Effects on the Surface and Luminescence Properties of GaAs by SF6 Plasma Passivation
Abstract
:1. Introduction
2. Experiment
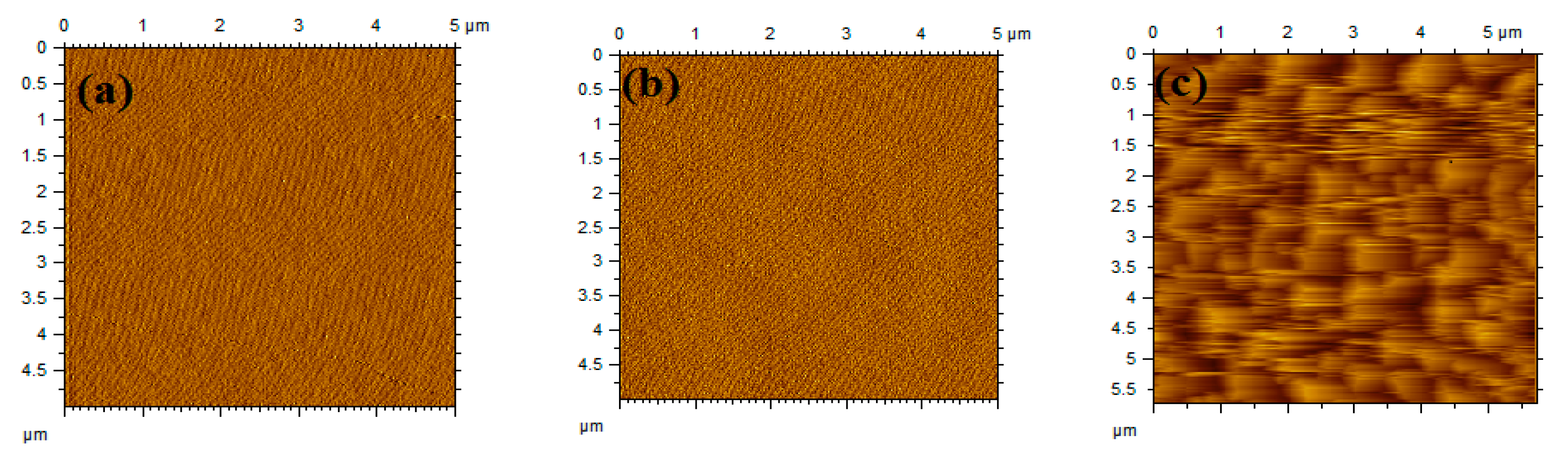
3. Measurement and Analysis
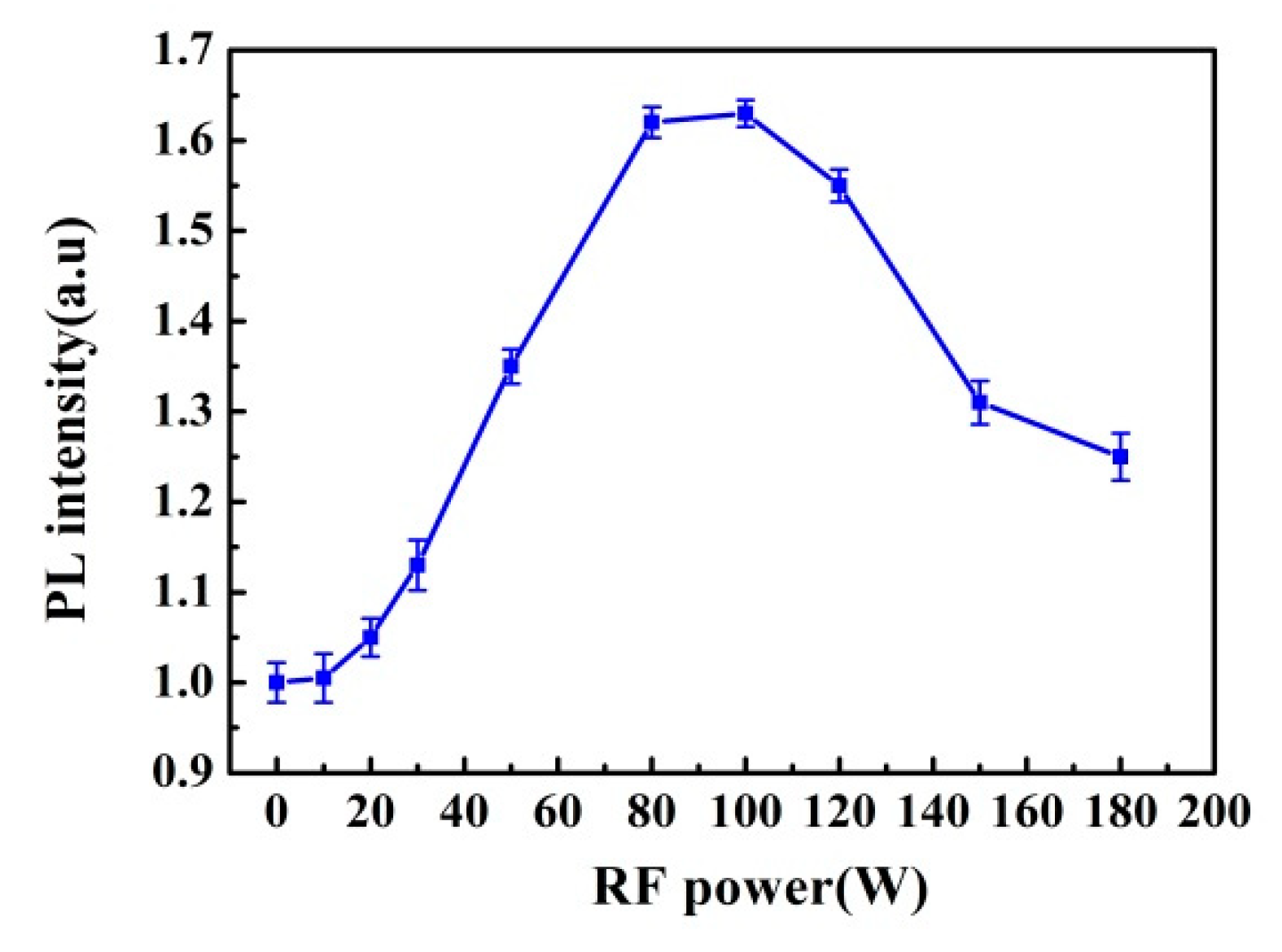
3.1. RF Power Effect
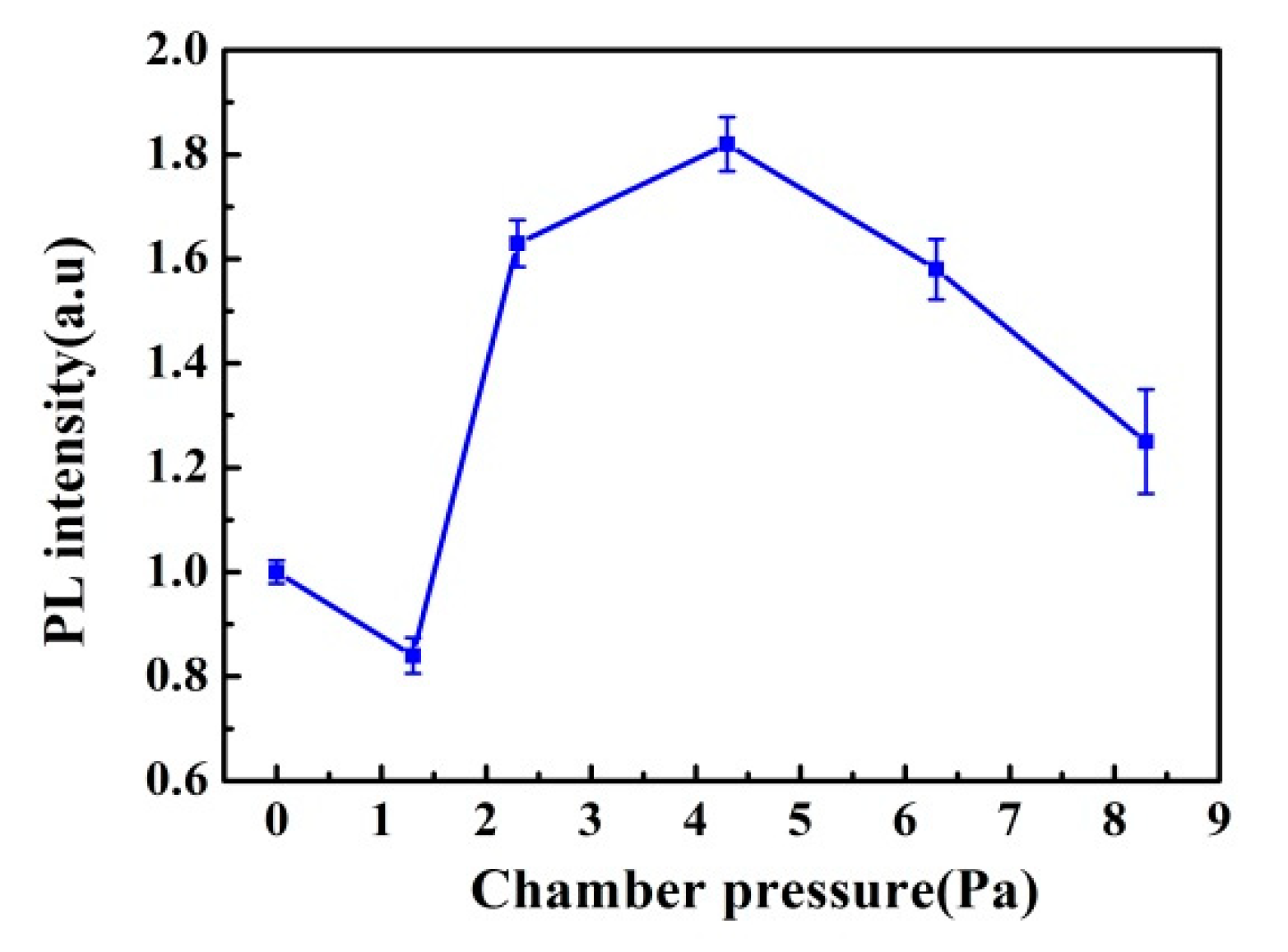
3.2. Chamber Pressure Effect
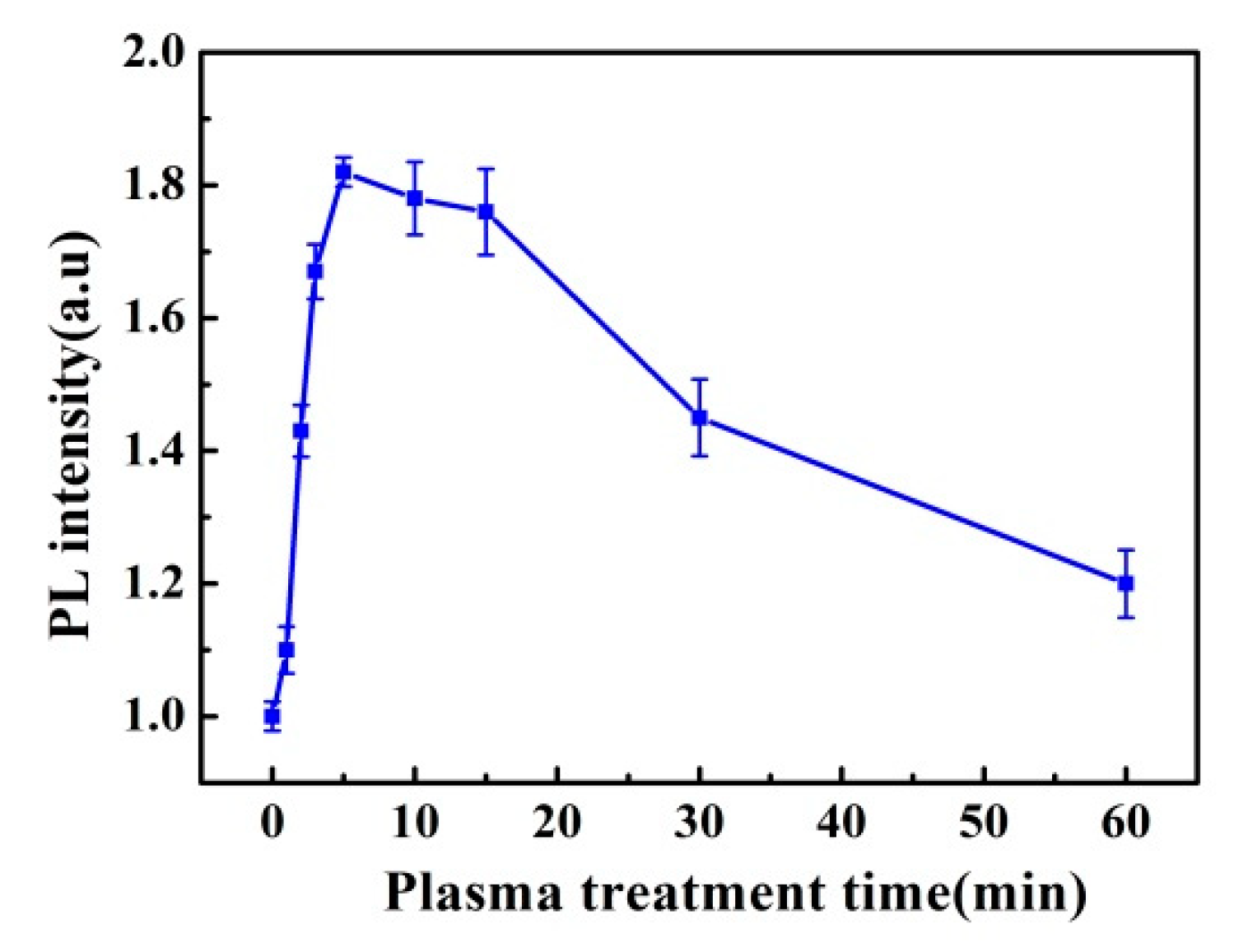
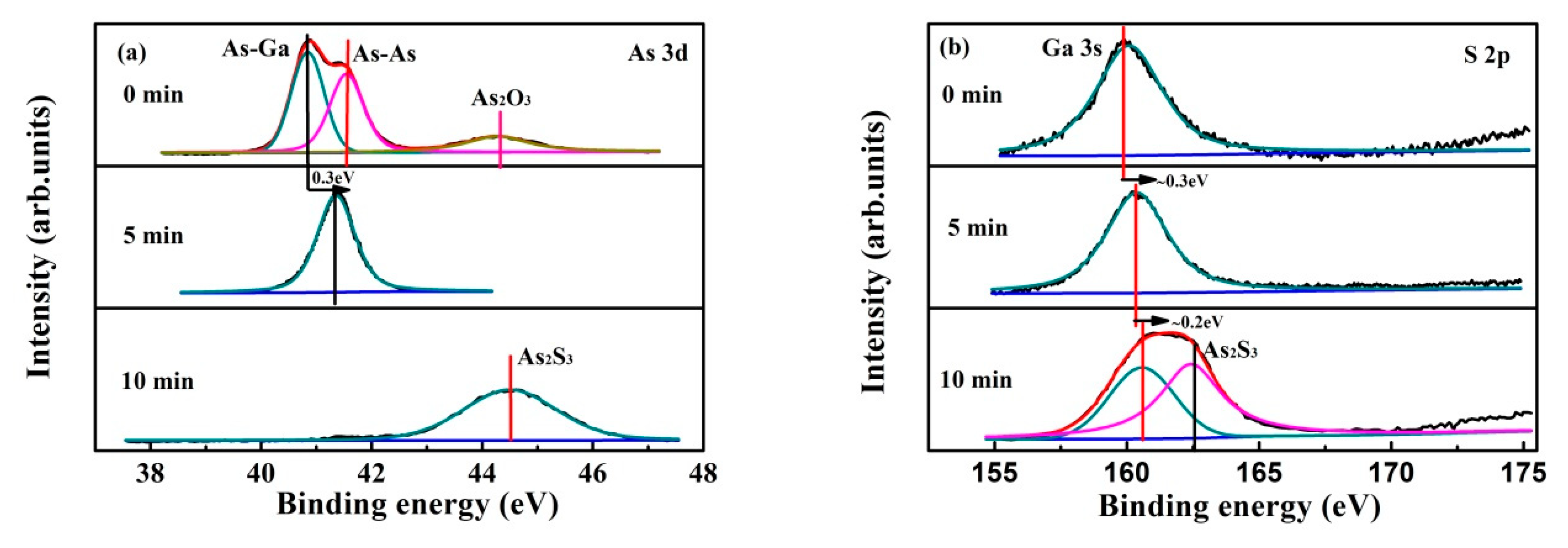
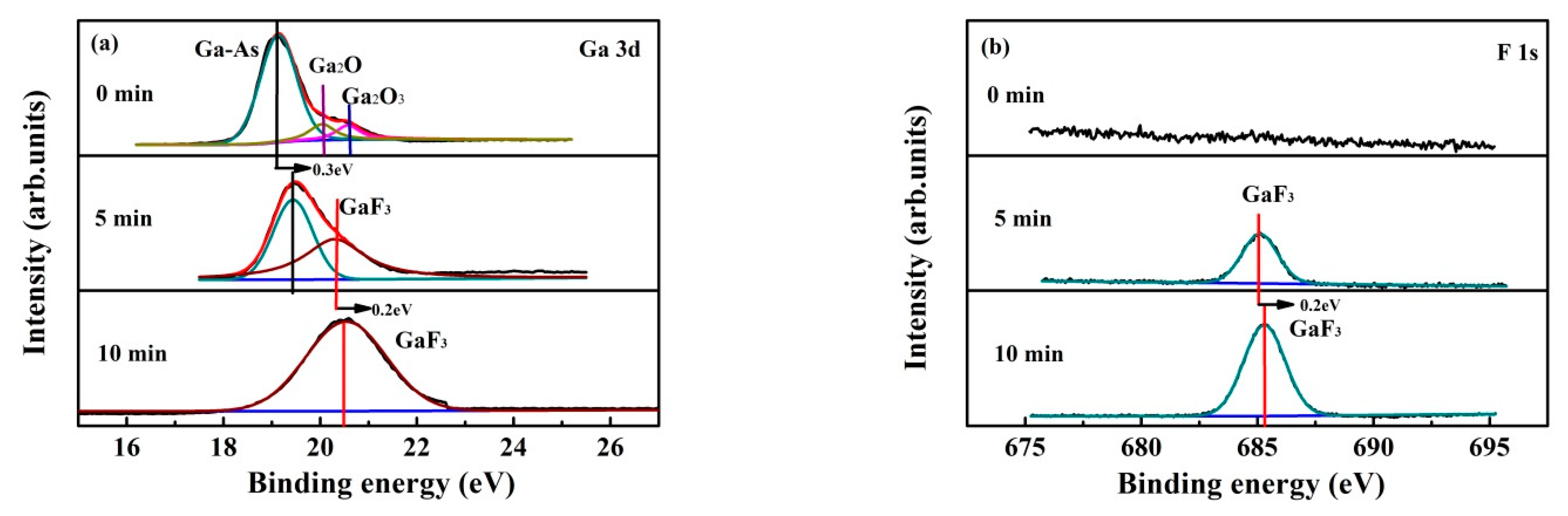
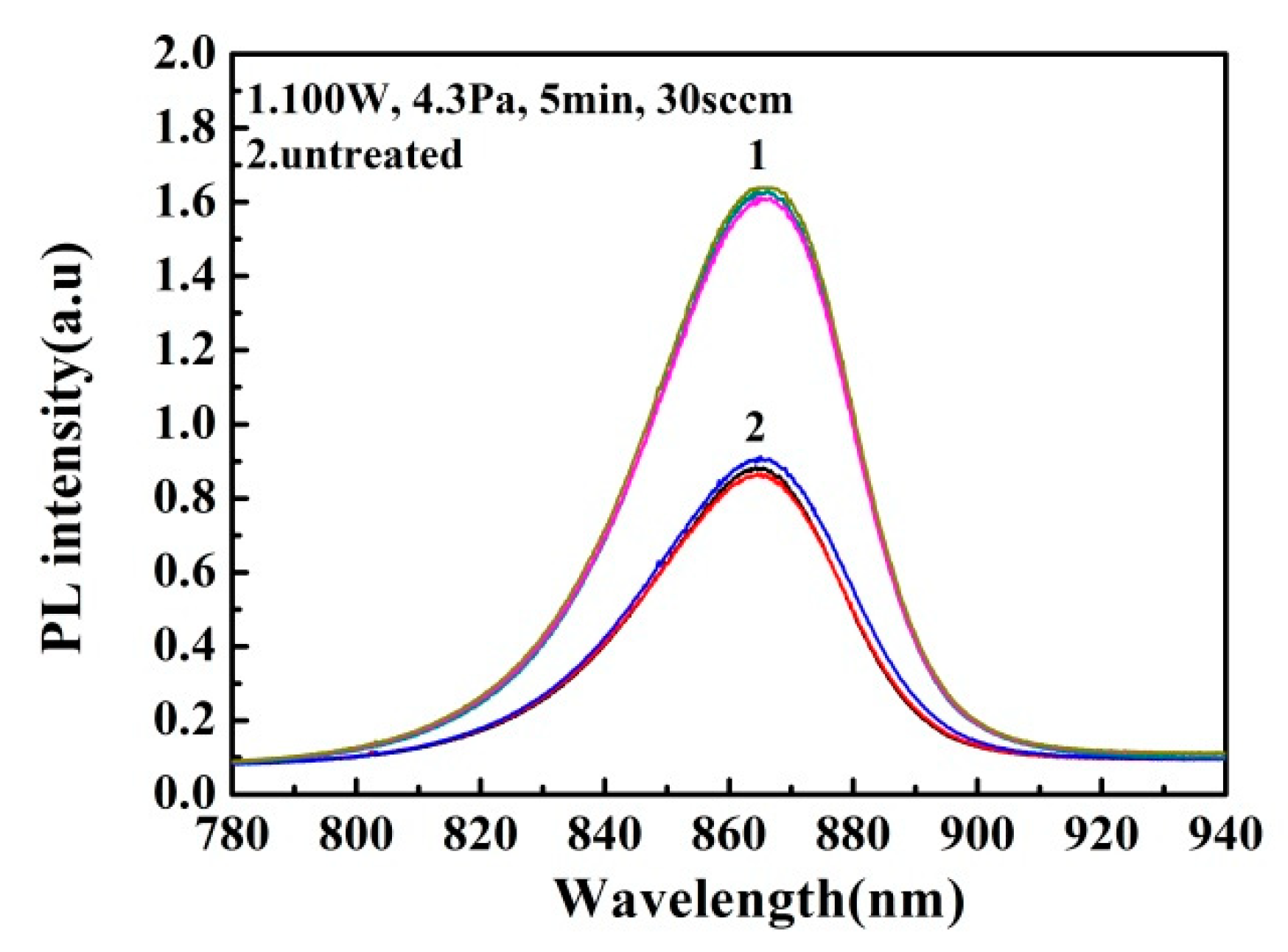
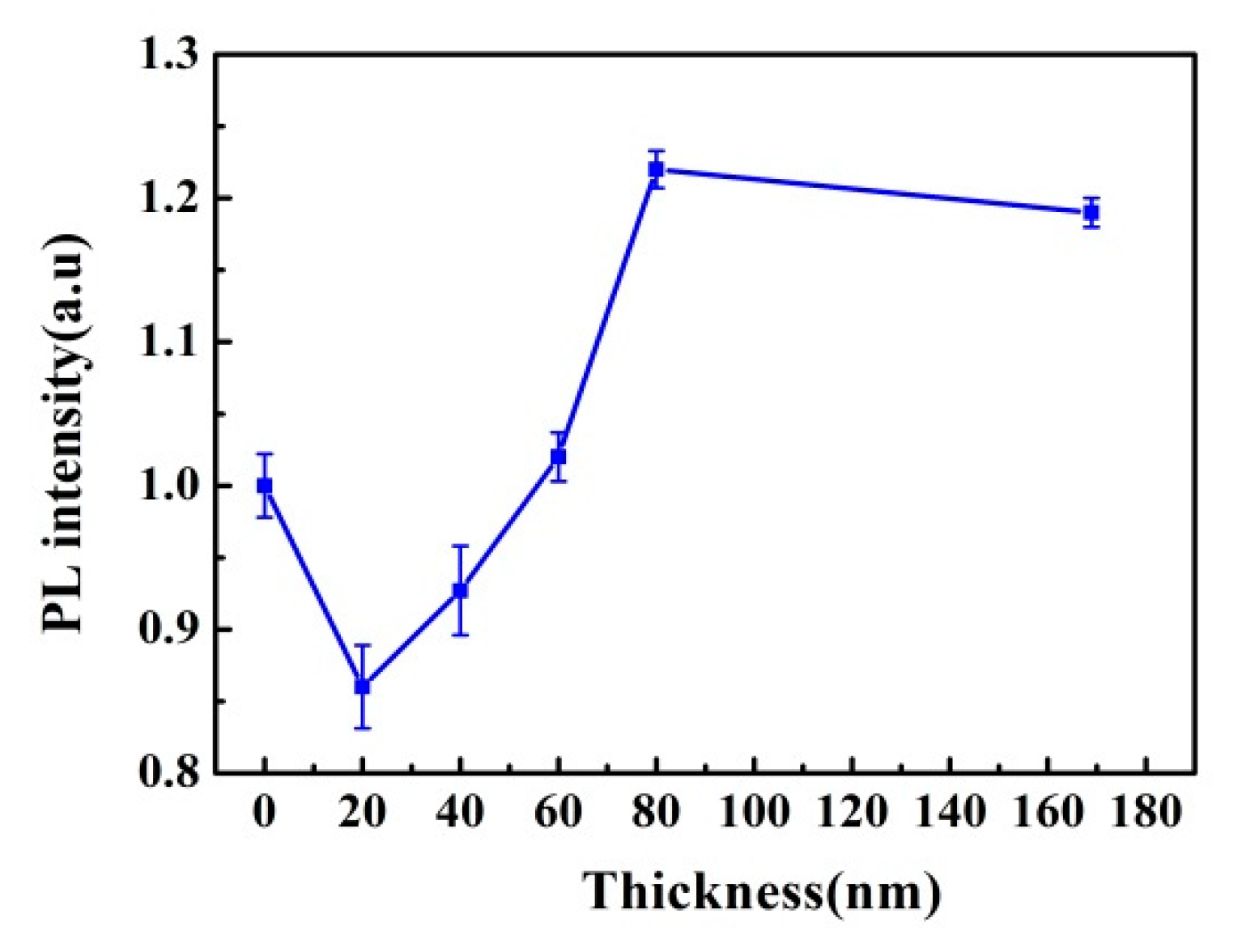
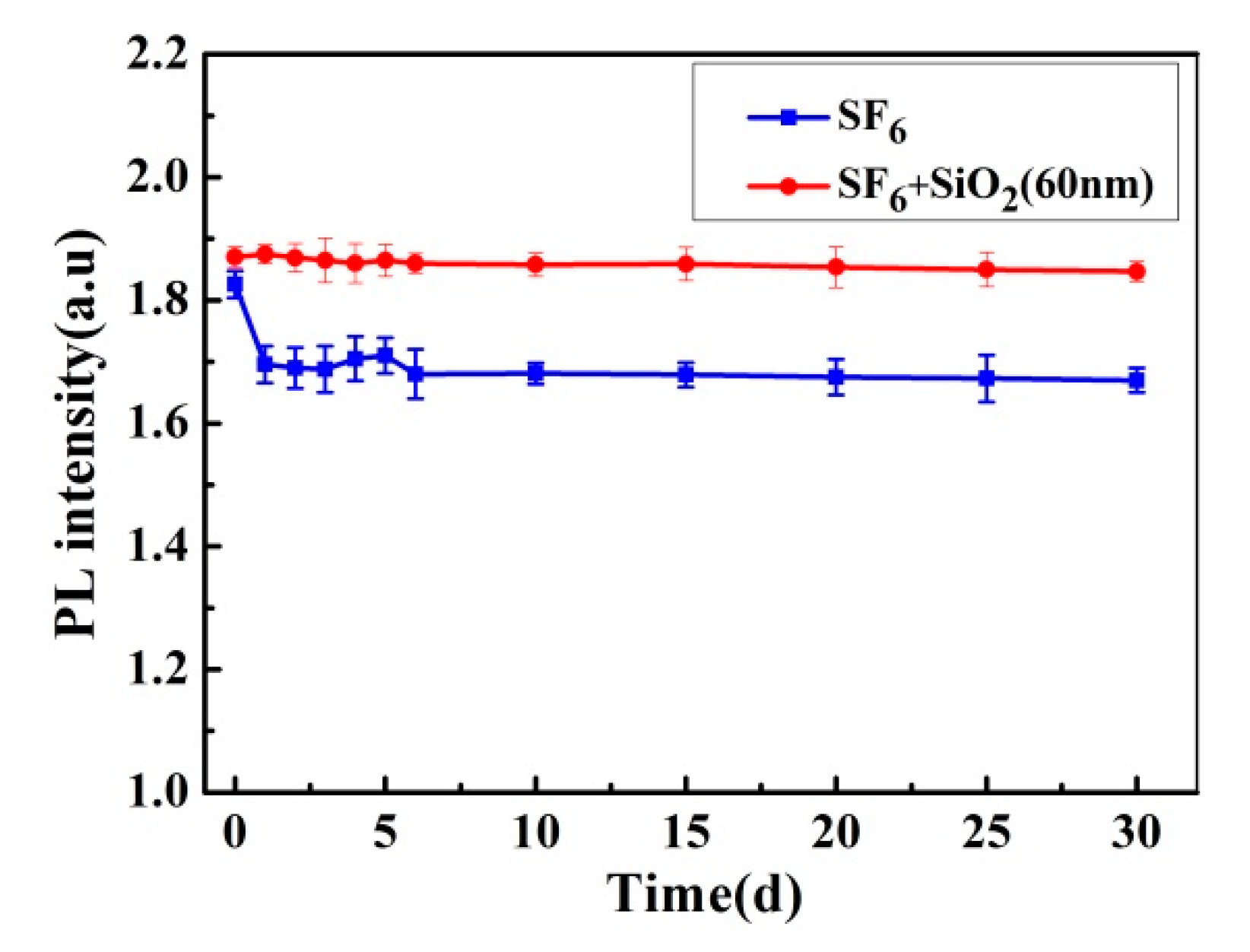
3.3. Plasma Treatment Time Effect
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lu, H.; Xu, J.; Liu, L.; Wang, L.; Lai, P.; Tang, W. Improved interfacial quality of GaAs metal-oxide-semiconductor device with NH3-plasma treated yittrium-oxynitride as interfacial passivation layer. Microelectron. Reliab. 2016, 56, 17–21. [Google Scholar] [CrossRef]
