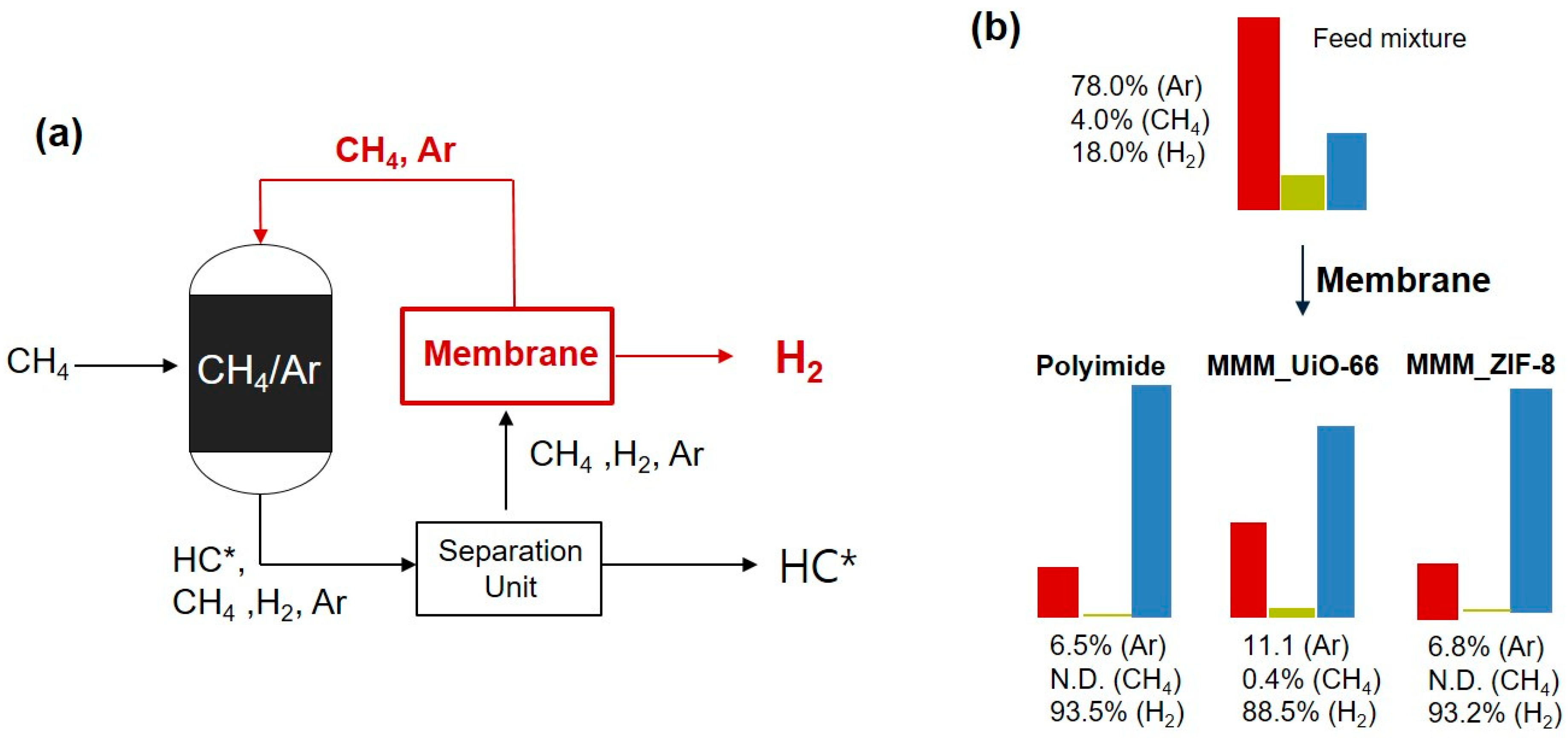
3.4. Multiple Gas Mixture Permeations Through MMMs
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, S.; Lu, G.; Millar, G.J. Carbon dioxide reforming of methane to produce synthesis gas over metal-supported catalysts: State of the art. Energy Fuels 1996, 10, 896–904. [Google Scholar] [CrossRef]
- York, A.P.; Xiao, T.; Green, M.L. Brief overview of the partial oxidation of methane to synthesis gas. Top. Catal. 2003, 22, 345–358. [Google Scholar] [CrossRef]
- Leung, D.Y.; Caramanna, G.; Maroto-Valer, M.M. An overview of current status of carbon dioxide capture and storage technologies. Renew. Sustain. Energy Rev. 2014, 39, 426–443. [Google Scholar] [CrossRef]
- Bernardo, P.; Drioli, E.; Golemme, G. Membrane gas separation: A review/state of the art. Ind. Eng. Chem. Res. 2009, 48, 4638–4663. [Google Scholar] [CrossRef]
- Baker, R.W.; Low, B.T. Gas separation membrane materials: A perspective. Macromolecules 2014, 47, 6999–7013. [Google Scholar] [CrossRef]
- Yang, H.; Xu, Z.; Fan, M.; Gupta, R.; Slimane, R.B.; Bland, A.E.; Wright, I. Progress in carbon dioxide separation and capture: A review. J. Environ. Sci. 2008, 20, 14–27. [Google Scholar] [CrossRef]
- Galizia, M.; Chi, W.S.; Smith, Z.P.; Merkel, T.C.; Baker, R.W.; Freeman, B.D. 50th anniversary perspective: Polymers and mixed matrix membranes for gas and vapor separation: A review and prospective opportunities. Macromolecules 2017, 50, 7809–7843. [Google Scholar] [CrossRef]
- Vu, D.Q.; Koros, W.J.; Miller, S.J. Effect of condensable impurity in CO2/CH4 gas feeds on performance of mixed matrix membranes using carbon molecular sieves. J. Membr. Sci. 2003, 221, 233–239. [Google Scholar] [CrossRef]
- Goh, P.; Ismail, A.; Sanip, S.; Ng, B.; Aziz, M. Recent advances of inorganic fillers in mixed matrix membrane for gas separation. Sep. Purif. Technol. 2011, 81, 243–264. [Google Scholar] [CrossRef]
- Chuah, C.Y.; Goh, K.; Yang, Y.; Gong, H.; Li, W.; Karahan, H.E.; Guiver, M.D.; Wang, R.; Bae, T.H. Harnessing filler materials for enhancing biogas separation membranes. Chem. Rev. 2018, 118, 8655–8769. [Google Scholar] [CrossRef]
- Vinh-Thang, H.; Kaliaguine, S. Predictive models for mixed-matrix membrane performance: A review. Chem. Rev. 2013, 113, 4980–5028. [Google Scholar] [CrossRef] [PubMed]
- Aroon, M.; Ismail, A.; Matsuura, T.; Montazer-Rahmati, M. Performance studies of mixed matrix membranes for gas separation: A review. Sep. Purif. Technol. 2010, 75, 229–242. [Google Scholar] [CrossRef]
- Bastani, D.; Esmaeili, N.; Asadollahi, M. Polymeric mixed matrix membranes containing zeolites as a filler for gas separation applications: A review. J. Ind. Eng. Chem. 2013, 19, 375–393. [Google Scholar] [CrossRef]
- Khdhayyer, M.R.; Esposito, E.; Fuoco, A.; Monteleone, M.; Giorno, L.; Jansen, J.C.; Attfield, M.P.; Budd, P.M. Mixed matrix membranes based on UiO-66 MOFs in the polymer of intrinsic microporosity PIM-1. Sep. Purif. Technol. 2017, 173, 304–313. [Google Scholar] [CrossRef]
- Ghalei, B.; Sakurai, K.; Kinoshita, Y.; Wakimoto, K.; Isfahani, A.P.; Song, Q.; Doitomi, K.; Furukawa, S.; Hirao, H.; Kusuda, H.; et al. Enhanced selectivity in mixed matrix membranes for CO2 capture through efficient dispersion of amine-functionalized MOF nanoparticles. Nat. Energy 2017, 2, 17086. [Google Scholar] [CrossRef]
- Fuoco, A.; Khdhayyer, M.R.; Attfield, M.P.; Esposito, E.; Jansen, J.C.; Budd, P.M. Synthesis and Transport Properties of Novel MOF/PIM-1/MOF Sandwich Membranes for Gas Separation. Membranes 2017, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Nataraj, S.; Roussenova, M.V.; Tan, J.C.; Hughes, D.J.; Li, W.; Bourgoin, P.; Alam, M.A.; Cheetham, A.K.; Al-Muhtaseb, S.A.; et al. Zeolitic imidazolate framework (ZIF-8) based polymer nanocomposite membranes for gas separation. Energy Environ. Sci. 2012, 5, 8359–8369. [Google Scholar] [CrossRef]
- Sanchez-Lainez, J.; Zornoza, B.; Friebe, S.; Caro, J.; Cao, S.; Sabetghadam, A.; Seoane, B.; Gascon, J.; Kapteijn, F.; le Guillouzer, C.; et al. Influence of ZIF-8 particle size in the performance of polybenzimidazole mixed matrix membranes for pre-combustion CO2 capture and its validation through interlaboratory test. J. Membr. Sci. 2016, 515, 45–53. [Google Scholar] [CrossRef]