- Edmonds, M.; Kent, T.; Chagarov, E.; Sardashti, K.; Droopad, R.; Chang, M.; Kachian, J.; Park, J.H.; Kummel, A. Passivation of InGaAs (001)-(2 × 4) by self-limiting chemical vapor deposition of a silicon hydride control layer. J. Am. Chem. Soc. 2015, 137, 8526–8533. [Google Scholar] [CrossRef] [PubMed]
- Dalapati, G.K.; Tong, Y.; Loh, W.Y.; Mun, H.K.; Cho, B.J. Impact of interfacial layer control using Gd2O3 in HfO2 gate dielectric on GaAs. Appl. Phys. Lett. 2007, 90, 1897–1904. [Google Scholar] [CrossRef]
- Lebedev, M.V.; Kunitsyna, E.V.; Calvet, W.; Mayer, T.; Jaegermann, W. Sulfur passivation of GaSb (100) surfaces: Comparison of aqueous and alcoholic sulfide solutions using synchrotron radiation photoemission spectroscopy. J. Phys. Chem. C 2013, 117, 15996–16004. [Google Scholar] [CrossRef]
- Ghita, R.; Negrila, C.; Cotirlan, C.; Logofatu, C. On the passivation of GaAs surface by sulfide compounds. Dig. J. Nanomater. Bios. 2013, 8, 1335–1344. [Google Scholar]
- Lucero, A.T.; Byun, Y.-C.; Qin, X.; Cheng, L.; Kim, H.; Wallace, R.M.; Kim, J. Formation of a ZnO/ZnS interface passivation layer on (NH4)2S treated In0.53Ga0.47As: Electrical and in-situ X-ray photoelectron spectroscopy characterization. J. Appl. Phys. 2016, 55, 08PC02. [Google Scholar] [CrossRef]
- Kim, S.-H.; Kim, G.-S.; Kim, J.-K.; Park, J.-H.; Shin, C.; Choi, C.; Yu, H.-Y. Fermi-level unpinning using a Ge-passivated metal-interlayer-semiconductor structure for non-alloyed ohmic contact of high-electron-mobility transistors. IEEE Electron Device Lett. 2015, 36, 884–886. [Google Scholar] [CrossRef]
- Lambert, R.W.; Ayling, T.; Hendry, A.F.; Carson, J.M.; Barrow, D.A.; McHendry, S.; Scott, C.J.; McKee, A.; Meredith, W. Facet-passivation processes for the improvement of Al-containing semiconductor laser diodes. J. Lightw. Technol. 