- Smith, S.J.; Ladewig, B.P.; Hill, A.J.; Lau, C.H.; Hill, M.R. Post-synthetic Ti exchanged UiO-66 metal-organic frameworks that deliver exceptional gas permeability in mixed matrix membranes. Sci. Rep. 2015, 5, 7823. [Google Scholar] [CrossRef]
- Anjum, M.W.; Vermoortele, F.; Khan, A.L.; Bueken, B.; de Vos, D.E.; Vankelecom, I.F. Modulated UiO-66-based mixed-matrix membranes for CO2 separation. ACS Appl. Mater. Interfaces 2015, 7, 25193–25201. [Google Scholar] [CrossRef]
- Askari, M.; Chung, T.-S. Natural gas purification and olefin/paraffin separation using thermal cross-linkable co-polyimide/ZIF-8 mixed matrix membranes. J. Membr. Sci. 2013, 444, 173–183. [Google Scholar] [CrossRef]
- Wijenayake, S.N.; Panapitiya, N.P.; Versteeg, S.H.; Nguyen, C.N.; Goel, S.; Balkus, K.J., Jr.; Musselman, I.H.; Ferraris, J.P. Surface cross-linking of ZIF-8/polyimide mixed matrix membranes (MMMs) for gas separation. Ind. Eng. Chem. Res. 2013, 52, 6991–7001. [Google Scholar] [CrossRef]
- Pan, Y.; Liu, Y.; Zeng, G.; Zhao, L.; Lai, Z. Rapid synthesis of zeolitic imidazolate framework-8 (ZIF-8) nanocrystals in an aqueous system. Chem. Commun. 2011, 47, 2071–2073. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Chen, Z.; Song, Z.; Li, J.; Dong, J. Synthesis of ZIF-8 and ZIF-67 by Steam-Assisted Conversion and an Investigation of Their Tribological Behaviors. Angew. Chem. 2011, 123, 698–701. [Google Scholar] [CrossRef]
- Katz, M.J.; Brown, Z.J.; Colón, Y.J.; Siu, P.W.; Scheidt, K.A.; Snurr, R.Q.; Hupp, J.T.; Farha, O.K. A facile synthesis of UiO-66, UiO-67 and their derivatives. Chem. Commun. 2013, 49, 9449–9451. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, S. Metal–organic frameworks (MOFs). Chem. Soc. Rev. 2014, 43, 5415–5418. [Google Scholar]
- Basu, S.; Cano-Odena, A.; Vankelecom, I.F. MOF-containing mixed-matrix membranes for CO2/CH4 and CO2/N2 binary gas mixture separations. Sep. Purif. Technol. 2011, 81, 31–40. [Google Scholar] [CrossRef]