2006, 24, 956–961. [Google Scholar] [CrossRef]
- Iida, M.; Kaibe, H.T.; Okumura, T. Low-temperature fluorination of GaAs surface by CF4 plasma. J. Appl. Phys. 1991, 30, 1581–1584. [Google Scholar] [CrossRef]
- Taniguchi, M.; Murakawa, T.; Kajitani, Y. A novel passivation technique of GaAs power MESFETs. Appl. Surf. Sci. 1992, 56, 827–831. [Google Scholar] [CrossRef]
- Kim, G.-S.; Kim, S.-H.; Kim, J.-K.; Shin, C.; Park, J.-H.; Saraswat, K.C.; Cho, B.J.; Yu, H.-Y. Surface passivation of germanium using SF6 plasma to reduce source/drain contact resistance in germanium n-FET. IEEE Electron Device Lett. 2015, 36, 745–747. [Google Scholar]
- Kim, S.-H.; Kim, G.-S.; Kim, S.-W.; Kim, J.-K.; Choi, C.; Park, J.-H.; Choi, R.; Yu, H.-Y. Non-alloyed ohmic contacts on gaas using metal-interlayer-semiconductor structure with SF6 plasma treatment. IEEE Electron Device Lett. 2016, 37, 373–376. [Google Scholar] [CrossRef]
- Polydorou, E.; Zeniou, A.; Tsikritzis, D.; Soultati, A.; Sakellis, I.; Gardelis, S.; Papadopoulos, T.A.; Briscoe, J.; Palilis, L.C.; Kennou, S. Surface passivation effect by fluorine plasma treatment on ZnO for efficiency and lifetime improvement of inverted polymer solar cells. J. Mater. Chem. A 2016, 4, 11844–11858. [Google Scholar] [CrossRef] [Green Version]
- Dittrich, T.; John, S.; John, W. Characterization of the n-GaAs surface after CF4, SF6, CCl2F2, and CCl2F2:O2 plasma treatment by photoreflectance. Phys. Status Solidi 1991, 128, 435–446. [Google Scholar] [CrossRef]
- Budz, H.; Biesinger, M.; LaPierre, R. Passivation of GaAs by octadecanethiol self-assembled monolayers deposited from liquid and vapor phases. J. Vac. Sci. Technol. B Nanotechnol. Microelectron. Mater. Process. Meas. Phenom. 2009, 27, 637–648. [Google Scholar] [CrossRef]
- Li, Y.; Wee, A.; Huan, C.; Zheng, J. Ion-induced nitridation of GaAs (100) surface. Appl. Surf. Sci. 2001, 174, 275–282. [Google Scholar] [CrossRef]
- Ruppalt, L.B.; Cleveland, E.R.; Champlain, J.G.; Bennett, B.R.; Boos, J.B.; Prokes, S.M. Electronic properties of atomic-layer-deposited high-k dielectrics on GaAb (001) with hydrogen plasma pretreatment. J. Vac. Sci. Technol. B Nanotechnol. Microelectron. Mater. Process. Meas. Phenom. 2015, 33, 04E102-5. [Google Scholar]
- Varekamp, P.; Simpson, W.; Shuh, D.; Durbin, T.; Chakarian, V.; Yarmoff, J. Electronic structure of GaF3 films grown on GaAs via exposure to XeF2. Phys. Rev. B 1994, 50, 14267. [Google Scholar] [CrossRef]
- Marshall, G.M.; Lopinski, G.P.; Bensebaa, F.; Dubowski, J.J. Surface dipole layer potential induced IR absorption enhancement inn-alkanethiol sams on GaAs (001). Langmuir 2009, 25, 13561–13568. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Dubowski, J.J. Solvent-mediated self-assembly of hexadecanethiol on GaAs (001). Appl. Surf. Sci. 2014, 299, 66–72. [Google Scholar] [CrossRef]
- Liu, L.N.; Choi, H.W.; Xu, J.P.; Lai, P.T. Passivation of oxide traps and interface states in GaAs metal-oxide-semiconductor capacitor by LaTaON passivation layer and fluorine incorporation. Appl. Phys. Lett. 2015, 107, 213501. [Google Scholar] [CrossRef]
| Parameter | Value |
|---|---|
| SF6 Gas purity | 99.999% |
| Background vacuum | 2.8 × 10−4 Pa |
| RF power | 10–180 W |
| Chamber pressure | 0.3–8.3 Pa |
| Treatment time | 1–60 min |
| SF6/N2 gas flow rate | 18/6 sccm |