- Fu, Y.-J.; Liao, K.-S.; Hu, C.-C.; Lee, K.-R.; Lai, J.-Y. Development and characterization of micropores in carbon molecular sieve membrane for gas separation. Microporous Mesoporous Mater. 2011, 143, 78–86. [Google Scholar] [CrossRef]
- Zornoza, B.; Tellez, C.; Coronas, J.; Gascon, J.; Kapteijn, F. Metal organic framework based mixed matrix membranes: An increasingly important field of research with a large application potential. Microporous Mesoporous Mater. 2013, 166, 67–78. [Google Scholar] [CrossRef]
- Nijem, N.; Wu, H.; Canepa, P.; Marti, A.; Balkus, K.J., Jr.; Thonhauser, T.; Li, J.; Chabal, Y.J. Tuning the gate opening pressure of metal–organic frameworks (MOFs) for the selective separation of hydrocarbons. J. Am. Chem. Soc. 2012, 134, 15201–15204. [Google Scholar] [CrossRef]
- Wu, H.; Chua, Y.S.; Krungleviciute, V.; Tyagi, M.; Chen, P.; Yildirim, T.; Zhou, W. Unusual and highly tunable missing-linker defects in zirconium metal–organic framework UiO-66 and their important effects on gas adsorption. J. Am. Chem. Soc. 2013, 135, 10525–10532. [Google Scholar] [CrossRef] [PubMed]
- Robeson, L.M. The upper bound revisited. J. Membr. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Bromberg, L.; Cohn, D.; Rabinovich, A.; Alexeev, N. Plasma catalytic reforming of methane. Int. J. Hydrogen Energy 1999, 24, 1131–1137. [Google Scholar] [CrossRef]
- Freeman, B.; Yampolskii, Y.; Pinnau, I. Materials Science of Membranes for Gas and Vapor Separation; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Saberi, M.; Dadkhah, A.; Hashemifard, S. Modeling of simultaneous competitive mixed gas permeation and CO2 induced plasticization in glassy polymers. J. Membr. Sci. 2016, 499, 164–171. [Google Scholar] [CrossRef]
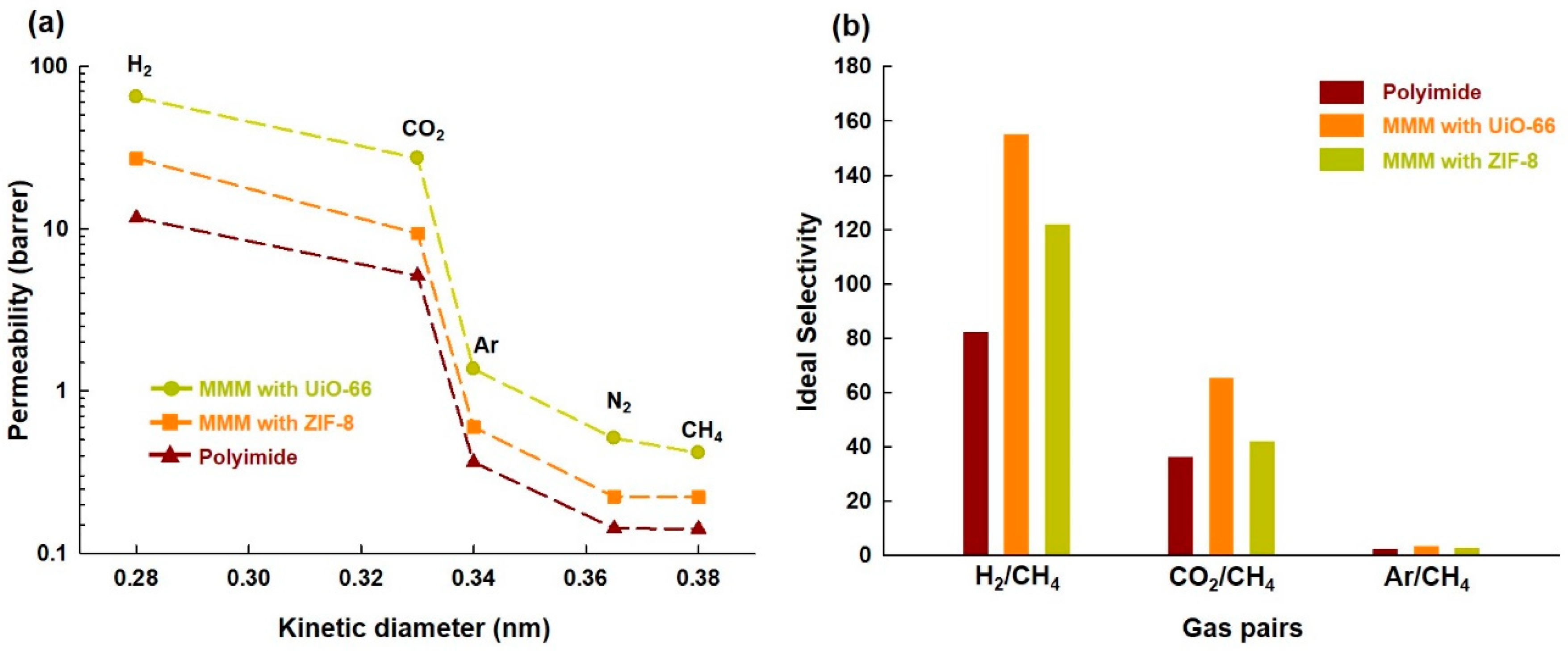
| Sample | Gases | Diffusivity (cm2/s) | Solubility (cm3(STP)/cm3cmHg) | Permeability (barrer) |
|---|---|---|---|---|
| PI | H2 | N.D. * | N.D. * | 11.7 |
| CO2 | 8.22 × 10−9 | 6.24 × 108 | 5.1 | |