| Sample temperature | 250 °C |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Gao, X.; Zhang, X.; Qiao, Z.; Zhang, J.; Zhou, L.; Bo, B. Effects on the Surface and Luminescence Properties of GaAs by SF6 Plasma Passivation. Crystals 2018, 8, 339. https://doi.org/10.3390/cryst8090339
Xu Y, Gao X, Zhang X, Qiao Z, Zhang J, Zhou L, Bo B. Effects on the Surface and Luminescence Properties of GaAs by SF6 Plasma Passivation. Crystals. 2018; 8(9):339. https://doi.org/10.3390/cryst8090339
Chicago/Turabian StyleXu, Yumeng, Xin Gao, Xiaolei Zhang, Zhongliang Qiao, Jing Zhang, Lu Zhou, and Baoxue Bo. 2018. "Effects on the Surface and Luminescence Properties of GaAs by SF6 Plasma Passivation" Crystals 8, no. 9: 339. https://doi.org/10.3390/cryst8090339
APA StyleXu, Y., Gao, X., Zhang, X., Qiao, Z., Zhang, J., Zhou, L., & Bo, B. (2018). Effects on the Surface and Luminescence Properties of GaAs by SF6 Plasma Passivation. Crystals, 8(9), 339. https://doi.org/10.3390/cryst8090339