| Ar | 2.83 × 10−8 | 1.28 × 107 | 0.36 | |
| N2 | 8.97 × 10−9 | 1.58 × 107 | 0.14 | |
| CH4 | 2.81 × 10−9 | 5.04 × 107 | 0.14 | |
| MMM(UiO-66(15%)) | H2 | N.D. * | N.D. * | 64.4 |
| CO2 | 7.10 × 10−9 | 3.81 × 109 | 27.1 | |
| Ar | 1.51 × 10−7 | 9.01 × 106 | 1.4 | |
| N2 | 6.92 × 10−9 | 7.40 × 107 | 0.51 | |
| CH4 | 1.25 × 10−9 | 3.32 × 108 | 0.42 | |
| MMM(ZIF-8(15%)) | H2 | N.D. * | N.D. * | 27.1 |
| CO2 | 8.02 × 10−9 | 1.16 × 109 | 9.3 | |
| Ar | 1.13 × 10−8 | 5.31 × 107 | 0.60 | |
| N2 | 6.68 × 10−9 | 3.34 × 107 | 0.22 | |
| CH4 | 1.66 × 10−9 | 1.33 × 108 | 0.22 |
| Membrane | Mixture Gas Permeability (barrer) | Mixture Gas Selectivity | Ideal Gas Selectivity | ||||||
|---|---|---|---|---|---|---|---|---|---|
| H2 | Ar | CH4 | H2/CH4 | H2/Ar | Ar/CH4 | H2/CH4 | H2/Ar | Ar/CH4 | |
| PI | 9.1 (−22%) | 0.15 (−58%) | N.D. * | - | 62.3 | - | 85.7 | 32.5 | 2.6 |
| MMM (UiO-66) | 18.3 (−72%) | 0.52 (−63%) | 0.37 (−12%) | 49.2 | 34.9 | 1.4 | 153.3 | 46.0 | 3.3 |
| MMM (ZIF-8) | 14.5 (−46%) | 0.24 (−60%) | N.D. * | - | 59.4 | - | 123.2 | 45.2 | 2.7 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, E.Y.; Kim, H.S.; Kim, D.; Kim, J.; Lee, P.S. Preparation of Mixed Matrix Membranes Containing ZIF-8 and UiO-66 for Multicomponent Light Gas Separation. Crystals 2019, 9, 15. https://doi.org/10.3390/cryst9010015
Kim EY, Kim HS, Kim D, Kim J, Lee PS. Preparation of Mixed Matrix Membranes Containing ZIF-8 and UiO-66 for Multicomponent Light Gas Separation. Crystals. 2019; 9(1):15. https://doi.org/10.3390/cryst9010015
Chicago/Turabian StyleKim, Eun Young, Hyun Su Kim, Donghwi Kim, Jinsoo Kim, and Pyung Soo Lee. 2019. "Preparation of Mixed Matrix Membranes Containing ZIF-8 and UiO-66 for Multicomponent Light Gas Separation" Crystals 9, no. 1: 15. https://doi.org/10.3390/cryst9010015
APA StyleKim, E. Y., Kim, H. S., Kim, D., Kim, J., & Lee, P. S. (2019). Preparation of Mixed Matrix Membranes Containing ZIF-8 and UiO-66 for Multicomponent Light Gas Separation. Crystals, 9(1), 15. https://doi.org/10.3390/cryst9010015





